Effect of Potassium Salt Addition on Silver Precipitation During Hydrothermal Synthesis of Argentojarosites
Abstract
1. Introduction
2. Materials and Methods
- Iron (III) sulfate (Fe2(SO4)3·9H2O), over 98 wt%;
- Potassium sulfate (K2SO4), over 98 wt%;
- Silver nitrate (AgNO3), over 99 wt%.
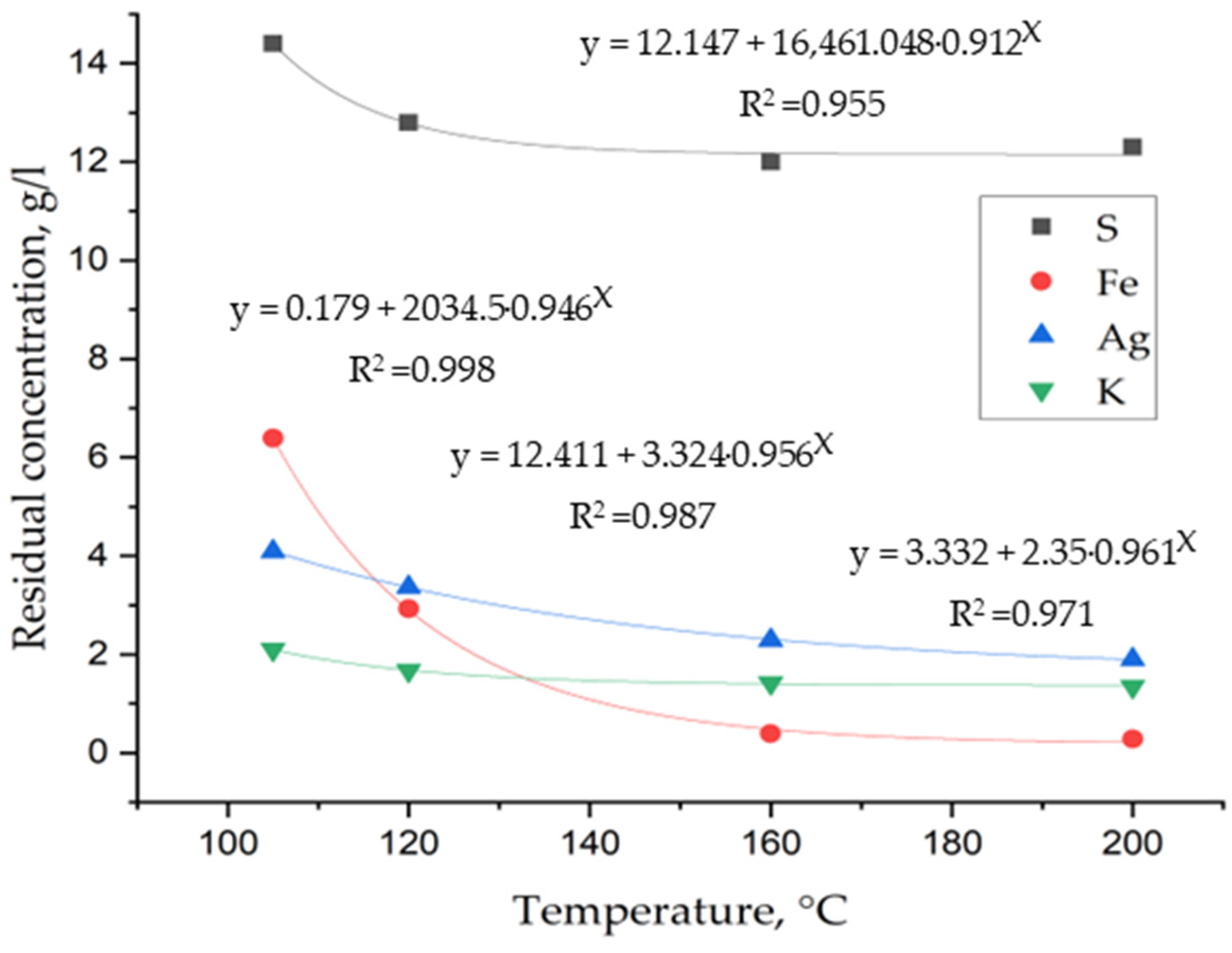
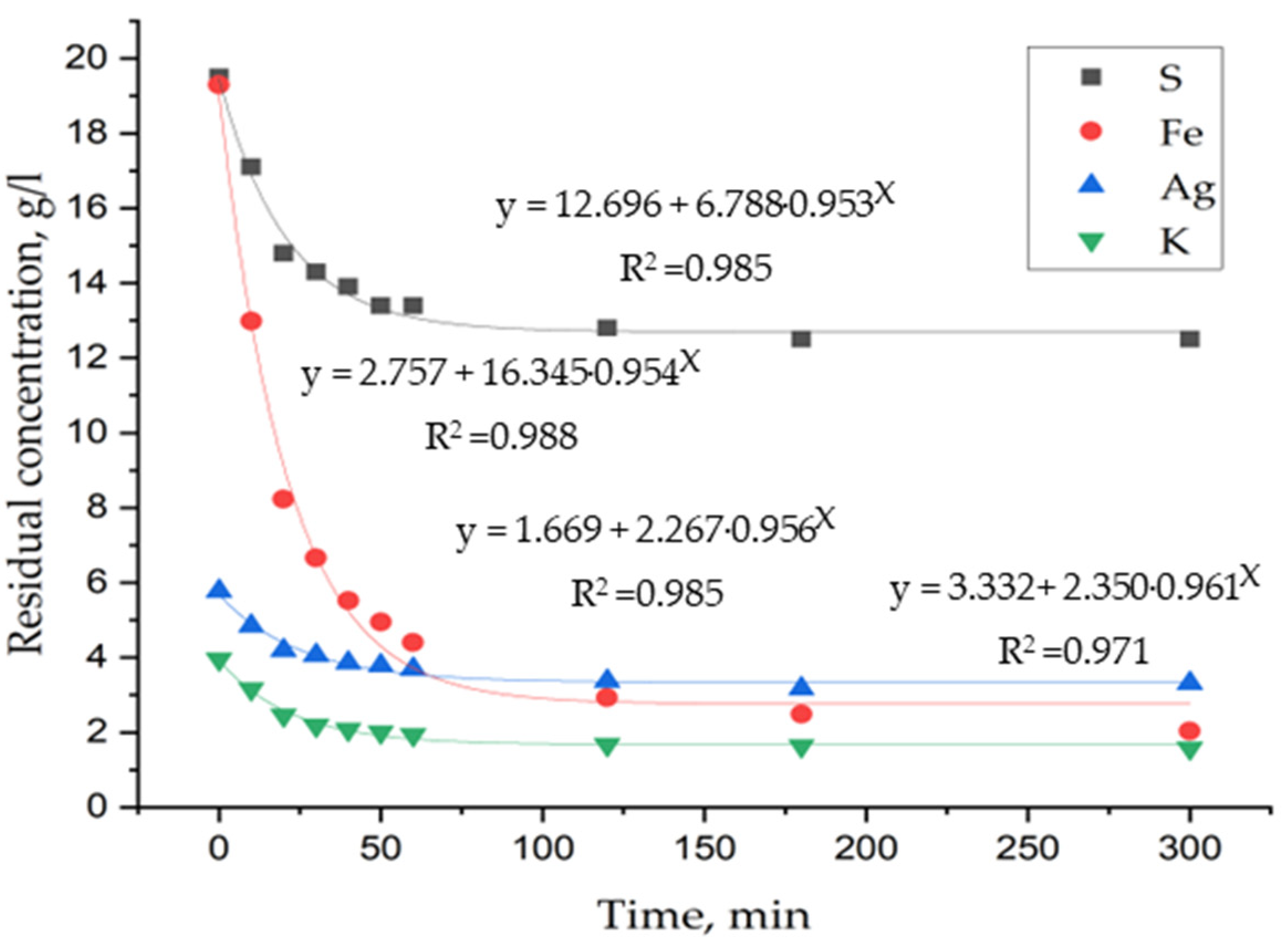
3. Results and Discussion
3.1. Laboratory Tests on Hydrothermal Synthesis of Jarosites
3.2. Determination of Thermodynamic Parameters
- h—constant depending on the dielectric permittivity of the solution ε and temperature T;
- z+, z–—charges of cation and anion;
- I—ionic strength of the solution;
- a—distance of the greatest convergence of electric centers of ions;
- B—parameter depending on temperature and dielectric permittivity of the medium;
- C—a constant coefficient that takes into account the decrease in dielectric permittivity near the ion as a result of the polarization of dipole molecules of the solvent.
