Optimization of Electrical Conductivity and Hardness in Al-1Si Alloy Through Mg/Fe Alloying and Heat Treatment
Abstract
1. Introduction
2. Experimental Process
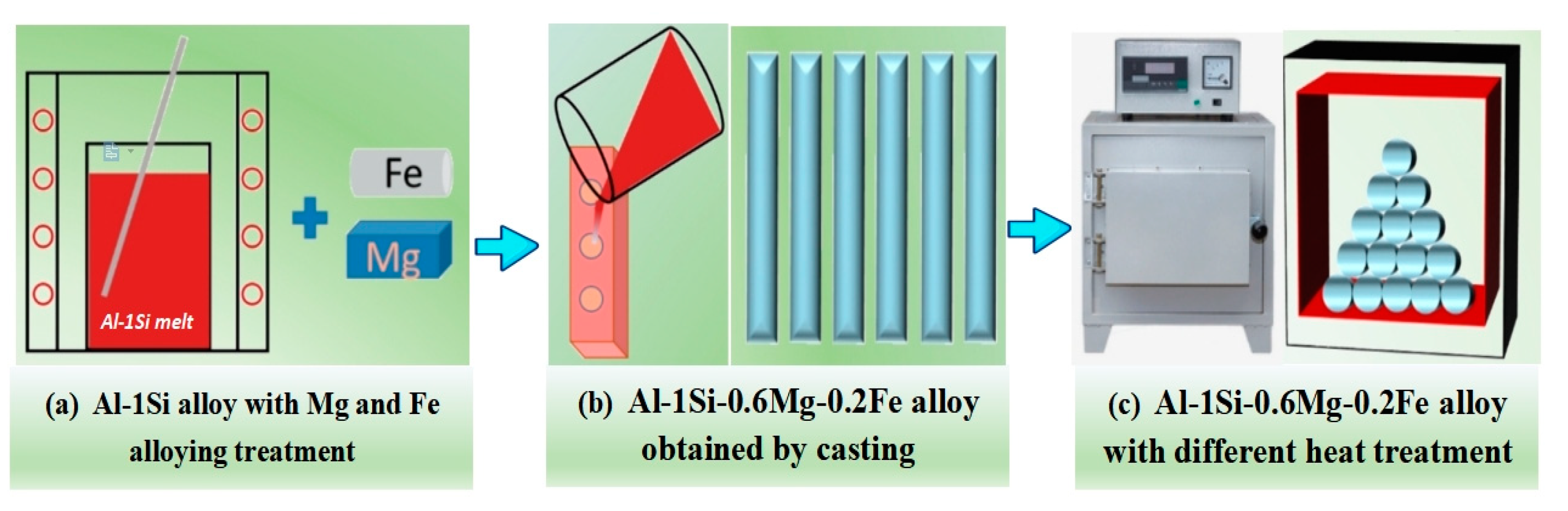
2.1. Sample Preparation
2.2. Performance and Microstructure Analysis
3. Results and Discussion
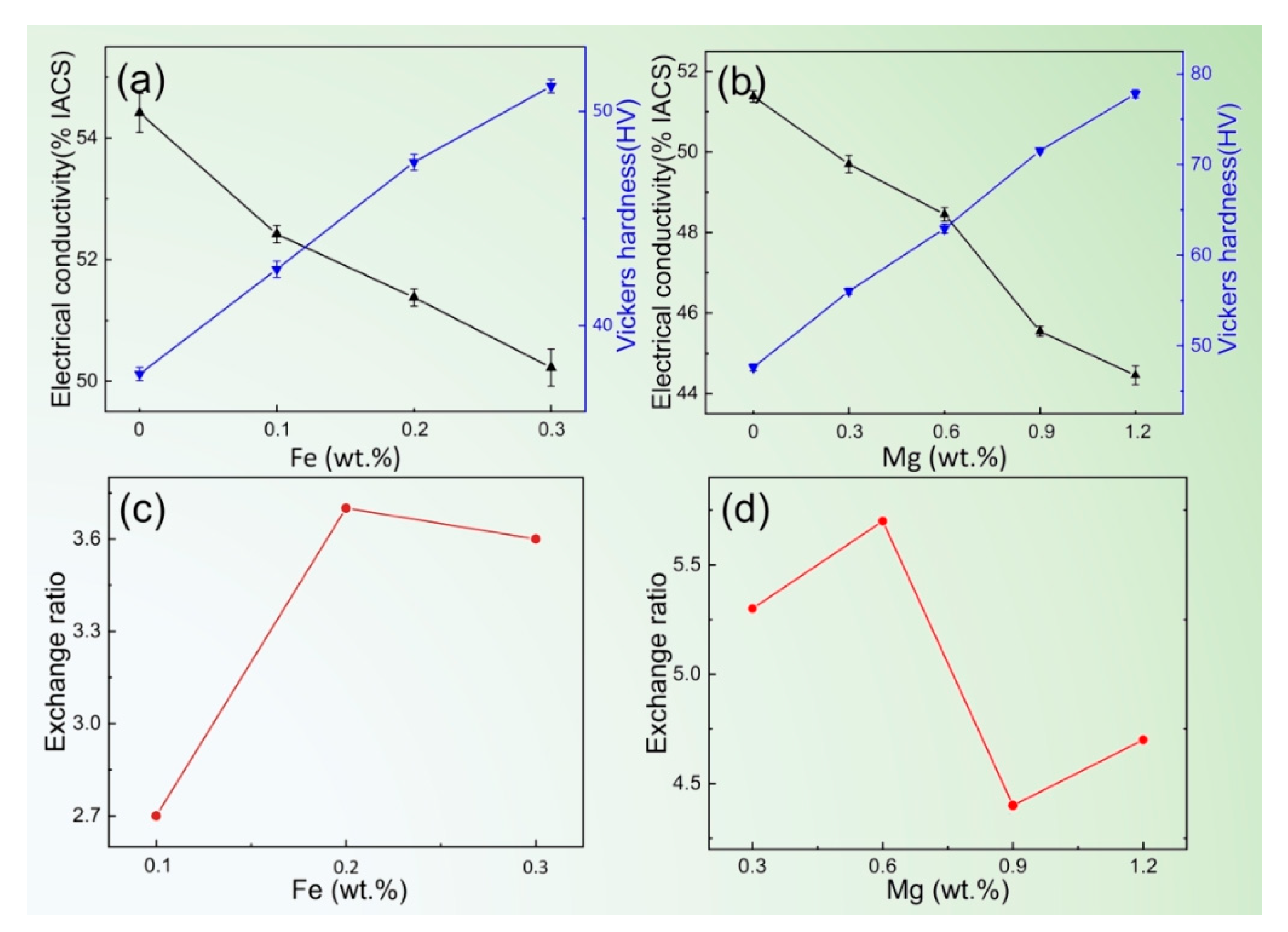
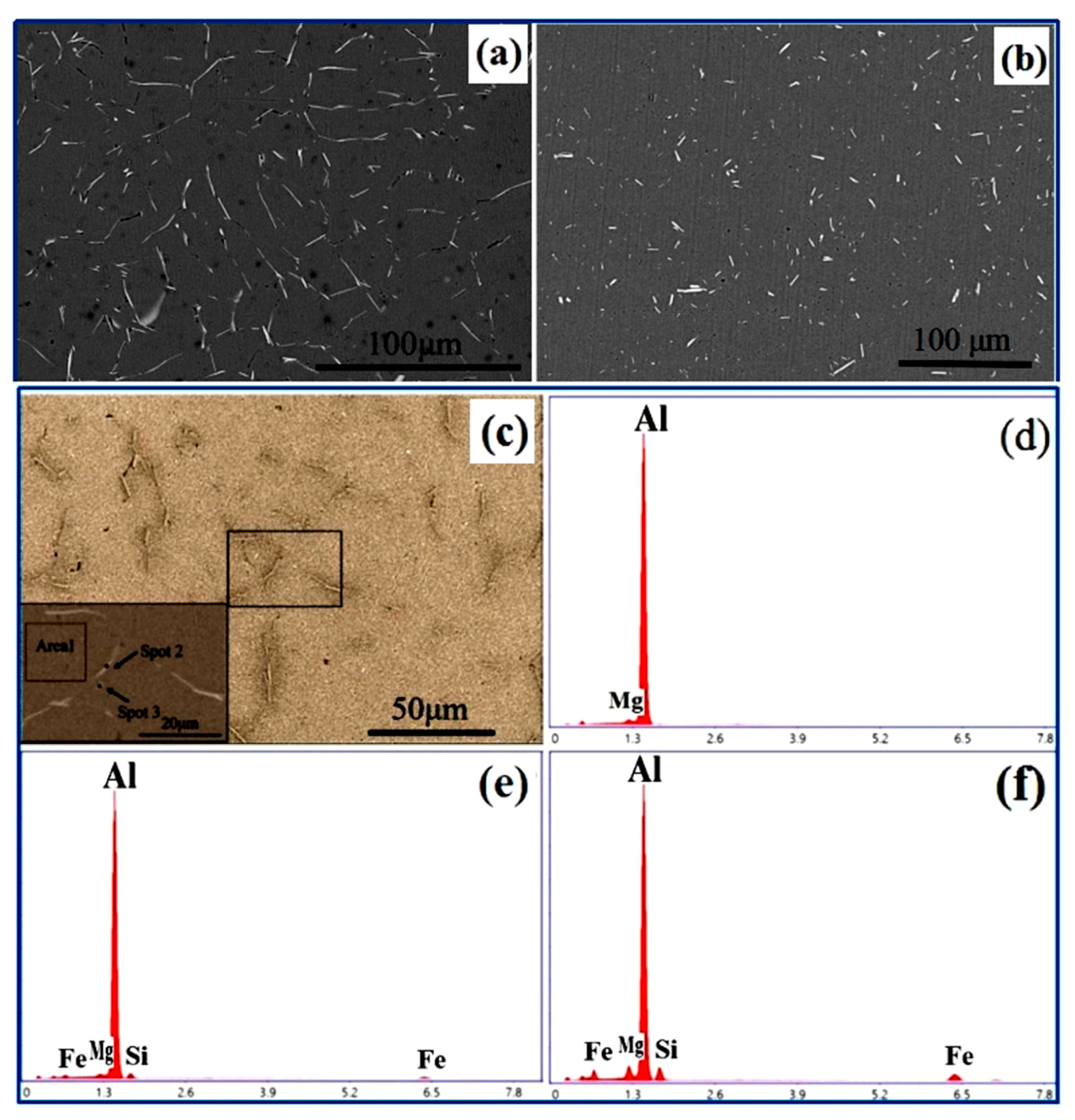
3.1. Effect of Mg and Fe Alloying on EC and HV of Al-1Si Alloy
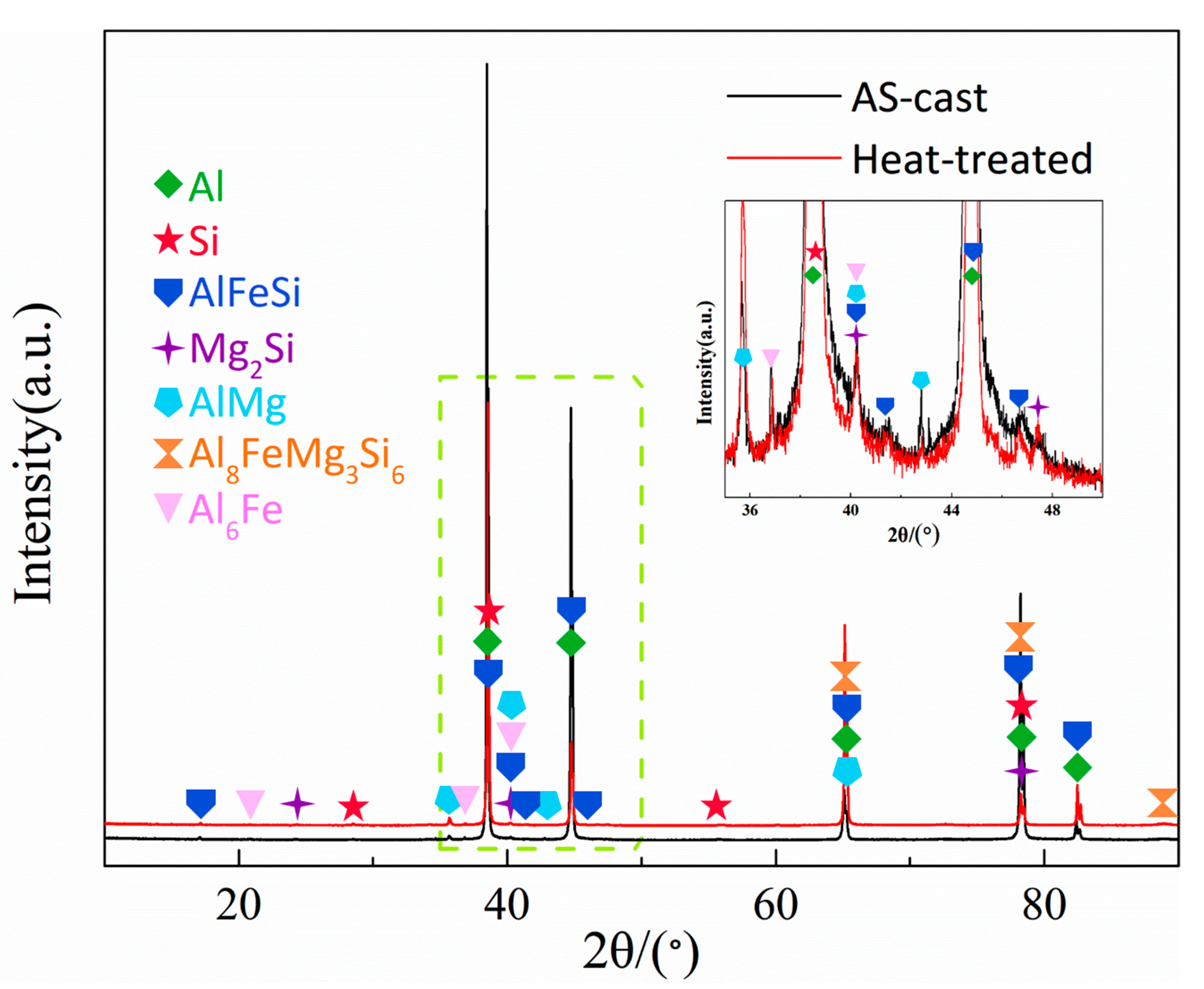
3.2. Effect of Heat Treatment on EC and HV of Al-1Si-0.6Mg-0.2Fe Alloy
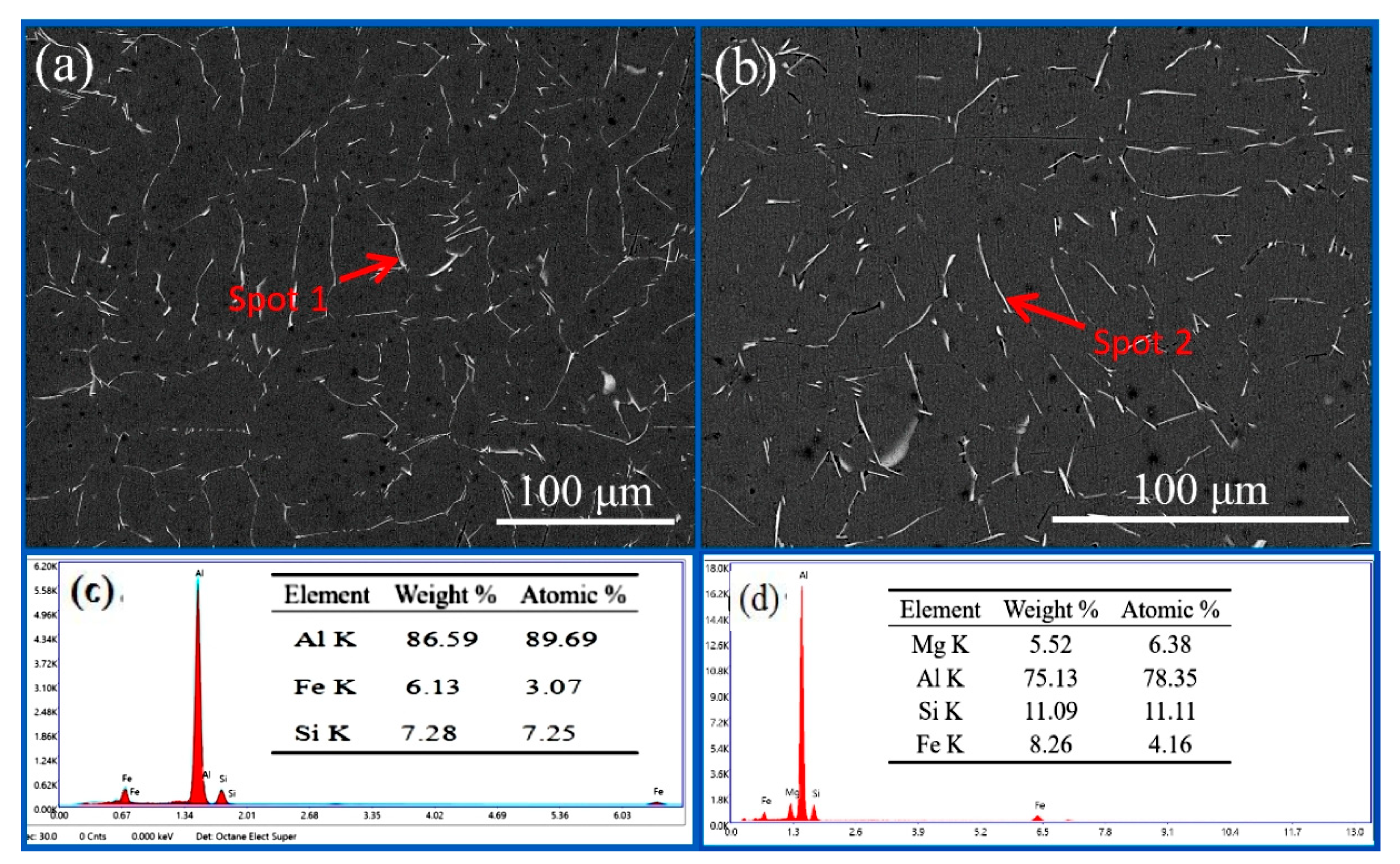
3.3. Discussion
4. Conclusions
- The optimum contents of Mg and Fe in the Al-1Si alloy are 0.6% and 0.2%, respectively. During the solidification process of the Al-1Si-0.6Mg-0.2Fe alloy, the content of the Al8FeMg3Si6 phase is the highest, and in this condition, it can promote more solution Si precipitate with the form of Mg2Si, and we can control the size and state of Mg2Si to make it maintain a good lattice match with the aluminum matrix. Furthermore, it is found that Al8FeMg3Si6 intermediate product grabbers Fe, resulting in forming slighter AlFeSi, which is helpful to achieve the optimal trade-off between EC and HV.
- For Al-1Si-0.6Mg-0.2Fe alloy, after 550 °C/2 h + 210 °C/24 h heat treatment, due to Mg2Si and Si re-solution and recrystallization, part of the FIMCs fused. In this condition, EC and HV reached 54.5% IACS and 79.8 HV, and compared with the Al-1Si alloy, the EC of the Al-1Si-0.6Mg-0.2Fe alloy is increased by 0.2% and the HV is increased by 111.1%, which achieves the optimizing EC and HV.