The Effect of Heat Treatment and Cold Forging on the Mechanical Properties of SCr420 Low-Alloy Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of SCr420 Low-Alloy Steel
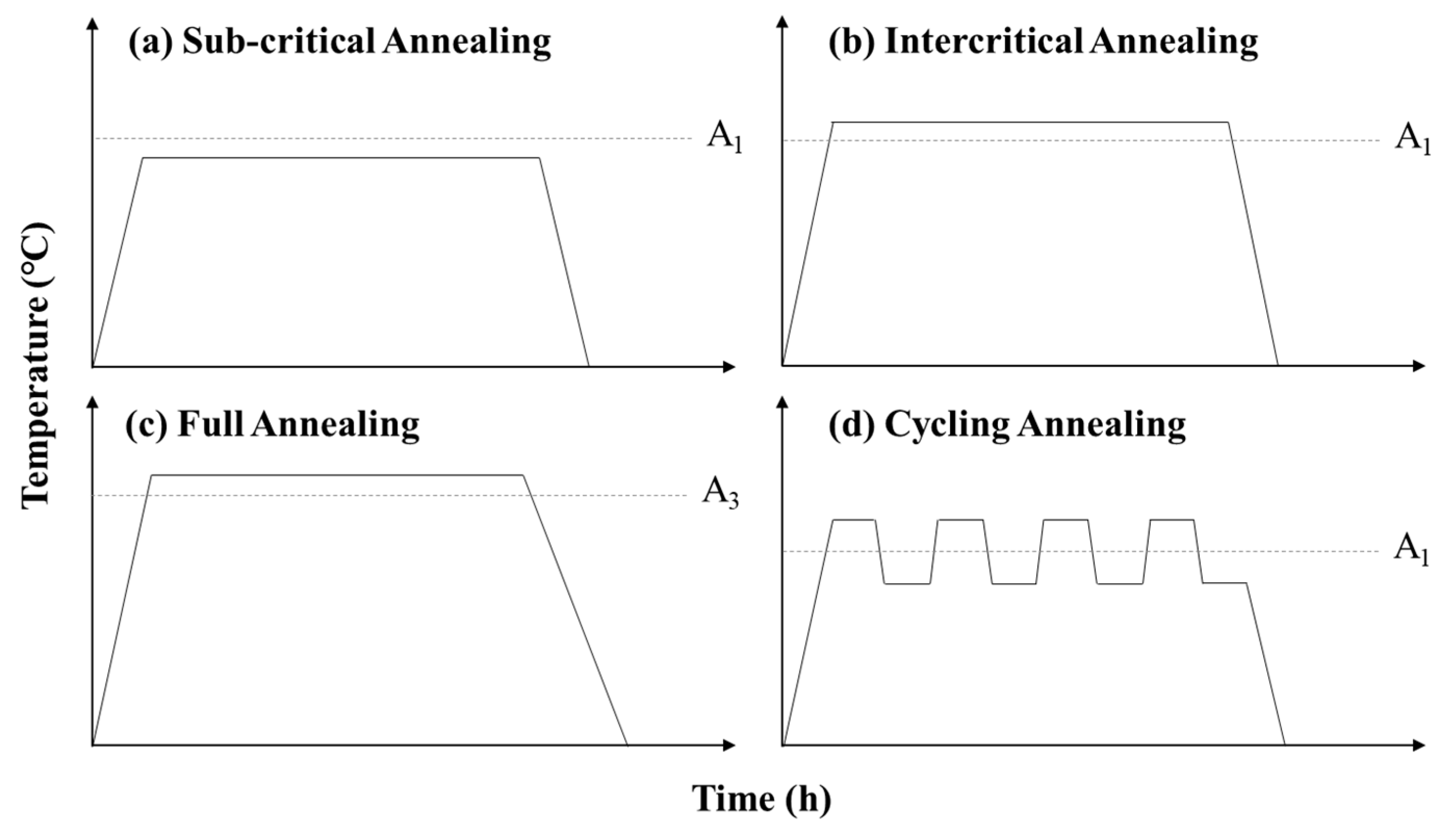
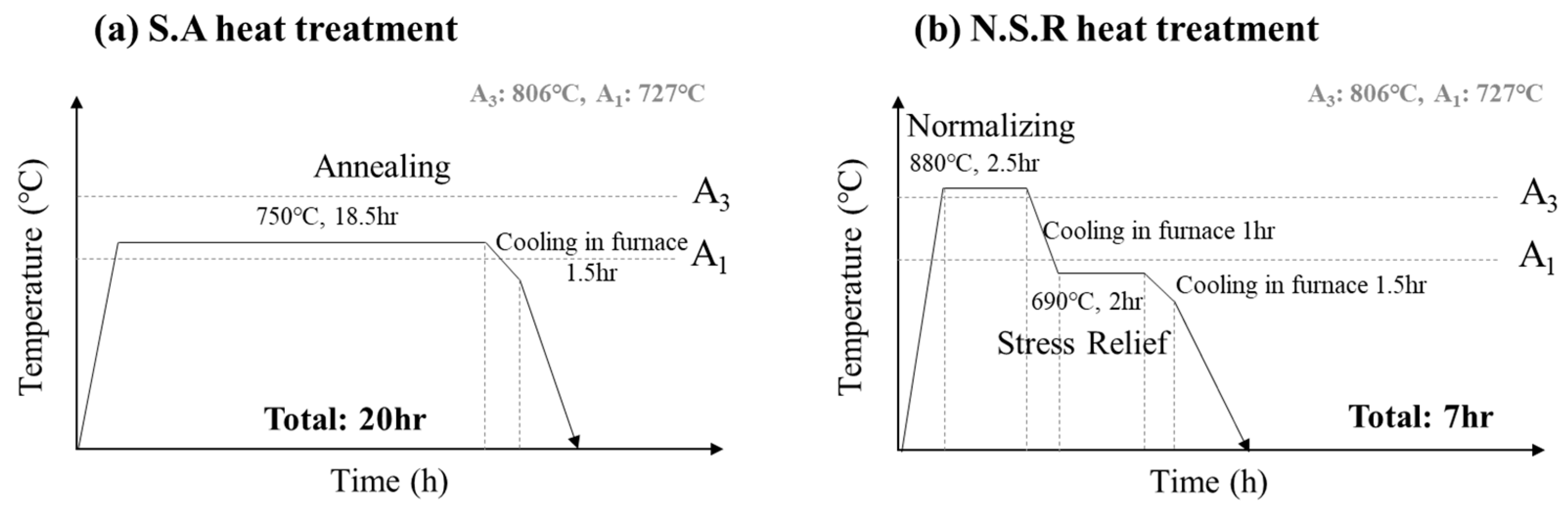
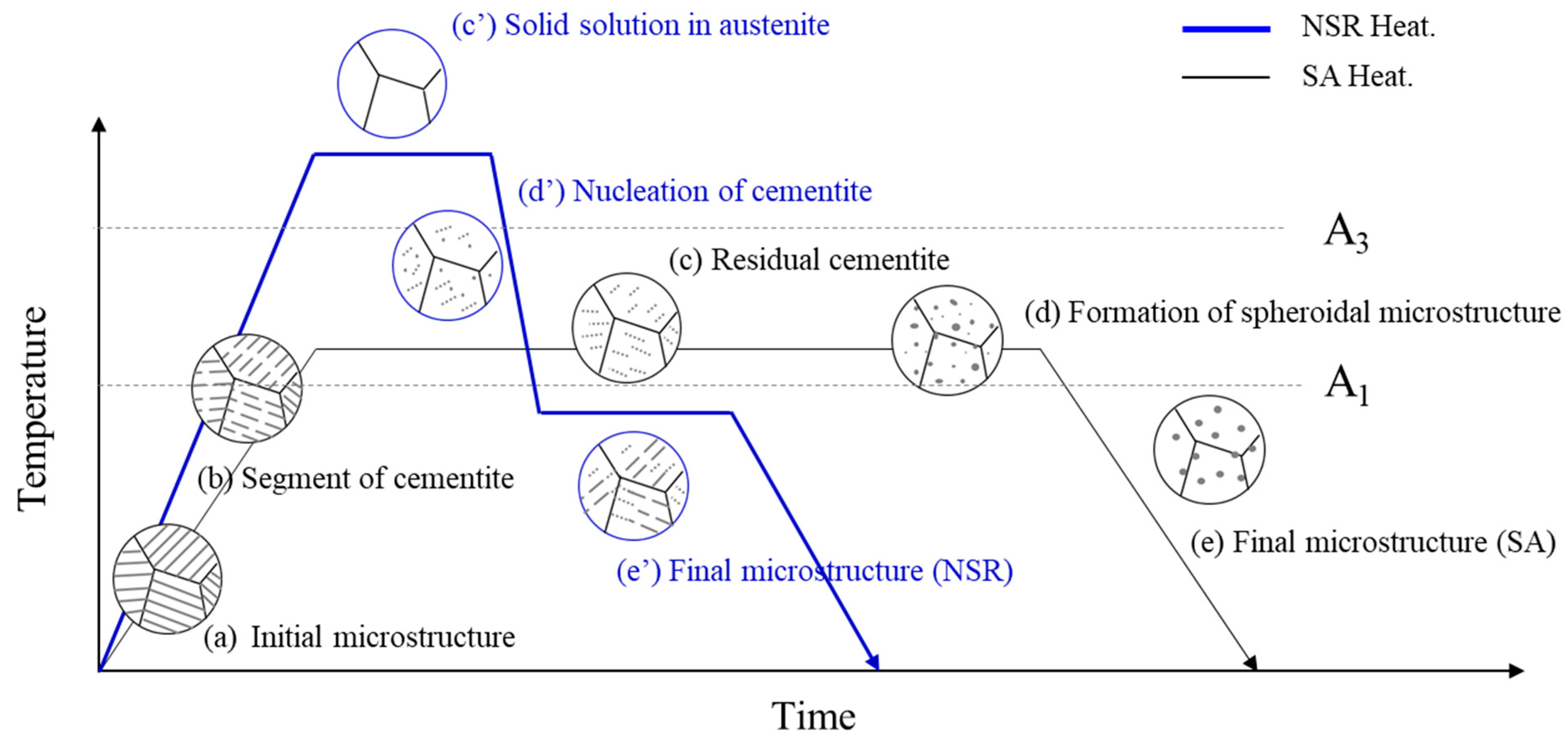
2.2. Development of the NSR Heat Treatment
2.3. Microstructures and Mechanical Properties
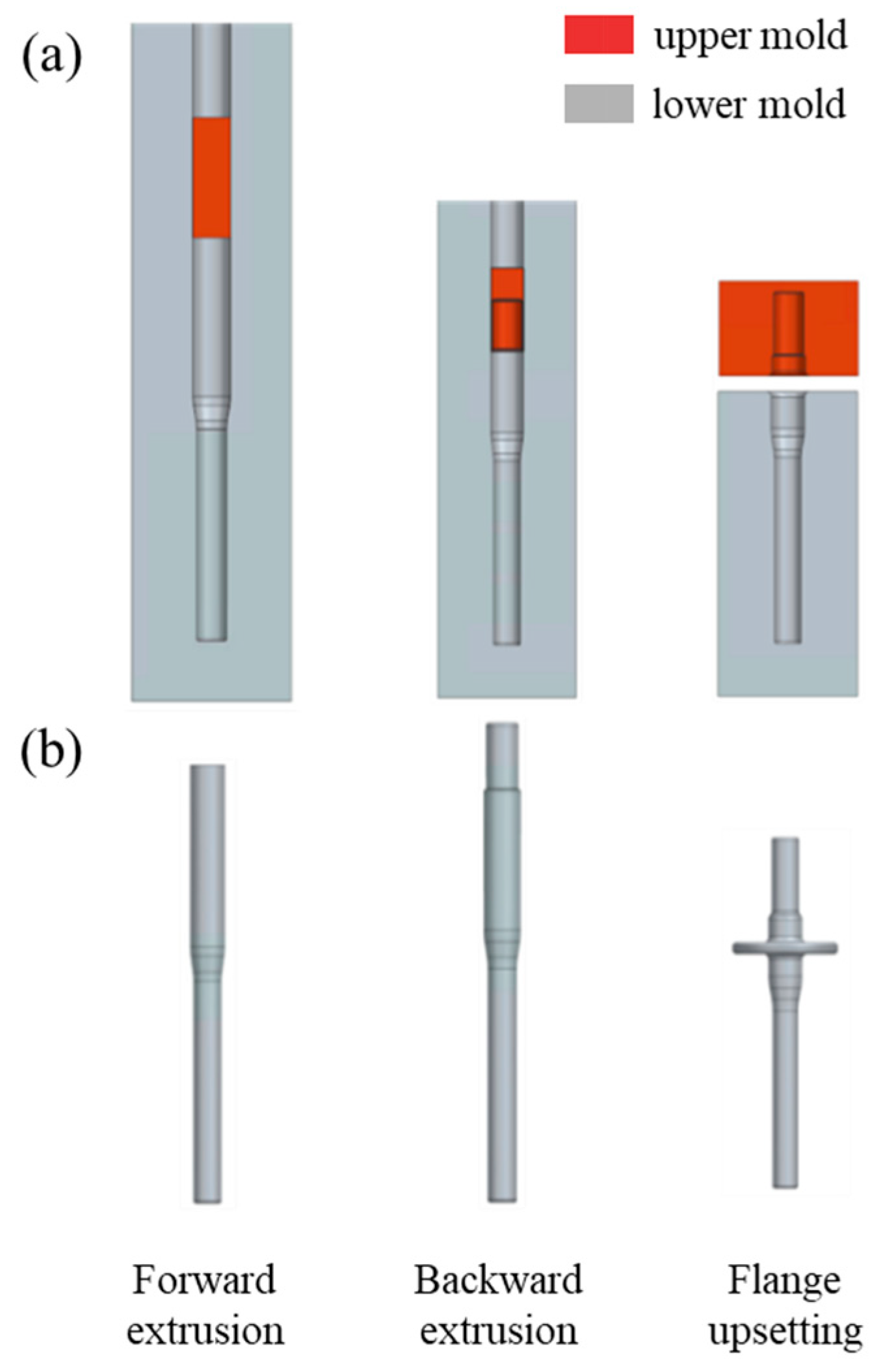
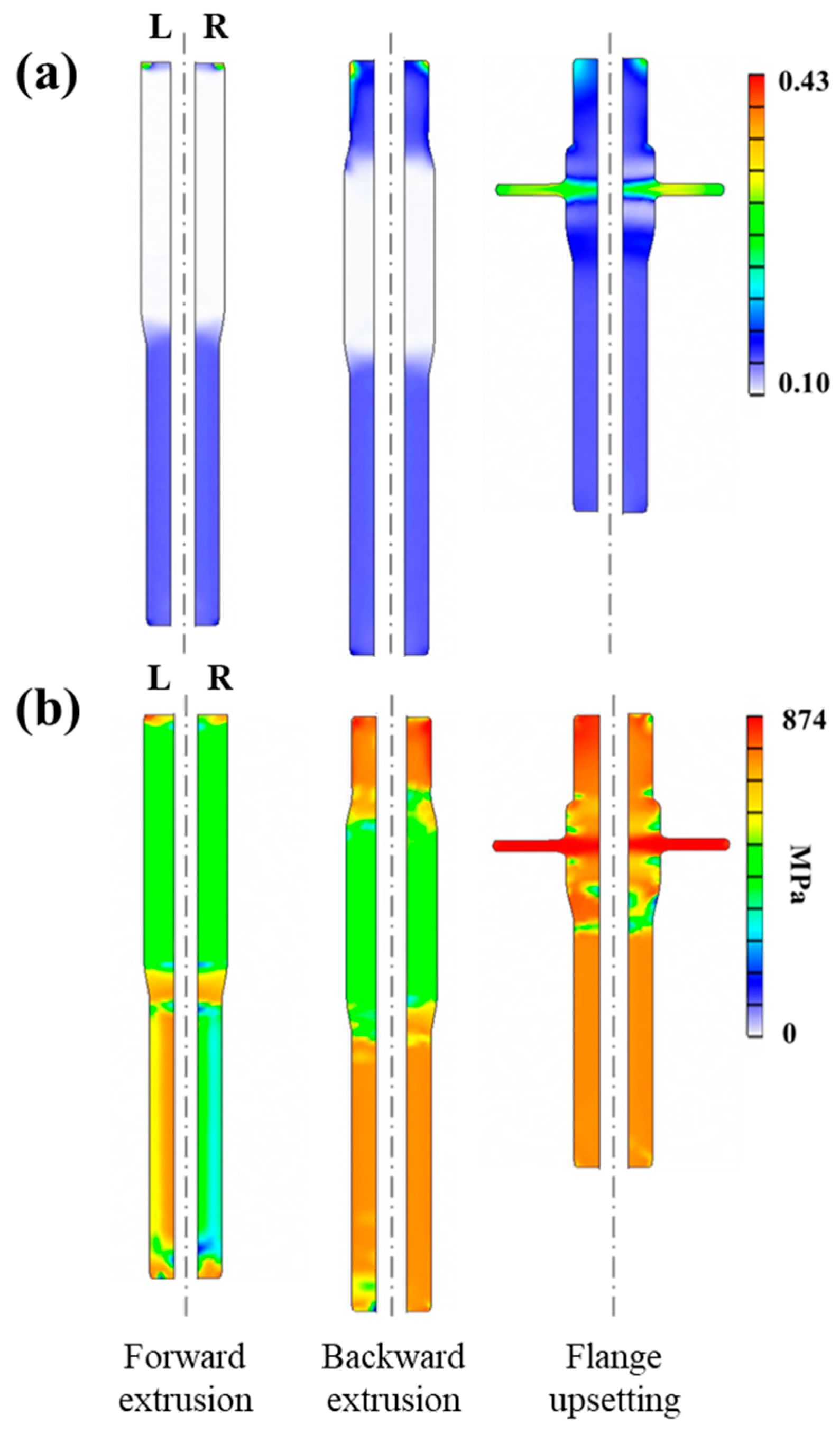
2.4. Finite Element Analysis of Cold Forging Formability
3. Results and Discussion
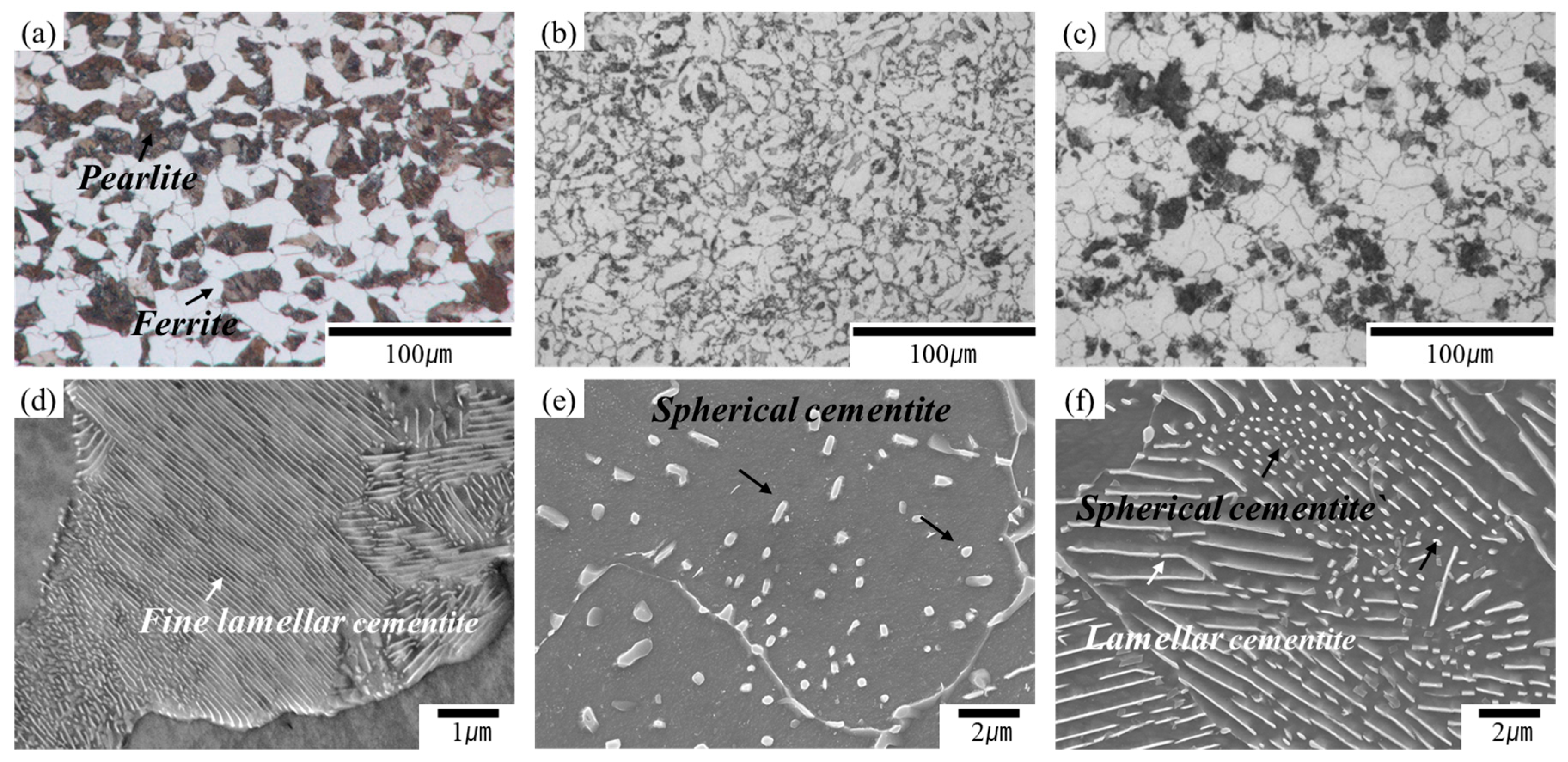
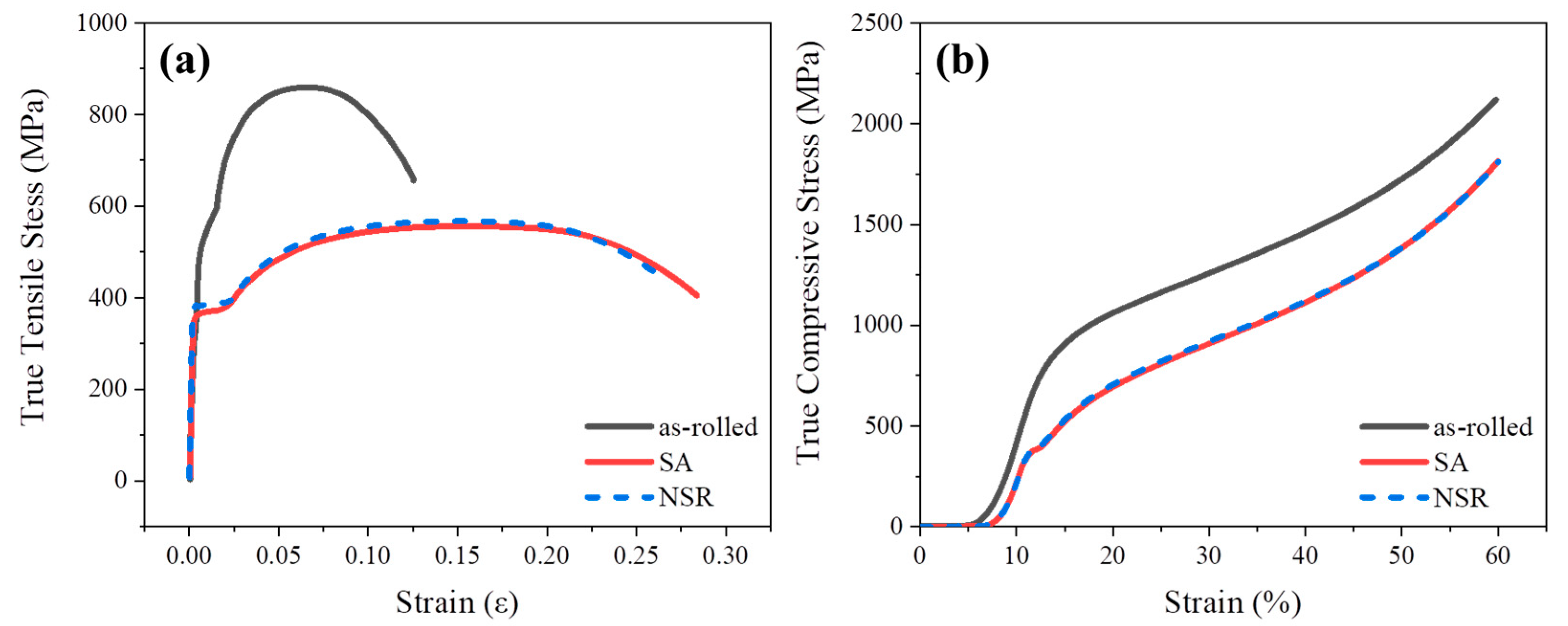
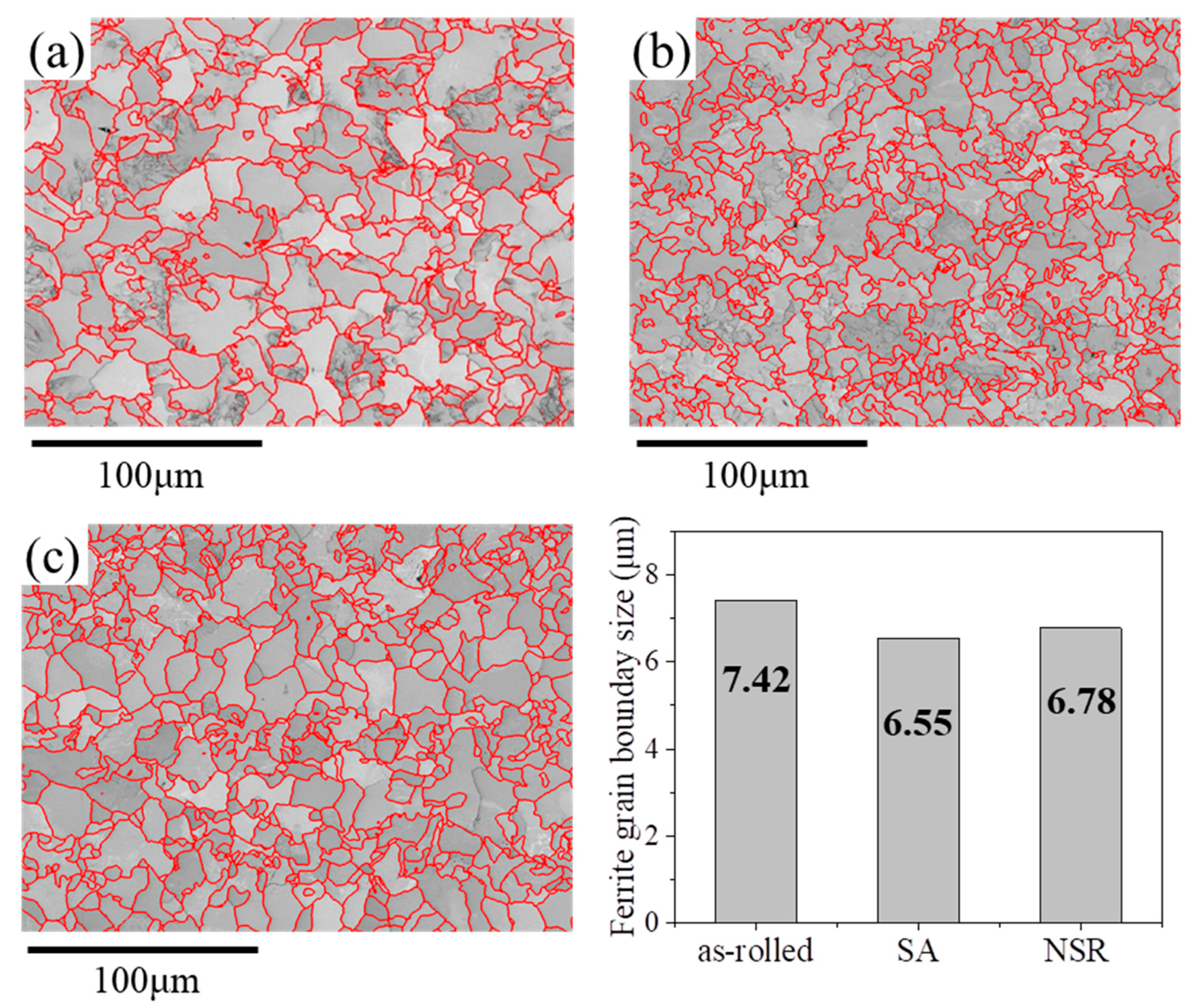
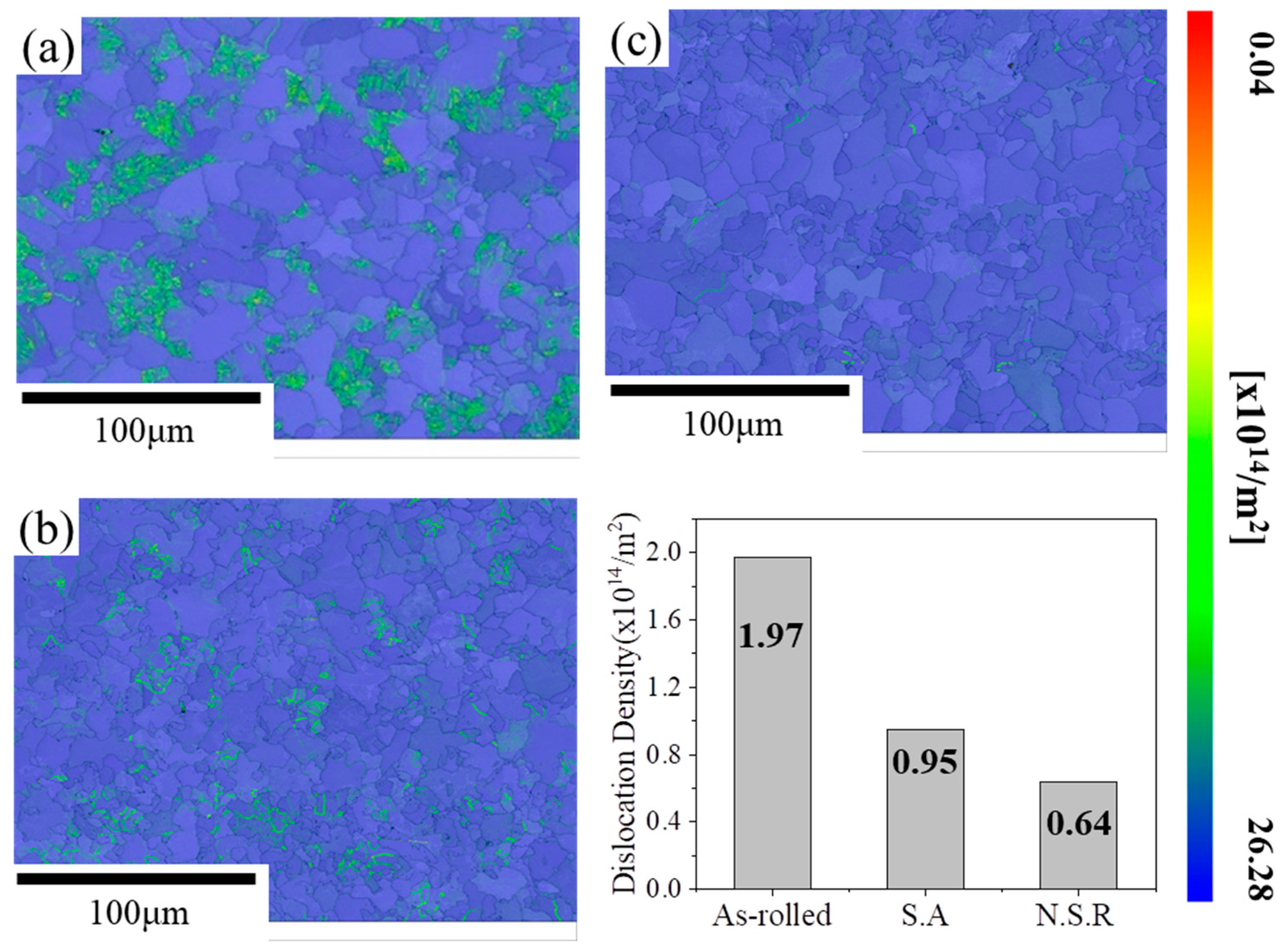
3.1. Microstructures and Mechanical Properties
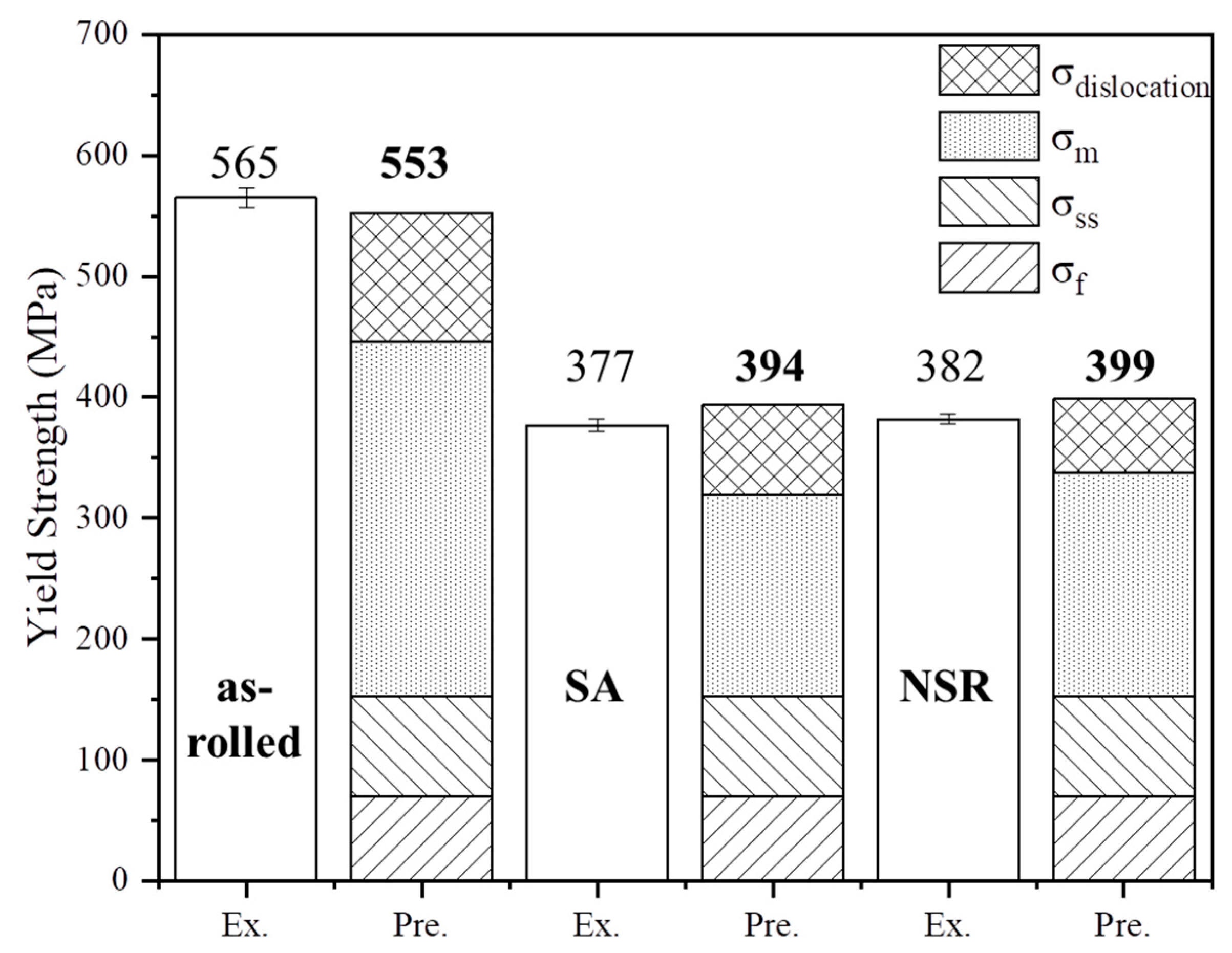
3.2. On the Strength Mechanisms
3.3. Results of Cold Forging Formability Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar]
- Ghosh, M.; Ghosh, A.; Roy, A. Renewable and sustainable materials in automotive industry. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 162–179. [Google Scholar]
