Sb2S3/Sb2O3 Heterojunction for Improving Photoelectrochemical Properties of Sb2S3 Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Sb2S3/Sb2O3 Heterojunction
2.3. Characterization
2.4. PEC Measurements
3. Results and Discussion
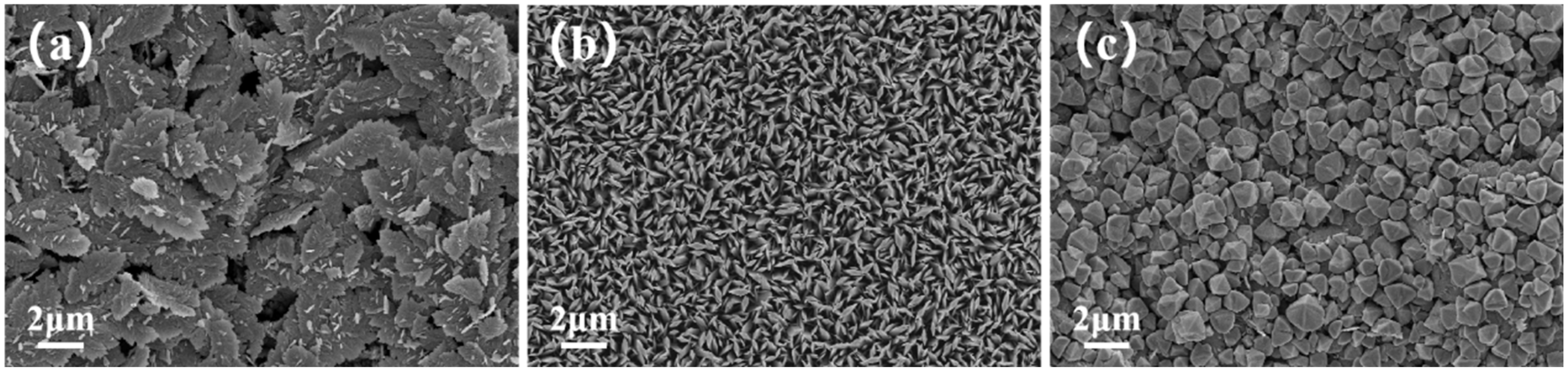
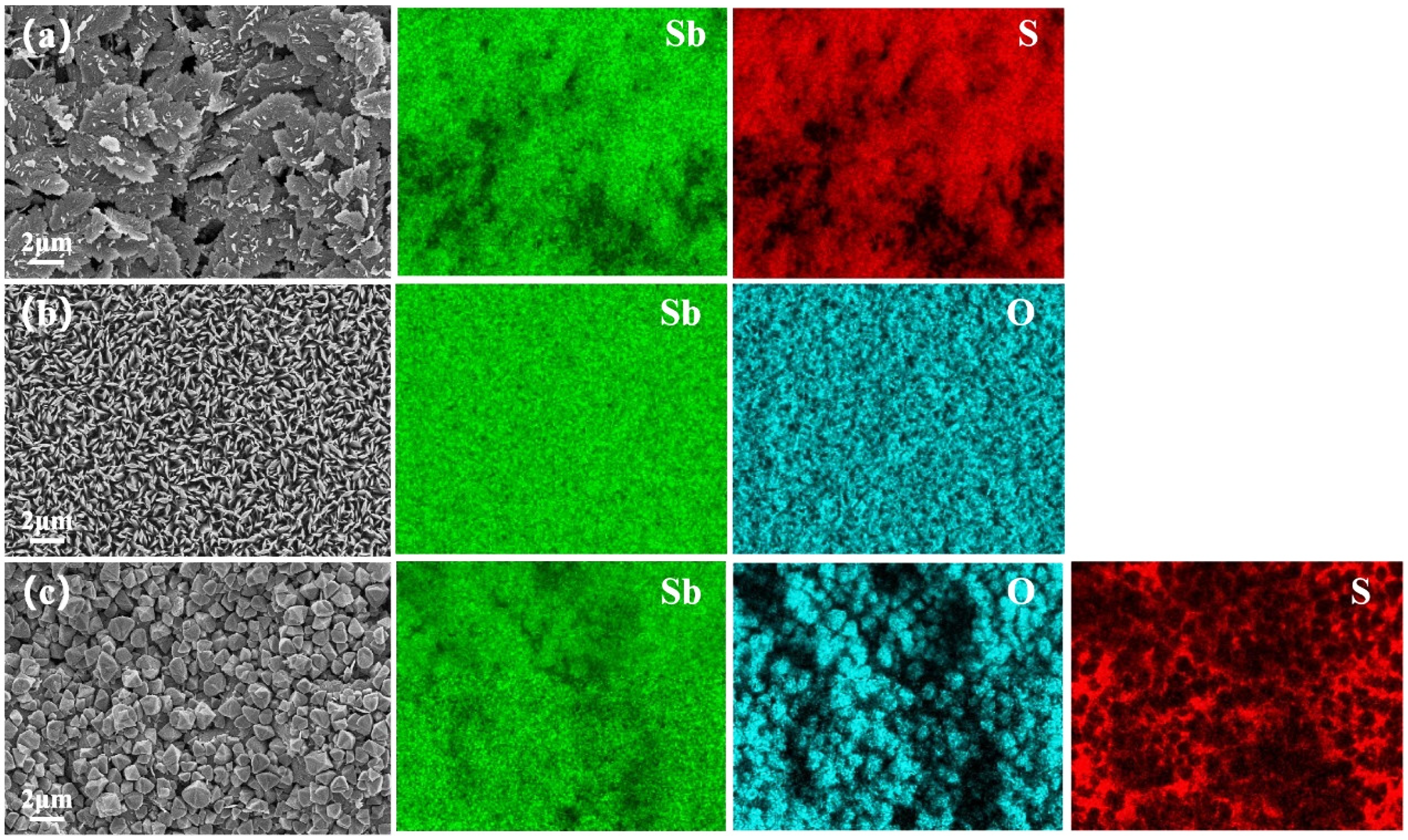
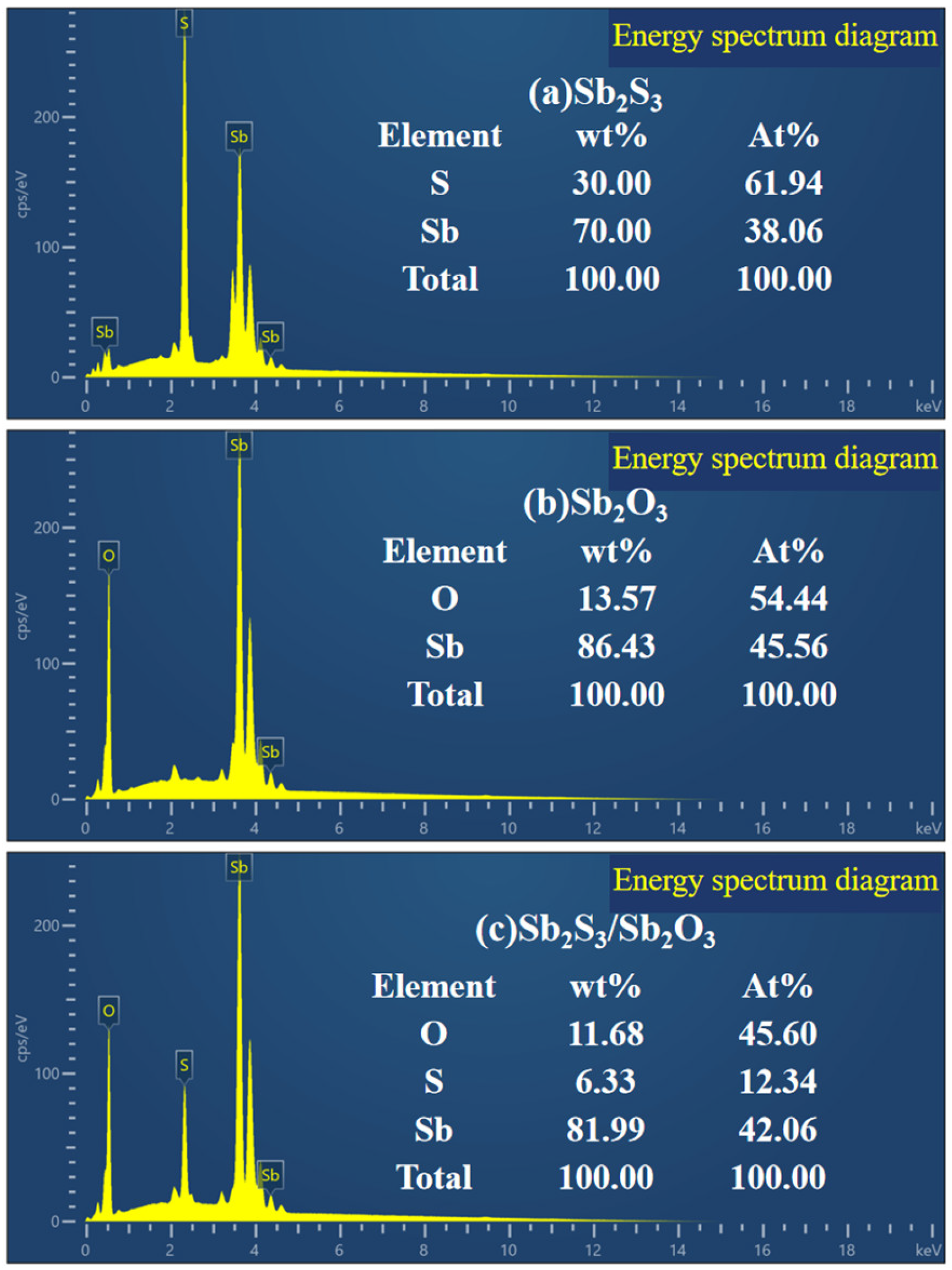
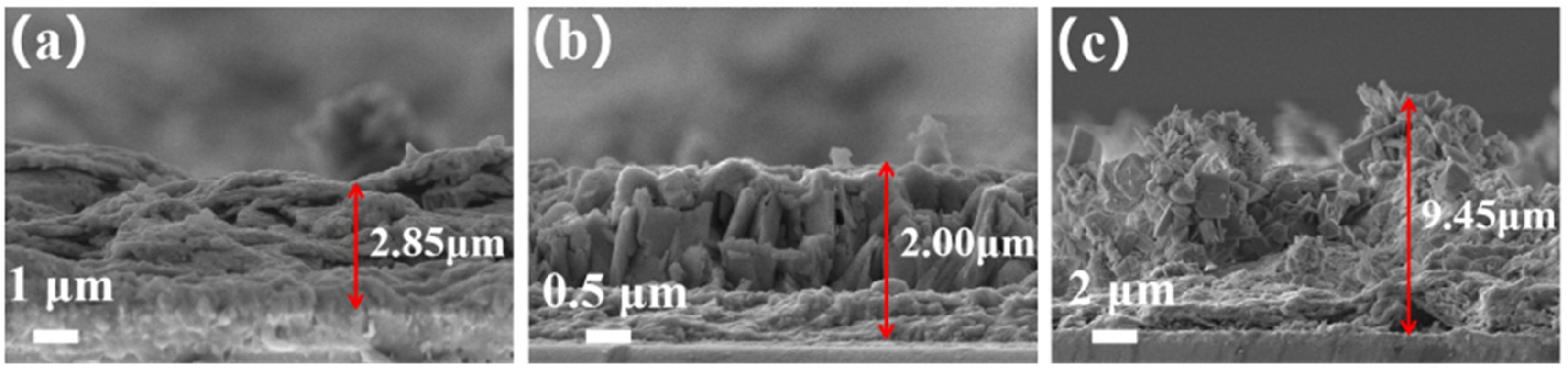
3.1. Characterization of the Phase Structure of Sb2S3 and the Sb2S3/Sb2O3 Heterojunction
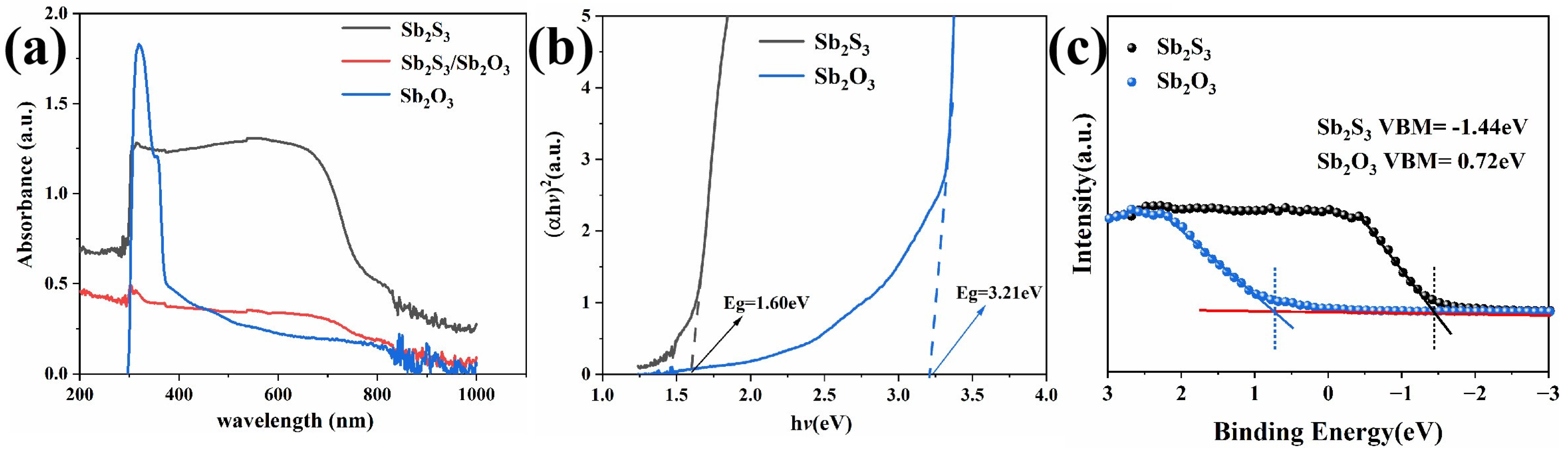
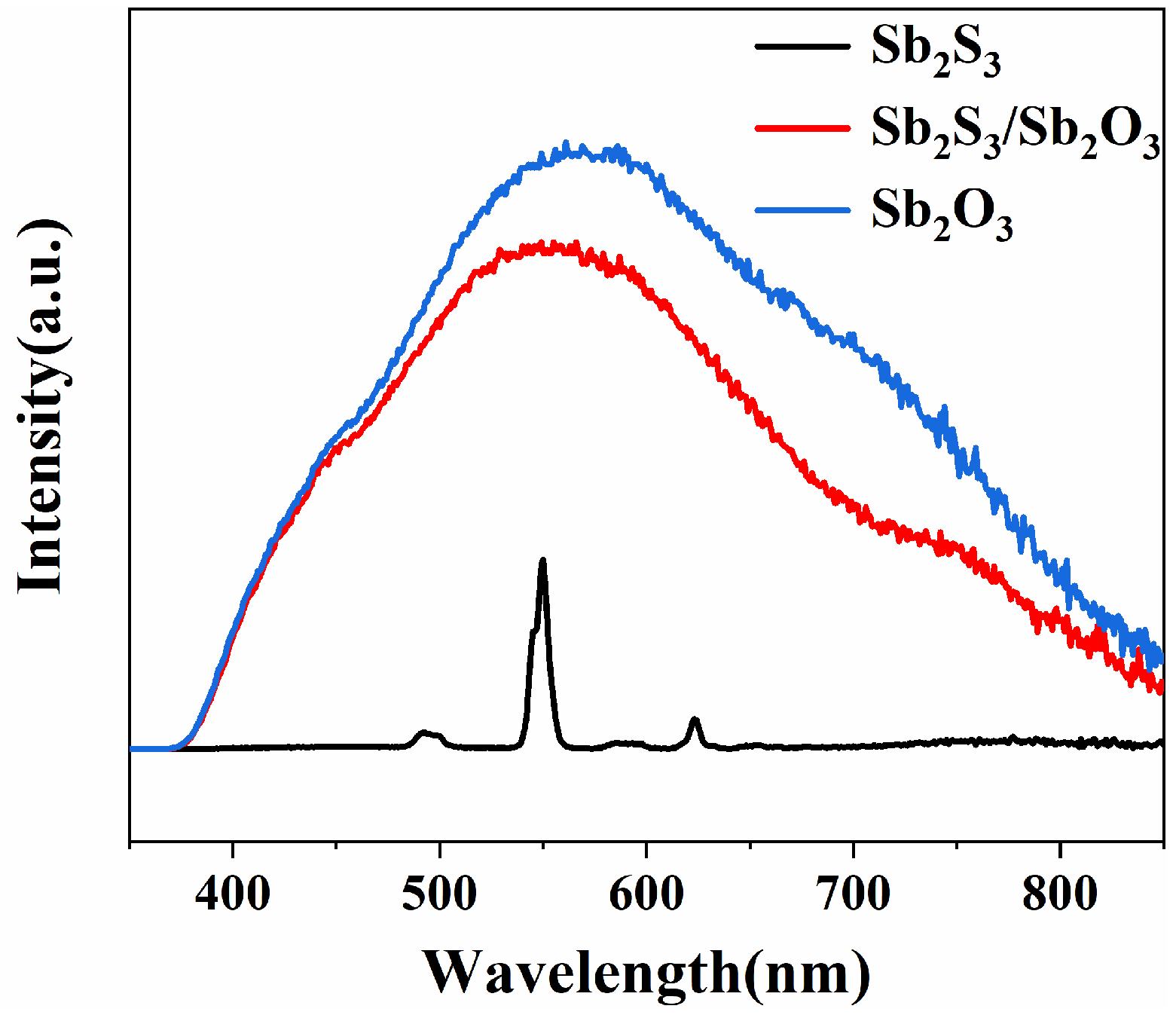
3.2. Analysis of Optical Properties of Sb2S3 and the Sb2S3/Sb2O3 Heterojunction
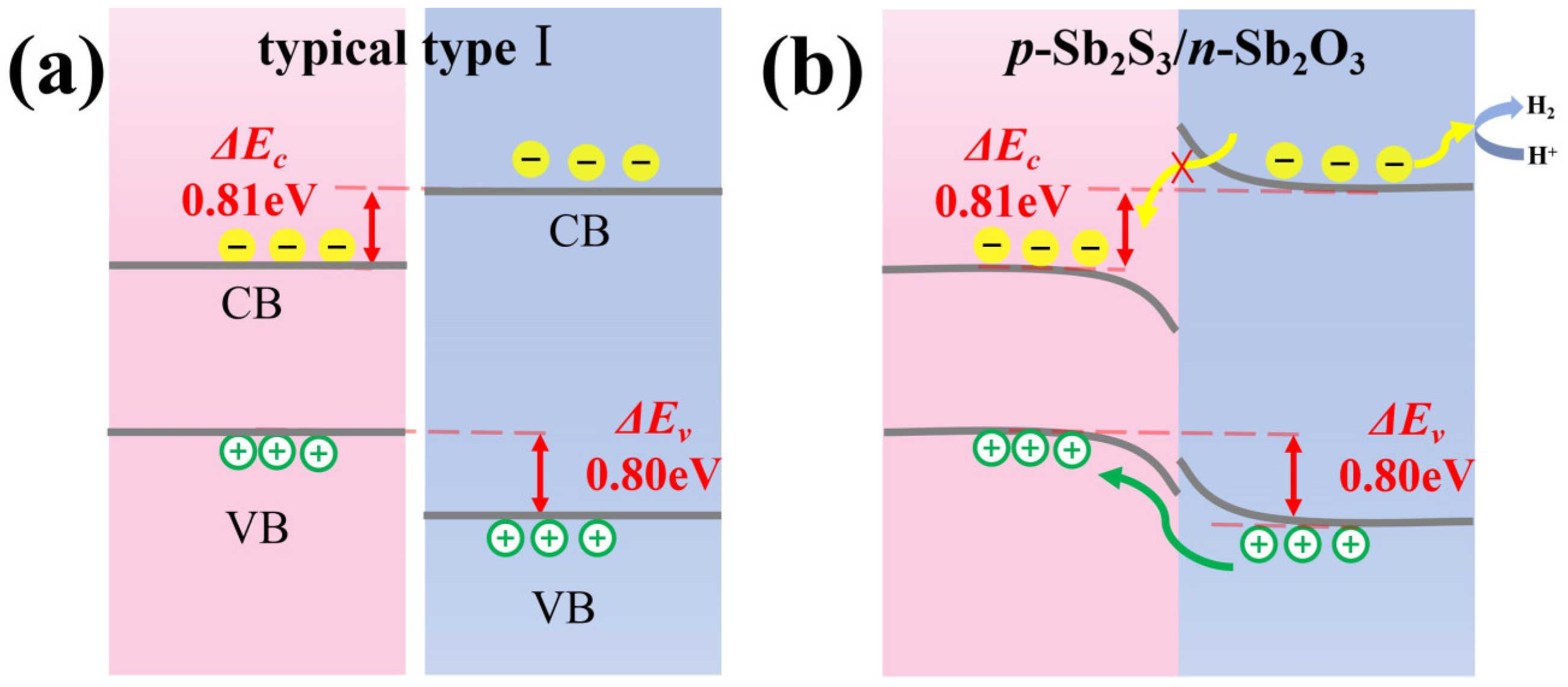
3.3. Schematic Diagram of the Energy Band of the Sb2S3/Sb2O3 Heterojunction
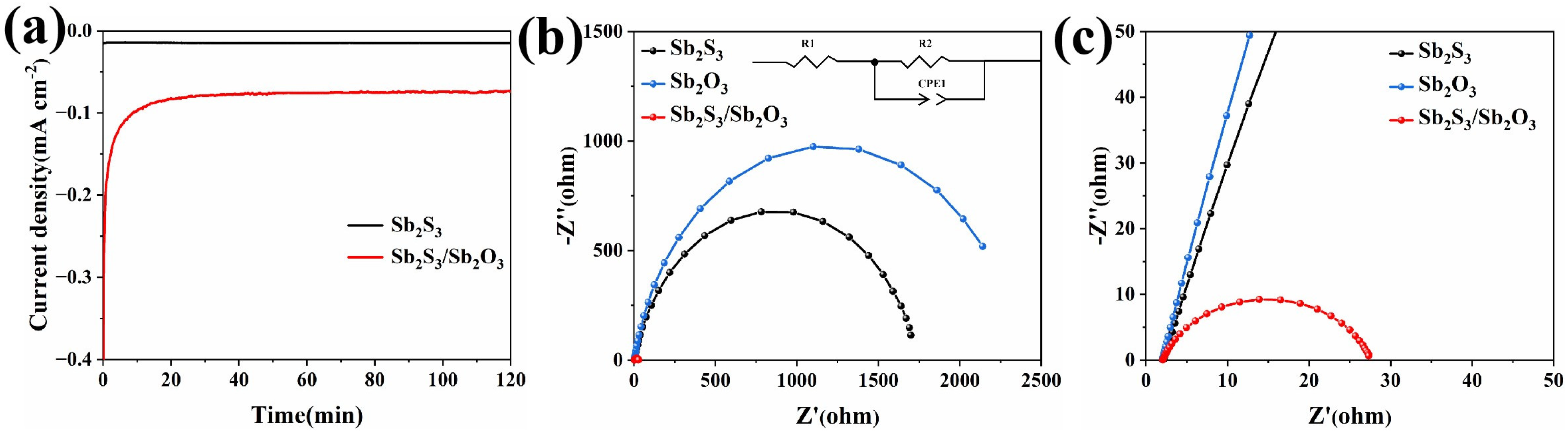
3.4. Photoelectrochemical Characterization of Sb2S3 and the Sb2S3/Sb2O3 Heterojunction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Li, F.; Xiang, Q. Advances and challenges in the modification of photoelectrode materials for photoelectrocatalytic water splitting. Mater. Horiz. 2024, 11, 1638–1657. [Google Scholar] [CrossRef] [PubMed]
- Suri, D.; Das, S.; Choudhary, S.; Venkanna, G.; Sharma, B.; Afroz, M.A.; Tailor, N.K.; Joshi, R.; Satapathi, S.; Tripathi, K. Enigma of Sustainable CO2 Conversion to Renewable Fuels and Chemicals Through Photocatalysis, Electrocatalysis, and Photoelectrocatalysis: Design Strategies and Atomic Level Insights. Small 2025, 21, 2408981. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Huang, K.F.; Tang, Z.Y.; Wang, J.H. Photoelectrocatalytic organic synthesis: A versatile method for the green production of building-block chemicals. J. Mater. Chem. A 2023, 11, 3281–3296. [Google Scholar] [CrossRef]
- Le, P.-A.; Trung, V.D.; Nguyen, P.L.; Bac Phung, T.V.; Natsuki, J.; Natsuki, T. The current status of hydrogen energy: An overview. RSC Adv. 2023, 13, 28262–28287. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, Y.; Zhang, X.; Yang, H.; Xie, R.; Zhang, S.; Lv, Y.; Xiong, L. Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production. Molecules 2024, 29, 289. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Su, J. Sb2S3 surface modification for improved photoelectrochemical water splitting performance of BiVO4 photoanode. J. Photonics Energy 2021, 11, 016502. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen MA, A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Kondrotas, R.; Chen, C.; Tang, J. Sb2S3 Solar Cells. Joule 2018, 2, 857–878. [Google Scholar] [CrossRef]
- Shah, U.A.; Chen, S.; Khalaf, G.M.G.; Jin, Z.; Song, H. Wide Bandgap Sb2S3 Solar Cells. Adv. Funct. Mater. 2021, 31, 2100265. [Google Scholar] [CrossRef]
- Myagmarsereejid, P.; Ingram, M.; Batmunkh, M.; Zhong, Y.L. Doping Strategies in Sb2S3 Thin Films for Solar Cells. Small 2021, 17, 2100241. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Deng, H.; Wu, K.; Wang, G.; Hong, J.; Wang, W.; Wu, J.; Zhou, H.; Xia, Y.; Cheng, S. Self-powered Sb2S3 photodetectors with efficient carrier transport enabled by compact vertical TiO2 nanorods. Opt. Lett. 2024, 49, 6533–6536. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liu, X.; Man, J.; Ou, Q.; Zheng, X.; Liu, Z.; Zhu, T.; Wang, H.E. 2D/1D PbI2/Sb2S3 van der Waals heterojunction for highly sensitive and broadband photodetectors. J. Mater. Chem. C 2024, 12, 3353–3364. [Google Scholar] [CrossRef]
- Lian, X.; Li, S.; Jiang, J.; Huang, W.; He, N.; Liu, X.; Wu, J.; Wang, Z.; Zhu, G.; Wang, L. Plasmonically-enhanced reconfigurable photodetector based on graphene/Sb2S3 heterojunction. Appl. Phys. Express 2025, 18, 015003. [Google Scholar] [CrossRef]
- Ijaz, M.; Shahzad Ahmad, K.; Nawaz, F.; Makawana, B.; Gupta, R.K.; Abdel-Maksoud, M.A.; Al-Qahtani, W.H.; Malik, A. Nanocomposite Material Sb2S3:SnS:MnS2 its Fabrication and Utilization as Efficient Electrode for Energy Storage in Supercapacitor. ChemistrySelect 2024, 9, e202403513. [Google Scholar] [CrossRef]
- Jaffri, S.B.; Ahmad, K.S.; Al-Hawadi, J.S.; Panchal, H.; Gupta, R.K.; Ashraf, G.A.; Okla, M.K. Harnessing sustainable energy: Synthesizing a trinary chalcogenide semiconductor BaS:Sb2S3:CuS for enhanced performance in supercapacitor devices and electro-catalysis. J. Energy Storage 2024, 103, 114527. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Wan, Z.; Chen, C.; Cao, W.; Naveed, F.; Kuang, J.; Cheng, L.; Lei, L.; Chen, J.; et al. PbSe-Induced Sb2S3 Crystallization and Interface Band Optimization for High-Efficiency Bulk Heterojunction Sb2S3 Solar Cells. Adv. Funct. Mater. 2025, 23, 2420361. [Google Scholar] [CrossRef]