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sauvage, X.; Bobruk, E.V.; Murashkin, M.Y.; Nasedkina, Y.; Enikeev, N.; Valiev, R. Optimization of electrical conductivity and strength combination by structure design at the nanoscale in Al-Mg-Si alloys. Acta Mater. 2015, 98, 355–366. [Google Scholar] [CrossRef]
- Guo, W.; Chen, X.; Liu, P.; Yu, G.; Fu, S.; Fan, J.; Zhou, H.; Liu, H. Effects of Er Additions on the Microstructure, Mechanical Properties, and Electrical Conductivity of the Al-0.4Fe-0.05Si Alloy. Adv. Eng. Mater. 2021, 23, 2000955. [Google Scholar] [CrossRef]
- Ye, H.; Cui, X.; Li, X.; Cui, H.; Zhang, B.; Li, H.; Pan, Y.; Feng, R.; Wu, Y.; Liu, X. Fabrication of hypoeutectic Al-4Si alloy with high electrical conductivity, high plasticity, and medium strength by the dual treatment of Al matrix and eutectic Si microstructure. J. Alloys Compd. 2021, 885, 161117. [Google Scholar] [CrossRef]
- Cui, X.; Ye, H.; Liu, H.; Li, X.; Man, Q.; Li, H.; Cui, H.; Feng, R.; Pan, Y. The improvement mechanism of good matching between electrical conductivity and mechanical properties for Al-4Si-0.8Mg-0.6Fe alloy. J. Alloys Compd. 2023, 938, 168275. [Google Scholar] [CrossRef]
