Electrochemical and Tribological Behavior of Dual-Phase Steels Obtained from a Commercial-Grade API 5CT Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Heat Treatments
2.2. Microstructural Characterisation
2.3. Sliding Wear Testing
2.4. Corrosion Test
3. Results
3.1. Microstructural Examination
3.2. Coefficient of Friction (CO)
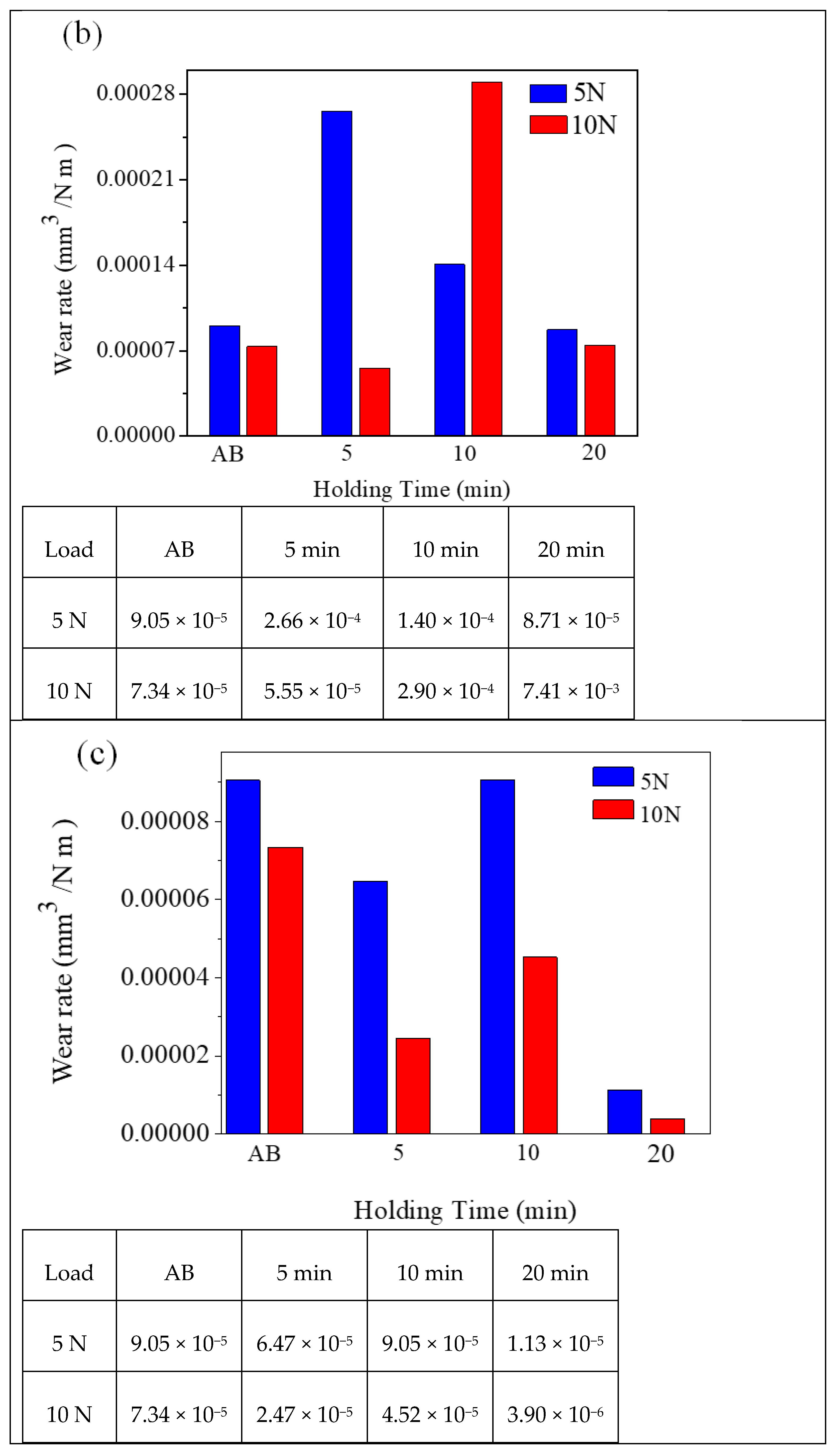
3.3. Wear Rate and Wear Coefficient K
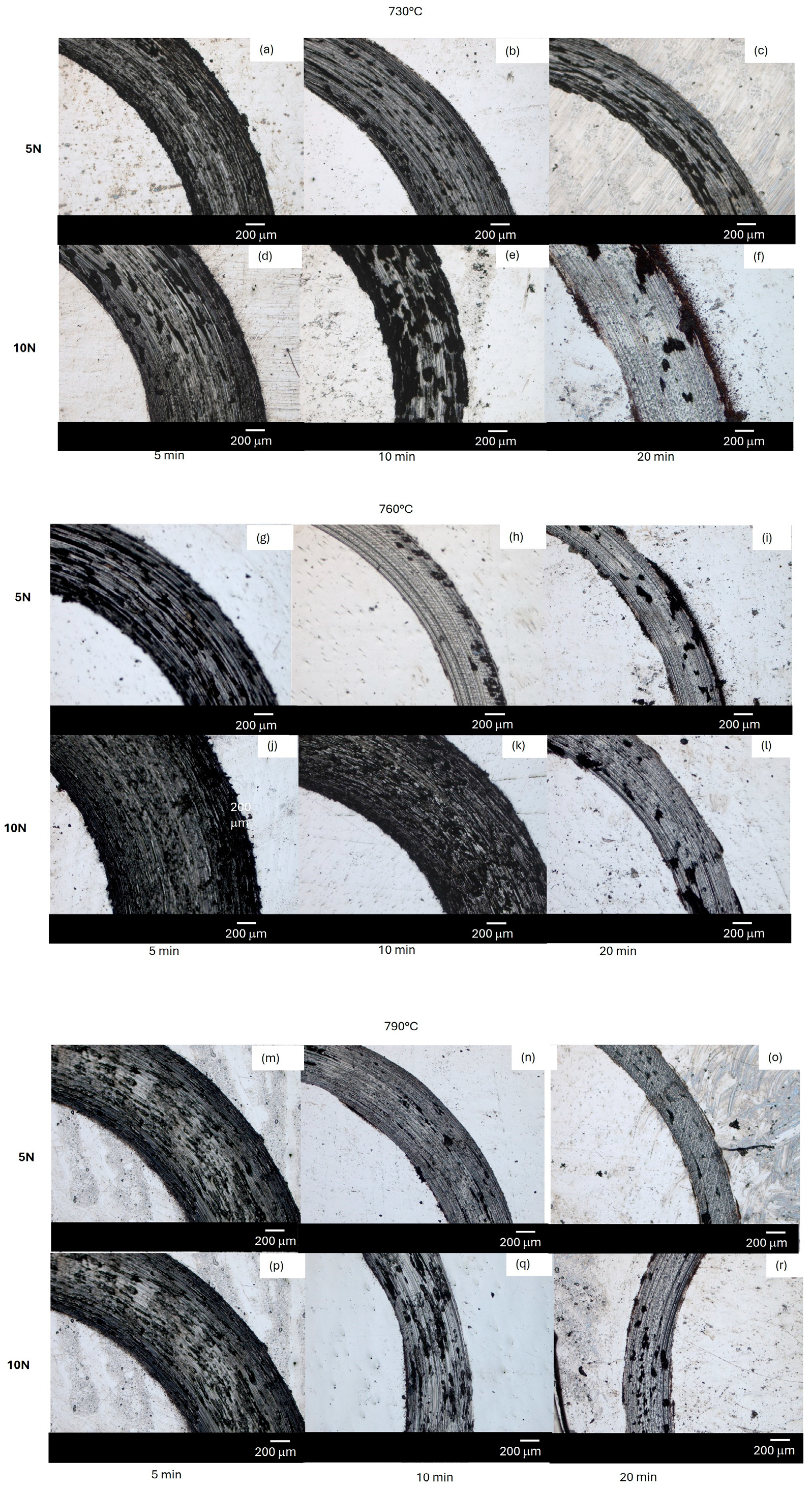
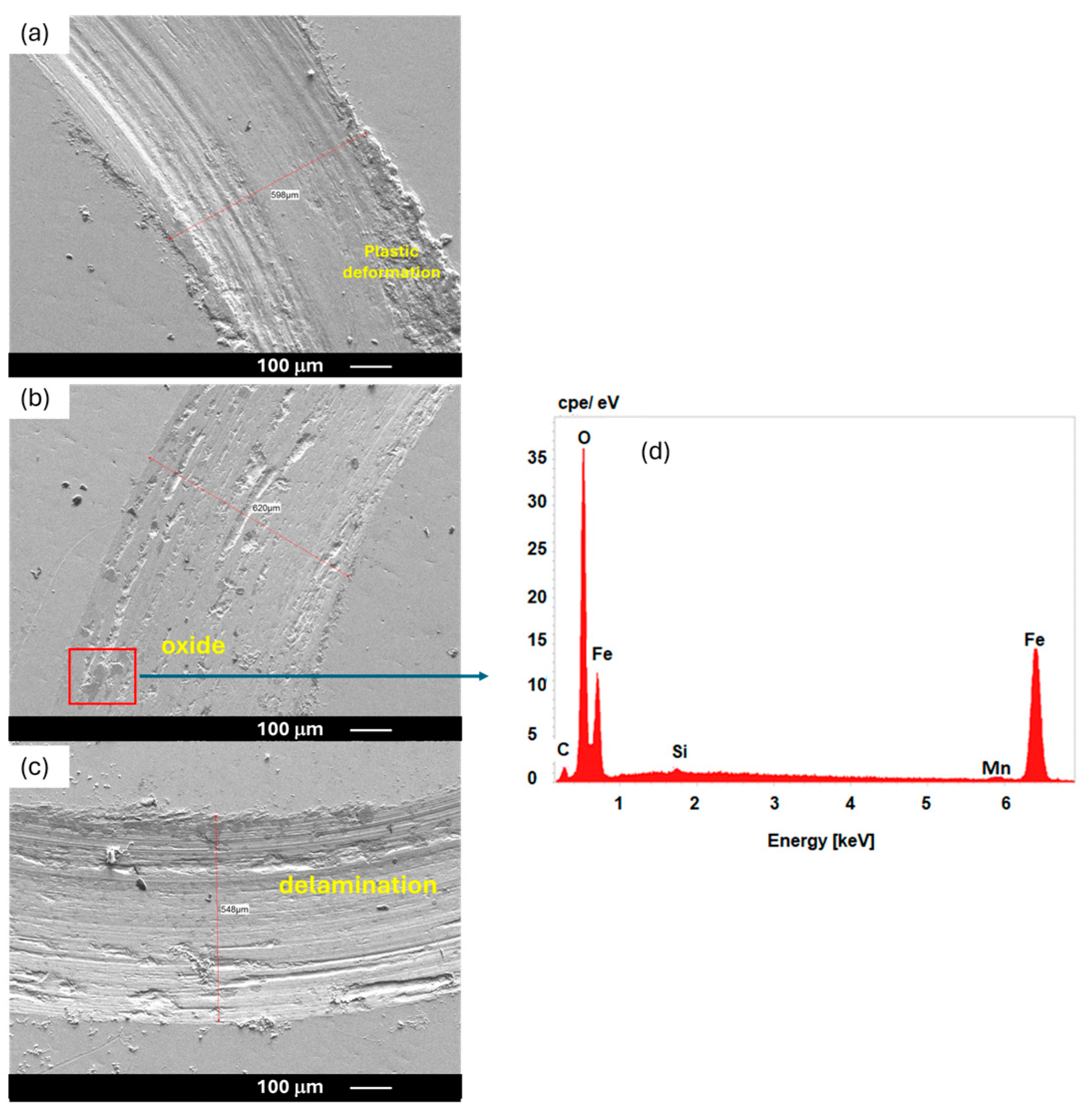
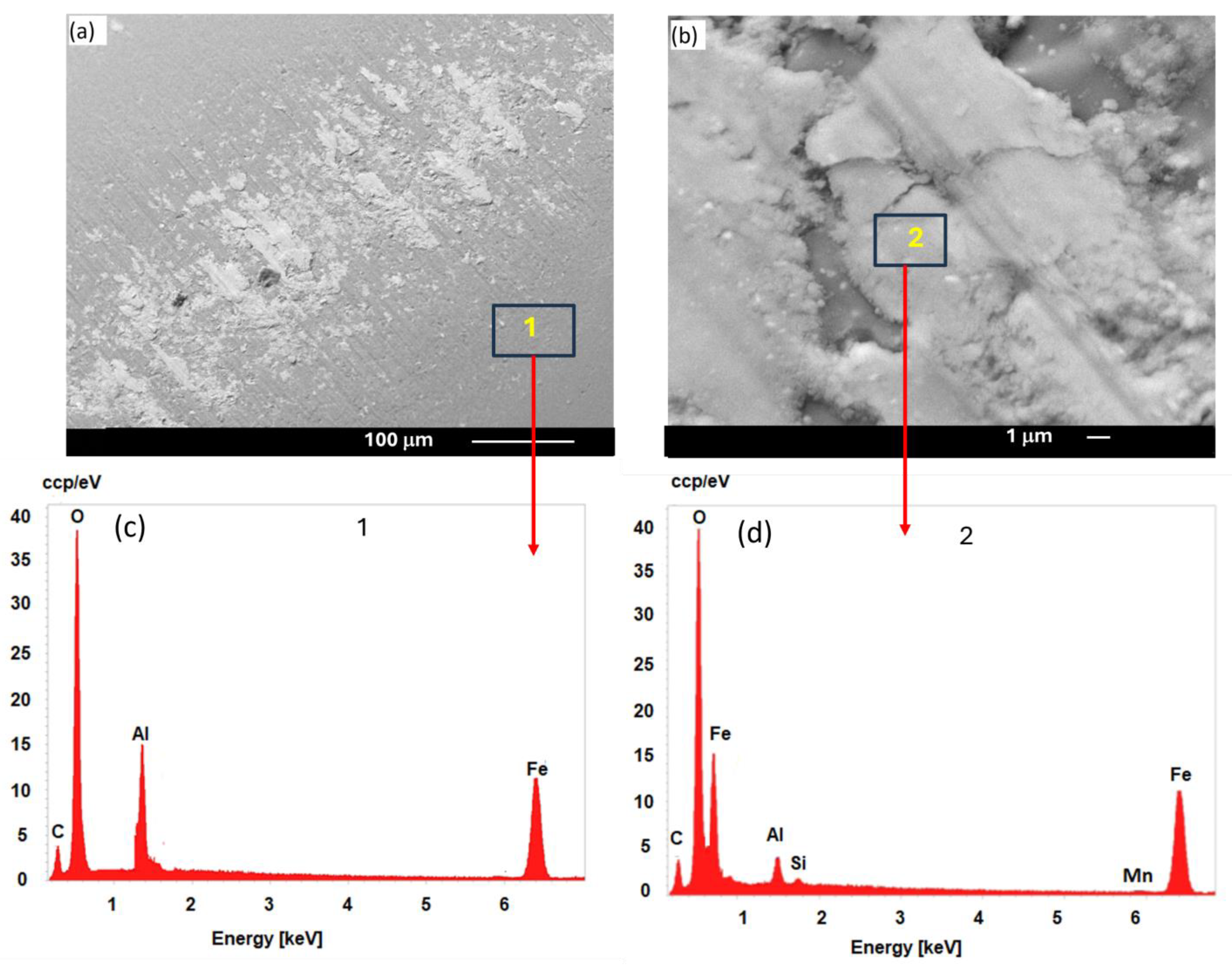
3.4. Optical and SEM Micrograph of Contact Surface
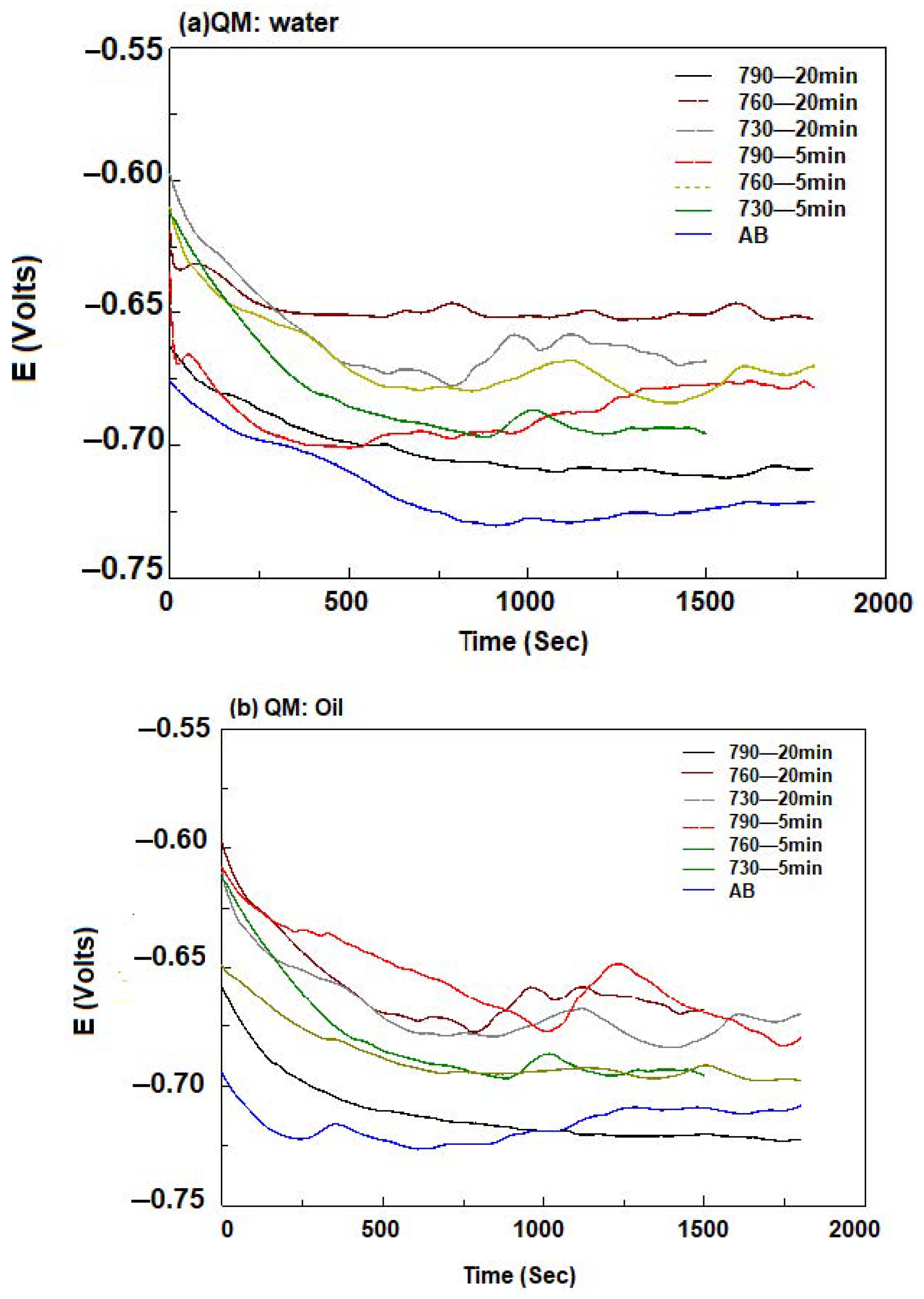
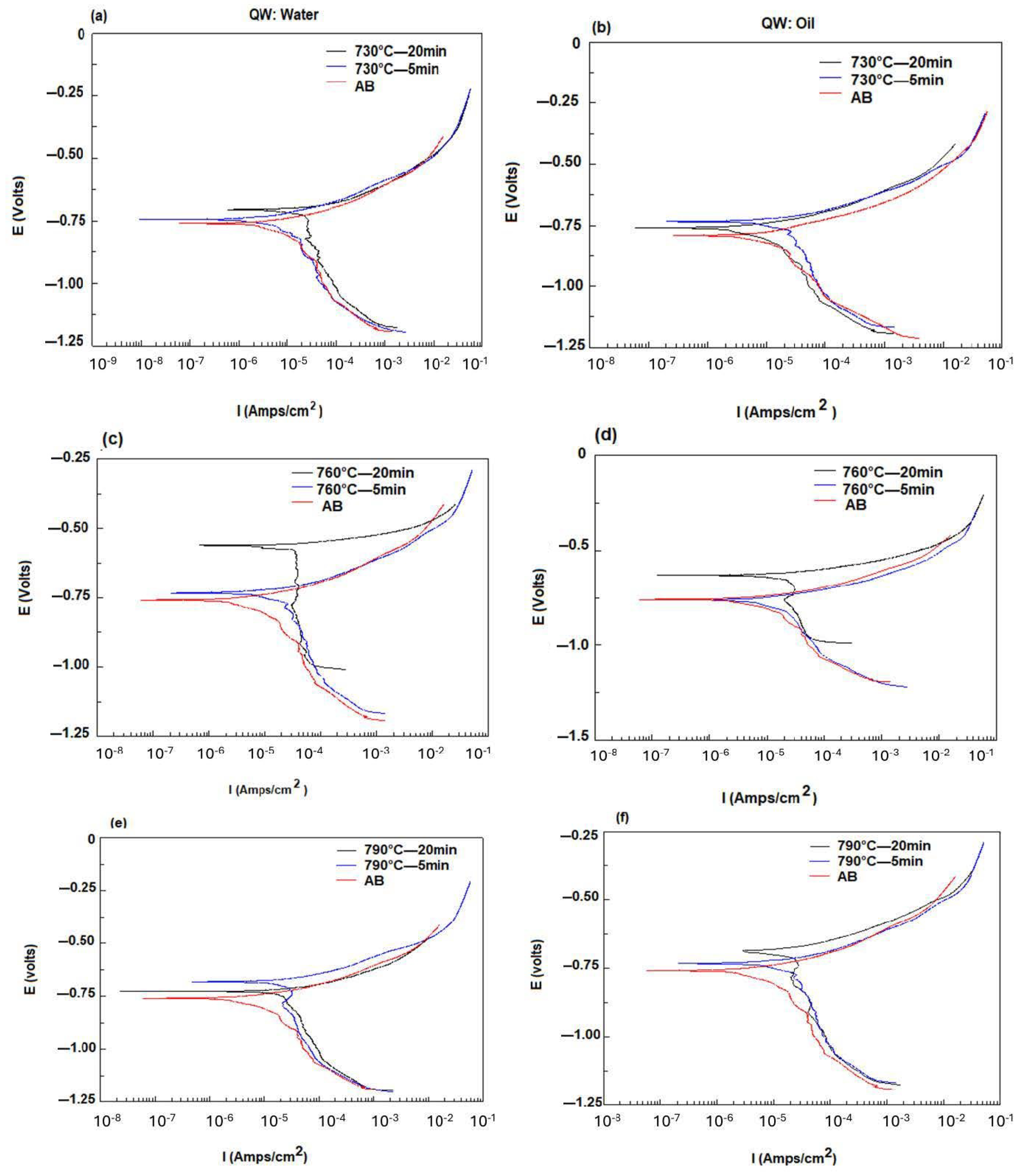
3.5. Electrochemical Behavior of API5CT
4. Conclusions
- (1)
- Hardness increases with the increase in VFSP and the reduction in grain size. The higher the percentage of martensite and the smaller the grains, the greater the hardness.
- (2)
- The friction coefficient is directly proportional to the VFSP.
- (3)
- For samples with low VFSP (30% or less), there is significant wear compared with high-VFSP samples.
- (4)
- As the VFSP decreases, the oxidative and abrasion wear increases. The formation of a compact oxide film (iron oxide) can effectively reduce wear, especially adhesion wear. For low VFSP, compact oxide layers are more likely to form and remain on worn surfaces.
- (5)
