Optimizing Recycling Processes for Mixed LFP/NMC Lithium-Ion Batteries: A Comparative Study of Acid-Excess and Acid-Deficient Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
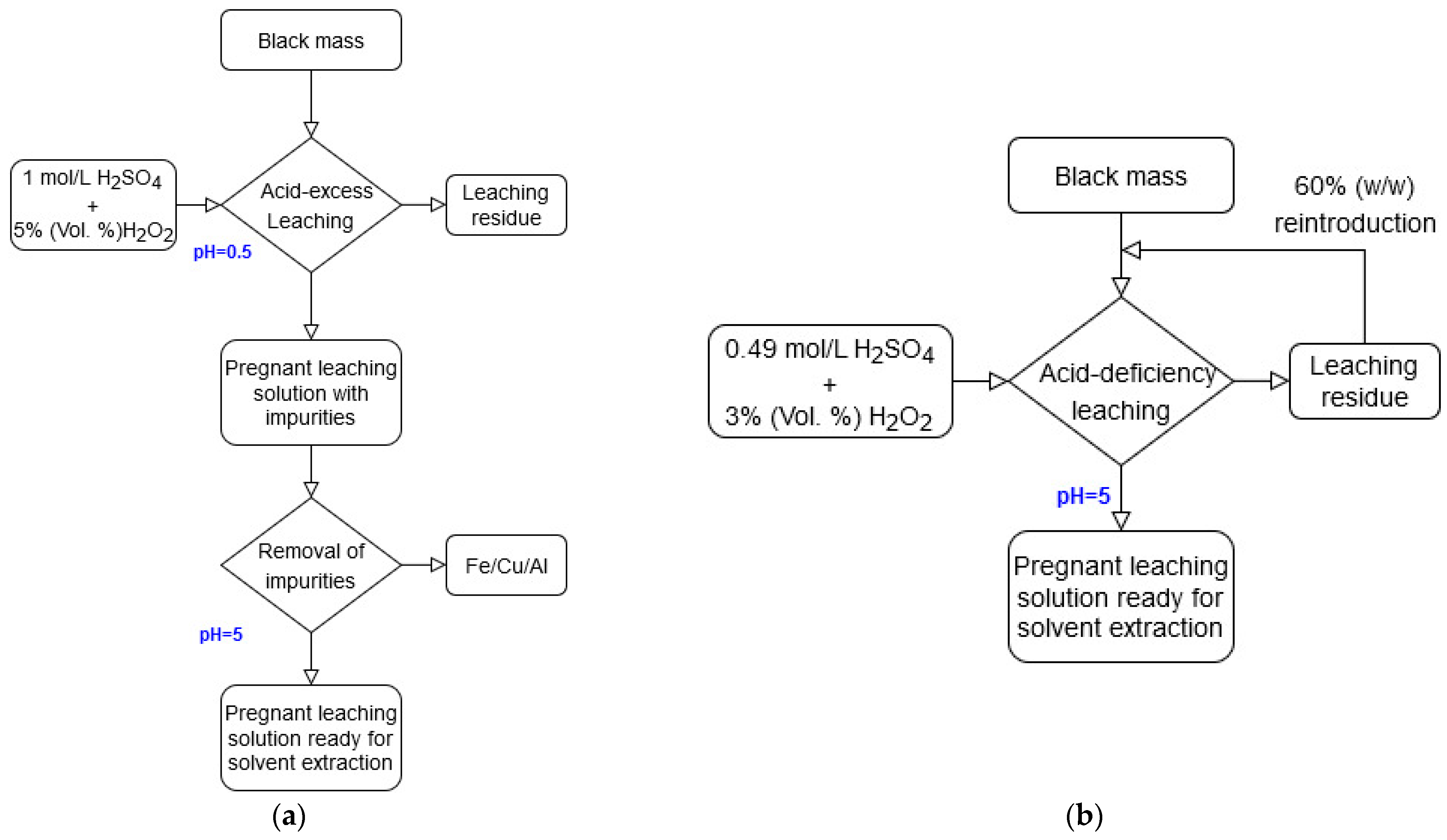
2.1.1. Leaching
2.1.2. Solvent Extraction
2.2. Methods
2.2.1. Analytical Methods
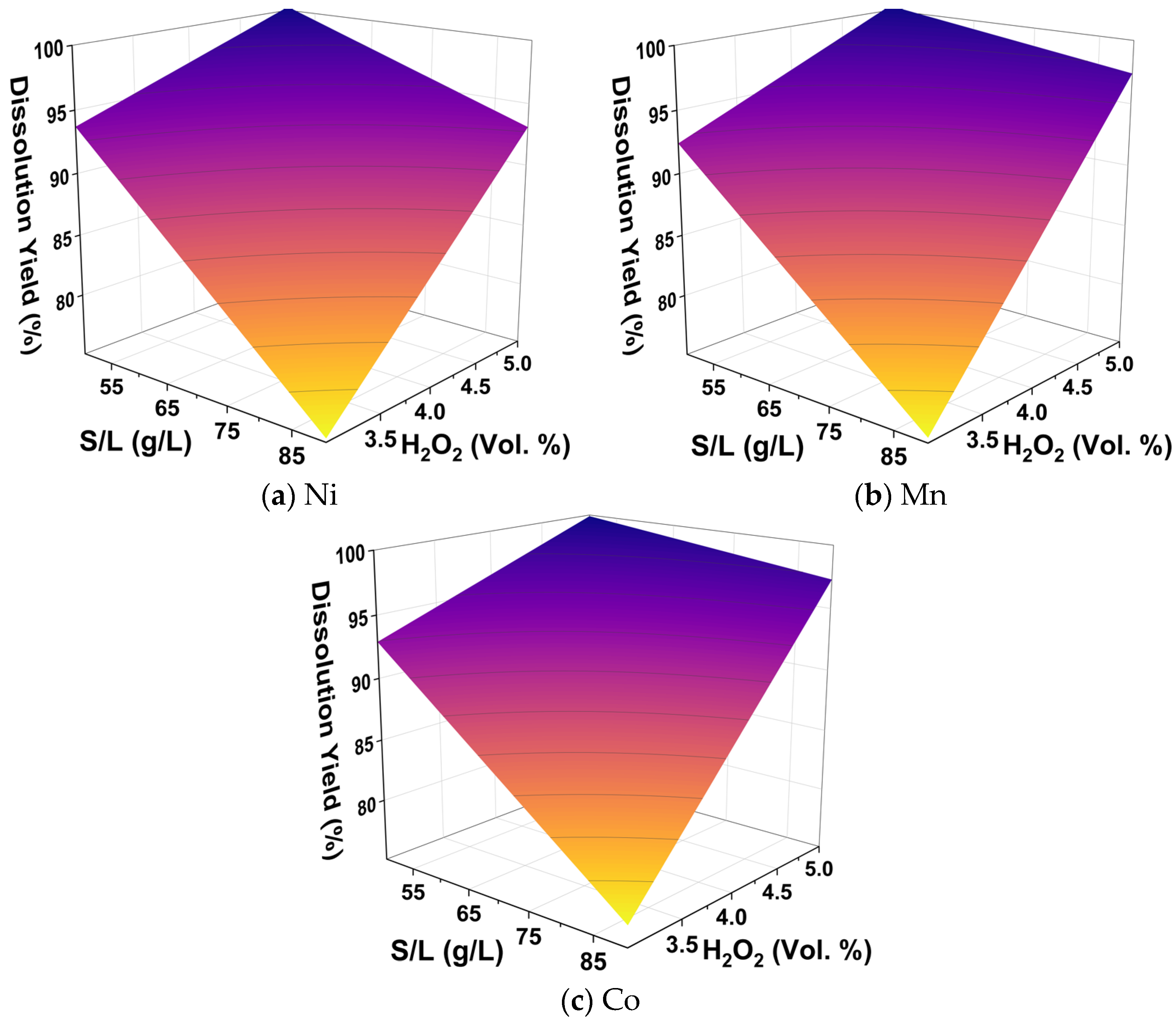
2.2.2. Design of Experiments