- Karabay, S. Modification of AA-6201 alloy for manufacturing of high conductivity and extra high conductivity wires with property of high tensile stress after artificial aging heat treatment for all-aluminum alloy conductors. Mater. Des. 2006, 27, 821–832. [Google Scholar] [CrossRef]
- Zhao, Q.; Qian, Z.; Cui, X.; Wu, Y.; Liu, X. Optimizing microstructures of dilute Al-Fe-Si alloys designed with enhanced electrical conductivity and tensile strength. J. Alloys Compd. 2015, 650, 768–776. [Google Scholar] [CrossRef]
- Zhao, Q.; Qian, Z.; Cui, X.; Wu, Y.; Liu, X. Influences of Fe, Si, and homogenization on electrical conductivity and mechanical properties of dilute Al-Mg-Si alloy. J. Alloys Compd. 2016, 666, 50–57. [Google Scholar] [CrossRef]
- Kuijpers NC, W.; Kool, W.H.; Koenis, P.T.G.; Nilsen, K.E.; Todd, I.; Van der Zwaag, S. Assessment of different techniques for quantification of α-Al(FeMn)Si and β-AlFeSi intermetallics in AA 6xxx alloys. Mater. Charact. 2002, 49, 409–420. [Google Scholar] [CrossRef]
- Fu, J.; Yang, Z.; Deng, Y.; Wu, Y.F.; Lu, J.Q. Influence of Zr addition on precipitation evolution and performance of Al-Mg-Si alloy conductor. Mater. Charact. 2020, 159, 110021. [Google Scholar] [CrossRef]
- Yuan, W.; Liang, Z. Effect of Zr addition on properties of Al-Mg-Si aluminum alloy used for all aluminum alloy conductor. Mater. Des. 2011, 32, 4195–4200. [Google Scholar] [CrossRef]
- Jing, L.; Cheng, W.; Gan, J. Influencing mechanisms of alloying elements on the electrical conductivity of pure aluminum. Mater. Rep. 2021, 35, 24101–24106. [Google Scholar]