- The predominant wear mechanisms were mild oxidation at lower values and severe adhesion at higher values of VFSP.
- (6)
- The increase in the percentage of martensite in dual-phase steel will give a lower corrosion rate. This shows that the percentage of martensite is inversely proportional to the corrosion rate.
- (7)
- Results revealed that intercritical annealing at temperatures of 730, 760, and 790 °C is a suitable option for the increase in the wear resistance of the API 5CT dual-phase steel.
- (8)
- Dual-phase steel API 5CT is susceptible to micro-galvanic corrosion when exposed to an NaCl solution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linares, C.A.G.; Aguilar, M.d.J.S.; Guerra, J.G.; Luevanos, A.M. Efecto del Tratamiento Térmico sobre la Microestructura y Resistencia al Desgaste de un Acero API. Cienc. Lat. Rev. Científica Multidiscip. 2024, 8, 5593–5607. [Google Scholar]
- Hara, T.; Semba, H.; Amaya, H. Pipe and Tube Steels for Oil and Gas Industry and Thermal Power Plant. In Encyclopedia of Materials: Metals and Alloys; Caballero, F.G., Ed.; Elsevier: Oxford, UK, 2022; pp. 140–152. [Google Scholar]
- Abdelnabe, J.P.; Tuckart, W.R.; Crespo, M.I.; Prieto, G. Mechanical properties and galling resistance of API grade steels: A comparative study. Wear 2025, 560–561, 205593. [Google Scholar] [CrossRef]
