Numerical and Experimental Study of Packed Bed Heat Transfer on the Preheating of Manganese Ore with Air up to 600 °C
Abstract
1. Introduction
2. Materials and Methods
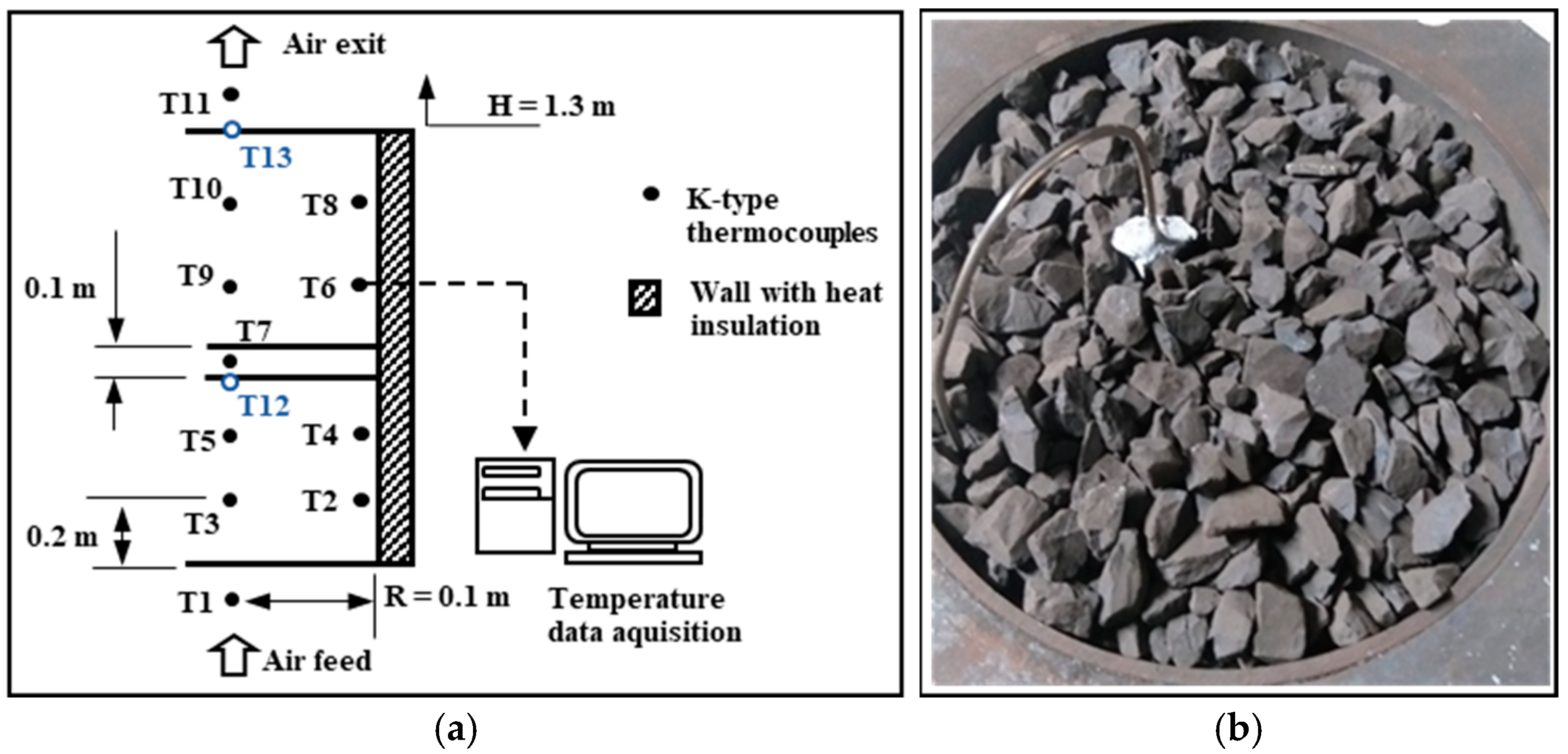
2.1. Experimental Set-Up
2.2. One-Dimensional Three-Phase Model
2.3. Packed Bed Characterization and Materials Transport Properties
2.4. Heat Transfer Coefficients
2.5. Consideration of Radiative Heat Transfer
2.6. Heat Transfer Efficiency
3. Results
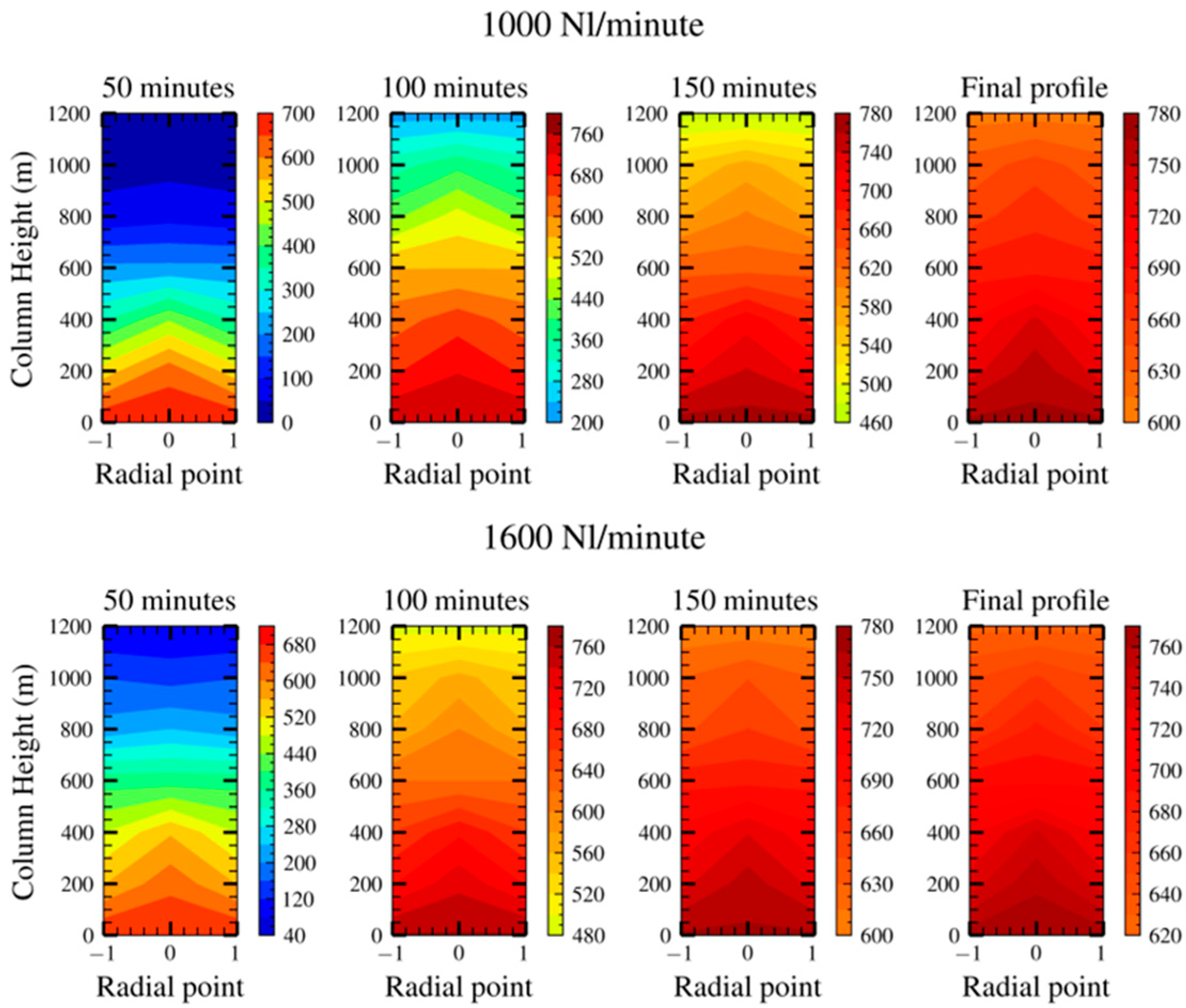
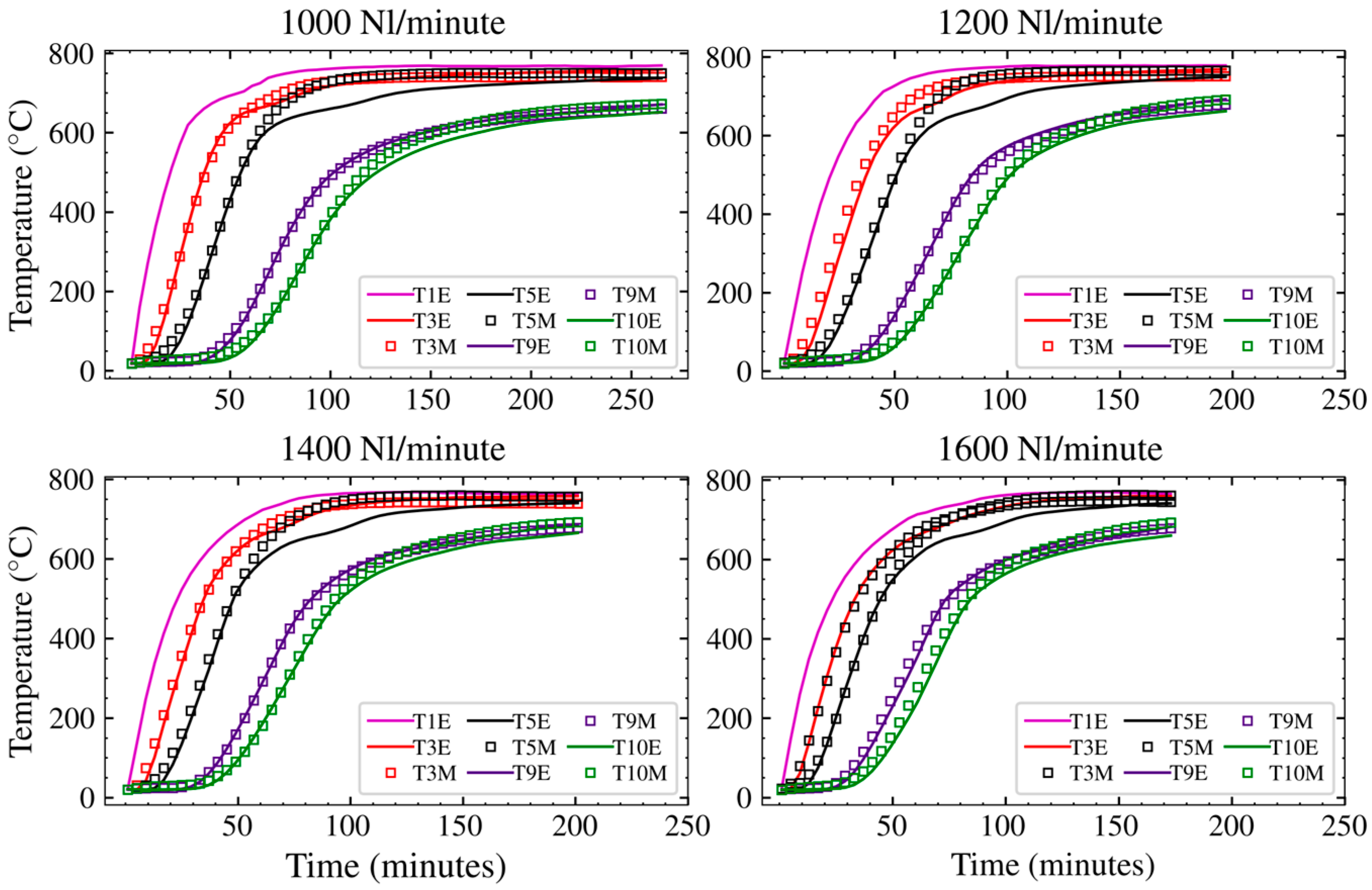
3.1. Experimental Results
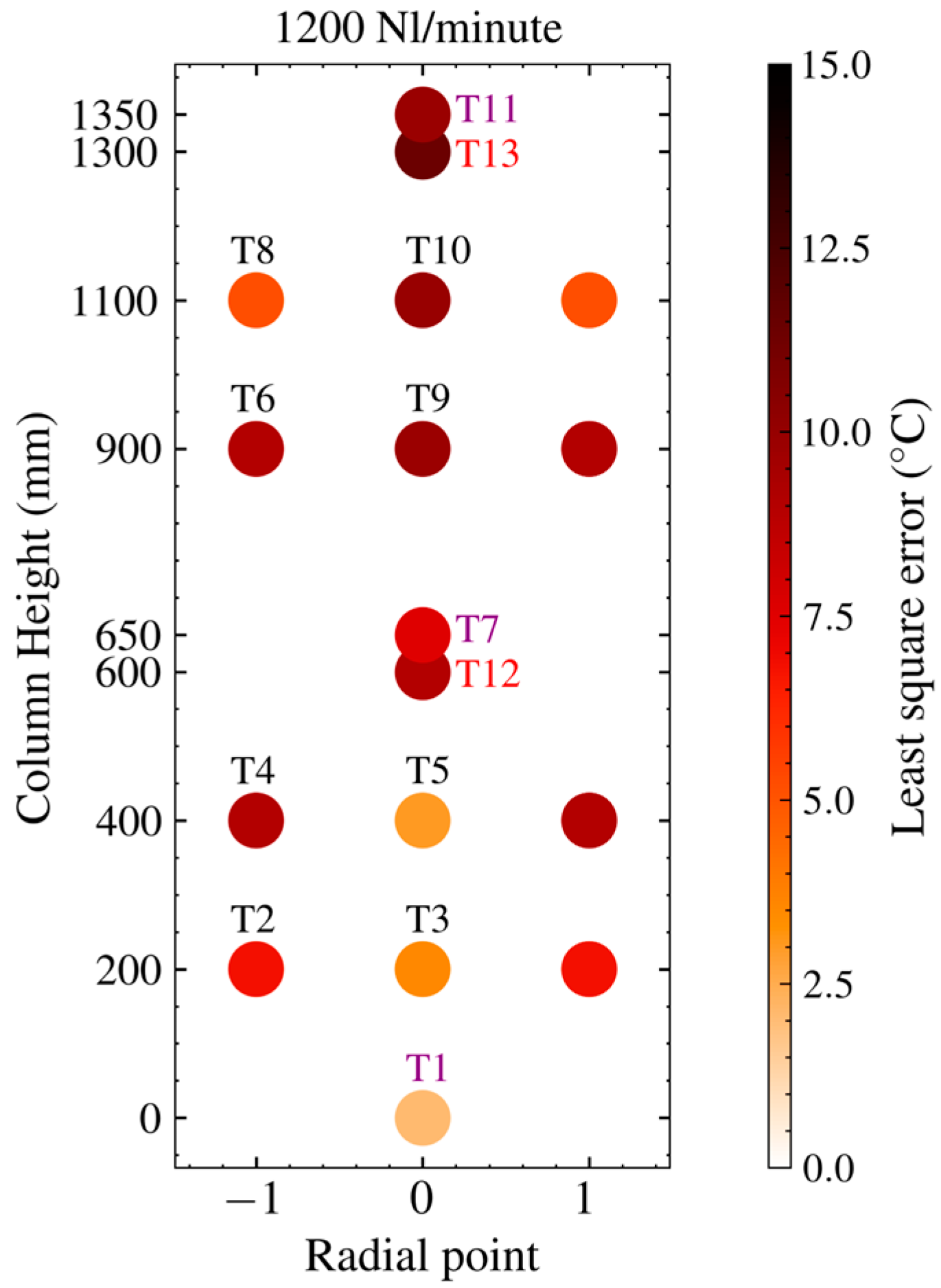
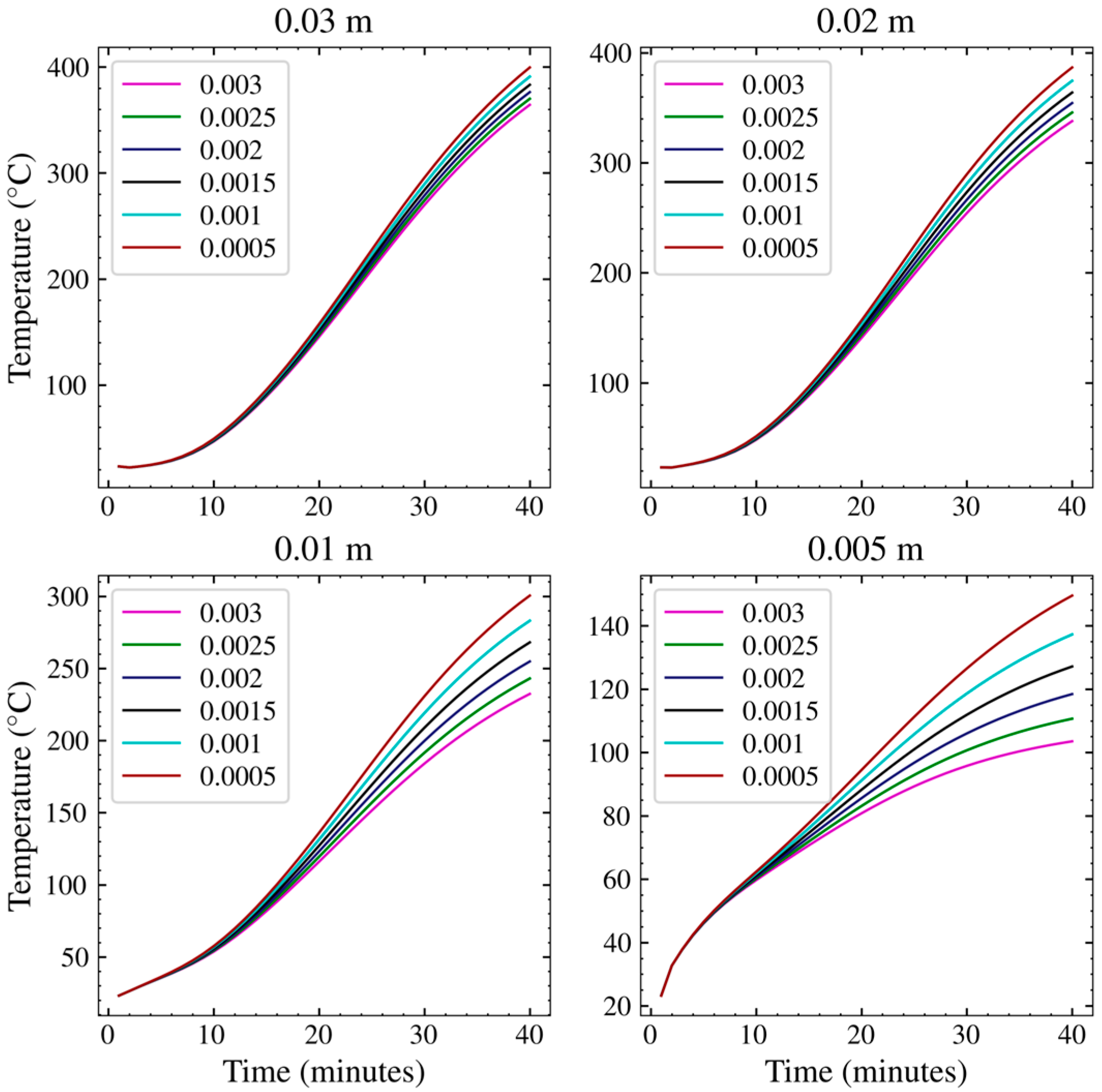
3.2. Grid Dependence and Model Verification
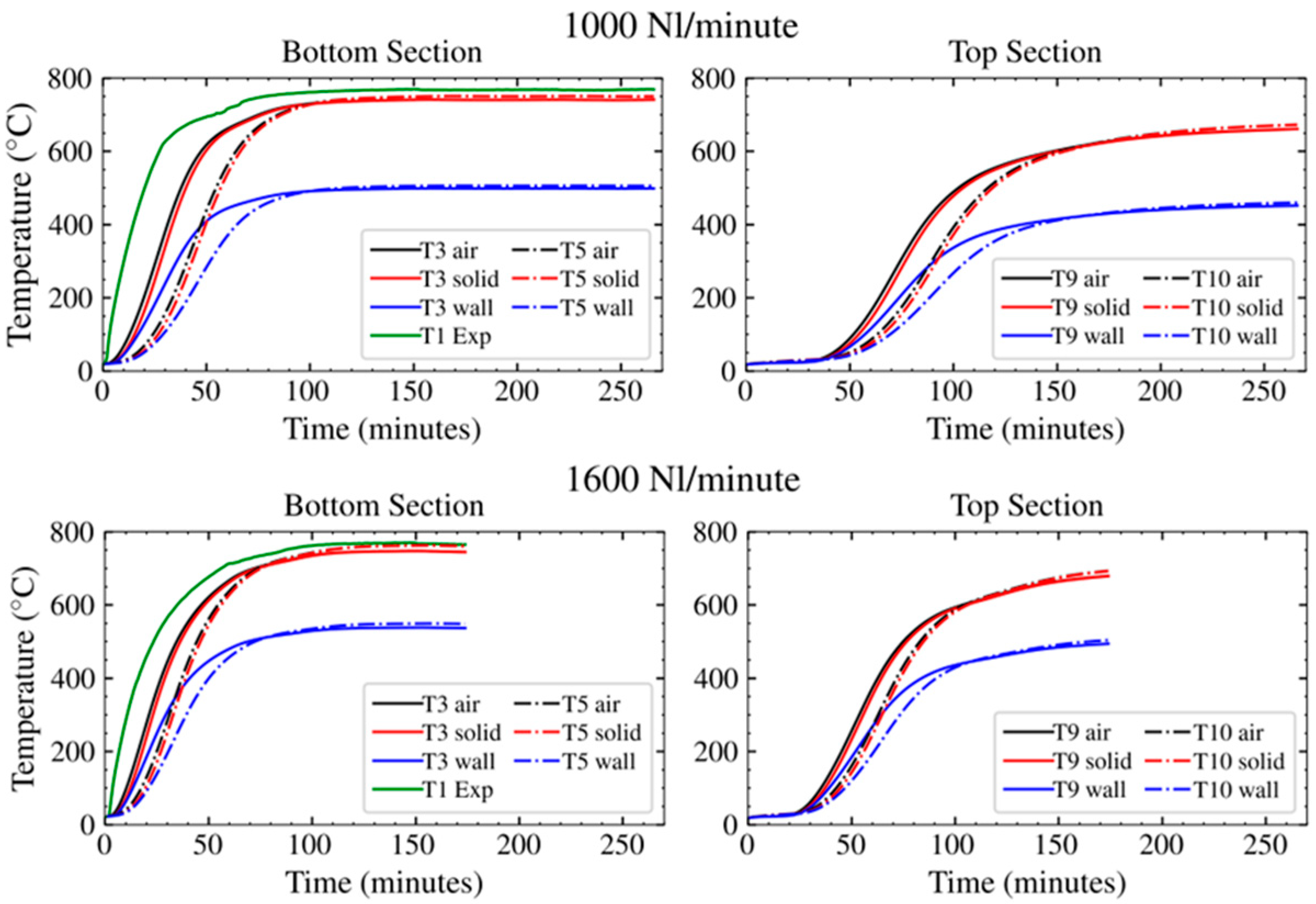
3.3. Model Output
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Thermal diffusivity of the solid packed bed materials | |
| As | Surface area to volume ratio of a packed bed |
| Surface area to volume ratio the packed bed encompassing column | |
| Dimensionless Biot number | |
| Specific heat capacity | |
| Dimensionless restrictive Fredrich Lewy Number (Courent number) | |
| Equivalent particle diameter | |
| Mean particle diameter (m) | |
| D | Column diameter (m) |
| Packing height | |
| Heat transfer coefficient at the solid-fluid interphase | |