3. Results and Discussion
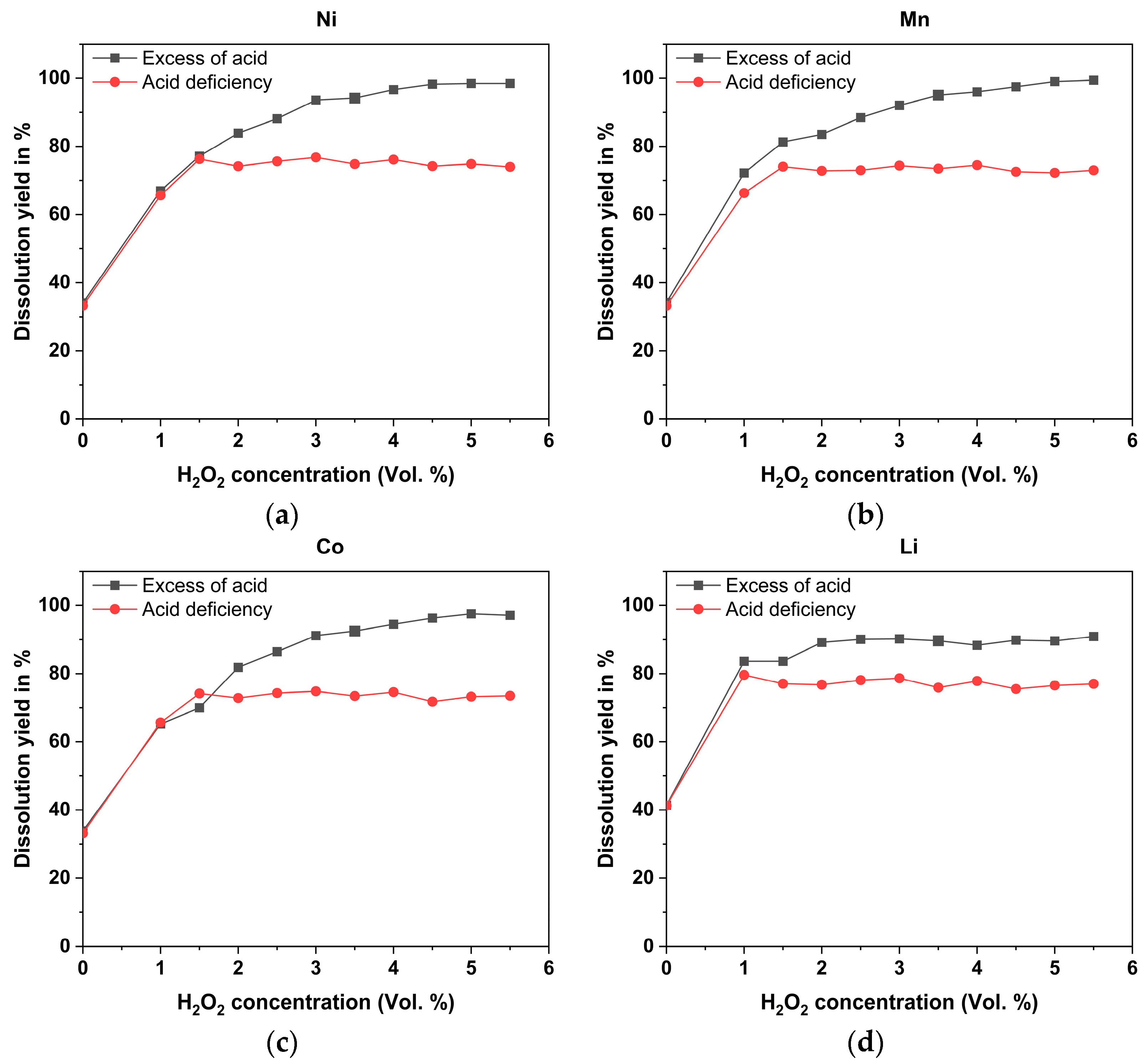
3.1. Leaching
3.1.1. Acid-Excess Leaching
3.1.2. Acid-Deficient Leaching
3.1.3. Residue Reintroduction Under Acid-Deficient Leaching: A Strategy to Increase Leaching Yields
3.1.4. Comparison of Acid-Excess Leaching and Acid-Deficient Leaching with Leaching Residue Reintroduction
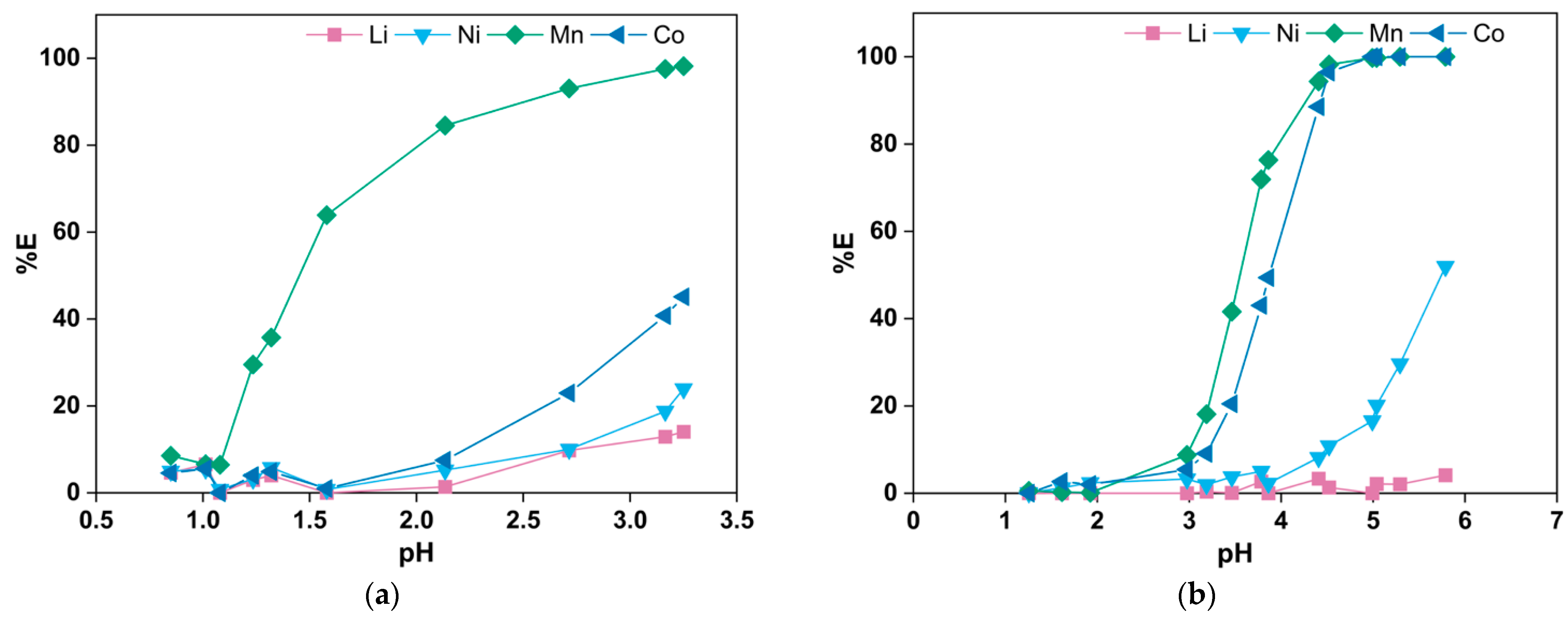