- Kumar, P.; Thomas, J.P.; Kharytonau, D.S.; Gradone, A.; Gilli, N.; You, S.; Leung, K.T.; Morandi, V.; Vomiero, A. Cadmium-free electron transport layers for hydrothermally processed semitransparent Sb2S3 solar cells. Nano Energy 2025, 134, 110539. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, X.; Dai, G.; Wang, X.; Wang, H.; Chen, T.; Wang, K.; Xiao, X.; Li, J. Surface treatment processed electron transport layers for efficient Sb2S3 solar cells. Chem. Eng. J. 2024, 500, 156907. [Google Scholar] [CrossRef]
- Chihi, A. Synthesis of Sb2S3:Eu thin films as a catalyst for the efficient photocatalytic degradation of rhodamine B (RhB) dye under visible light. RSC Adv. 2025, 15, 5681–5697. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Wang, J.; Yang, Z.; Li, Y.; Zhang, L.; Zhang, G. Built-in electric field intensified by SPR effect to drive charge separation over Ag-AgI/Sb2S3 heterojunction for high-efficiency photocatalytic metronidazole mineralization. Sep. Purif. Technol. 2024, 346, 127443. [Google Scholar] [CrossRef]
- Farhana, M.A.; Manjceevan, A.; Bandara, J. Recent advances and new research trends in Sb2S3 thin film based solar cells. J. Sci.Adv. Mater. Devices 2023, 8, 100533. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Liu, Z. Novel WO3/Sb2S3 Heterojunction Photocatalyst Based on WO3 of Different Morphologies for Enhanced Efficiency in Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 9684–9691. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, Ç.; Zunger, A. n-type doping of oxides by hydrogen. Appl. Phys. Lett. 2002, 81, 73–75. [Google Scholar] [CrossRef]