| Heat transfer coefficient at the wall-fluid interphase | |
| Dimensionless Nusselt number | |
| Dimensionless Prandtl number | |
| Column radius | |
| Dimensionless Reynolds number | |
| Stanton number (numerical discretization) | |
| Fluid temperature | |
| Solid temperature | |
| Wall temperature | |
| Fluid ambient temperature | |
| Thermal conductivity | |
| Dynamic fluid viscosity | |
| Density | |
| Bed average porosity | |
| Discretization length (m) | |
| Heat transfer efficiency of the transfer system |
References
- Bale, S.; Tiwari, S.S.; Nandakumar, K.; Joshi, J.B. Effect of Schmidt number and D/d ratio on mass transfer through gas-solid and liquid-solid packed beds: Direct numerical simulations. Powder Technol. 2019, 354, 529–539. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Bu, S.; Zeng, M.; Wang, Q.; Nakayama, A. Experimental analysis of forced convective heat transfer in novel structured packed beds of particles. Chem. Eng. Sci. 2012, 71, 126–137. [Google Scholar] [CrossRef]
- Gordon, Y.; Nell, J.; Yaroshenko, Y. Manganese Ore Thermal Treatment Prior to Smelting. KnE Eng. 2018, 3, 71–86. [Google Scholar] [CrossRef]
- Steenkamp, J.D.; Chetty, D.; Singh, A.; Hockaday, S.A.C.; Denton, G.M. From Ore Body to High Temperature Processing of Complex Ores: Manganese—A South African Perspective. JOM 2020, 72, 3422–3435. [Google Scholar] [CrossRef]