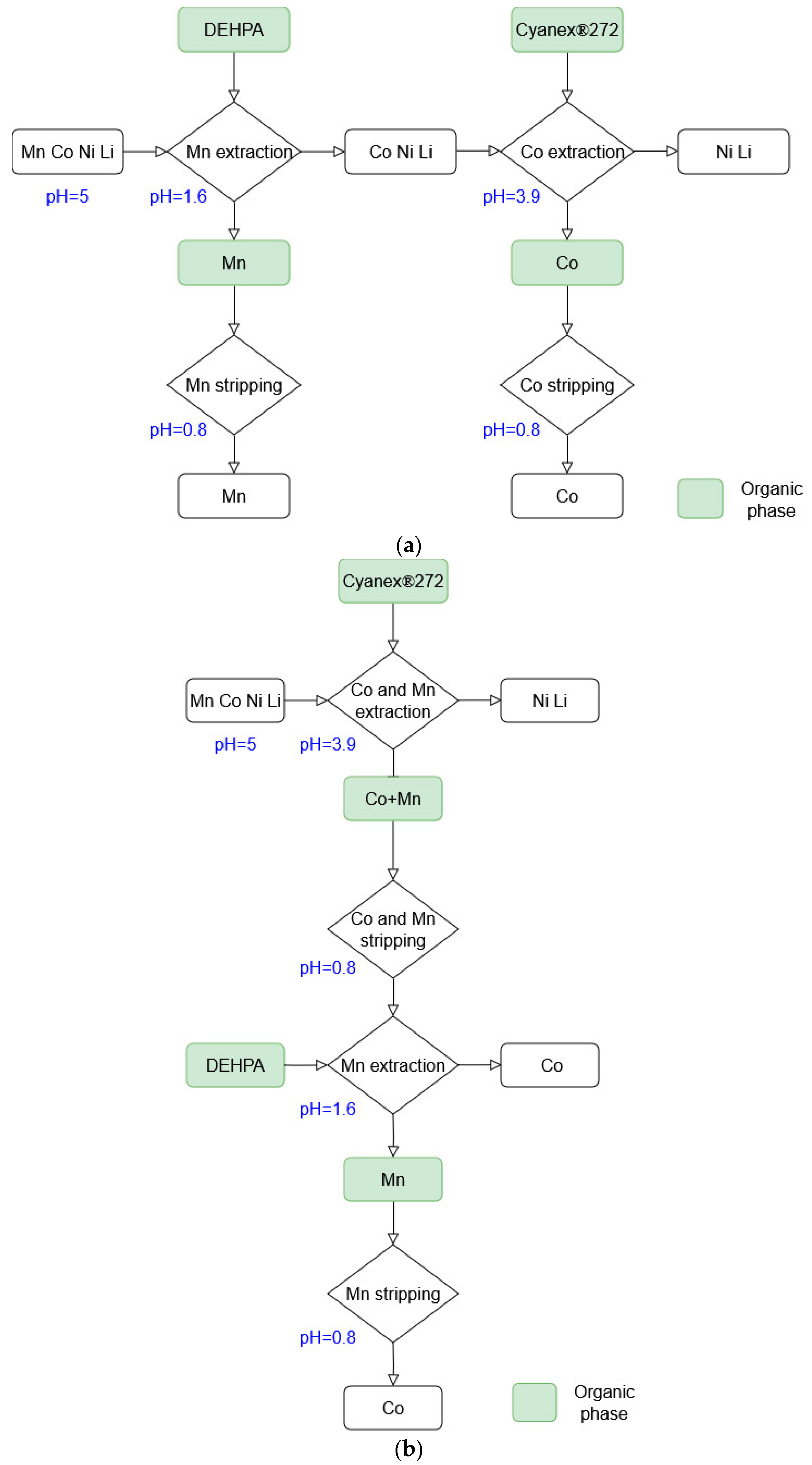
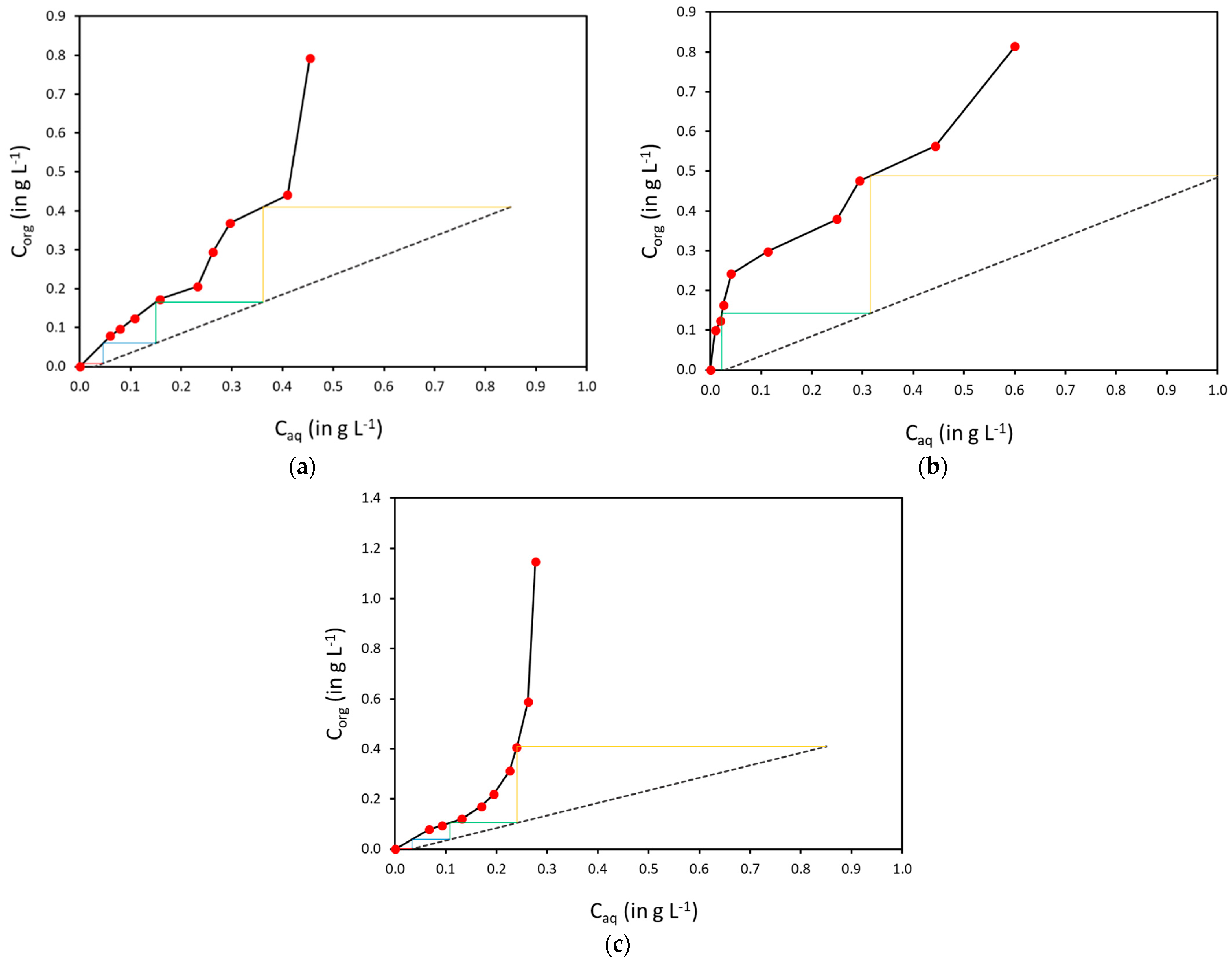
3.2. Solvent Extraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries: Technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Mennik, F.; Dinç, N.İ.; Burat, F. Selective recovery of metals from spent mobile phone lithium-ion batteries through froth flotation followed by magnetic separation procedure. Results Eng. 2023, 17, 100868. [Google Scholar] [CrossRef]
- Shen, K.; Yuan, C.; Hauschild, M. Direct recycling of lithium ion batteries from electric vehicles for closed-loop life cycle impact mitigation. CIRP Ann. 2023, 72, 13–16. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Srivastava, V.; Rantala, V.; Mehdipour, P.; Kauppinen, T.; Tuomikoski, S.; Heponiemi, A.; Runtti, H.; Tynjälä, P.; Simões Dos Reis, G.; Lassi, U. A comprehensive review of the reclamation of resources from spent lithium-ion batteries. Chem. Eng. J. 2023, 474, 145822. [Google Scholar] [CrossRef]
- Regulation (EU) 2023/1542 of the European Parliament and of the Council. 2023. Available online: https://eur-lex.europa.eu/eli/reg/2023/1542/oj (accessed on 13 January 2025).