- Tigau, N. Structure and electrical conduction of Sb2O3 thin films. Cryst. Res. Technol. 2006, 41, 1106–1111. [Google Scholar] [CrossRef]
- Yao, X.; Zuo, J.; Wang, Y.-J.; Song, N.-N.; Li, H.-H.; Qiu, K. Enhanced Photocatalytic Degradation of Perfluorooctanoic Acid by Mesoporous Sb2O3/TiO2 Heterojunctions. Front. Chem. 2021, 9, 690520. [Google Scholar] [CrossRef]
- Nalina, M.; Messaddeqa, Y.; Ribeiroa, S.; Poulainb, M.; Briois, V.; Sud, P. Photosensitivity in Antimony Based Glasses. J. Optoelectron. Adv. Mater. 2001, 3, 553–558. [Google Scholar]
- Cao, G.; Su, J.; Lu, J.; Zhang, J.; Liu, Q. Preparation of Sb2O3-loaded Activated Carbon Particle Electrode and Its Electrocatalytic Degradation of Methyl Orange in a Three-dimensional Electrode Cell. J. Chin. Chem. Soc. 2012, 59, 583–588. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ning, X.; Lei, J.; Shao, J.; Wang, W.; Huang, Y.; Hou, B. Sb2S3/Sb2O3 modified TiO2 photoanode for photocathodic protection of 304 stainless steel under visible light. Appl. Surf. Sci. 2018, 462, 155–163. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, J.; Wang, Y.; Pan, Y.; Xiao, B.; Ouyang, N.; Liu, F. Sb2O3/Sb2S3 Heterojunction Composite Thin Film Photoanode Prepared via Chemical Bath Deposition and Post-Sulfidation. J. Electrochem. Soc. 2018, 165, H1052–H1058. [Google Scholar] [CrossRef]