- Hamuyuni, J.; Saarenmaa, J.; Mäkelä, P.; Pekkala, O.; Binder, C.; Rannantie, S.; Lindgren, M. Pretreatment of Manganese Ore for Improved Energy Efficiency and Smelting Furnace Stability. In Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI), Trondheim, Norway, 27–29 September 2021. [Google Scholar] [CrossRef]
- Julia, N.; Hecquet, A.; Nussbaum, G.; Blancher, S.; Amalric, A. Pre-Heating Manganese Ore in a Pilot-Scale Rotary Kiln. In Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI), Trondheim, Norway, 27–29 September 2021. [Google Scholar] [CrossRef]
- Khama, M.; Reynolds, Q.G.; Xakalashe, B.X.; Sarkar, A.; Safarian, J. Computational reacting flow models for the pre-reduction of lumpy Nchwaning manganese ore with hydrogen. In Proceedings of the Sustainable Pyrometallurgy-Surviving Today and Thriving Tomorrow, Johannesburg, South Africa, 11–14 March 2024. [Google Scholar]
- Sarkar, A.; Schanche, T.L.; Wallin, M.; Safarian, J. Evaluating the Reaction Kinetics on the H2 Reduction of a Manganese Ore at Elevated Temperatures. J. Sustain. Metall. 2024, 10, 2085–2103. [Google Scholar] [CrossRef]
- Bale, S.; Tiwari, S.; Sathe, M.; Berrouk, A.S.; Nandakumar, K.; Joshi, J. Direct numerical simulation study of end effects and D/d ratio on mass transfer in packed beds. Int. J. Heat Mass Transf. 2018, 127, 234–244. [Google Scholar] [CrossRef]