- Luo, K.; Wang, Z.; Meng, L.; Guo, Z. Removal of iron for aluminum recovery from scrap aluminum alloy by supergravity separation with manganese addition. Chem. Eng. Process. Process Intensif. 2022, 173, 108841. [Google Scholar] [CrossRef]
- Chen, H.; Lu, J.; Kong, Y.; Li, K.; Yang, T.; Meingast, A.; Yang, M.; Lu, Q.; Du, Y. Atomic-scale investigation of the crystal structure and interfaces of the β′ precipitate in Al-Mg-Si alloys. Acta Mater. 2020, 185, 193–203. [Google Scholar] [CrossRef]
- Matsuda, K.; Sakaguchi, Y.; Miyata, Y.; Uetani, Y.; Sato, T.; Kamio, A.; Ikeno, S. Precipitation sequence of various kinds of metastable phases in Al-1.0mass% Mg2Si-0.4mass% Si alloy. J. Mater. Sci. 2000, 35, 179–189. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-precipitate clusters and precipitation processes in Al-Mg-Si alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Zandbergen, M.W.; Xu, Q.; Cerezo, A.; Smith, G.D. Study of precipitation in Al-Mg-Si alloys by Atom Probe Tomography I. Microstructural changes as a function of aging temperature. Acta Mater. 2015, 101, 136–148. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Y.; Ning, H.; Wang, B. Effects of Zr and Co on the microstructure and mechanical properties of NiAl-based alloys. J. Alloys Compd. 2021, 883, 160815. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, J.; Cui, J.; Chen, D.; Ren, J.; Wang, T.; Chang, H.; Tai, P.; Zhang, L.; Tian, Y.; et al. Microstructural evolution, strengthening, and high thermal conductivity mechanisms of FeCrV-based medium-entropy alloys with Laves phase precipitation formed by adding minimal Ti. Mater. Charact. 2023, 200, 112860. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, F.; Dong, J. Potential for carbide dispersive precipitation strengthening Ni-based alloy for high temperature. J. Mater. Res. Technol. 2023, 23, 4860–4865. [Google Scholar] [CrossRef]
- Lee, K.; Song, Y.; Kim, S.; Kim, M.; Seol, J.; Cho, K.; Choi, H. Genetic design of new aluminum alloys to overcome strength-ductility trade-off dilemma. J. Alloys Compd. 2023, 947, 169546. [Google Scholar] [CrossRef]
- Liu, J.; Scales, R.J.; Li, B.-S.; Goode, M.; Young, B.A.; Hu, J.; Wilkinson, A.J.; Armstrong, D.E. Controlling microstructure and mechanical properties of Ti-V-Cr-Nb-Ta refractory high entropy alloys through heat treatments. J. Alloys Compd. 2023, 932, 167651. [Google Scholar] [CrossRef]
- Guerra-López, F.V.; Bedolla-Jacuinde, A.; León-Patiño, C.A.; Vázquez-Ramos, M. The effect of small additions of Nb and Ti on the sliding wear behavior of a Co-30Cr-5Mo alloy. Wear 2023, 522, 204846. [Google Scholar] [CrossRef]