- ε—dielectric constant of sulfuric acid.
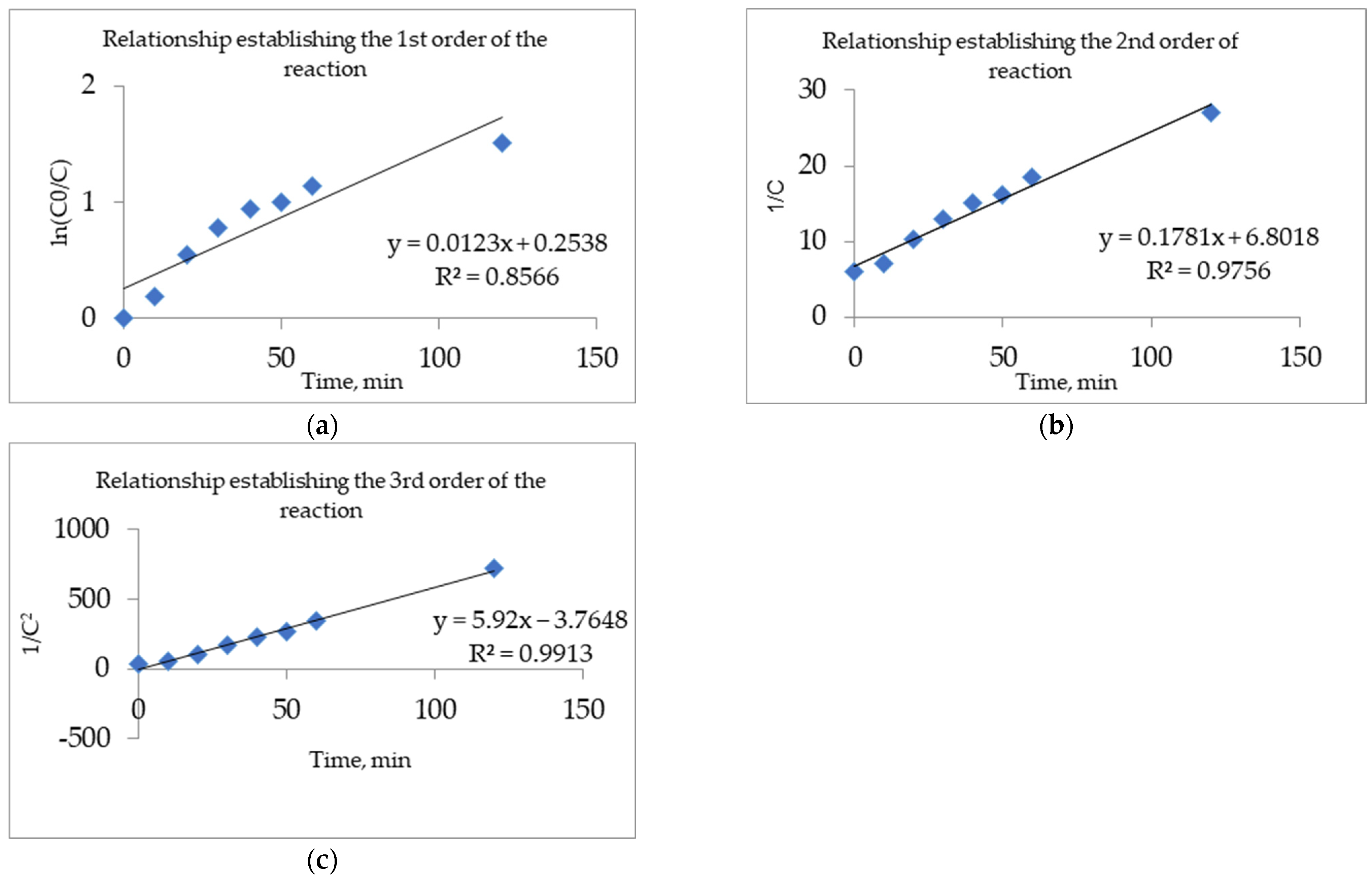
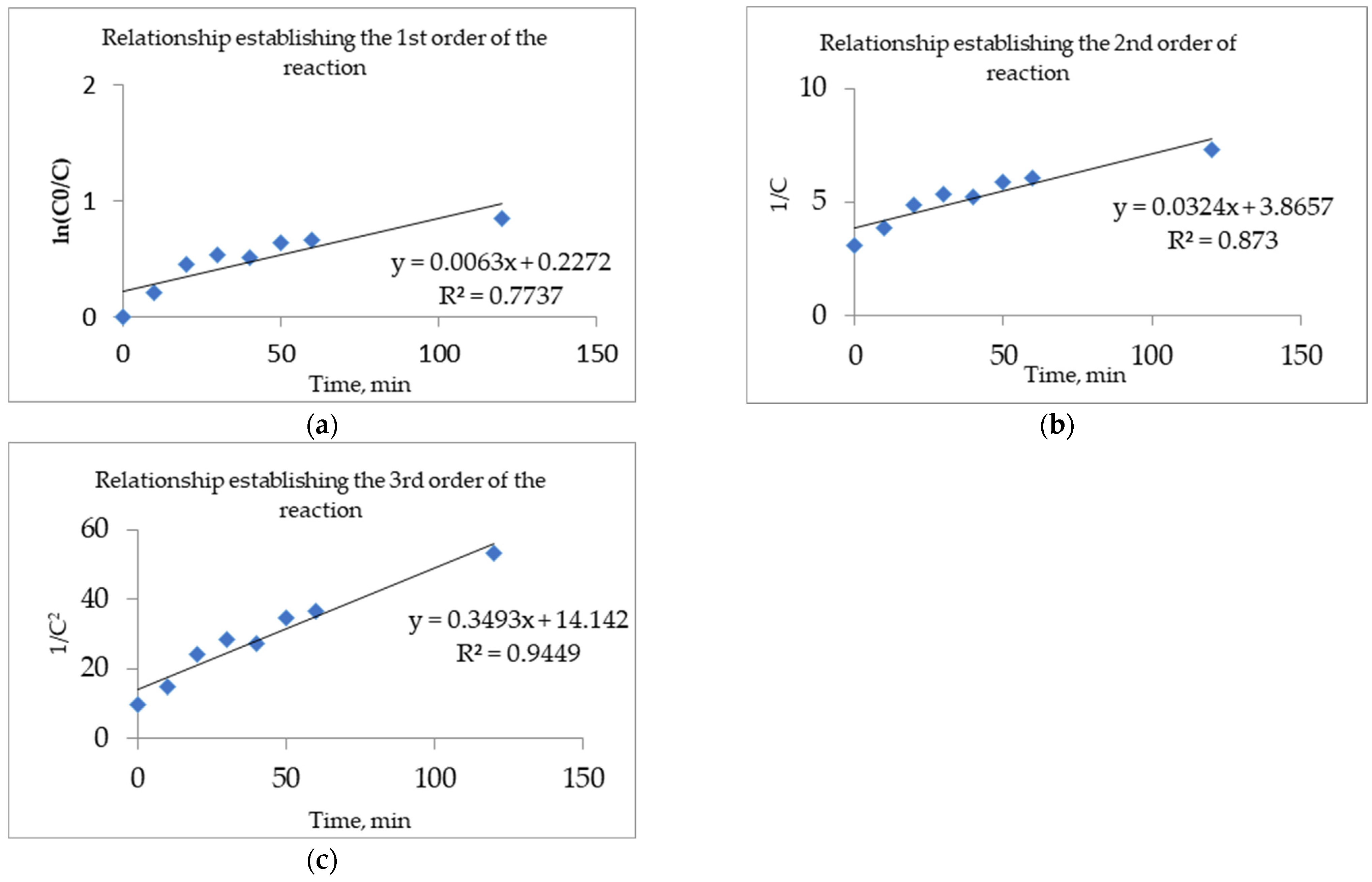
3.3. Determination of Kinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Option | Value |
|---|---|
| Temperature | 105 °C, 120 °C, 160 °C, 200 °C |
| The speed of mixing | 600 rpm 1 |
| Nitrogen partial pressure | 0.2 MPa |
| Time | 0–300 min 2 |
| Operating medium | sulfuric acid |
| Exp. | Temperature | Time | Element Content in the Final Solution, g/L | ||||
|---|---|---|---|---|---|---|---|
| № | °C | min | K | Ag | Fe | S | pH |
| 1 | 105 | 120 | 1.91 | - | 8.02 | 14.70 | 0.87 |
| 2 | 120 | 0 | 3.80 | - | 18.14 | 19.70 | 1.24 |
| 3 | 120 | 10 | 3.18 | - | 15.10 | 17.80 | 1.09 |
| 4 | 120 | 20 | 2.41 | - | 10.50 | 15.70 | 0.96 |
| 5 | 120 | 30 | 1.97 | - | 8.33 | 14.70 | 0.87 |
| 6 | 120 | 40 | 1.79 | - | 7.10 | 14.30 | 0.85 |
| 7 | 120 | 50 | 1.65 | - | 6.66 | 14.20 | 0.83 |
| 8 | 120 | 60 | 1.56 | - | 5.82 | 13.90 | 0.78 |
| 9 | 120 | 120 | 1.08 | - | 4.00 | 13.00 | 0.73 |
| 10 | 120 | 180 | 0.90 | - | 3.09 | 12.93 | 0.73 |
| 11 | 120 | 300 | 0.77 | - | 2.65 | 12.90 | 0.72 |
| 12 | 160 | 120 | 0.45 | - | 0.83 | 12.60 | 0.69 |
| 13 | 200 | 120 | 0.28 | - | 0.74 | 12.30 | 0.67 |
| 14 | 105 | 120 | - | 1.24 | 10.50 | 13.19 | 0.86 |
| 15 | 120 | 0 | - | 4.84 | 18.00 | 15.81 | 1.23 |
| 16 | 120 | 10 | - | 2.96 | 14.50 | 14.44 | 1.07 |
| 17 | 120 | 20 | - | 1.77 | 11.40 | 13.58 | 0.97 |
| 18 | 120 | 30 | - | 1.35 | 10.50 | 13.27 | 0.89 |
| 19 | 120 | 40 | - | 1.23 | 10.70 | 13.18 | 0.88 |
| 20 | 120 | 50 | - | 0.69 | 9.50 | 12.79 | 0.85 |
| 21 | 120 | 60 | - | 0.52 | 9.22 | 12.67 | 0.82 |
| 22 | 120 | 120 | - | 0.18 | 7.66 | 12.42 | 0.82 |
| 23 | 120 | 180 | - | 0.12 | 7.40 | 12.38 | 0.79 |