- Han, Z.Y.; Zhang, M.D.; Xu, H.F.; Dong, H.; Cao, W.Q. Research and application of high performance automobile steel. Iron Steel 2016, 51, 1. [Google Scholar]
- Wang, C.Y.; Yang, J.; Chang, Y.; Cao, W.Q.; Dong, H. Development trend and challenge of advanced high strength automobile steels. Iron Steel 2019, 54, 1. [Google Scholar]
- McKelvey, S.A.; Fatemi, A. Surface finish effect on fatigue behavior of forged steel. Int. J. Fatigue 2012, 36, 130–145. [Google Scholar]
- Chen, S.; Qin, Y.; Chen, J.G.; Choy, C.M. A forging method for reducing process steps in the forming of automotive fasteners. Int. J. Mech. Sci. 2018, 137, 1–14. [Google Scholar]
- Bilbao, O.; Loizaga, I.; Alonso, J.; Girot, F.; Torregaray, A. 42CrMo4 steel flow behavior characterization for high temperature closed dies hot forging in automotive components applications. Heliyon 2023, 9, e22256. [Google Scholar] [CrossRef]
- Douthit, T.J.; Van Tyne, C.J. The effect of nitrogen on the cold forging properties of 1020 steel. J. Mater. Process. Technol. 2005, 160, 335–347. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Hosford, W.F. Spheroidization cycles for medium carbon steels. Metall. Mater. Trans. A 2002, 33, 1255–1261. [Google Scholar] [CrossRef]
- Onodera, S.; Sawai, K. Current cold-forging techniques for the manufacture of complex precision near-net-shapes. J. Mater. Process. Technol. 1992, 35, 385–396. [Google Scholar] [CrossRef]
- Miroslaw, S.; Grzegorz, W.; Lukasz, W.; Tomasz, B. Effect of Annealing Time and Temperature Parameters on the Microstructure, Hardness, and Strain-Hardening Coefficients of 42CrMo4 Steel. Materials 2020, 13, 2022. [Google Scholar] [CrossRef]
- Yokoyama, K.; Yamamoto, A.; Yamamoto, J. On the Annealing-Temperature and-Time for Spheroidization of Wire Rods for Cold Forming. Tetsu-to-Hagane 1963, 49, 774–780. [Google Scholar]
- Kada, O.; Fujita, T.; Hashimura, M. Development of Advanced Analysis System for the Production of Parts with Bar and Wire Rod; Nippon Steel Technical Report, No. 103; Nippon Steel: Tokyo, Japan, 2013; pp. 121–126. [Google Scholar]