- Çarpinlioğlu, M.Ö.; Özahi, E. A simplified correlation for fixed bed pressure drop. Powder Technol. 2008, 187, 94–101. [Google Scholar] [CrossRef]
- Das, S.; Deen, N.G.; Kuipers, J.A.M. A DNS study of flow and heat transfer through slender fixed-bed reactors randomly packed with spherical particles. Chem. Eng. Sci. 2017, 160, 1–19. [Google Scholar] [CrossRef]
- Reddy, R.K.; Joshi, J.B. CFD modeling of pressure drop and drag coefficient in fixed and expanded beds. Chem. Eng. Res. Des. 2008, 86, 444–453. [Google Scholar] [CrossRef]
- Jiang, P.-X.; Ren, Z.-P. Numerical investigation of forced convection heat transfer in porous media using a thermal non-equilibrium model. Int. J. Heat Fluid Flow 2001, 22, 102–110. [Google Scholar] [CrossRef]
- Liu, S.; Ahmadi-Senichault, A.; Pozzobon, V.; Lachaud, J. Multi-scale investigation of heat and momentum transfer in packed-bed TES systems up to 800 K. Appl. Energy 2024, 366, 123285. [Google Scholar] [CrossRef]
- Teruel, F.E.; Díaz, L. Calculation of the interfacial heat transfer coefficient in porous media employing numerical simulations. Int. J. Heat Mass Transf. 2013, 60, 406–412. [Google Scholar] [CrossRef]