- Xu, R.; Wang, W.; Wang, K.; Dai, Q. Finite element simulation of residual stress distribution during selective laser melting of Mg-Y-Sm-Zn-Zr alloy. Mater. Today Commun. 2023, 35, 105571. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; You, J.; Qiu, D.; Gao, Y.; Xu, H.; Xu, J.; Wang, H.-Y. Substantial grain refinement of Al-Mn-Si alloys mediated by collaborative effect of Al-5Ti-1B refiner and sub-rapid solidification. J. Mater. Sci. Technol. 2024, 187, 230–239. [Google Scholar] [CrossRef]
- Fan, W.; Bai, Y.; Zuo, G.; Hao, H. Enhanced grain refinement and mechanical properties of AZ91 alloy by a novel Al-5.1V-2.3B refiner containing VB2 and VB particles. Mater. Des. 2023, 225, 111474. [Google Scholar] [CrossRef]
- Que, Z.; Mendis, C.L. Formation of θ-Al13Fe4 and the multi-step phase transformations to α-Al8Fe2Si, β-Al5FeSi, and δ-Al4FeSi2 in Al-20Si-0.7Fe alloy. Intermetallics 2020, 127, 106960. [Google Scholar] [CrossRef]
- Que, Z.; Mendis, C.L. Heterogeneous nucleation and phase transformation of Fe-rich intermetallic compounds in Al-Mg-Si alloys. J. Alloys Compd. 2020, 836, 155515. [Google Scholar] [CrossRef]
- Que, Z.; Wang, Y.; Fan, Z. Heterogeneous nucleation of eutectic structure in Al-Mg-Si alloys. Metall. Mater. Trans. A 2020, 51, 2697–2702. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, D.; Yan, X.; Chen, Y.; Jia, Z.; Zhang, D.; Han, M.; Wang, X.; Liu, G.; Liu, X.; et al. Insights into the dual effects of Ti on the grain refinement and mechanical properties of hypoeutectic Al-Si alloys. J. Mater. Sci. Technol. 2024, 189, 44–59. [Google Scholar] [CrossRef]