- Park, J.Y.; Ko, W.S.; Park, K.B.; Kang, G.B.; Im, H.T.; Kwon, H.G. Effect of cooling rate on phase transformation behavior during isothermal annealing of SCr420 steel. Steel Res. Int. 2022, 93, 2100711. [Google Scholar]
- Alza, V.A. Spheroidizing in steels: Processes, mechanisms, kinetic and microstructure—A review. IOSR J. Mech. Civ. Eng. 2021, 18, 63–81. [Google Scholar]
- Hwang, H.; De Cooman, B.C. Influence of the initial microstructure on the spheroidization of SAE 52100 bearing steel. Steel Res. Int. 2016, 87, 112–125. [Google Scholar]
- ASTM E562-19; Standard Test Method for Determining Volume Fraction by Systematic Manual Point Counting. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Vander Voort, G.F.; Roosz, A. Measurement of the interlamellar spacing of pearlite. Metallography 1984, 17, 1–17. [Google Scholar]
- Kang, B.S.; Ku, T.W. Process Modification and Numerical Simulation for an Outer Race of a CV Joint using Multi-Stage Cold Forging. Trans. Mater. Process. 2014, 23, 211–219. [Google Scholar]
- Anand, L.; Gurland, J. Effect of internal boundaries on the yield strengths of spheroidized steels. Metall. Trans. A 1976, 7, 191–197. [Google Scholar]
- Karadeniz, E. Influence of different initial microstructure on the process of spheroidization in cold forging. Mater. Des. 2008, 29, 251–256. [Google Scholar]
- Shanmugam, S.; Ramisetti, N.K.; Misra, R.D.K.; Mannering, T.; Panda, D.; Jansto, S. Effect of cooling rate on the microstructure and mechanical properties of Nb-microalloyed steels. Mater. Des. 2007, 460, 335–343. [Google Scholar]
- Hall, E.O. Variation of hardness of metals with grain size. Nature 1954, 173, 948–949. [Google Scholar]
- O’Donnelly, B.E.; Baker, T.N. Strengthening in low carbon pearlitic steels. Mater. Sci. Eng. 1986, 84, 131–135. [Google Scholar]
- O’Donnelly, B.E.; Reuben, R.L.; Baker, T.N. Quantitative assessment of strengthening parameters in ferrite-pearlite steels from microstructural measurements. Metals Technol. 1984, 11, 45–51. [Google Scholar]