- Prabhakar, R.R.; Moehl, T.; Siol, S.; Suh, J.; Tilley, S.D. Sb2S3/TiO2 Heterojunction Photocathodes: Band Alignment and Water Splitting Properties. Chem. Mater. 2020, 32, 7247–7253. [Google Scholar] [CrossRef]
- Prabhakar, R.R.; Septina, W.; Siol, S.; Moehl, T.; Wick-Joliat, R.; Tilley, S.D. Photocorrosion-resistant Sb2Se3 photocathodes with earth abundant MoSx hydrogen evolution catalyst. J. Mater. Chem. A 2017, 5, 23139–23145. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Liu, Y.; Tang, D.; Liu, F.; Lai, Y. Facile synthesis and photoelectrochemical characterization of Sb2O3 nanoprism arrays. J. Alloy. Compd. 2017, 727, 469–474. [Google Scholar] [CrossRef]
- Song, D.; Liu, Y.; Sun, C.; Zhao, Y.; Zhu, T.; Yang, R.; Song, Y.; Zhao, J. Designed composite catalysts of Cu-doped BiOCl nanosheets on CuFe-PBA cubes for enhanced photoelectrochemical H2 evolution activity in neutral medium. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132592. [Google Scholar] [CrossRef]
- Han, T.; Luo, M.; Liu, Y.; Lu, C.; Ge, Y.; Xue, X.; Dong, W.; Huang, Y.; Zhou, Y.; Xu, X. Sb2S3/Sb2Se3 heterojunction for high-performance photodetection and hydrogen production. J. Colloid Interface Sci. 2022, 628, 886–895. [Google Scholar] [CrossRef]
- Zhang, L.; Xin, C.; Jin, W.; Sun, Q.; Wang, Y.; Wang, J.; Hu, X.; Miao, H. Novel Sb2S3-xSex photocathode decorated NiFe-LDH hole blocking layer with enhanced photoelectrochemical performance. Appl. Surf. Sci. 2023, 639, 158184. [Google Scholar] [CrossRef]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G. Limitations