- Cuenca Quiñonez, S.J.; Cevallos Ibarra, N.D.; Bravo López, E.B. Desarrollo de procedimiento de fabricación de roscas según API 5B–API 5CT: Development of thread manufacturing procedure according TO API 5B–API 5CT. Rev. Científica Multidiscip. G-Nerando 2024, 5, 240–253. [Google Scholar] [CrossRef]
- Shaanxi World Iron & Steel Co. Una breve introducción de API 5L, API 5B, API 5CT y API 5D. World Iron & Steel, 6 March 2018. [Google Scholar]
- Terán, G.; Capula-Colindres, S.; Velazques, J.C.; Gonzalez-Arevalo, N.E.; Santillan, E.S.; Angeles-Herrera, D.; Cervantes-Tobon, A. Effect of the Aging Process in the Failure Pressure Estimation in an API 5L Gr. B Cracked Pipeline Using Finite Element Modeling. Coatings 2025, 15, 29. [Google Scholar]
- Wu, Q.; He, S.; Hu, P.; Liu, Y.; Zhang, Z.; Fan, C.; Fan, R.; Zhong, N. Effect of finish rolling temperature on microstructure and mechanical properties of X80 pipeline steel by on-line quenching. Mater. Sci. Eng. A 2023, 862, 144496. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, L.; Jakhar, A. To Investigate the Effect of Heat Treatment on API X70 Pipeline Steel. Natl. Acad. Sci. Lett. 2024, 47, 521–528. [Google Scholar] [CrossRef]
- API Specification 5CT, Specification for Casing and Tubing, 9th Edition 2011. Available online: https://www.octalsteel.com/pdf/api-spec-5ct-specification-for-casing-and-tubing.pdf (accessed on 31 December 2024).