- Vanderbruggen, A.; Hayagan, N.; Bachmann, K.; Ferreira, A.; Werner, D.; Horn, D.; Peuker, U.; Serna-Guerrero, R.; Rudolph, M. Lithium-Ion Battery Recycling─Influence of Recycling Processes on Component Liberation and Flotation Separation Efficiency. ACS EST Eng. 2022, 2, 2130–2141. [Google Scholar] [CrossRef]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef]
- Fan, X.; Song, C.; Lu, X.; Shi, Y.; Yang, S.; Zheng, F.; Huang, Y.; Liu, K.; Wang, H.; Li, Q. Separation and recovery of valuable metals from spent lithium-ion batteries via concentrated sulfuric acid leaching and regeneration of LiNi1/3Co1/3Mn1/3O2. J. Alloys Compd. 2021, 863, 158775. [Google Scholar] [CrossRef]
- Guimarães, L.F.; Botelho Junior, A.B.; Espinosa, D.C.R. Sulfuric acid leaching of metals from waste Li-ion batteries without using reducing agent. Miner. Eng. 2022, 183, 107597. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, X.; Cao, H.; Lin, X.; Liu, C.; Sun, Y.; Zhang, Y.; Sun, Z. Selective Recovery of Lithium from Spent Lithium Iron Phosphate Batteries: A Sustainable Process. Green Chem. 2016, 18, 1839–1854. [Google Scholar] [CrossRef]
- Li, H.; Xing, S.; Liu, Y.; Li, F.; Guo, H.; Kuang, G. Recovery of lithium, iron and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 8017–8024. [Google Scholar] [CrossRef]
- Benjamasutin, P.; Promphan, R. Determination of Optimal Parameters for the Application of Hydrogen Peroxide as Reducing Agent in the Leaching Process. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2022. [Google Scholar]
- Chen, X.; Cao, L.; Kang, D.; Li, J.; Zhou, T.; Ma, H. Recovery of valuable metals from mixed types of spent lithium ion batteries. Part II: Selective extraction of lithium. Waste Manag. 2018, 80, 198–210. [Google Scholar] [CrossRef]
- Zou, Y. Leaching of NMC industrial black mass in the presence of LFP. Sci. Rep. 2024, 14, 10818. [Google Scholar] [CrossRef]
- Azimi, G.; Chan, K.H. A review of contemporary and emerging recycling methods for lithium-ion batteries with a focus on NMC cathodes. Resour. Conserv. Recycl. 2024, 209, 107825. [Google Scholar] [CrossRef]
- Mehdi, A. Oxfordenergy. Insight-145-Lithium-Price-Volatility. 2024. Available online: https://www.oxfordenergy.org/wpcms/wp-content/uploads/2024/02/Insight-145-Lithium-Price-Volatility.pdf (accessed on 4 November 2024).
- Nadimi, H.; Jalalian Karazmoudeh, N. Leaching of Co, Mn and Ni Using H2O2 in Sulfuric Acid Medium from Mobile Phone LIBs. J. Inst. Eng. India Ser. D 2020, 101, 111–116. [Google Scholar] [CrossRef]
- Zou, Y.; Chernyaev, A.; Seisko, S.; Sainio, J.; Lundström, M. Removal of iron and aluminum from hydrometallurgical NMC-LFP recycling process through precipitation. Miner. Eng. 2024, 218, 109037. [Google Scholar] [CrossRef]
- Hubert, P.; Chagnes, A.; Jradi, S.; Clerget, L. Selective Metal Recovery: Innovating Leaching from Lfp-NMC Cathode Mixtures in Lithium-Ion Batteries. In Electrochemical Society Meeting Abstracts 245; The Electrochemical Society, Inc.: San Francisco, CA, USA, 2024. [Google Scholar]
- Cox, D.R.; Reid, N. The Theory of the Design of Experiments; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Narukulla, S.; Bogadi, S.; Tallapaneni, V.; Sanapalli, B.K.R.; Sanju, S.; Khan, A.A.; Malik, A.; Barai, H.R.; Mondal, T.K.; Karri, V.V.S.R.; et al. Comparative study between the Full Factorial, Box–Behnken, and Central Composite Designs in the optimization of metronidazole immediate release tablet. Microchem. J. 2024, 207, 111875. [Google Scholar] [CrossRef]