- Morrison, W.B. The effect of grain size on the stress-strain relationship in low-carbon steel. ASM Trans. Quart. 1966, 59, 824–846. [Google Scholar]
- Mintz, B. Importance of ky (Hall-Petch slope) in determining strength of steels. Metals Technol. 1984, 11, 265–272. [Google Scholar]
- Hall, E.O. The deformation and ageing of mild steel: III discussion of results. Proc. Phys. Soc. Sect. B 1951, 64, 747. [Google Scholar]
- Dolzhenko, A.; Pydrin, A.; Gaidar, S.; Kaibyshev, R.; Belyakov, A. Microstructure and strengthening mechanisms in an HSLA steel subjected to Tempforming. Metals 2021, 12, 48. [Google Scholar] [CrossRef]
- Ray, K.K.; Mondal, D. The effect of interlamellar spacing on strength of pearlite in annealed eutectoid and hypoeutectoid plain carbon steels. Acta Metall. Mater. 1991, 39, 2201–2208. [Google Scholar]
- Xiong, Z.; Timokhina, I.; Pereloma, E. Clustering, nano-scale precipitation and strengthening of steels. Prog. Mater. Sci. 2021, 118, 100764. [Google Scholar]
- Modi, O.P.; Desmukh, N.; Mondal, D.P.; Jha, A.K.; Yegneswaran, A.H.; Khaira, H.K. Effect of interlamellar spacing on the mechanical properties of 0.65% C steel. Mater. Charact. 2001, 46, 347–352. [Google Scholar]
- Speich, G.R.; Schwoeble, A.J.; Leslie, W.C. Elastic constants of binary iron-base alloys. Metall. Trans. 1972, 3, 2031–2037. [Google Scholar]

| C | Si | Mn | Cr |
|---|---|---|---|
| 0.22 | 0.25 | 0.80 | 1.20 |
| Properties | Unit | Value |
|---|---|---|
| Young’s modulus | GPa | 210 |
| Poisson’s ratio | - | 0.3 |
| Density | g/cm3 | 7.85 |
| Thermal expansion coefficient | 1/°C | 1.2 × 10−5 |
| Mechanical Properties | Tensile Properties | Compressive Stress (MPa) | ||||
|---|---|---|---|---|---|---|
| Y.S 1 (MPa) | U.T.S 2 (MPa) | Elongation (%) | 20% | 40% | 60% | |
| As-rolled | 565 ± 11 | 842 ± 15 | 17 ± 0.7 | 1170 ± 14 | 1520 ± 11 | 2119 ± 19 |
| SA | 362 ± 6 | 556 ± 5 | 30 ± 1.3 | 805 ± 11 | 1169 ± 15 | 1810 ± 17 |
| NSR | 382 ± 5 | 567 ± 8 | 28 ± 1.4 | 810 ± 12 | 1174 ± 17 | 1815 ± 14 |
| Ky | As-rolled | SA | NSR |
|---|---|---|---|
| Unit: MPa/µm1/2 | 601 | 411 | 430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Hwang, S.; Lee, S.; Jang, B. The Effect of Heat Treatment and Cold Forging on the Mechanical Properties of SCr420 Low-Alloy Steel. Metals 2025, 15, 336. https://doi.org/10.3390/met15030336
Lim J, Hwang S, Lee S, Jang B. The Effect of Heat Treatment and Cold Forging on the Mechanical Properties of SCr420 Low-Alloy Steel. Metals. 2025; 15(3):336. https://doi.org/10.3390/met15030336
Chicago/Turabian StyleLim, Jaehan, Soonhong Hwang, Sangwon Lee, and Byounglok Jang. 2025. "The Effect of Heat Treatment and Cold Forging on the Mechanical Properties of SCr420 Low-Alloy Steel" Metals 15, no. 3: 336. https://doi.org/10.3390/met15030336
APA StyleLim, J., Hwang, S., Lee, S., & Jang, B. (2025). The Effect of Heat Treatment and Cold Forging on the Mechanical Properties of SCr420 Low-Alloy Steel. Metals, 15(3), 336. https://doi.org/10.3390/met15030336