- Liu, M.; Wang, C.H.; Dai, Y.C.; Li, X.; Cao, G.H.; Russell, A.M.; Liu, Y.H.; Dong, X.M.; Zhang, Z.H. Effect of quenching and tempering process on sulfide stress cracking susceptibility in API-5CT-C110 casing steel. Mater. Sci. Eng. A 2017, 688, 378–387. [Google Scholar] [CrossRef]
- Alcantar-Martínez, L.M.; Ruiz-Trabolsi, P.A.; Tadeo-Rosas, R.; Miranda-Hernandez, J.G.; Cabrera-Sierra, R.; Velazquez, J.C.; Hernandez-Sanchez, E. Improving the Surface Properties of an API 5L Grade B Pipeline Steel by Applying the Boriding Process—Part II: On the Changes in the Mechanical Properties. Coatings 2023, 13, 470. [Google Scholar] [CrossRef]
- Nakata, H.; Kami, C.; Mathuo, N. Development of API X80 Grade Electric Resistance Welding Line Pipe with Excellent Low Temperature Toughness. JFE Technical Report 2008, 12, 27–31. [Google Scholar]
- Sharma, L.; Dhiman, A.; Jakhar, A.; Sharma, K. Experimental Investigation on Corrosion Behaviour of Heat-Treated API X70 Pipeline Steel. Adv. Mater. Sci. 2024, 24, 70–91. [Google Scholar] [CrossRef]
- Slimani, A.; Lemmadi, F.Z.; Hamdi, I.; Bentrah, H. Heat treatment effect on the corrosion rate, microstructure and mechanical properties of X60 pipeline steel. Stud. Eng. Exact Sci. 2024, 5, e12315. [Google Scholar] [CrossRef]
- Krauss, G. Martensite in steel: Strength and structure. Mater. Sci. Eng. A 1999, 273–275, 40–57. [Google Scholar] [CrossRef]
- Badinier, G.; Sinclair, C.W.; Allain, S.; Danoix, F.; Goune, M. The Mechanisms of Transformation and Mechanical Behavior of Ferrous Martensite. In Reference Module in Materials Science and Materials Engineering; Elsevier: Oxford, UK, 2017; pp. 1–34. [Google Scholar]
- Kot, R.A.; Bramfitt, B.L. Fundamentals of Dual-phase Steels: Proceedings of a Symposium. In Proceedings of the 110th AIME Annual Meeting, Chicago, IL, USA, 23–24 February 1981. [Google Scholar]
- Davies, R.G. Influence of martensite composition and content on the properties of dual phase steels. Metall. Trans. A 1978, 9, 671–679. [Google Scholar] [CrossRef]
- Bleck, W. Cold-rolled, high-strength sheet steels for auto applications. JOM 1996, 48, 26–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Huang, G.; Wang, Y.; Li, Q.; He, J. Influence of Martensite/Bainite Dual Phase-Content on the Mechanical Properties of EA4T High-Speed Axle Steel. Materials 2023, 16, 4657. [Google Scholar] [CrossRef]
- Çiftçi, Y.S.; Aygun, Z.; Gecu, R. Annealing temperature-dependent microstructure, mechanical, and tribological properties of intercritically heat-treated dual-phase steel. Mater. Res. Express 2024, 11, 116511. [Google Scholar] [CrossRef]
- Fekete, J.R.; Stibich, A.M.; Shi, M.F. A Comparison of the Response of HSLA and Dual Phase Sheet Steel in Dynamic Crush; SAE Technical Paper Series International Body Engineering Conference & Exposition; SAE International: Warrendale, PA, USA, 2001; pp. 1–7. [Google Scholar]
- Zhang, Y.; Jourani, A. Effect of Martensite Volume Fraction on Oxidative and Adhesive Wear. Materials 2021, 14, 2964. [Google Scholar] [CrossRef]
- Tasan, C.C.; Diehl, M.; Yan, D.; Bechtold, M.; Roters, F.; Schemmann, L.; Zheng, C.; Peranio, N.; Ponge, D.; Koyama, M.; et al. An Overview of Dual-Phase Steels: Advances in Microstructure-Oriented Processing and Micromechanically Guided Design. Annu. Rev. Mater. Res. 2015, 45, 391–431. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhu, X.; Zhang, L. Effect of martensite morphology and volume fraction on the low-temperature impact toughness of dual-phase steels. Mater. Sci. Eng. A 2022, 832, 142424. [Google Scholar] [CrossRef]
- Shi, P.; Ren, Y.; Zhang, X.; Wang, H.; Chen, J.; Pei, Y.; Yan, J.; Zheng, H.; Zhang, J. Role of martensite morphology on mechanical response of dual-phase steel produced by partial reversion from martensite. Mater. Sci. Eng. A 2024, 893, 146116. [Google Scholar] [CrossRef]
- Davenport, A.T. Formable HSLA and Dual-Phase Steels: Proceedings of a Symposium; Metallurgical Society of AIME: Wilkes-Barre, PA, USA, 1979. [Google Scholar]
- Guerra Linares, C.A.; Aguilar, M.d.J.S.; Guerra, J.G.; Luevanos, A.M. Efecto del Tratamiento Térmico de Recocido Intercritico sobre la Microestructura y su Resistencia a Corrosión y Desgaste de un Acero Grado PJ55. Cienc. Cierta 2022, 70, 139–155. [Google Scholar]
- Trevisiol, C.; Jourani, A.; Bouvier, S. Effect of abrasive particle size on friction and wear behaviour of various microstructures of 25CD4 steel. J. Phys. Conf. Ser. 2017, 843, 012073. [Google Scholar] [CrossRef]
- de la Concepcióna, V.L.; Lorusso, H.N.; Svoboda, H.G. Effect of carbon content on microstructure and mechanical properties of dual phase steels. Procedia Mater. Sci. 2015, 8, 1047–1056. [Google Scholar] [CrossRef]
- Zambrano, O.A.; Gomez, J.A.; Coronado, J.J.; Rodriguez, S.A. The sliding wear behaviour of steels with the same hardness. Wear 2019, 418–419, 201–207. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Gou, H.; Chen, S.; Wu, D.; Wei, H.; Chong, X.; Li, Z.; Wu, X.; Shan, Q. Improving the impact wear properties of medium carbon steel by adjusting microstructure under alternating quenching in water and air. Wear 2023, 512–513, 204531. [Google Scholar] [CrossRef]
- Thakare, A.S.; Butee, S.P.; Kambale, K.R. Microstructural aspects of tensile strength, toughness and wear for 34CrMo4 steel. Mater. Today Proc. 2021, 43, 2985–2992. [Google Scholar] [CrossRef]
- Viáfara, C.C.; Sinatora, A. Influence of hardness of the harder body on wear regime transition in a sliding pair of steels. Wear 2009, 267, 425–432. [Google Scholar] [CrossRef]
- Hanief, M.; Charoo, M.S. Archard’s wear law revisited to measure accurate wear coefficient considering actual sliding velocity. Mater. Today Proc. 2021, 47, 5598–5600. [Google Scholar] [CrossRef]
- Handoko, W.; Pahlevani, F.; Hossain, R.; Sahajwalla, V. Stress-Induced Phase Transformation and Its Correlation with Corrosion Properties of Dual-Phase High Carbon Steel. J. Manuf. Mater. Process. 2019, 3, 55. [Google Scholar] [CrossRef]
- Castillo Gutiérrez, D.E., II; Moncaleano, A.; Baracaldo, R.R. Caracterización microestructural y mecánica de aceros de fase dual (ferrita-martensita), obtenidos mediante procesos térmicos y termomecánicos. Ingeniare. Rev. Chil. De Ing. 2018, 26, 430–439. [Google Scholar] [CrossRef]
- Snape, E. Roles of Composition and Microstructure in Sulfide Cracking of Steel. Corrosion 1968, 24, 261–282. [Google Scholar] [CrossRef]
- Abouei, V.; Saghafian, H.; Kheirandish, S.; Ranjbar, K. An investigation of the wear behaviour of 0.2% C dual phase steels. J. Mater. Process. Technol. 2008, 203, 107–112. [Google Scholar] [CrossRef]
- Tyagi, R.; Nath, S.K.; Ray, S. Dry sliding friction and wear in plain carbon dual phase steel. Metall. Mater. Trans. A 2001, 32, 359–367. [Google Scholar] [CrossRef]
- Xu, X.; van der Zwaag, S.; Xu, W. The effect of ferrite–martensite morphology on the scratch and abrasive wear behaviour of a dual phase construction steel. Wear 2016, 348–349, 148–157. [Google Scholar] [CrossRef]
- Salamci, E.; Candan, S.; Kabakci, F. Effect of microstructure on corrosion behavior of dual-phase steels. Kovove Mater. 2017, 55, 133–139. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Vinaya; Madhusudhan, R.; Sah, R.; Manjini, S. Mechanical and Electrochemical Behavior of Dual-Phase Steels Having Varying Ferrite–Martensite Volume Fractions. J. Mater. Eng. Perform. 2019, 28, 3600–3613. [Google Scholar]
- Sarkar, P.P.; Kumar, P.; Kumar Manna, M.; Chakraborti, P.C. Microstructural influence on the electrochemical corrosion behaviour of dual-phase steels in 3.5% NaCl solution. Mater. Lett. 2005, 59, 2488–2491. [Google Scholar] [CrossRef]
- Osorio, W.R.; Peixoto, L.C.; Garcia, L.R.; Garcia, A. Electrochemical corrosion response of a low carbon heat treated steel in a NaCl solution. Mater. Corros. 2009, 60, 804–812. [Google Scholar] [CrossRef]
- Keleştemur, O.; Yıldız, S. Effect of various dual-phase heat treatments on the corrosion behavior of reinforcing steel used in the reinforced concrete structures. Constr. Build. Mater. 2009, 23, 78–84. [Google Scholar] [CrossRef]
- Nadlene, R.; Esah, H.; Norliana, S.; Mohd Irwan, M.A. Study on the Effect of Volume Fraction of Dual Phase Steel to Corrosion Behaviour and Hardness. Int. J. Mech. Mechatron. Eng. 2011, 50, 393–396. [Google Scholar]
- ISO 11960:2014; Petroleum and Natural Gas Industries-Steel Pipes for Use as Casing or Tubing for Wells. ISO: Genewa, Switzerland, 2014.
- E407-07; Standard Practice for Microetching Metals and Alloy. ASTM: West Conshohocken, PA, USA, 2015.
- ASTM G99-17; Standard Test Method for ASTM International Wear Testing with a Pin-on-Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 2016.