| Alloy Composition (wt.%) | Second Phase (wt.%) | Intermediate Products (wt.%) | ||||
|---|---|---|---|---|---|---|
| Si | Mg2Si | β-AlFeSi | α-AlFeSi | Al8FeMg3Si6 | Al3Fe | |
| Al-1Si-.3Mg-0.1Fe | 0.78 | 0.47 | 0.37 | 0.08 | 0.92 | \ |
| Al-1Si-0.6Mg-0.1Fe | 0.60 | 0.95 | 0.37 | 0.10 | 0.92 | \ |
| Al-1Si-0.9Mg-0.1Fe | 0.43 | 1.42 | 0.37 | 0.12 | 0.92 | \ |
| Al-1Si-1.2 Mg-0.1Fe | 0.26 | 1.89 | 0.37 | 0.15 | 0.45 | \ |
| Al-1Si-0.3Mg-0.2Fe | 0.73 | 0.47 | 0.74 | 0.40 | 1.15 | \ |
| Al-1Si-0.6Mg-0.2Fe | 0.55 | 0.95 | 0.74 | 0.42 | 1.83 | \ |
| Al-1Si-0.9Mg-0.2Fe | 0.38 | 1.42 | 0.74 | 0.44 | 1.09 | \ |
| Al-1Si-1.2 Mg-0.2Fe | 0.21 | 1.89 | 0.74 | 0.46 | 0.19 | \ |
| Al-1Si-0.3Mg-0.3Fe | 0.68 | 0.47 | 1.10 | 0.73 | 1.16 | \ |
| Al-1Si-0.6Mg-0.3Fe | 0.50 | 0.95 | 1.10 | 0.74 | 1.73 | \ |
| Al-1Si-0.9Mg-0.3Fe | 0.33 | 1.42 | 1.10 | 0.76 | 0.83 | \ |
| Al-1Si-1.2 Mg-0.3Fe | 0.15 | 1.89 | 1.10 | 0.78 | \ | 0.04 |
| Mismatch Degree (%) | Mismatch Degree (%) | ||
|---|---|---|---|
| (01) Al5FeSi | (111) Mg2Si | 0.7 | (222) Al0.5Fe3Si0.5 | (400) Mg2Si | 4.0 |
| (111) Al5FeSi | (200) Mg2Si | 3.0 | (400) Al0.5Fe3Si0.5 | (420) Mg2Si | 0.7 |
| (211) Al5FeSi | (220) Mg2Si | 2.7 | (331) Al0.5Fe3Si0.5 | (422) Mg2Si | 1.2 |
| (22) Al5FeSi | (311) Mg2Si | 3.9 | (422) Al0.5Fe3Si0.5 | (511) Mg2Si | 4.5 |
| (112) Al5FeSi | (222) Mg2Si | 2.4 | (422) Al0.5Fe3Si0.5 | (440) Mg2Si | 4.0 |
| (103) Al5FeSi | (422) Mg2Si | 2.6 | (440) Al0.5Fe3Si0.5 | (600) Mg2Si | 4.5 |