of the Tauc Plot Method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Wei, X.; Li, Q.; Zhang, D.; Wang, Y.; Liu, E.; Miao, H. CdSe Quantum Dots Supported on Sb2S3 Nanorods as S-Scheme Heterojunction Photoanode in Photoelectrochemical Cells. ACS Appl. Nano Mater. 2024, 7, 24213–24223. [Google Scholar] [CrossRef]
- Kraut, E.A.; Grant, R.W.; Waldrop, J.R.; Kowalczyk, S.P. Precise Determination of the Valence-Band Edge in X-Ray Photoemission Spectra: Application to Measurement of Semiconductor Interface Potentials. Phys. Rev. Lett. 1980, 44, 1620–1623. [Google Scholar] [CrossRef]
- Luo, M.; Lu, C.; Liu, Y.; Han, T.; Ge, Y.; Zhou, Y.; Xu, X. Band alignment of type-I SnS2/Bi2Se3 and type-II SnS2/Bi2Te3 van der Waals heterostructures for highly enhanced photoelectric responses. Sci. China Mater. 2022, 65, 1000–1011. [Google Scholar] [CrossRef]
- Tangi, M.; Mishra, P.; Tseng, C.-C.; Ng, T.K.; Hedhili, M.N.; Anjum, D.H.; Alias, M.S.; Wei, N.; Li, L.-J.; Ooi, B.S. Band Alignment at GaN/Single-Layer WSe2 Interface. ACS Appl. Mater. Interfaces 2017, 9, 9110–9117. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.A.; Bae, C.; Joe, J.; Yang, H.; Kim, S.; Park, J.H.; Shin, H. Heterojunction Photoanode of Atomic-Layer-Deposited MoS2 on Single-Crystalline CdS Nanorod Arrays. ACS Appl. Mater. Interfaces 2019, 11, 37586–37594. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Lu, Y.; Du, K.; Zheng, H.; Mao, L.; Li, H.; Ma, Z.; Wang, R.; Zhang, J. Interfacial Mediation by Sn And S Vacancies of p—SnS/n—ZnIn2S4 for Enhancing Photocatalytic Hydrogen Evolution with New Scheme of Type—I Heterojunction. Adv. Funct. Mater. 2023, 33, 2304072. [Google Scholar] [CrossRef]