| 24 | 120 | 300 | - | 0.02 | 7.00 | 12.31 | 0.78 |
| 25 | 160 | 120 | - | 0.01 | 6.31 | 12.38 | 0.74 |
| 26 | 200 | 120 | - | 0.01 | 2.16 | 12.38 | 0.64 |
| 27 | 105 | 120 | 2.10 | 4.09 | 6.39 | 14.40 | 0.79 |
| 28 | 120 | 0 | 3.95 | 5.77 | 19.30 | 19.50 | 1.15 |
| 29 | 120 | 10 | 3.16 | 4.85 | 12.99 | 17.10 | 0.99 |
| 30 | 120 | 20 | 2.46 | 4.19 | 8.23 | 14.80 | 0.85 |
| 31 | 120 | 30 | 2.19 | 4.06 | 6.66 | 14.30 | 0.82 |
| 32 | 120 | 40 | 2.08 | 3.86 | 5.52 | 13.90 | 0.79 |
| 33 | 120 | 50 | 2.01 | 3.79 | 4.95 | 13.40 | 0.77 |
| 34 | 120 | 60 | 1.94 | 3.70 | 4.40 | 13.40 | 0.76 |
| 35 | 120 | 120 | 1.67 | 3.37 | 2.93 | 12.80 | 0.73 |
| 36 | 120 | 180 | 1.64 | 3.17 | 2.49 | 12.50 | 0.70 |
| 37 | 120 | 300 | 1.58 | 3.31 | 2.03 | 12.50 | 0.68 |
| 38 | 160 | 120 | 1.42 | 2.29 | 0.39 | 12.00 | 0.65 |
| 39 | 200 | 120 | 1.35 | 1.90 | 0.28 | 12.30 | 0.64 |
Appendix B
| Series No. | K | Ag | H3O |
|---|---|---|---|
| 1 | 0.92 | 0.00 | 0.08 |
| 2 | 0.00 | 0.80 | 0.20 |
| 3 1 | 0.66 | 0.34 | 0.00 |
| Reaction Order | Integrated Rate Law | Plot Needed for Linear Fit of Rate Data |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 |
References
- Shahalov, A.A.; Ospanov, E.A.; Bakhvalov, S.S.; Fomenko, I.V. Development of comprehensive hydrometallurgical technology for polymetallic concentrates and intermediate feedstock. Tsvetnye Met. 2020, 9, 99–104. [Google Scholar] [CrossRef]
- Padilla, R.; Rodríguez, G.; Ruiz, M.C. Copper and arsenic dissolution from chalcopyrite-enargite concentrate by sulfidation and pressure leaching in H2SO4–O2. Hydrometallurgy 2010, 100, 152–156. [Google Scholar] [CrossRef]
- Kachor, O.L.; Sarapulova, G.I.; Bogdanov, A.V. Investigation of the Possibility of Immobilization of Mobile Forms of Arsenic in Technogenic Soils. J. Min. Inst. 2019, 239, 596–602. [Google Scholar] [CrossRef]
