Nickel Extraction from Olivine: Effect of Carbonation Pre-Treatment †
Abstract
:1. Introduction
2. Experimental Section
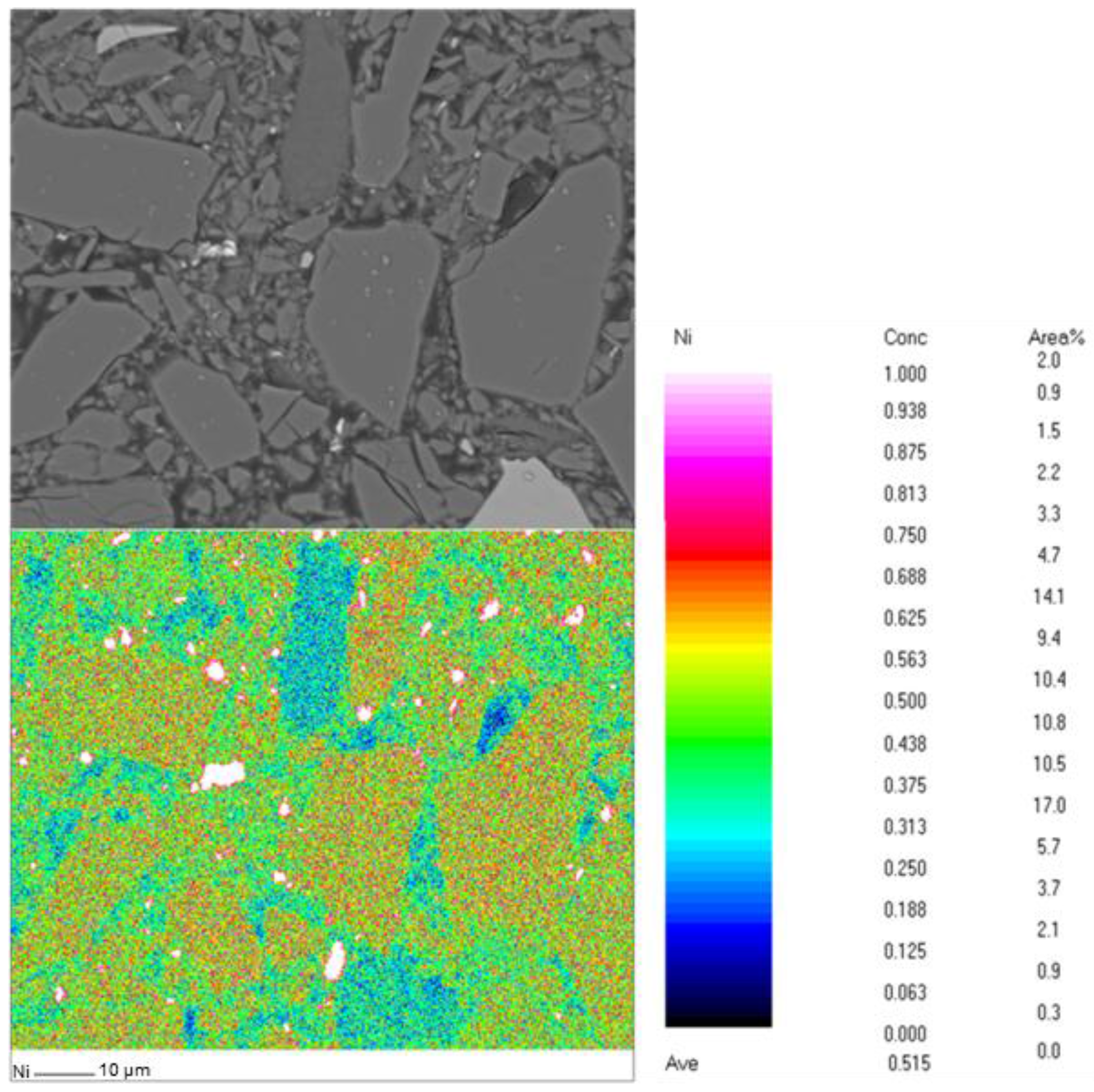
2.1. Olivine Characterization
| Element | Mass % |
|---|---|
| Mg | 27.2 |
| Si | 20.7 |
| Fe | 3.7 |
| Ni | 0.27 |
| Cr | 0.24 |
| Al | 0.17 |
| Ca | 0.17 |
| Mn | 0.09 |
| Co | 0.02 |

2.2. Carbonation
| Parameters | Tested Values |
|---|---|
| Temperature | 150–185–200 °C |
| Solids loading | 50–100–200 g/800 mL |
| Residence time | 4–24–48–72 h |
| NaCl concentration | 0–1–2 M |
| NaHCO3 concentration | 0–0.64–2.5 M |
2.3. Leaching
2.4. Analytical Methods