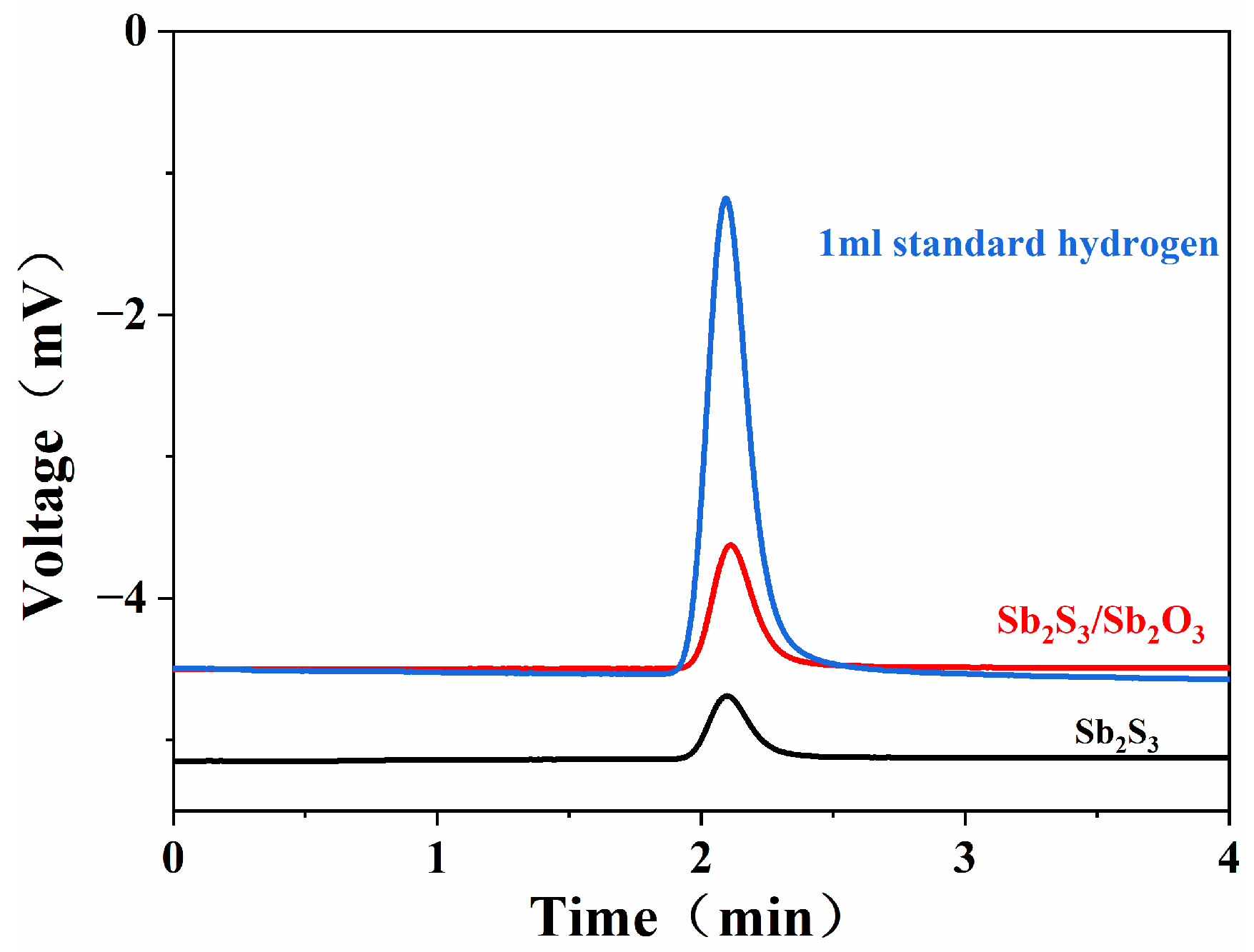
| Sample | Time of Appearance of Hydrogen Peak | Chromatographic Peak Area | Hydrogen Volume/mL | Hydrogen Precipitation rate/mL cm−2 h−1 |
|---|---|---|---|---|
| 1 mL standard hydrogen | 2.039 | 40.8321 | 1.000 | \ |
| Sb2S3 | 2.098 | 4.9891 | 0.122 | 0.081 |
| Sb2S3/Sb2O3 | 2.111 | 9.9438 | 0.244 | 0.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.; Yang, J.; Cui, Z.; Tan, R.; Li, T.; Xu, B.; Li, S.; Yang, B. Sb2S3/Sb2O3 Heterojunction for Improving Photoelectrochemical Properties of Sb2S3 Thin Films. Metals 2025, 15, 478. https://doi.org/10.3390/met15050478
Tan H, Yang J, Cui Z, Tan R, Li T, Xu B, Li S, Yang B. Sb2S3/Sb2O3 Heterojunction for Improving Photoelectrochemical Properties of Sb2S3 Thin Films. Metals. 2025; 15(5):478. https://doi.org/10.3390/met15050478
Chicago/Turabian StyleTan, Honglei, Jia Yang, Zhaofeng Cui, Renjie Tan, Teng Li, Baoqiang Xu, Shaoyuan Li, and Bin Yang. 2025. "Sb2S3/Sb2O3 Heterojunction for Improving Photoelectrochemical Properties of Sb2S3 Thin Films" Metals 15, no. 5: 478. https://doi.org/10.3390/met15050478
APA StyleTan, H., Yang, J., Cui, Z., Tan, R., Li, T., Xu, B., Li, S., & Yang, B. (2025). Sb2S3/Sb2O3 Heterojunction for Improving Photoelectrochemical Properties of Sb2S3 Thin Films. Metals, 15(5), 478. https://doi.org/10.3390/met15050478