- Selivanov, E.N.; Novikov, D.O.; Belyaev, V.V.; Skopov, G.V. Distribution of arsenic betwenn the pyrometallurgical products of copper-zinc concentrate. Tsvetnye Met. 2020, 1, 14–18. [Google Scholar] [CrossRef]
- Strauss, J.A.; Bazhko, V.; Ventruti, G.; Liguo, X.; Gomez, M.A. Arsenic behavior during the treatment of refractory gold ores via POX: Characterization of Fe-AsO4-SO4 precipitates. Hydrometallurgy 2021, 203, 105616. [Google Scholar] [CrossRef]
- Aleksandrova, N.; Afanasova, A.V.; Aburova, V.A. “Invisible” noble metals in carbonaceous rocks and beneficiation products: Feasibility of detection and coarsening. Min. Sci. Technol. 2024, 9, 231–242. [Google Scholar] [CrossRef]
- Pyagay Igor, N.; Svahina, Y.A.; Titova, M.E.; Miroshnichenko, V.V.; Dronova, V.R. Effect of Hydrogel Molar Composition on the Synthesis of LTA-type Zeolites in the Utilization of Technogenic Silica Gel. Silicon 2024, 16, 4811–4819. [Google Scholar] [CrossRef]
- Litvinenko, V.S.; Petrov, E.I.; Vasilevskaya, D.V.; Yakovenko, A.V.; Naumov, I.A.; Ratnikov, M.A. Assessment of the role of the state in the management of mineral resources. J. Min. Inst. 2023, 259, 95–111. [Google Scholar] [CrossRef]
- Savinova, Y.A.; Tsemekhman, L.S.; Popov, V.A. Composition of the solid products of oxidizing roasting of non-ferrous metal sulfide ore concentrates: Comparative analysis. Tsvetnye Met. 2018, 11, 27–35. [Google Scholar] [CrossRef]
- Tsemekhman, L.S.; Paretskiy, V.M. Modern processing techniques for copper-nickel Sulphide concentrates: A review. Tsvetnye Met. 2020, 1, 24–32. [Google Scholar] [CrossRef]