- Nikkhah, S.; Mirzadeh, H.; Zamani, M. Fine tuning the mechanical properties of dual phase steel via thermomechanical processing of cold rolling and intercritical annealing. Mater. Chem. Phys. 2019, 230, 1–8. [Google Scholar] [CrossRef]
- Kuang, S.; Kang, Y.-L.; Yu, H.; Liu, R.-D. Effect of continuous annealing parameters on the mechanical properties and microstructures of a cold rolled dual phase steel. Int. J. Miner. Metall. Mater. 2009, 16, 159–164. [Google Scholar] [CrossRef]
- Huyan, F.; Hedström, P.; Borgenstam, A. Modelling of the Fraction of Martensite in Low-alloy Steels. Mater. Today Proc. 2015, 2, S561–S564. [Google Scholar] [CrossRef]
- Monkova, K.; Zetkova, I.; Kucerova, L.; Zetek, M.; Monka, P.; Dana, M. Study of 3D printing direction and effects of heat treatment on mechanical properties of MS1 maraging steel. Arch. Appl. Mech. 2019, 89, 791–804. [Google Scholar] [CrossRef]
- Xiao, X.; Shi, G.; Zhang, S.; Wang, Q. Effect of Cooling Path on Microstructure Features and Tensile Properties in a Low Carbon Mo-V-Ti-N Steel. Metals 2018, 8, 677. [Google Scholar] [CrossRef]
- Kong, Z.; Zhang, J.; Li, H.; Kong, N. Effects of continuous annealing process parameters on the microstructure and mechanical properties of dual phase steel. Steel Res. Int. 2018, 89, 1800034. [Google Scholar] [CrossRef]
- Gholamalipour, S.; Jamaati, R.; Hosseinipour, S.J. The effect of high-temperature intercritical annealing and rolling strain on the development of trimodal microstructure in low-carbon steel. J. Mater. Res. Technol. 2025, 34, 1529–1538. [Google Scholar] [CrossRef]
- Garcia-Mateo, C. Bainite and Martensite: Developments and Challenges. Metals 2018, 8, 966. [Google Scholar] [CrossRef]
- Miles, M.P.; Nelson, T.W.; Gunter, C.; Liu, F.C.; Fourment, L.; Mathis, T. Predicting recrystallized grain size in friction stir processed 304L stainless steel. J. Mater. Sci. Technol. 2019, 35, 491–498. [Google Scholar] [CrossRef]
- Fonstein, N. 7-Dual-phase steels. In Automotive Steels, Design, Metallurgy, Processing and Applications; Rana, R., Singh, S.B., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 169–216. [Google Scholar]
- Kocatepe, K.; MCerah; Erdogan, M. Effect of martensite volume fraction and its morphology on the tensile properties of ferritic ductile iron with dual matrix structures. J. Mater. Process. Technol. 2006, 178, 44–51. [Google Scholar] [CrossRef]
- Basantia, S.K.; Bhattacharya, A.; Khutia, N.; Das, D. Influence of microstructural parameters on nanohardness of various dual-phase steels: Experiment, Finite Element simulation and Statistical analysis. Mater. Today Commun. 2022, 30, 103125. [Google Scholar] [CrossRef]
- Maffei, B.; Salvatore, W.; Valentini, R. Dual-phase steel rebars for high-ductile r.c. structures, Part 1: Microstructural and mechanical characterization of steel rebars. Eng. Struct. 2007, 29, 3325–3332. [Google Scholar] [CrossRef]
- Han, R.; Yang, G.; Zhao, G.; Sun, X.; Zhu, X. Strengthening mechanism and three-body impact abrasive wear behavior of the hot-rolled air-cooling martensitic wear resistant steel. J. Mater. Res. Technol. 2023, 24, 3023–3032. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, J.; Jiang, S.; Cao, A.; Yao, Y.; Sun, Y.; Li, S.; Zhang, Z. Study of the Effects on the Strengthening Mechanism and Wear Behavior of Wear-Resistant Steel of Temperature Controlling in Heat Treatment. Nanomaterials 2024, 14, 1171. [Google Scholar] [CrossRef]
