Exotic Alloys Obtained by Condensation of Metallic Vapors
Abstract
:1. Introduction
2. Ohmic Heating Evaporation
Ground Based System


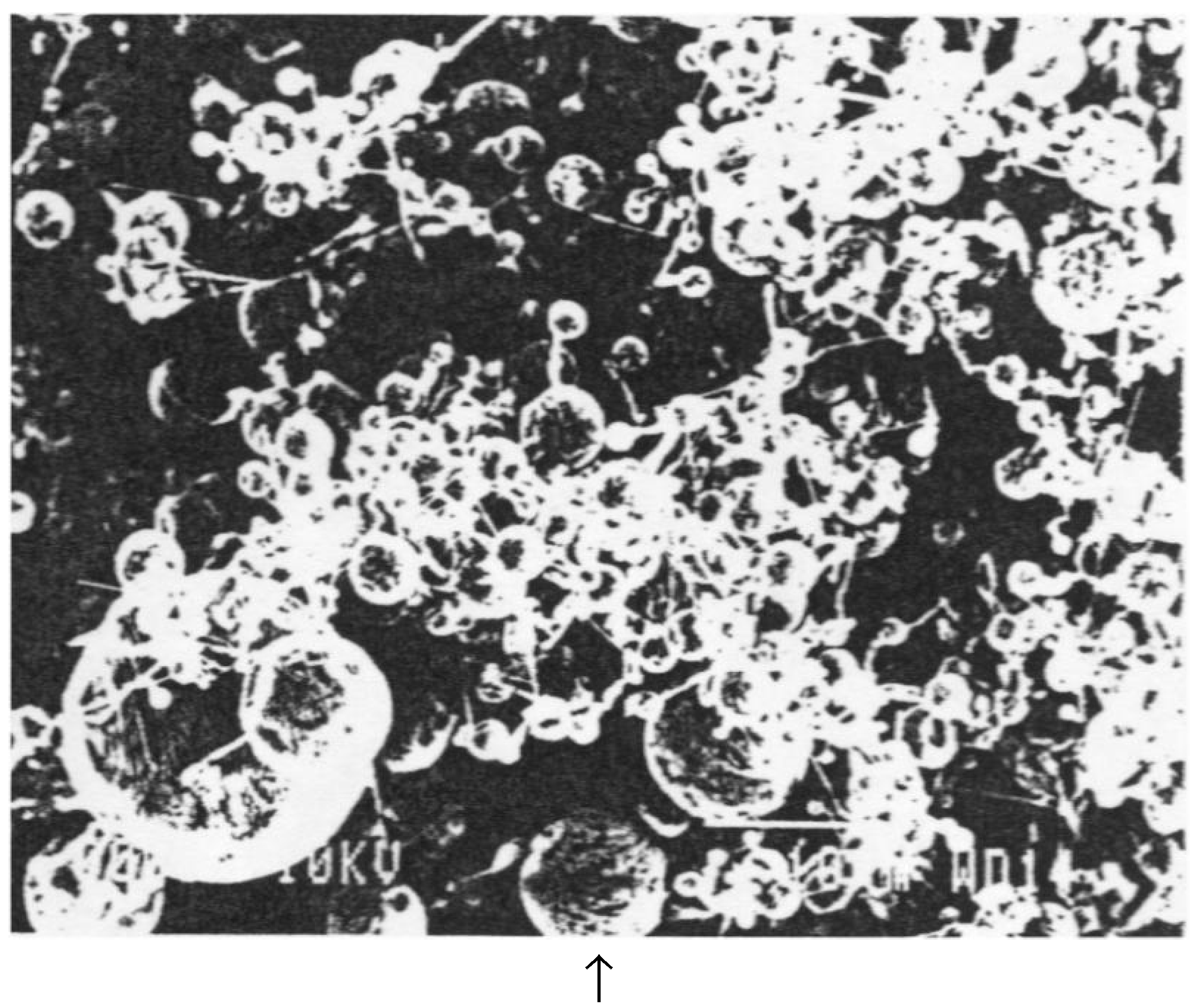

3. Evaporation by Laser Beam Abrasion
3.1. Laser Characteristics
| Parameters | Nominal values | Values used |
|---|---|---|
| Wavelength (nm) | 248 | 248 |
| Maximum energy per pulse (mJ) | 450 | 120 to 240 |
| Mean energy maximum (W) | 80 | 1 to 2.5 |
| Repetition rate (pps) | 200 | 10 |
| Length of pulse (ns) | 12 to 20 | 12 to 20 |
| Size of the beam (mm) | 8 − 12 × 25 | 8 − 12 × 25 |
| Divergence of the beam (mrad) | 1 × 3 | 1 × 3 |