- Ivanik, S.A.; Ilyukhin, D.A. Flotation extraction of elemental sulfur from gold-bearing cakes. J. Min. Inst. 2020, 242, 202–208. [Google Scholar] [CrossRef]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Value-added strategies for the sustainable handling, disposal, or value-added use of copper smelter and refinery wastes. J. Hazard. Mater. 2021, 403, 123602. [Google Scholar] [CrossRef] [PubMed]
- Gorlanov, E.S.; Leontev, L.I. Directions in the technological development of aluminium pots. J. Min. Inst. 2024, 266, 246–259. [Google Scholar]
- Telyakov, N.M.; Darin, A.A.; Luganov, V.A. Prospects of biotechnologies application in metallurgy and enrichment. J. Min. Inst. 2016, 217, 113. [Google Scholar]
- Zaytsev, P.V.; Kravchenko, N.A. Hydrometallurgical recovery of copper and silver from flotation concentrates obtained from mixed ore. Tsvetnye Met. 2020, 9, 84–91. [Google Scholar] [CrossRef]
- Zaytsev, P.V.; Shneerson, Y.M. Autoclave processing of copper-bearing materials. Tsvetnye Met. 2016, 1, 26–31. [Google Scholar] [CrossRef][Green Version]
- Kritskii, A.V.; Tretyak, M.A.; Karimov, K.A.; Naboichenko, S.S. Autoclave treatment of cakes after pressure oxidation leaching of chalcopyrite concentrates. Izv. Vuzov. Tsvetnaya Metall. 2020, 13–18. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Bello, R.; Padilla, P.R. Removal of arsenic from enargite rich copper concentrates. In Materials Processing Fundamentals; Springer: Cham, Switzerland, 2013; pp. 249–256. [Google Scholar] [CrossRef]
- Bolobov, V.I.; Fomenko, I.V.; Shakhalov, A.A.; Ospanov, E.A. Corrosion resistance of metallic construction materials in the products of pressure leaching of sulfide copper concentrates. Tsvetnye Met. 2019, 4, 60–66. [Google Scholar] [CrossRef]