| (123) Al8Fe2Si | (111) Mg2Si | 0.4 | (440) Al0.5Fe3Si0.5 | (620) Mg2Si | 0.7 |
| (125) Al8Fe2Si | (200) Mg2Si | 1.1 | (440) Al0.5Fe3Si0.5 | (533) Mg2Si | 4.4 |
| (228) Al8Fe2Si | (220) Mg2Si | 0.4 | (620) Al0.5Fe3Si0.5 | (622) Mg2Si | 5.5 |
| (152) Al8Fe2Si | (311) Mg2Si | 0.3 | (620) Al0.5Fe3Si0.5 | (444) Mg2Si | 1.3 |
| (154) Al8Fe2Si | (222) Mg2Si | 0.9 | (620) Al0.5Fe3Si0.5 | (551) Mg2Si | 1.7 |
| (111) Al0.5Fe3Si0.5 | (200) Mg2Si | 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Liu, H.; Wang, Y.; Lu, C.; Shi, W.; Tie, D. Optimization of Electrical Conductivity and Hardness in Al-1Si Alloy Through Mg/Fe Alloying and Heat Treatment. Metals 2025, 15, 317. https://doi.org/10.3390/met15030317
Cui X, Liu H, Wang Y, Lu C, Shi W, Tie D. Optimization of Electrical Conductivity and Hardness in Al-1Si Alloy Through Mg/Fe Alloying and Heat Treatment. Metals. 2025; 15(3):317. https://doi.org/10.3390/met15030317
Chicago/Turabian StyleCui, Xiaoli, Houyun Liu, Yan Wang, Chao Lu, Wenqing Shi, and Di Tie. 2025. "Optimization of Electrical Conductivity and Hardness in Al-1Si Alloy Through Mg/Fe Alloying and Heat Treatment" Metals 15, no. 3: 317. https://doi.org/10.3390/met15030317
APA StyleCui, X., Liu, H., Wang, Y., Lu, C., Shi, W., & Tie, D. (2025). Optimization of Electrical Conductivity and Hardness in Al-1Si Alloy Through Mg/Fe Alloying and Heat Treatment. Metals, 15(3), 317. https://doi.org/10.3390/met15030317