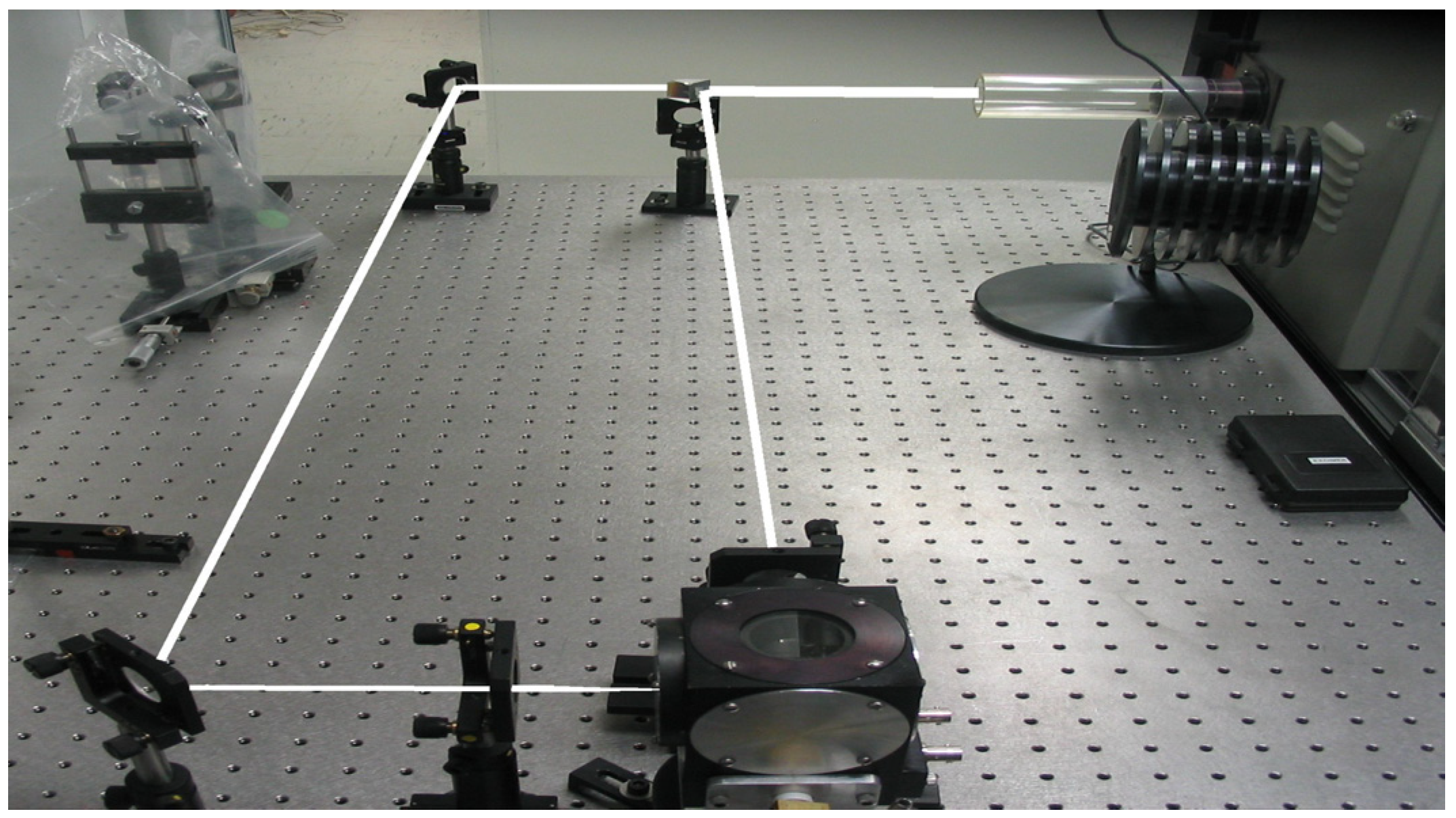
3.2. Alloy of Al and Tungsten

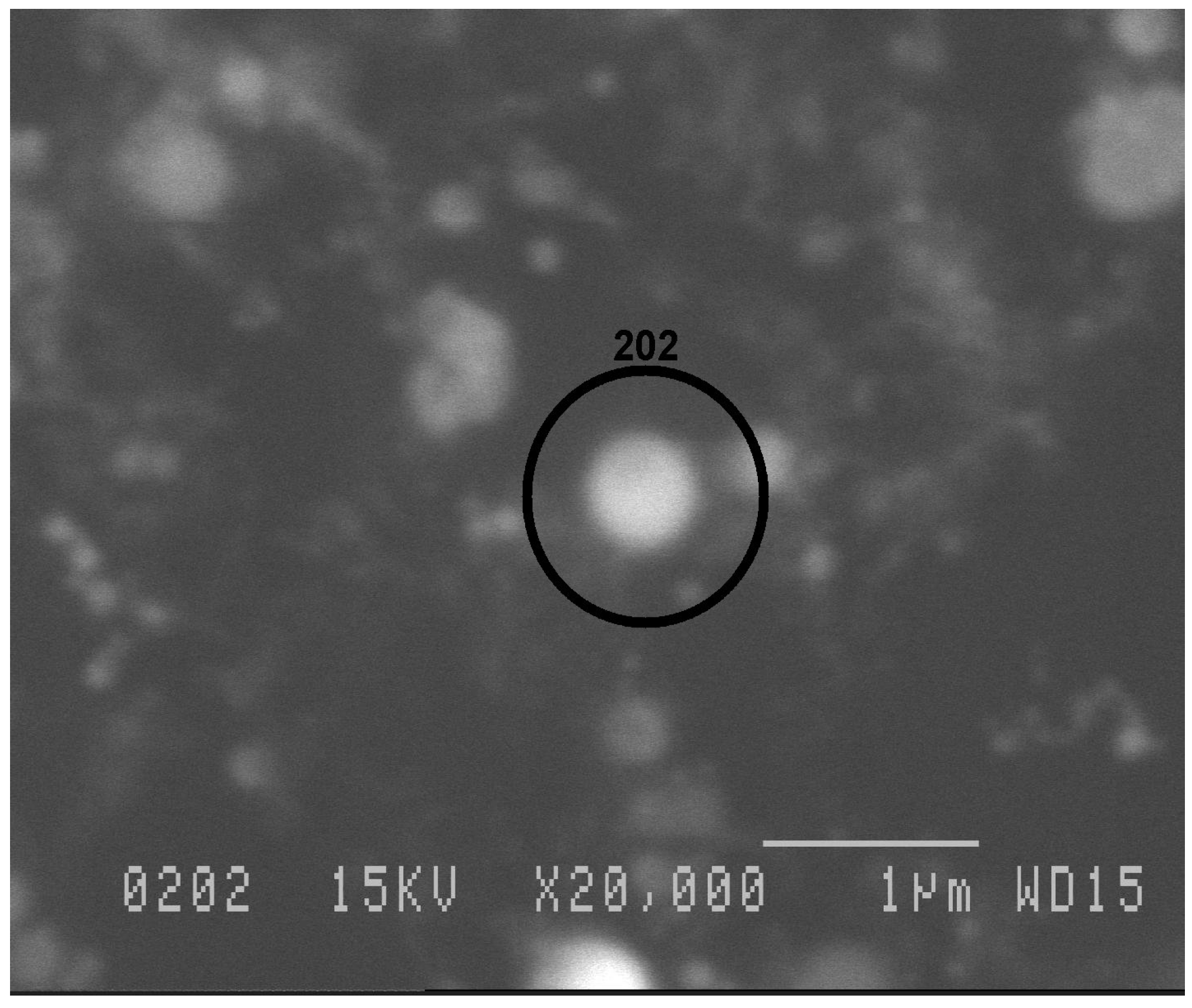

4. Concluding Remarks
Acknowledgments
References
- Deladurantaye, P.; Rioux, C.; Slobodrian, R.J. Effect of gravity on the growth of fractal aggregates. Solitons Fractals 1997, 8, 1693–1709. [Google Scholar] [CrossRef]
- Simulated and Real Low Gravity Fractal Aggregates. Available online: http://adsabs.harvard.edu/full/2001ESASP.454..779S (accessed on 13 September 2013).
- Slobodrian, R.J.; Rioux, C.; Piché, M. A review of metallic fractal aggregates. OJMetal 2011, 1, 17–24. [Google Scholar] [CrossRef]
- Leclerc, J.-C.; Piché, P.B.M.; McCarthy, N.; Rioux, C.; Slobodrian, R.J. Alloys of aluminium and tungsten in the micrometer scale. J. Alloys Metals 2008, 452, L1–L4. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Slobodrian, R.J. Exotic Alloys Obtained by Condensation of Metallic Vapors. Metals 2013, 3, 312-318. https://doi.org/10.3390/met3030312
Slobodrian RJ. Exotic Alloys Obtained by Condensation of Metallic Vapors. Metals. 2013; 3(3):312-318. https://doi.org/10.3390/met3030312
Chicago/Turabian StyleSlobodrian, R. J. 2013. "Exotic Alloys Obtained by Condensation of Metallic Vapors" Metals 3, no. 3: 312-318. https://doi.org/10.3390/met3030312
APA StyleSlobodrian, R. J. (2013). Exotic Alloys Obtained by Condensation of Metallic Vapors. Metals, 3(3), 312-318. https://doi.org/10.3390/met3030312