- Chernyaev, A.; Zou, Y.; Wilson, B.P.; Lundström, M. The interference of copper, iron and aluminum with hydrogen peroxide and its effects on reductive leaching of LiNi1/3Mn1/3Co1/3O2. Sep. Purif. Technol. 2022, 281, 119903. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A closed loop process for recycling spent lithium ion batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Chen, W.-S.; Ho, H.-J. Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods. Metals 2018, 8, 321. [Google Scholar] [CrossRef]
- Billy, E.; Joulié, M.; Laucournet, R.; Boulineau, A.; De Vito, E.; Meyer, D. Dissolution Mechanisms of LiNi1/3Mn1/3Co1/3O2 Positive Electrode Material from Lithium-Ion Batteries in Acid Solution. ACS Appl. Mater. Interfaces 2018, 10, 16424–16435. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Karazhiyan, H.; Razavi, S.M.A.; Phillips, G.O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011, 25, 915–920. [Google Scholar] [CrossRef]
- Vieceli, N.; Ottink, T.; Stopic, S.; Dertmann, C.; Swiontek, T.; Vonderstein, C.; Sojka, R.; Reinhardt, N.; Ekberg, C.; Friedrich, B.; et al. Solvent extraction of cobalt from spent lithium-ion batteries: Dynamic optimization of the number of extraction stages using factorial design of experiments and response surface methodology. Sep. Purif. Technol. 2023, 307, 122793. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, S.; Song, S.; Sun, W.; Wang, L. Stepwise recycling of valuable metals from Ni-rich cathode material of spent lithium-ion batteries. Waste Manag. 2020, 102, 131–138. [Google Scholar] [CrossRef]
- Djoudi, N.; Le Page Mostefa, M.; Muhr, H. Precipitation of Cobalt Salts for Recovery in Leachates. Chem. Eng. Technol. 2019, 42, 1492–1499. [Google Scholar] [CrossRef]
- Vieceli, N.; Reinhardt, N.; Ekberg, C.; Petranikova, M. Optimization of Manganese Recovery from a Solution Based on Lithium-Ion Batteries by Solvent Extraction with D2EHPA. Metals 2020, 11, 54. [Google Scholar] [CrossRef]
- Locati, A.; Mikulić, M.; Rouquette, L.M.J.; Ebin, B.; Petranikova, M. Production of High Purity MnSO4·H2O from Real NMC111 Lithium-Ion Batteries Leachate Using Solvent Extraction and Evaporative Crystallization. Solvent Extr. Ion Exch. 2024, 1–22. [Google Scholar] [CrossRef]
- Rodrigues, I.R.; Deferm, C.; Binnemans, K.; Riaño, S. Separation of cobalt and nickel via solvent extraction with Cyanex-272: Batch experiments and comparison of mixer-settlers and an agitated column as contactors for continuous counter-current extraction. Sep. Purif. Technol. 2022, 296, 121326. [Google Scholar] [CrossRef]
- Pahla, G.; Ntuli, F.; Magwa, N. New experimental findings on the separation of cobalt and nickel from an ammonia-ammonium-based leach liquor using ammonium-saponified Cyanex 272. S. Afr. J. Chem. Eng. 2024, 49, 1–10. [Google Scholar] [CrossRef]
| Li | Ni | Mn | Co | Fe | P | O | D10 | D50 | D90 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LFP | Wt. % | 4.64 | 34.23 | 18.01 | 43.12 | 0.020 | 0.074 | 4.73 | |||
| Molar ratio | 1.00 | 0.92 | 0.87 | 4.03 | |||||||
| NMC | Wt. % | 7.71 | 49.41 | 4.30 | 6.78 | 31.80 | 0.019 | 0.094 | 7.53 | ||
| Molar ratio | 1.00 | 0.76 | 0.07 | 0.10 | 1.79 |
| H2SO4 (mol/L) | H2O2 % (Vol. %) | S/L (g/L) | %D(Ni) | %D(Mn) | %D(Co) | %D(Li) |
|---|---|---|---|---|---|---|
| 2 | 4 | 42.9 | 100.0 | 97.9 | 97.6 | 89.3 |
| 1 | 3 | 50 | 92.6 | 90.1 | 90.7 | 89.2 |
| 3 | 3 | 50 | 94.5 | 93.1 | 93.1 | 88.2 |
| 1 | 5 | 50 | 100.0 | 99.8 | 98.9 | 89.9 |
| 3 | 5 | 50 | 100.0 | 100.0 | 99.7 | 89.1 |
| 2 | 2.6 | 70 | 80.4 | 81.1 | 82.1 | 88.3 |
| 0.6 | 4 | 70 | 92.8 | 92.4 | 93.1 | 89.9 |
| 2 | 4 | 70 | 89.9 | 92.0 | 93.1 | 92.4 |
| 2 | 4 | 70 | 92.0 | 95.2 | 95.6 | 92.7 |
| 2 | 4 | 70 | 89.7 | 91.9 | 92.7 | 91.9 |
| 3.4 | 4 | 70 | 90.5 | 90.7 | 91.1 | 92.3 |