- Islas, H.; Flores, M.U.; Juárez, J.C.; Reyes, M.; Blanco, A.; Gutiérrez, E.J.; Aguilar, J.; Nolasco, M.C.; Rodríguez, I.; Reyes, I.A. Silver leaching from jarosite-type compounds using cyanide and non-cyanide lixiviants: A kinetic approach. Miner. Eng. 2021, 174, 107250. [Google Scholar] [CrossRef]
- Aleksandrova, T.; Nikolaeva, N.; Afanasova, A.; Romashev, A.; Aburova, V.; Prokhorova, E. Extraction of low-dimensional structures of noble and rare metals from carbonaceous ores using low-temperature and energy impacts at succeeding stages of raw material transformation. Minerals 2023, 13, 84. [Google Scholar] [CrossRef]
- Babcan, J. Synthesis of Jarosite, KFe3(SO4)2(OH)6. Geol. Zb. 1971, 22, 299–304. [Google Scholar]
- Fleming, C.A. Basic iron sulfate a potential killer in the processing of refractory gold concentrates by pressure oxidation. Min. Metall. Explor. 2010, 27, 81–88. [Google Scholar] [CrossRef]
- Adams, M.D. Gold Ore Processing: Project Development and Operations, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2016; 980p. [Google Scholar]
- Fedotov, P.K.; Senchenko, A.E.; Fedotov, K.V.; Burdonov, A.E. Studies of enrichment of sulfide and oxidized ores of gold deposits of the Aldan shield. J. Min. Inst. 2020, 242, 218. [Google Scholar] [CrossRef]
- Feshchenko, R.Y.; Erokhina, O.O.; Litavrin, I.O.; Ryaboshuk, S.V. Improvement of Oxidation Resistance of Arc Furnace Graphite Electrodes. Chernye Met. 2023, 7, 31–36. [Google Scholar] [CrossRef]
- Kritskii, A.V.; Naboichenko, S.S.; Klyshnikov, A.M.; Musaev, V.V. Pressure oxidative leaching of chalcopyrite concentrates: Influence of the process temperature on cakes cyanidation efficiency. Tsvetnye Met. 2020, 5, 25–29. [Google Scholar] [CrossRef]