- Van Antwerpen, W.; duToit, C.; Rousseau, P.G. A review of correlations to model the packing structure and effective thermal conductivity in packed beds of mono-sizes spherical particles. Nucl. Eng. Des. 2010, 240, 1803–1818. [Google Scholar] [CrossRef]
- Tangstad, M.; Ichihara, K.; Ringdalen, E. Pretreatment unit in ferromanganese production. In Proceedings of the Fourteenth International Ferroalloys Congress, Kiev, Ukraine, 31 May–4 June 2015; p. 8. [Google Scholar]
- Sarker, M.R.I.; Saha, M.; Rahman, M.S.; Beg, R.A. Recirculating metallic particles for the efficiency enhancement of concentrated solar receivers. Renew. Energy 2016, 96, 850–862. [Google Scholar] [CrossRef]
- Calderón, A.; Palacios, A.; Barreneche, C.; Segarra, M.; Prieto, C.; Rodriguez-Sanchez, A.; Fernández, A.I. High temperature systems using solid particles as TES and HTF material: A review. Appl. Energy 2018, 213, 100–111. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; Van Der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Daizo, H.; Octave, L. Fluidisation Engineering, 2nd ed.; Chemical Engineering; Massachusetts Institute of Technology: Cambridge, MA, USA, 1992. [Google Scholar]
- Reddy, R.K.; Joshi, J.B. CFD modeling of pressure drop and drag coefficient in fixed beds: Wall effects. Particuology 2010, 8, 37–43. [Google Scholar] [CrossRef]