3. Results and Discussion
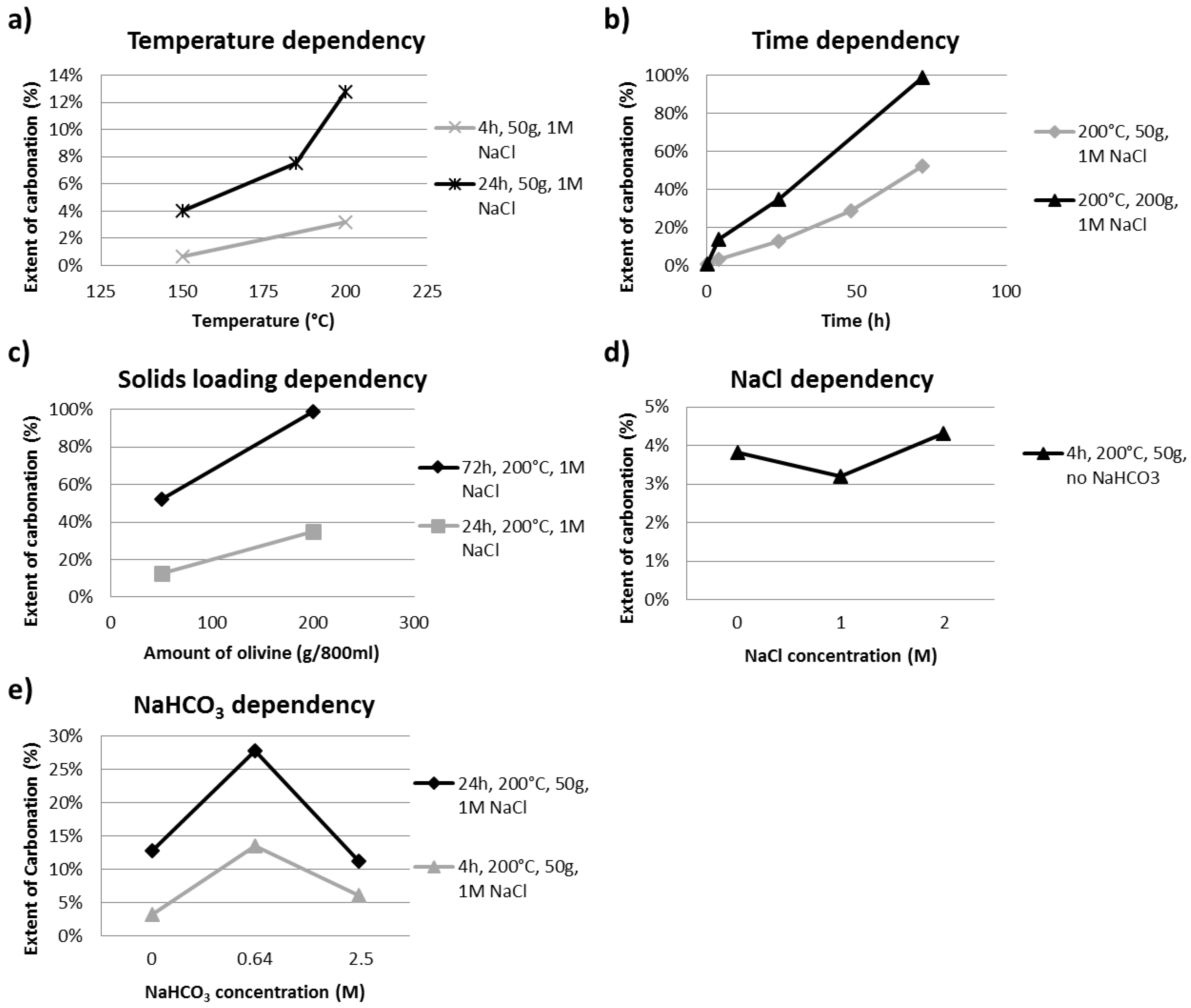
3.1. Influence of Carbonation Parameters
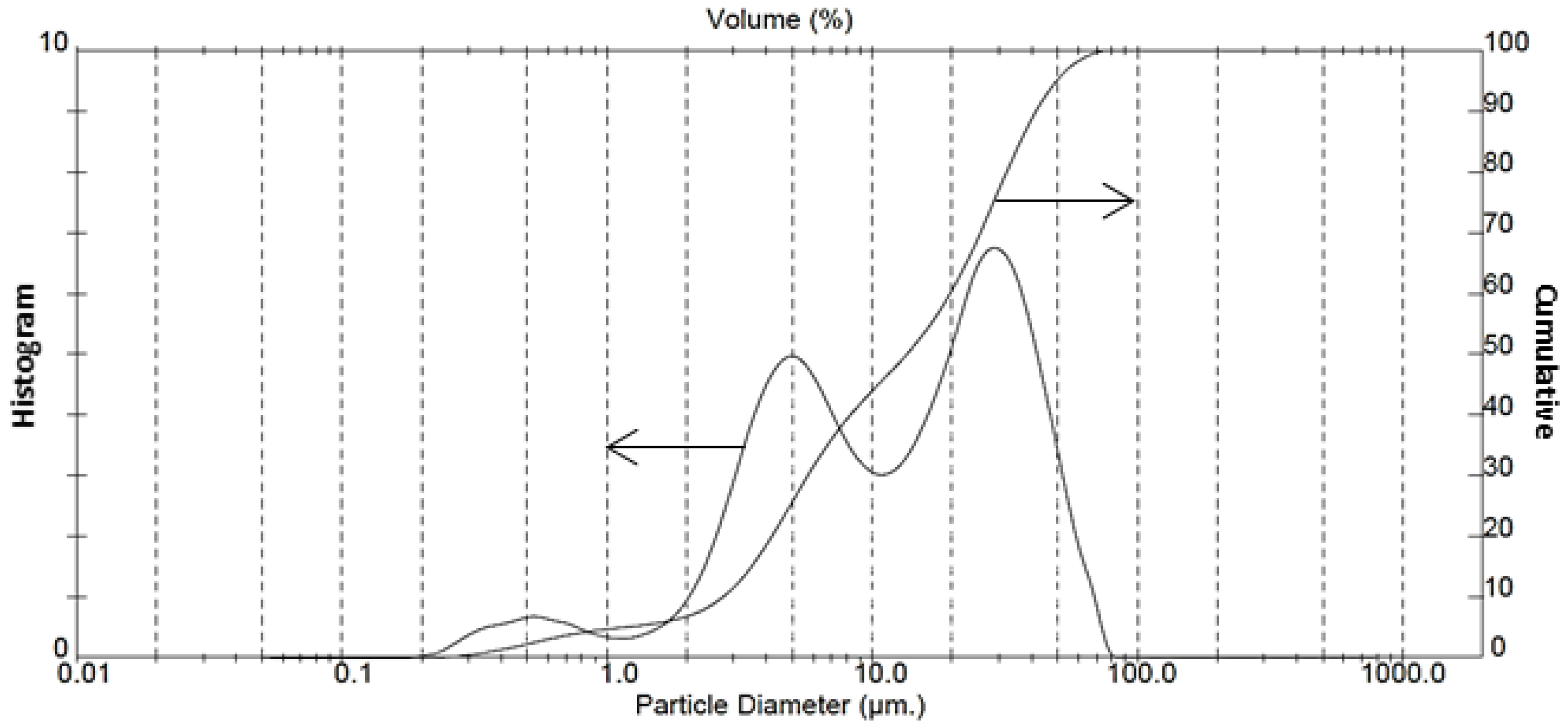
3.2. Characterization of Fully-Carbonated Olivine
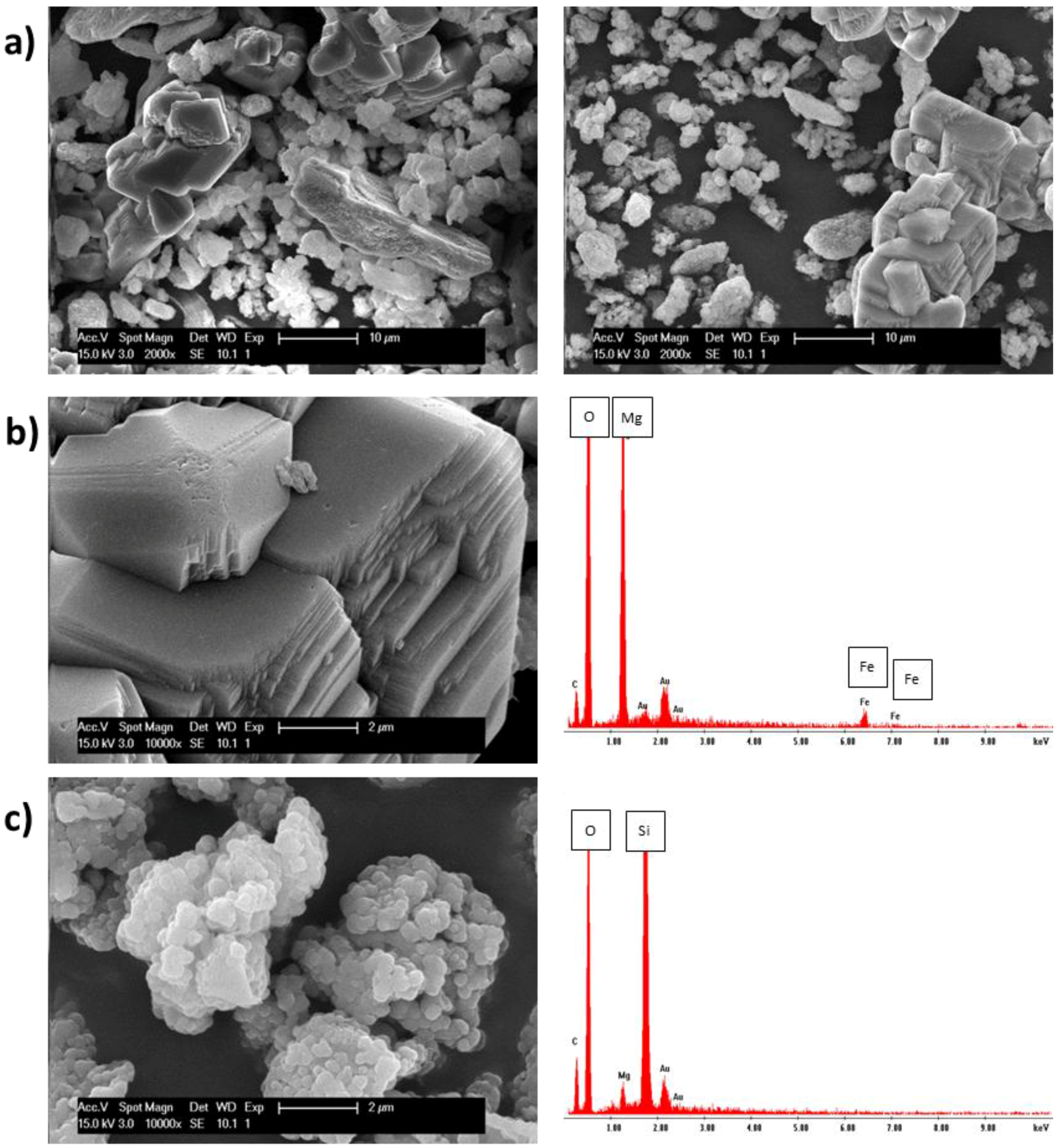
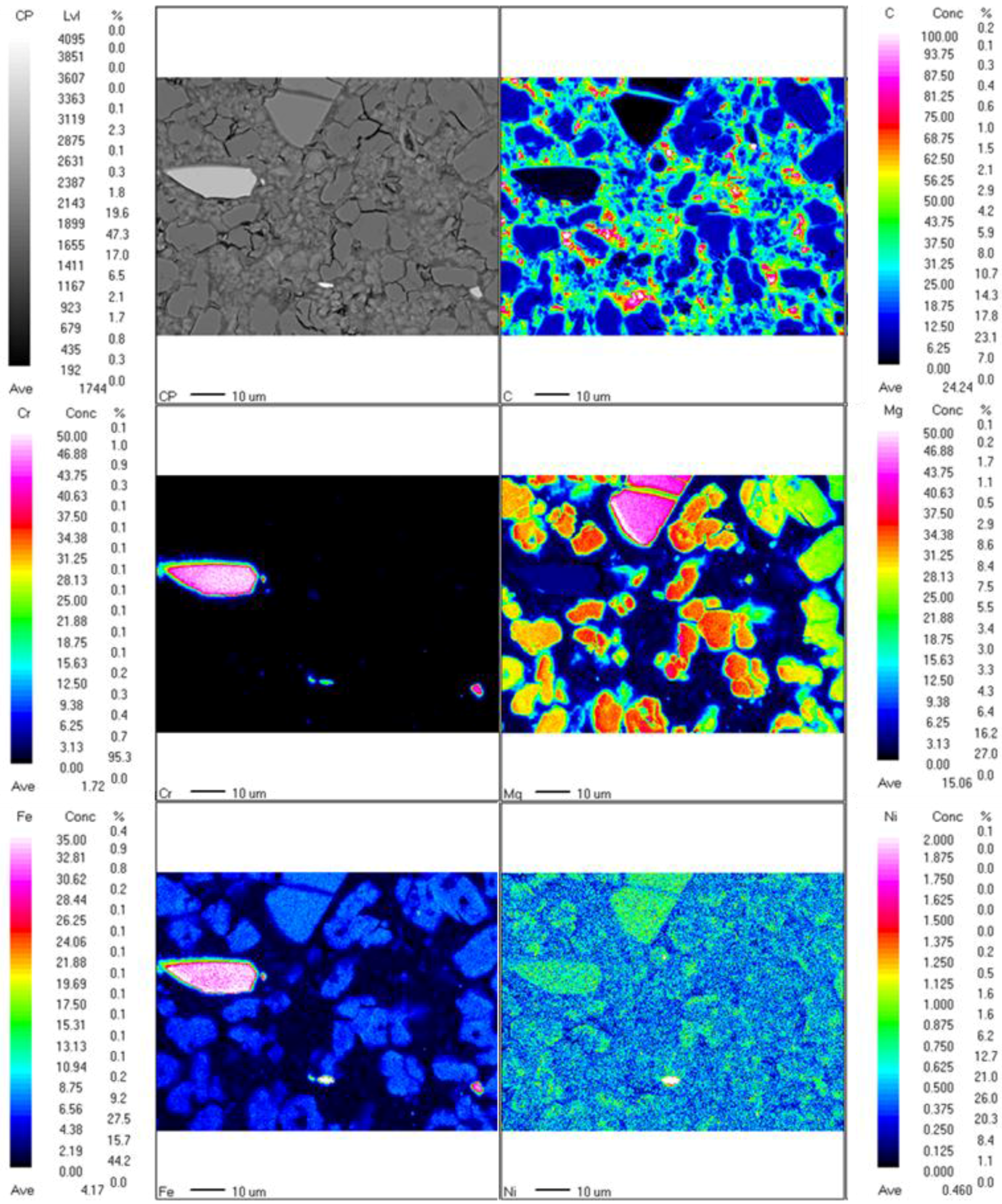
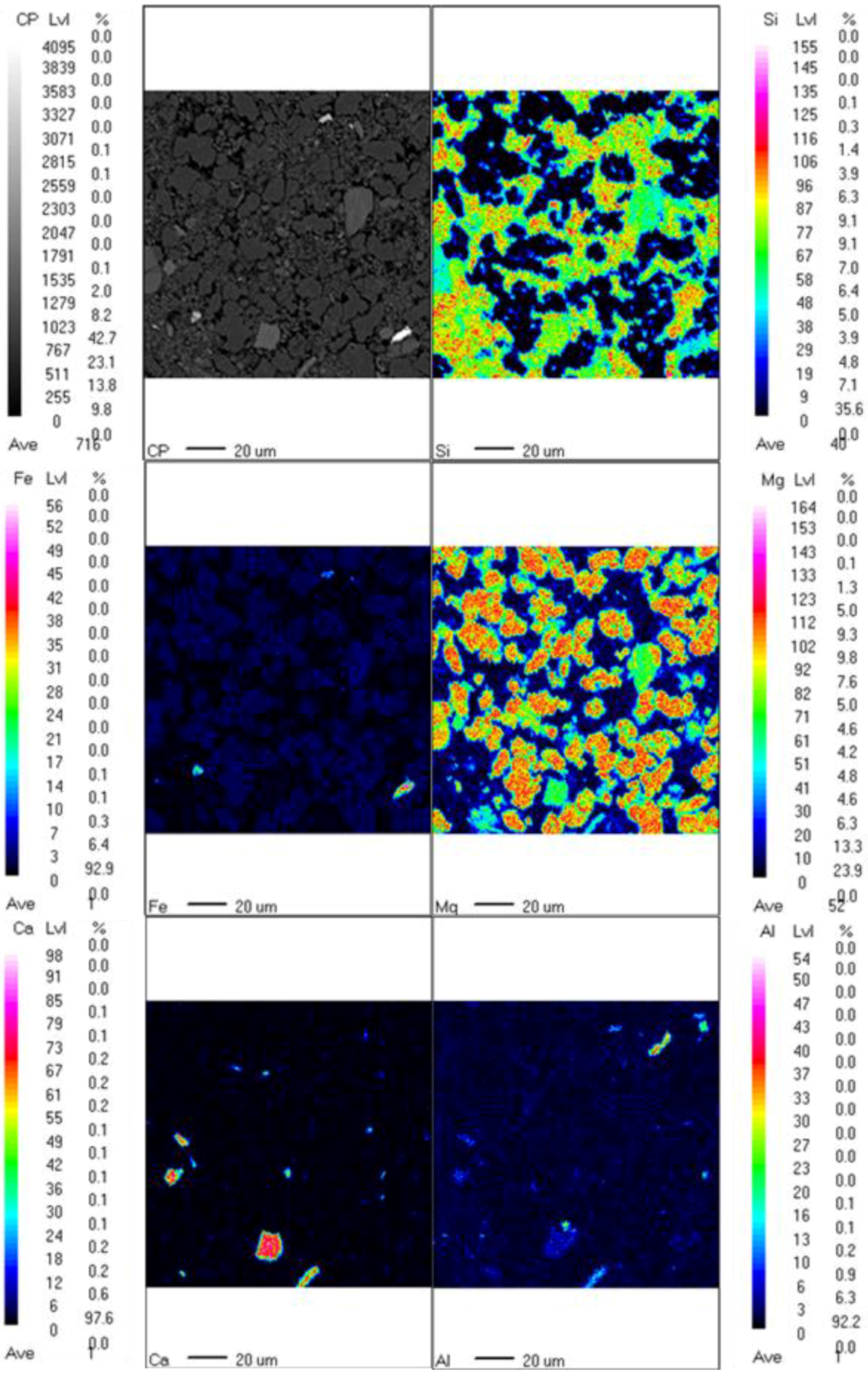
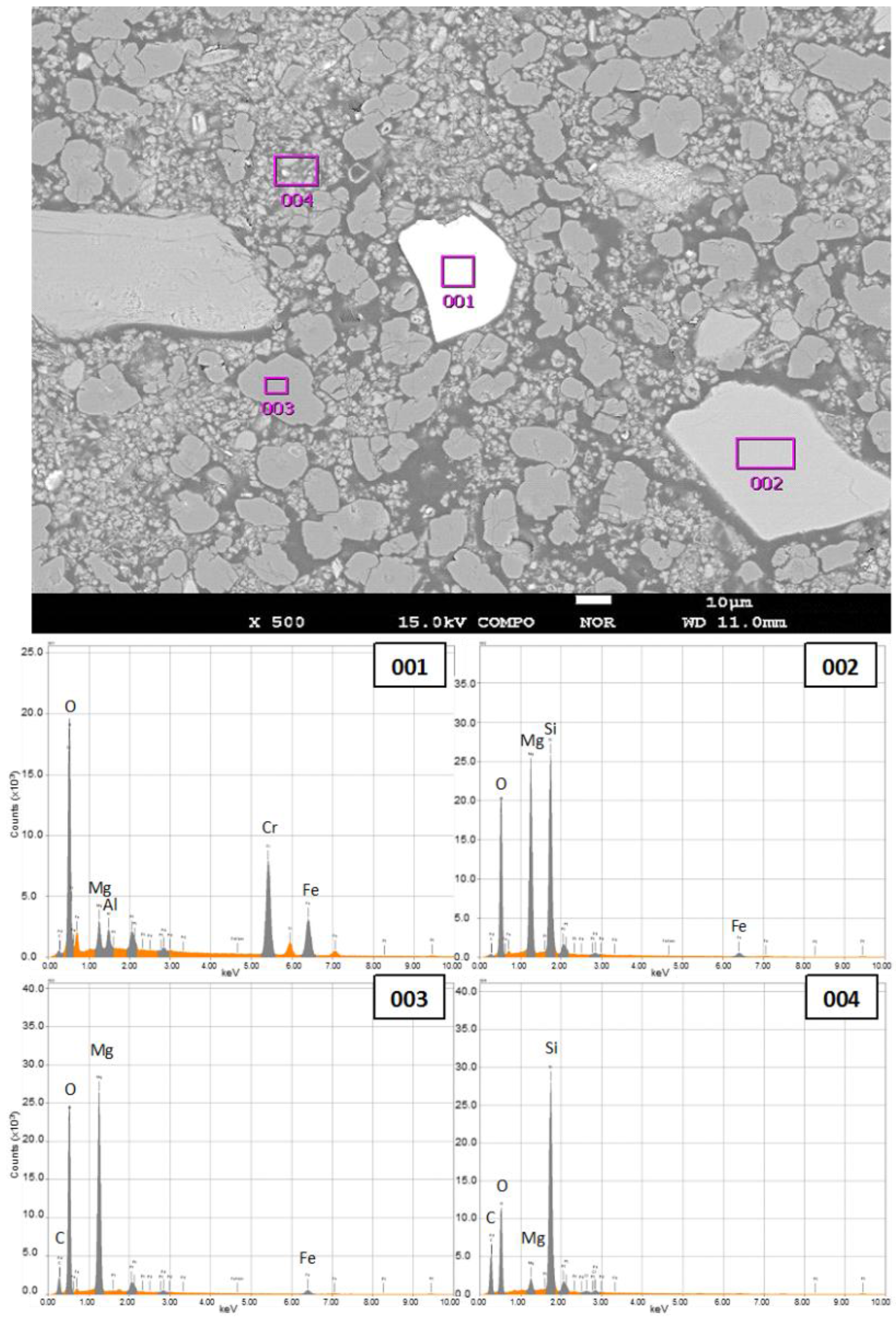
| Chemical Formula | 001 | 002 | 003 | 004 |
|---|---|---|---|---|
| C | 0.5 | 1.2 | 19.8 | 37.7 |
| MgO | 4.8 | 35.3 | 68.6 | 2.6 |
| SiO2 | 0 | 57.6 | 0 | 59.8 |
| Al2O3 | 3.3 | 0 | 0 | 0 |
| Cr2O3 | 55.5 | 5.8 | 0 | 0 |
| Fe2O3 | 35.9 | 0 | 11.6 | 0 |
3.3. Acid Leaching of Fresh and Carbonated Olivine