- Singh, P.P.; Ghosh, S.; Mula, S. Characterization of dry-sliding wear phenomena in a novel martensitic structure based low-carbon Nb and V alloyed steel. Tribol. Int. 2023, 189, 108959. [Google Scholar] [CrossRef]
- Modi, O.P.; Pandit, P.; Mondal, D.P.; Prasad, B.K. High-stress abrasive wear response of 0.2% carbon dual phase steel: Effects of microstructural features and experimental conditions. Mater. Sci. Eng. A 2007, 458, 303–311. [Google Scholar] [CrossRef]
- Tyagi, R.; Nath, S.K.; Ray, S. Effect of martensite content on friction and oxidative wear behavior of 0.42 Pct carbon dual-phase steel. Metall. Mater. Trans. A 2002, 33, 3479–3488. [Google Scholar] [CrossRef]
- León-Patiño, C.A.; García-Guerra, J.; Aguilar-Reyes, E.A. Tribological characterization of heat-treated Ni-P and Ni-P-Al2O3 composite coatings by reciprocating sliding tests. Wear 2019, 426–427, 330–340. [Google Scholar] [CrossRef]
- Bahrami, A.; Mousavi Anijdan, S.H.; Golozar, M.A.; Shamanian, M.; Varahram, N. Effects of conventional heat treatment on wear resistance of AISI H13 tool steel. Wear 2005, 258, 846–851. [Google Scholar] [CrossRef]
- So, H.; Yu, D.S.; Chuang, C.Y. Formation and wear mechanism of tribo-oxides and the regime of oxidational wear of steel. Wear 2002, 253, 1004–1015. [Google Scholar] [CrossRef]
- Sakrani, S.B.; Sullivan, J.L. Iron oxide films in tribological surfaces of alloy steel. Proc. SPIE 1998, 3175, 175–179. [Google Scholar]
- Hokkirigawa, K.; Kato, K. An experimental and theoretical investigation of ploughing, cutting and wedge formation during abrasive wear. Tribol. Int. 1988, 21, 51–57. [Google Scholar] [CrossRef]
- Tang, A.; Liu, H.; Chen, R.; Liu, G.; Lai, Q.; Zhong, Y.; Wang, L.; Wang, J.; Lu, Q.; Shen, Y. Mesoscopic origin of damage nucleation in dual-phase steels. Int. J. Plast. 2021, 137, 102920. [Google Scholar] [CrossRef]
- Inman, I.A.; Datta, P.S. Studies of high temperature sliding wear of metallic dissimilar interfaces III: Incoloy MA956 versus Incoloy 800HT. Tribol. Int. 2010, 43, 2051–2071. [Google Scholar] [CrossRef]
- Lekatou, A.; Karantzalis, A.E.; Evangelou, A.; Gousia, V.; Kaptay, G.; Gasci, Z.; Baumli, P.; Simon, A. Aluminium reinforced by WC and TiC nanoparticles (ex-situ) and aluminide particles (in-situ): Microstructure, wear and corrosion behaviour. Mater. Des. (1980–2015) 2015, 65, 1121–1135. [Google Scholar] [CrossRef]
- Sue, J.A.; Troue, H.H. Friction and wear properties of titanium nitride coating in sliding contact with AISI 01 steel. Surf. Coat. Technol. 1990, 43–44, 709–720. [Google Scholar] [CrossRef]
- Kayaba, T.; Iwabuchi, A. Effect of the hardness of hardened steels and the action of oxides on fretting wear. Wear 1981, 66, 27–41. [Google Scholar] [CrossRef]
- Calderón, J.A.; Rossa Mattos, Ó.; Esteves Barcia, O. Análisis de la evolución del potencial de circuito abierto del cobalto en medio levemente alcalino. Rev. Fac. De Ing. Univ. De Antioq. 2006, 38, 20–30. [Google Scholar] [CrossRef]
- Zadeh, M.K.; Yeganeh, M.; Shoushtari, M.T.; Ramezanalizadeh, H.; Seidi, F. Microstructure, corrosion behavior, and biocompatibility of Ti-6Al-4 V alloy fabricated by LPBF and EBM techniques. Mater. Today Commun. 2022, 31, 103502. [Google Scholar] [CrossRef]