- Ringdalen, E.; Gjøvik, J.E.; Larssen, T.A.; Tangstad, M. Pretreatment of Manganese Ores in Different Gas-Atmospheres—A Method to Reduce Energy Consumption and CO2 Emissions in Mn-Alloy Production. In Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI), Trondheim, Norway, 27–29 September 2021. [Google Scholar] [CrossRef]
- Mertens, N.; Alobaid, F.; Frigge, L.; Epple, B. Dynamic simulation of integrated rock-bed thermocline storage for concentrated solar power. Sol. Energy 2014, 110, 830–842. [Google Scholar] [CrossRef]
- Yang, B.; Bai, F.; Wang, Y.; Wang, Z. Study on standby process of an air-based solid packed bed for flexible high-temperature heat storage: Experimental results and modelling. Appl. Energy 2019, 238, 135–146. [Google Scholar] [CrossRef]
- Esence, T.; Bruch, A.; Molina, S.; Stutz, B.; Fourmigué, J.-F. A review on experience feedback and numerical modeling of packed-bed thermal energy storage systems. Sol. Energy 2017, 153, 628–654. [Google Scholar] [CrossRef]
- Zanganeh, G.; Pedretti, A.; Zavattoni, S.; Barbato, M.; Steinfeld, A. Packed-bed thermal storage for concentrated solar power—Pilot-scale demonstration and industrial-scale design. Sol. Energy 2012, 86, 3084–3098. [Google Scholar] [CrossRef]