3.3.1. Inorganic Acids
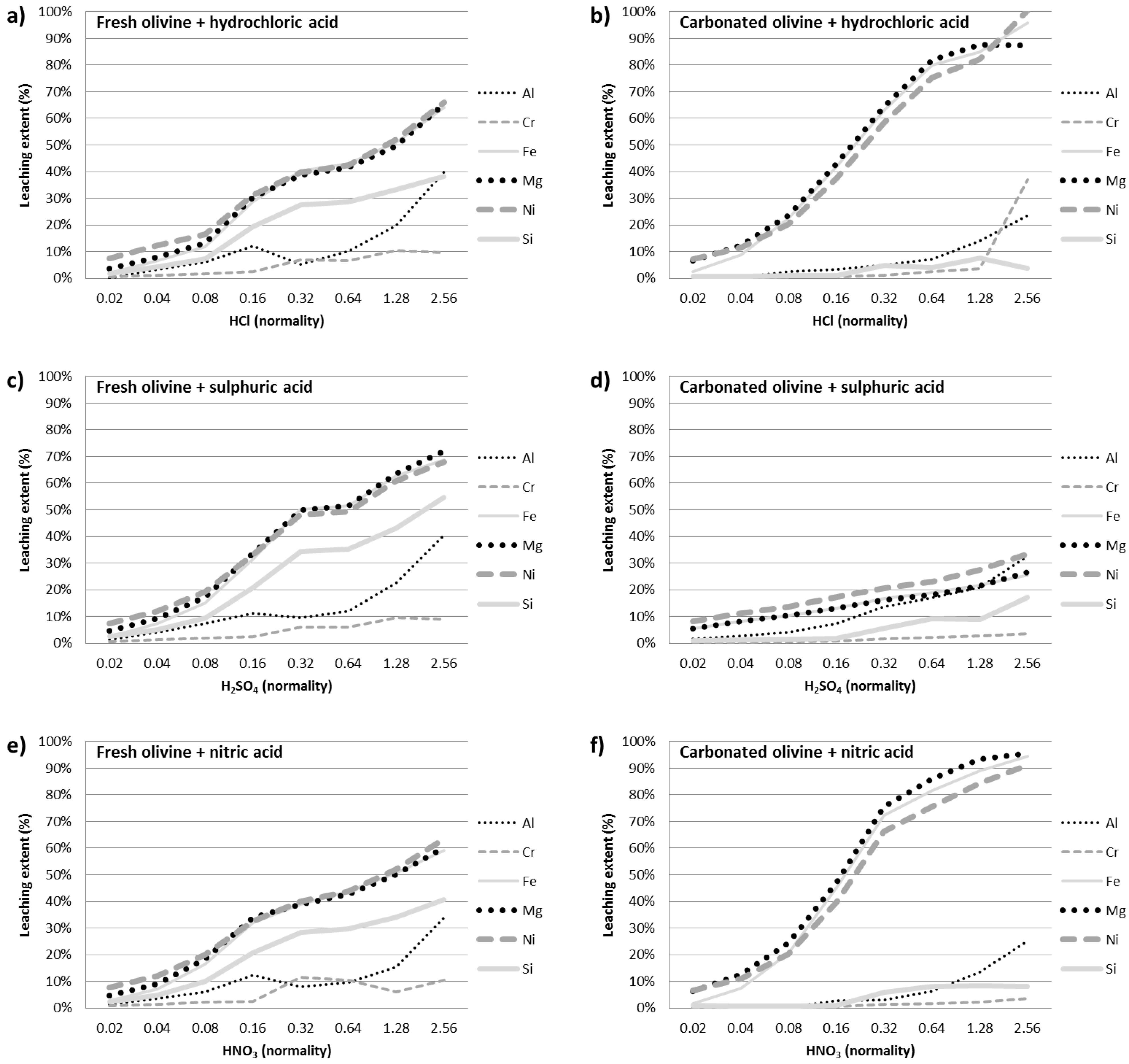
| Leaching Ratio φ (Carbonated/Fresh) | HCl | HNO3 | H2SO4 | Leaching Ratio φ (Carbonated/Fresh) | Lactic | Citric | Formic |
|---|---|---|---|---|---|---|---|
| 0.02 N | 0.99 | 0.85 | 1.11 | 0.25 N | 1.01 | 1.15 | 0.46 |
| 0.04 N | 0.93 | 0.92 | 0.92 | 0.5 N | 1.18 | 1.25 | 0.46 |
| 0.08 N | 1.23 | 1.01 | 0.72 | 1.0 N | 1.38 | 1.16 | 0.47 |
| 0.16 N | 1.20 | 1.22 | 0.52 | 2.0 N | 1.48 | 1.00 | 0.57 |
| 0.32 N | 1.47 | 1.65 | 0.42 | 4.0 N * | 1.35 | - | 0.68 |
| 0.64 N | 1.77 | 1.72 | 0.47 | ||||
| 1.28 N | 1.58 | 1.62 | 0.45 | ||||