- Ochoa, N.; Vega, C.; Pebere, N.; Lacaze, J.; Brito, J.L. CO2 corrosion resistance of carbon steel in relation with microstructure changes. Mater. Chem. Phys. 2015, 156, 198–205. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Z.; Lu, L.; Huang, Y.; Li, X. Correlation of micro-galvanic corrosion behavior with corrosion rate in the initial corrosion process of dual phase steel. J. Mater. Res. Technol. 2021, 15, 3310–3320. [Google Scholar] [CrossRef]
- Fontana, M.G.; Greene, N.D. Corrosion Engineering; Mc Graw Hill: New York, NY, USA, 1986. [Google Scholar]
- Fushimi, K.; Yanagisawa, K.; Nakanishi, T.; Hasegawa, Y.; Kawano, T.; Kimura, M. Microelectrochemistry of dual-phase steel corroding in 0.1 M sulfuric acid. Electrochim. Acta 2013, 114, 83–87. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.; Zhou, Y.; He, X.; Wang, C.; Ke, W. Influence of the secondary phase on micro galvanic corrosion of low carbon bainitic steel in NaCl solution. Mater. Charact. 2018, 139, 401–410. [Google Scholar] [CrossRef]
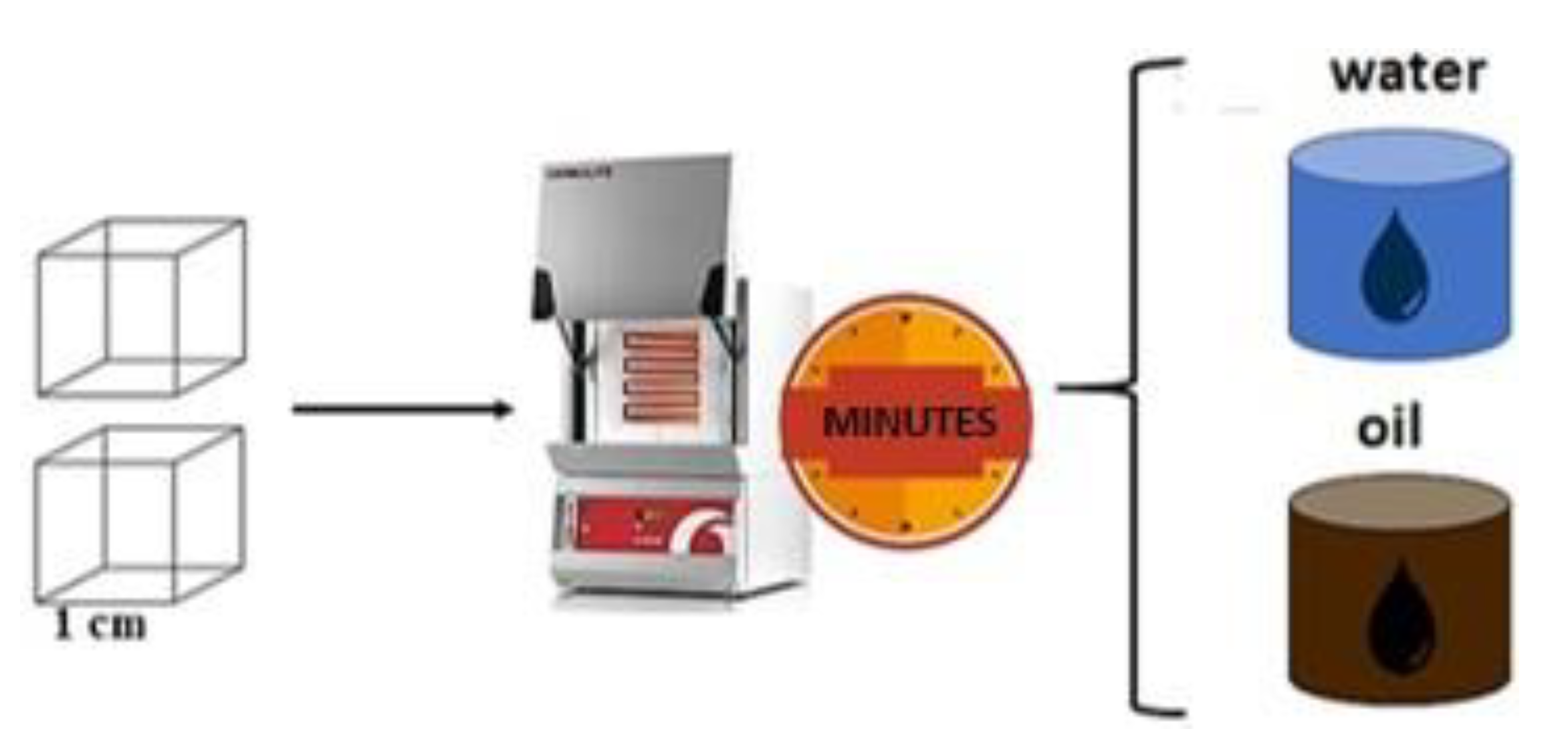


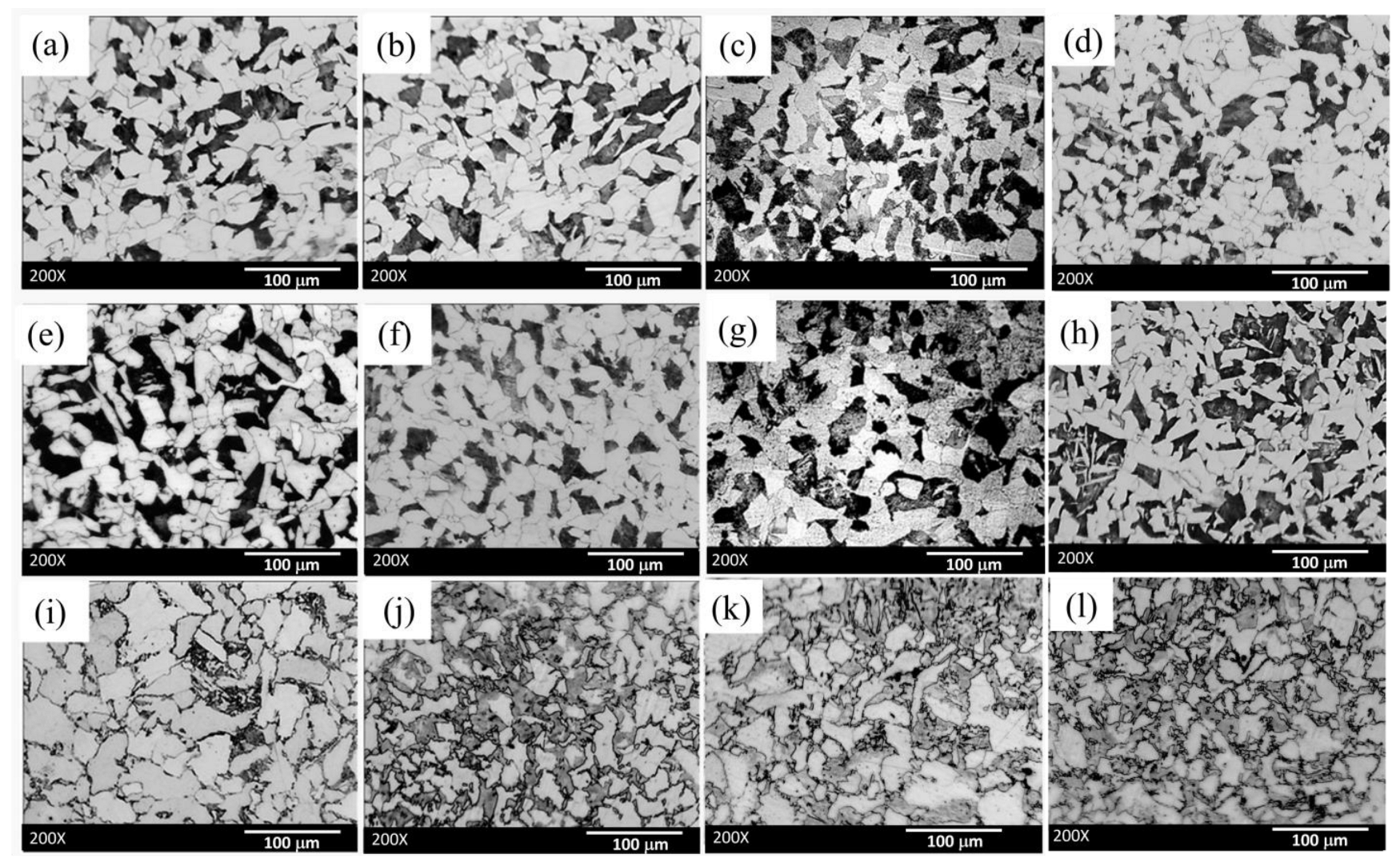

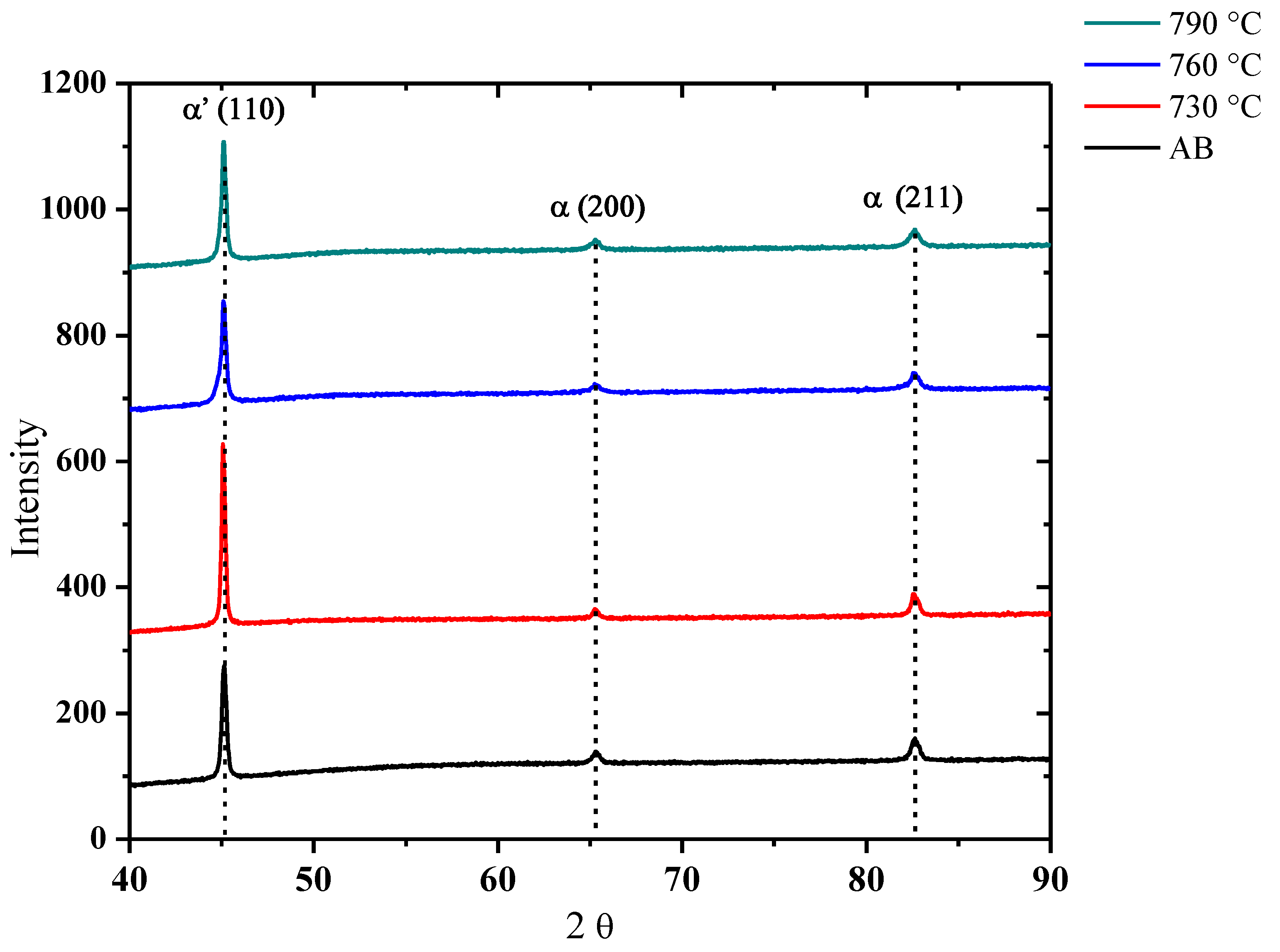
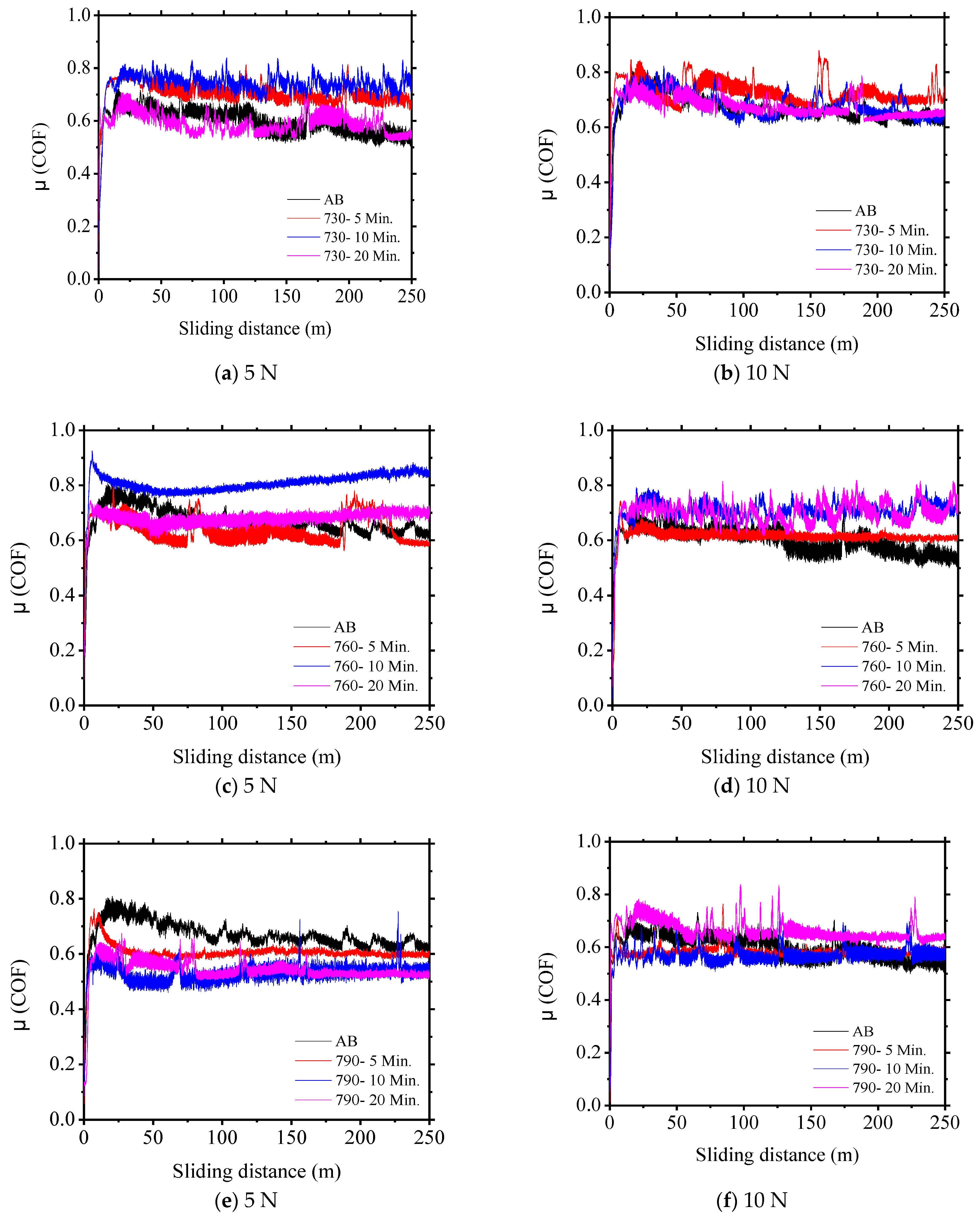


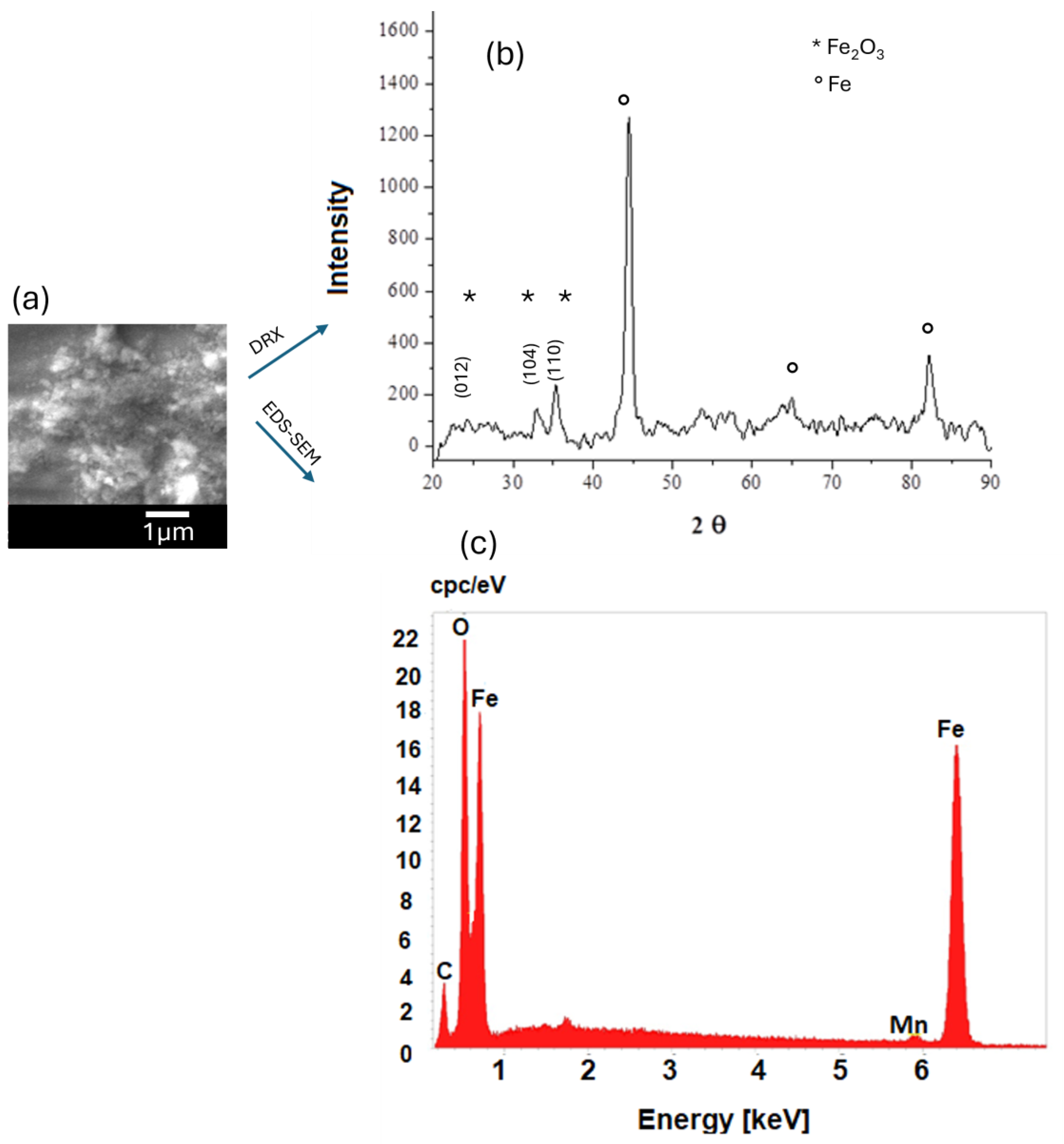
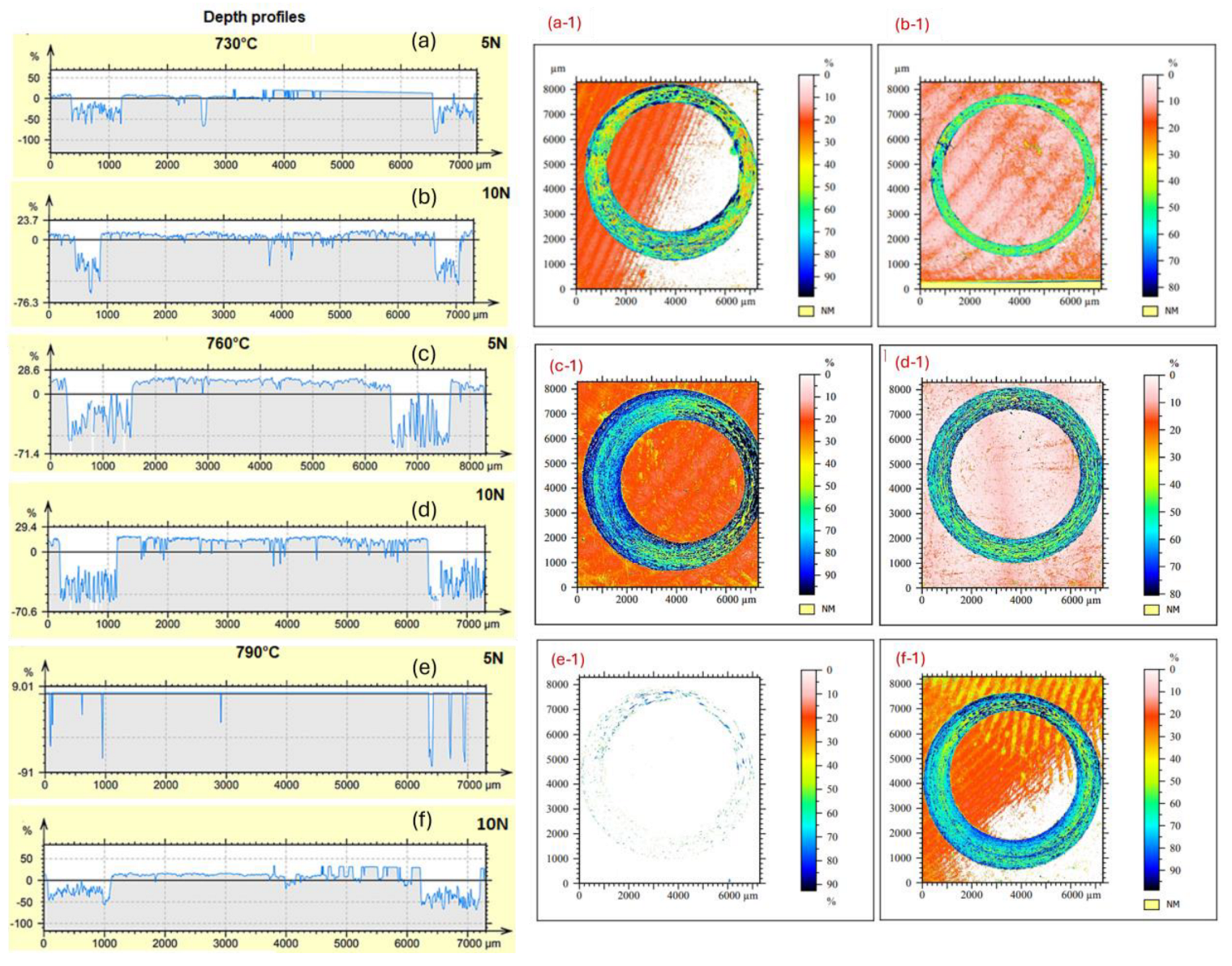

| Sample | C | Si | Cr | Ni | Mn |
|---|---|---|---|---|---|
| API 5CT Steel (AB) | 0.23 | 0.26 | ≤0.10 | ≤0.08 | ≤1.3 |
| T (°C) | Time (min) | ASTM-E112 (G) |
|---|---|---|
| Base steel (AB) | - | 6 |
| 730 | 2 | 7 |
| 5 | 8 | |
| 10 | 8 | |
| 20 | 8 | |
| 760 | 2 | 7 |
| 5 | 8 | |
| 10 | 8 | |
| 20 | 9 | |
| 790 | 2 | 8 |
| 5 | 8 | |
| 10 | 9 | |
| 20 | 9 |
| T (°C) | Holding Time (min) | Cooling | VFSP (vol%) | F (vol%) |
|---|---|---|---|---|
| 730 | 2 | water | 23.82 | 76.17 |
| 730 | 5 | water | 37.04 | 62.96 |
| 730 | 10 | water | 37.75 | 62.25 |
| 730 | 20 | water | 41.33 | 58.67 |
| 730 | 2 | oil | 13.25 | 86.75 |
| 730 | 5 | oil | 15.82 | 84.18 |
| 730 | 10 | oil | 19.79 | 80.21 |
| 730 | 20 | oil | 27.48 | 72.52 |
| 760 | 2 | water | 25.8 | 74.2 |
| 760 | 5 | water | 36.95 | 63.05 |
| 760 | 10 | water | 40.6 | 59.4 |
| 760 | 20 | water | 45.03 | 54.97 |
| 760 | 2 | oil | 15.19 | 84.81 |
| 760 | 5 | oil | 16.23 | 83.77 |
| 760 | 10 | oil | 19.79 | 80.21 |
| 760 | 20 | oil | 28.56 | 71.44 |
| 790 | 2 | water | 34.54 | 65.46 |
| 790 | 5 | Water | 33.71 | 66.29 |
| 790 | 10 | water | 45.87 | 54.13 |
| 790 | 20 | water | 50.61 | 49.39 |
| 790 | 2 | oil | 25.14 | 74.86 |
| 790 | 5 | oil | 27.56 | 72.44 |
| 790 | 10 | oil | 36.54 | 63.46 |
| 790 | 20 | oil | 38.52 | 61.48 |
| T. RI. (°C) | t. RI. (min) | HRB | HVF | HVM | HV |
|---|---|---|---|---|---|
| 730 | 2 | 92 | 234.8 | 245.9 | 240.4 |
| 730 | 5 | 93 | 225.5 | 241.8 | 233.7 |
| 730 | 10 | 95 | 229.3 | 245.6 | 237.5 |
| 730 | 20 | 104 | 238.0 | 254.1 | 246.0 |
| 760 | 2 | 94 | 201.5 | 214.1 | 207.8 |
| 760 | 5 | 103 | 235.0 | 274.3 | 254.7 |
| 760 | 10 | 107 | 298.3 | 412.7 | 355.5 |
| 760 | 20 | 109 | 306.3 | 376.0 | 341.1 |
| 790 | 2 | 95 | 227.3 | 254.2 | 240.8 |
| 790 | 5 | 107 | 240.8 | 285.0 | 262.9 |
| 790 | 10 | 112 | 260.7 | 419.0 | 339.9 |
| 790 | 20 | 113 | 352.0 | 445.2 | 398.6 |
| AB | STT | 91 | 227.7 | 245.5 | 236.6 |