- Hänchen, M.; Brückner, S.; Steinfeld, A. High-temperature thermal storage using a packed bed of rocks—Heat transfer analysis and experimental validation. Appl. Therm. Eng. 2011, 31, 1798–1806. [Google Scholar] [CrossRef]
- Jorge, L.M.D.M.; Jorge, R.M.M.; Giudici, R. Experimental and numerical investigation of dynamic heat transfer parameters in packed bed. Heat Mass Transf. 2010, 46, 1355–1365. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Cheng, Z.; Liu, Y.; Chen, Y.; Wang, Q. Experimental and numerical study on pressure drop and heat transfer performance of grille-sphere composite structured packed bed. Appl. Energy 2018, 227, 719–730. [Google Scholar] [CrossRef]
- Hernández-Jiménez, F.; Pérez-Álvarez, R.; López-Quesada, G.; García-Gutiérrez, L.M. Effect of Biot number on the thermocline evolution of a packed bed TES system. J. Energy Storage 2025, 110, 115272. [Google Scholar] [CrossRef]


| Units | A | B | C | D | E | F | G | |
|---|---|---|---|---|---|---|---|---|
| 1.68 | 1.49 × 10−2 | −1.15 × 10−4 | 3.71 × 10−7 | −5.86 × 10−10 | 4.41 × 10−13 | −1.25 × 10−16 | ||
| 4.95 × 10−2 | 5.98 | −3.18 × 10−2 | 7.96 × 10−5 | −8.77 × 10−8 | −2.85 × 10−11 | 6.25 × 10−15 | ||
| 3.67 | 1.0 × 10−4 | 1.0 × 10−6 | −6.0 × 10−9 | 2.0 × 10−11 | −2.0 × 10−14 | 1.0 × 10−17 | ||
| 6.94 × 10−2 | 1.36 | 4.555 × 10−3 | −2.78 × 10−5 | 3.735 × 10−8 | −9.23 × 10−12 | −5.59 × 10−15 | ||
| Units | A | B | C | D | Formula | |