| 2.56 N | 1.52 | 1.43 | 0.49 |
3.3.2. Organic Acids

3.3.3. Sulfuric Acid Leaching Investigation
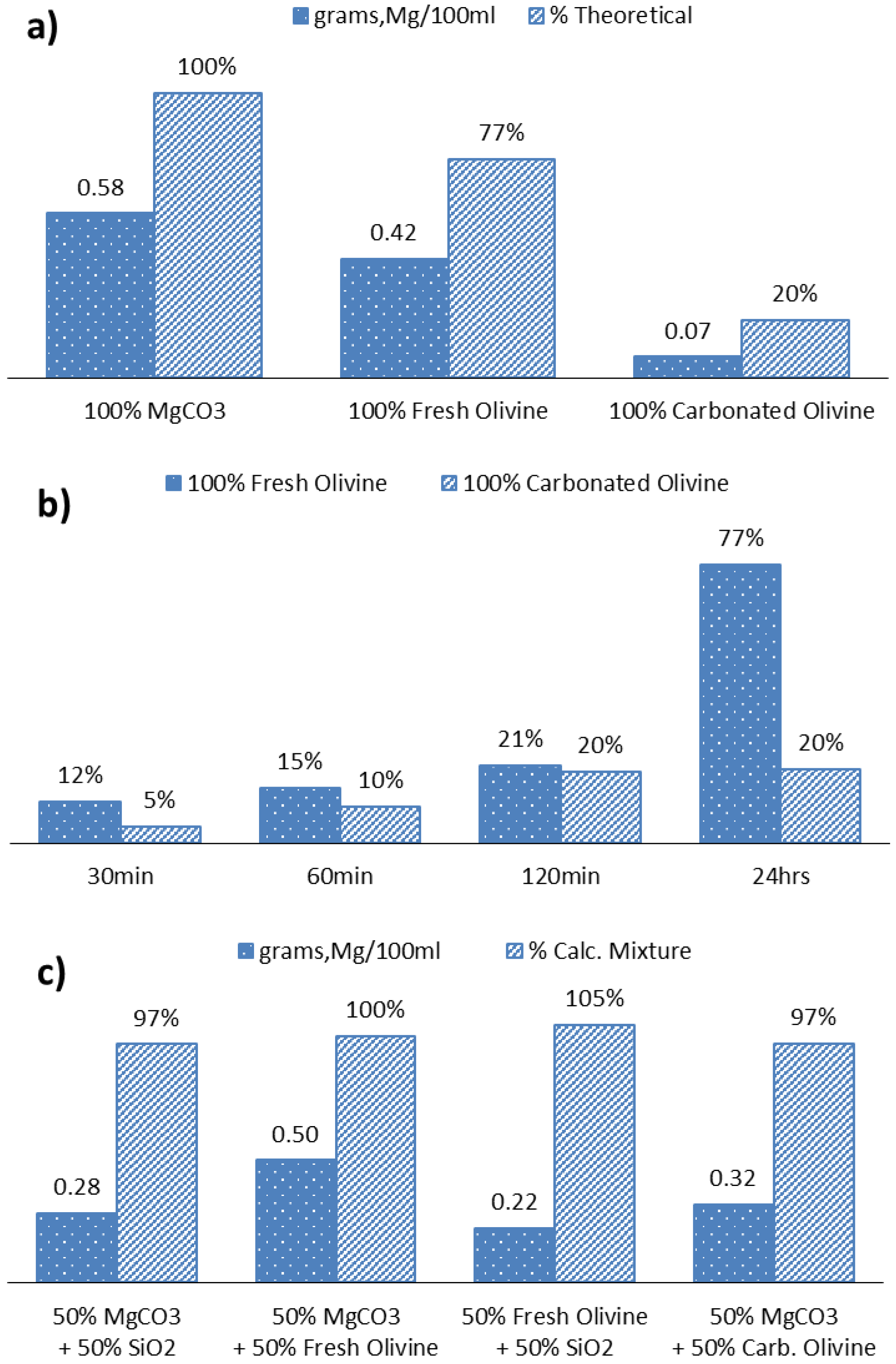
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goldie, R.J. The shortage of nickel. Prospectors & Developers Association of Canada 2004 Convention. Available online: http://www.webcitation.org/query?url=http%3A%2F%2Fwww.pdac.ca%2Fdocs%2Fdefault-source%2Fpublications---papers-presentations---conventions%2Ftechprgm-goldie.pdf%3Fsfvrsn%3D4&date=2015-07-26 (accessed on 26 July 2015).
- Kerfoot, D.G.E. Nickel. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 27, pp. 37–101. [Google Scholar]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review—Part I: sulphuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Zhai, Y.-C.; Mu, W.-N.; Liu, Y.; Xu, Q. A green process for recovering nickel from nickeliferous laterite ores. Trans. Nonferrous Metals Soc. China 2010, 20, s65–s70. [Google Scholar] [CrossRef]
- Chen, N.; Cao, Z.-F.; Zhong, H.; Fan, F.; Qiu, P.; Wang, M.-M. A novel approach for recovery of nickel and iron from nickel laterite ore. Metall. Res. Technol. 2015, 112, 306. [Google Scholar] [CrossRef]
- Harben, P.W.; Smith, C., Jr. Olivine. In Industrial Minerals & Rocks: Commodities, Markets, and Uses, 7th ed.; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; Society for Mining, Metallurgy and Exploration, Inc.: Littleton, CO, USA, 2006; pp. 679–684. [Google Scholar]
- Smyth, J.R.; Frost, D.J.; Nestola, F.; Holl, C.M.; Bromiley, G. Olivine hydration in the deep upper mantle: Effects of temperature and silica activity. Geophys. Res. Lett. 2006. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Dimaki, D. Heap Leaching of Poor Nickel Laterites by Sulphuric Acid at Ambient Temperature. In Hydrometallurgy ’94; Springer: Dordrecht, The Netherlands, 1994; pp. 193–208. [Google Scholar]
- Bodor, M.; Santos, R.M.; van Gerven, T.; Vlad, M. Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation—A review. Cent. Eur. J. Eng. 2013, 3, 566–584. [Google Scholar] [CrossRef]
- Sanna, A.; Hall, M.R.; Maroto-Valer, M. Post-processing pathways in carbon capture and storage by mineral carbonation (CCSM) towards the introduction of carbon neutral materials. Energy Environ. Sci. 2012, 5, 7781–7796. [Google Scholar] [CrossRef]
- Ahmad, W. Nickel laterites—Fundamentals of chemistry, mineralogy, weathering processes, formation, and exploration. VALE Inco-VITSL. Available online: http://www.scribd.com/doc/83755762/Nickel-Laterites-Vale-Inco-Oct-2008 (accessed on 7 July 2012).