| T (°C) | t (min) | QM | Load (N) | COF (µ) |
|---|---|---|---|---|
| AB | - | 5 | 0.80 | |
| AB | - | 10 | 0.72 | |
| 730 | 5 | water | 5 | 0.87 |
| 730 | 10 | water | 5 | 0.80 |
| 730 | 20 | water | 5 | 0.78 |
| 730 | 5 | oil | 10 | 0.81 |
| 730 | 10 | oil | 10 | 0.83 |
| 730 | 20 | oil | 10 | 0.69 |
| 760 | 5 | water | 5 | 0.76 |
| 760 | 10 | water | 5 | 0.87 |
| 760 | 20 | water | 5 | 0.72 |
| 760 | 5 | oil | 10 | 0.62 |
| 760 | 10 | oil | 10 | 0.79 |
| 760 | 20 | oil | 10 | 0.80 |
| 790 | 5 | water | 5 | 0.64 |
| 790 | 10 | water | 5 | 0.60 |
| 790 | 20 | water | 5 | 0.62 |
| 790 | 5 | oil | 10 | 0.60 |
| 790 | 10 | oil | 10 | 0.68 |
| 790 | 20 | oil | 10 | 0.70 |
| 790 °C water | ||||
| Holding time (min) | Ba (mV) | Bc (mV) | Io (A/cm2) | Eo (V) |
| AB | 54.134 | 219.92 | 5.51 × 10−5 | −0.7596 |
| 5 | 49.272 | 692.97 | 2.07 × 10−6 | −0.6820 |
| 20 | 93.452 | 593.83 | 1.46 × 10−6 | −0.7267 |
| oil | ||||
| 5 | 73.775 | 531.38 | 2.3772 × 10−5 | −0.73364 |
| 20 | 85.656 | 500.17 | 2.062 × 10−5 | −0.69195 |
| 760 °C water | ||||
| Holding time (min) | Ba (mV) | Bc (mV) | Io (A/cm2) | Eo (V) |
| AB | 54.134 | 219.92 | 5.51 × 10−5 | −0.7596 |
| 5 | 60.422 | 327.83 | 1.77 × 10−5 | −0.7335 |
| 20 | 26.574 | 805.06 | 1.36 × 10−6 | −0.5609 |
| oil | ||||
| 5 | 83.427 | 398.11 | 2.398 × 10−5 | −0.7636 |
| 20 | 53.908 | 1476.1 | 1.739 × 10−6 | −0.6300 |
| 730 °C water | ||||
| Holding time (min) | Ba (mV) | Bc (mV) | Io (A/cm2) | Eo (V) |
| AB | 54.134 | 219.92 | 5.51 × 10−5 | −0.7596 |
| 5 | 69.319 | 265.75 | 6.413 × 10−5 | −0.7434 |
| 20 | 30.962 | 364.42 | 1.453 × 10−6 | −0.7047 |
| oil | ||||
| 5 | 73.545 | 357.43 | 1.508 × 10−5 | −0.7631 |
| 20 | 60.194 | 212.33 | 8.7595 × 10−6 | −0.7407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Linares, C.; Soria-Aguilar, M.J.; García-Guerra, J.; Martínez-Luevanos, A.; Carrillo-Pedroza, F.R.; Gutíerrez-Castañeda, E.; Díaz-Guillén, J.C.; Acevedo Dávila, J.L.; González de la Cruz, J.M. Electrochemical and Tribological Behavior of Dual-Phase Steels Obtained from a Commercial-Grade API 5CT Steel. Metals 2025, 15, 319. https://doi.org/10.3390/met15030319
Guerra-Linares C, Soria-Aguilar MJ, García-Guerra J, Martínez-Luevanos A, Carrillo-Pedroza FR, Gutíerrez-Castañeda E, Díaz-Guillén JC, Acevedo Dávila JL, González de la Cruz JM. Electrochemical and Tribological Behavior of Dual-Phase Steels Obtained from a Commercial-Grade API 5CT Steel. Metals. 2025; 15(3):319. https://doi.org/10.3390/met15030319
Chicago/Turabian StyleGuerra-Linares, C., M. J. Soria-Aguilar, J. García-Guerra, A. Martínez-Luevanos, F. R. Carrillo-Pedroza, E. Gutíerrez-Castañeda, J. C. Díaz-Guillén, J. L. Acevedo Dávila, and J. M. González de la Cruz. 2025. "Electrochemical and Tribological Behavior of Dual-Phase Steels Obtained from a Commercial-Grade API 5CT Steel" Metals 15, no. 3: 319. https://doi.org/10.3390/met15030319
APA StyleGuerra-Linares, C., Soria-Aguilar, M. J., García-Guerra, J., Martínez-Luevanos, A., Carrillo-Pedroza, F. R., Gutíerrez-Castañeda, E., Díaz-Guillén, J. C., Acevedo Dávila, J. L., & González de la Cruz, J. M. (2025). Electrochemical and Tribological Behavior of Dual-Phase Steels Obtained from a Commercial-Grade API 5CT Steel. Metals, 15(3), 319. https://doi.org/10.3390/met15030319