|---|---|---|---|---|---|---|
| −2.099 × 10−8 | 7.64 × 10−5 | 2.45 × 10−3 | ||||
| −2.993 × 10−7 | 4.133 × 10−4 | 4.754 × 10−2 | 1002 | |||
| −11.298 × 10−11 | 4.417 × 10−8 | 1.748 | ||||
| 5.198 × 10−1 | −6.343 × 10−3 | 6.964 × 10−1 | −9.452 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambo, S.N.; Hockaday, L.; Seodigeng, T.; Reynolds, Q.G. Numerical and Experimental Study of Packed Bed Heat Transfer on the Preheating of Manganese Ore with Air up to 600 °C. Metals 2025, 15, 269. https://doi.org/10.3390/met15030269
Sambo SN, Hockaday L, Seodigeng T, Reynolds QG. Numerical and Experimental Study of Packed Bed Heat Transfer on the Preheating of Manganese Ore with Air up to 600 °C. Metals. 2025; 15(3):269. https://doi.org/10.3390/met15030269
Chicago/Turabian StyleSambo, Sifiso Nation, Lina Hockaday, Tumisang Seodigeng, and Quinn Gareth Reynolds. 2025. "Numerical and Experimental Study of Packed Bed Heat Transfer on the Preheating of Manganese Ore with Air up to 600 °C" Metals 15, no. 3: 269. https://doi.org/10.3390/met15030269
APA StyleSambo, S. N., Hockaday, L., Seodigeng, T., & Reynolds, Q. G. (2025). Numerical and Experimental Study of Packed Bed Heat Transfer on the Preheating of Manganese Ore with Air up to 600 °C. Metals, 15(3), 269. https://doi.org/10.3390/met15030269