- Thompson, J.F.H.; Barnes, S.J.; Dyke, J.M. The distribution of nickel and iron between olivine and magmatic sulfides in some natural assemblages. Can. Miner. 1984, 22, 55–66. [Google Scholar]
- Santos, R.M.; Verbeeck, W.; Knops, P.; Rijnsburger, K.; Pontikes, Y.; van Gerven, T. Integrated mineral carbonation reactor technology for sustainable carbon dioxide sequestration: “CO2 energy reactor”. Energy Proc. 2013, 37, 5884–5891. [Google Scholar] [CrossRef]
- Gadikota, G.; Matter, J.; Kelemen, P.; Park, A.-H.A. Chemical and morphological changes during olivine carbonation for CO2 storage in the presence of NaCl and NaHCO3. Phys. Chem. Chem. Phys. 2014, 16, 4679–4693. [Google Scholar] [CrossRef] [PubMed]
- Gadikota, G.; Swanson, E.J.; Zhao, H.; Park, A.-H.A. Experimental design and data analysis for accurate estimation of reaction kinetics and conversion for carbon mineralization. Ind. Eng. Chem. Res. 2014, 53, 6664–6676. [Google Scholar] [CrossRef]
- Julcour, C.; Bourgeois, F.; Bonfils, B.; Benhamed, I.; Guyot, F.; Bodénan, F.; Petiot, C.; Gaucher, É.C. Development of an attrition-leaching hybrid process for direct aqueous mineral carbonation. Chem. Eng. J. 2015, 262, 716–726. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Petallidou, K.C.; Vasiliades, M.A.; Delimitis, A.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. Carbon dioxide storage in olivine basalts: Effect of ball milling process. Powder Technol. 2015, 273, 220–229. [Google Scholar] [CrossRef]
- Oxtoby, D.W.; Freeman, W.A.; Block, T.F. Chemistry: Science of Change, 4th ed.; Brooks/Cole: Belmont, NY, USA, 2002. [Google Scholar]
- Béarat, H.; McKelvy, M.J.; Chizmeshya, A.V.G.; Gormley, D.; Nunez, R.; Carpenter, R.W.; Squires, K.; Wolf, G.H. Carbon sequestration via aqueous olivine mineral carbonation: Role of passivating layer formation. Environ. Sci. Technol. 2006, 40, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; O’Connor, W.K.; Gerdemann, S.J. Chemistry of aqueous mineral carbonation for carbon sequestration and explanation of experimental results. Environ. Prog. 2006, 25, 161–166. [Google Scholar] [CrossRef]
- De Windt, L.; Devillers, P. Modeling the degradation of Portland cement pastes by biogenic organic acids. Cem. Concr. Res. 2010, 40, 1165–1174. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Santos, R.M.; van Audenaerde, A.; Monballiu, A.; van Gerven, T.; Meesschaert, B. Chemoorganotrophic Bioleaching of Olivine for Nickel Recovery. Minerals 2014, 4, 553–564. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Rush, G.E.; Gerdemann, S.J.; Penner, L.R.; Nilsen, D.N. Aqueous mineral carbonation—Mineral availability, pretreatment, reaction parametrics, and process studies. National Energy Technology Laboratory, US DOE. Available online: http://www.webcitation.org/query?url=https%3A%2F%2Fwww.netl.doe.gov%2FFile%2520Library%2FResearch%2FCoal%2FNETLAlbanyAqueousMineralCarbonation.pdf&date=2015-07-26 (accessed on 26 July 2015).
- Larachi, F.; Daldoul, I.; Beaudoin, G. Fixation of CO2 by chrysotile in low-pressure dry and moist carbonation: Ex-situ and in-situ characterizations. Geochim. Cosmochim. Acta 2010, 74, 3051–3075. [Google Scholar] [CrossRef]
- Qafoku, O.; Kovarik, L.; Kukkadapu, R.K.; Ilton, E.S.; Arey, B.W.; Tucek, J.; Felmy, A.R. Fayalite dissolution and siderite formation in water-saturated supercritical CO2. Chem. Geol. 2012, 332–333, 124–135. [Google Scholar] [CrossRef]
- Lieftink, D.J.; Geus, J.W. The preparation of silica from the olivine process and its possible use as a catalyst support. J. Geochem. Explor. 1998, 62, 1–3. [Google Scholar] [CrossRef]
- Lazaro, A.; Brouwers, H.J.H.; Quercia, G.; Geus, J.W. The properties of amorphous nano-silica synthesized by the dissolution of olivine. Chem. Eng. J. 2012, 211–212, 112–121. [Google Scholar] [CrossRef]
- Wiedenmann, D.; Zaitsev, A.N.; Britvin, S.N.; Krivovichev, S.V.; Keller, J. Alumoåkermanite, (Ca,Na)2(Al,Mg,Fe2+)(Si2O7), a new mineral from the active carbonatite-nephelinite-phonolite volcano Oldoinyo Lengai, northern Tanzania. Miner. Mag. 2009, 73, 373–384. [Google Scholar] [CrossRef]
- Sanemasa, I.; Yoshida, M.; Ozawa, T. The dissolution of olivine in aqueous solution of inorganic acids. Bull. Chem. Soc. Jpn. 1972, 45, 1741–1746. [Google Scholar] [CrossRef]
- Terry, B. The acid decomposition of silicate minerals part I. Reactivities and modes of dissolution of silicates. Hydrometallurgy 1983, 10, 135–150. [Google Scholar] [CrossRef]
- Tzeferis, P.G.; Agatzini-Leonardou, S. Leaching of nickel and iron from Greek non-sulphide nickeliferous ores by organic acids. Hydrometallurgy 1994, 36, 345–360. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.M.; Van Audenaerde, A.; Chiang, Y.W.; Iacobescu, R.I.; Knops, P.; Van Gerven, T. Nickel Extraction from Olivine: Effect of Carbonation Pre-Treatment. Metals 2015, 5, 1620-1644. https://doi.org/10.3390/met5031620
Santos RM, Van Audenaerde A, Chiang YW, Iacobescu RI, Knops P, Van Gerven T. Nickel Extraction from Olivine: Effect of Carbonation Pre-Treatment. Metals. 2015; 5(3):1620-1644. https://doi.org/10.3390/met5031620
Chicago/Turabian StyleSantos, Rafael M., Aldo Van Audenaerde, Yi Wai Chiang, Remus I. Iacobescu, Pol Knops, and Tom Van Gerven. 2015. "Nickel Extraction from Olivine: Effect of Carbonation Pre-Treatment" Metals 5, no. 3: 1620-1644. https://doi.org/10.3390/met5031620
APA StyleSantos, R. M., Van Audenaerde, A., Chiang, Y. W., Iacobescu, R. I., Knops, P., & Van Gerven, T. (2015). Nickel Extraction from Olivine: Effect of Carbonation Pre-Treatment. Metals, 5(3), 1620-1644. https://doi.org/10.3390/met5031620