| 2 | 5.4 | 70 | 97.7 | 100.0 | 100.0 | 90.4 |
| 1 | 3 | 90 | 74.4 | 73.5 | 74.8 | 88.9 |
| 3 | 3 | 90 | 79.1 | 79.7 | 80.3 | 89.1 |
| 1 | 5 | 90 | 96.9 | 100.0 | 100.0 | 93.7 |
| 3 | 5 | 90 | 91.7 | 98.1 | 96.8 | 98.9 |
| 2 | 4 | 97.1 | 78.2 | 78.6 | 79.2 | 86.0 |
| Ni | Mn | Co | |
|---|---|---|---|
| 90.6 | 91.4 | 91.7 | |
| 6.1 | 7.5 | 6.9 | |
| 6.4 | 5.0 | 4.7 | |
| 2.8 | 3.5 | 3.4 | |
| 4 | 4 | 4 | |
| 70 | 70 | 70 | |
| F-value | 68.8 | 49.5 | 44.4 |
| p-value | <0.0001 | <0.0001 | <0.0001 |
| R2 | 0.94 | 0.92 | 0.91 |
| 0.93 | 0.90 | 0.89 | |
| RMSE | 2.17 | 2.64 | 2.62 |
| H2O2 in % (Vol. %) | S/L (g/L) | %D(Ni) | %D(Mn) | %D(Co) | %D(Li) |
|---|---|---|---|---|---|
| 4 | 48.4 | 78.6 | 78.7 | 76.6 | 81.2 |
| 3 | 50 | 77.9 | 75.7 | 74.9 | 79.4 |
| 5 | 50 | 79.0 | 76.2 | 76.3 | 78.8 |
| 2.9 | 70 | 76.3 | 74.5 | 76.1 | 80.0 |
| 4 | 70 | 78.5 | 76.4 | 77.7 | 80.3 |
| 4 | 70 | 77.7 | 79.2 | 79.4 | 80.3 |
| 5.1 | 70 | 75.4 | 73.1 | 75.1 | 79.1 |
| 3 | 90 | 71.5 | 72.3 | 72.8 | 80.2 |
| 5 | 90 | 76.5 | 76.7 | 79.2 | 81.2 |
| 4 | 91.6 | 75.5 | 77.0 | 78.1 | 80.3 |
| Average values (%) | 76.7 | 76.0 | 76.6 | 80.1 | |
| Standard deviations (%) | 2.2 | 2.2 | 2.1 | 0.8 | |
| RSD (%) | 2.9 | 2.9 | 2.7 | 1.0 | |
| %D(Ni) | %D(Mn) | %D(Co) | %D(Li) | |
|---|---|---|---|---|
| Experimental test for acid-deficient leaching without recirculation (S/L = 50 g/L) | 76.7 | 76.0 | 76.6 | 80.1 |
| Experimental test for acid-deficient with 60%(Wt. %) reintroduction(S/L = 66 g/L) * | 87.1 | 87.1 | 87.4 | 89.4 |
| Acid-deficient leaching with Calculation with 60%(Wt. %) recirculation after 1 reintroduction loop (S/L = 66 g/L) * | 87.4 | 86.9 | 87.4 | 89.7 |
| Calculation with acid-deficient leaching with 60%(Wt. %) recirculation after 7 reintroduction loops (S/L = 88 g/L) * | 89.2 | 88.8 | 89.1 | 91.0 |
| %D(Ni) | %D(Mn) | %D(Co) | %D(Li) | %D(Fe) | %D(Al) | %D(Cu) | |
|---|---|---|---|---|---|---|---|
| Experimental test of acid-excess leaching without reintroduction | 100 | 100 | 99.9 | 88.0 | 100 | 17 | 100 |
| Experimental test of acid-deficient leaching without reintroduction | 76.7 | 76.0 | 76.6 | 80.1 | 0 | 0 | 5.8 |
| Calculation under acid-deficient leaching with reintroduction | 89.2 | 88.8 | 89.1 | 91.0 | 0 | 0 | 13.3 |
| %D(Ni) | %D(Mn) | %D(Co) | %D(Li) | Number of Unit Operations | mol H2SO4 (per Liter of PLS) | mol H2O2 (per Liter of PLS) | mol NaOH (per Liter of PLS) | |
|---|---|---|---|---|---|---|---|---|
| (1) | 95.5 | 95.5 | 95.4 | 84 | 3 | 1 | 2.1 | 0.6 |
| (2) | 89.2 | 87 | 89 | 90.7 | 1 | 0.4 | 1.3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubert, P.; Noclain, A.; Jradi, S.; Chagnes, A. Optimizing Recycling Processes for Mixed LFP/NMC Lithium-Ion Batteries: A Comparative Study of Acid-Excess and Acid-Deficient Leaching. Metals 2025, 15, 74. https://doi.org/10.3390/met15010074
Hubert P, Noclain A, Jradi S, Chagnes A. Optimizing Recycling Processes for Mixed LFP/NMC Lithium-Ion Batteries: A Comparative Study of Acid-Excess and Acid-Deficient Leaching. Metals. 2025; 15(1):74. https://doi.org/10.3390/met15010074
Chicago/Turabian StyleHubert, Pierric, Angelina Noclain, Safi Jradi, and Alexandre Chagnes. 2025. "Optimizing Recycling Processes for Mixed LFP/NMC Lithium-Ion Batteries: A Comparative Study of Acid-Excess and Acid-Deficient Leaching" Metals 15, no. 1: 74. https://doi.org/10.3390/met15010074
APA StyleHubert, P., Noclain, A., Jradi, S., & Chagnes, A. (2025). Optimizing Recycling Processes for Mixed LFP/NMC Lithium-Ion Batteries: A Comparative Study of Acid-Excess and Acid-Deficient Leaching. Metals, 15(1), 74. https://doi.org/10.3390/met15010074