- Gordeev, D.V.; Fomenko, I.V.; Shneerson, Y.M.; Petrov, G.V. Processing of carbonaceous gold-containing concentrates by autoclave oxidation with the addition of nitric acid as a secondary oxidizer. Obogashchenie Rud 2023, 5, 18–24. [Google Scholar] [CrossRef]
- Soe, K.M.; Ruan, R.; Jia, Y.; Tan, Q.; Wang, Z.; Shi, J.; Zhong, C.; Sun, H. Influence of jarosite precipitation on iron balance in heap bioleaching at Monywa copper mine. J. Min. Inst. 2021, 247, 102–113. [Google Scholar] [CrossRef]
- Cruells, M.; Roca, A. Jarosites: Structure, Formation, Leaching, Environmental, and Applications. Metals 2023, 13, 1292. [Google Scholar] [CrossRef]
- Kotova, O.B.; Ustyugov, V.A.; Sun, S.; Ponaryadov, A.V. Mullite production: Phase transformations of kaolinite, thermodynamics of the process. J. Min. Inst. 2022, 254, 129–135. [Google Scholar] [CrossRef]
- Gaboreau, S.; Vieillard, P. Prediction of Gibbs free energies of formation of minerals of the alunitesupergroup. Geochim. Cosmochim. Acta. 2004, 68, 3307–3316. [Google Scholar] [CrossRef]
- Brichkin, V.N.; Kurtenkov, R.V.; Maksimova, R.I.; Bormotov, I.S. Regeneration and recycling of lime component in complex processing of kaolin raw materials. Obogashchenie Rud 2024, 4, 32–38. [Google Scholar] [CrossRef]
- Cogram, P.F.; Welch, M.D.; Hudson-Edwards, K.A. Uptake of Silver by Jarosite and Natrojarosite Family Compounds at 22 °C, 97 °C and 140 °C. Metals 2023, 13, 627. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Morris, C.; Rea, S.; Li, J.; Usher, K.M.; McDonald, R.G.; Hilario, F.; Hosken, T.; Jackson, M.; du Plessis, C.A. Biohydrometallurgical iron oxidation and precipitation: Part II—Jarosite precipitate characterisation and acid recovery by conversion to hematite. Hydrometallurgy 2014, 147, 264–272. [Google Scholar] [CrossRef]
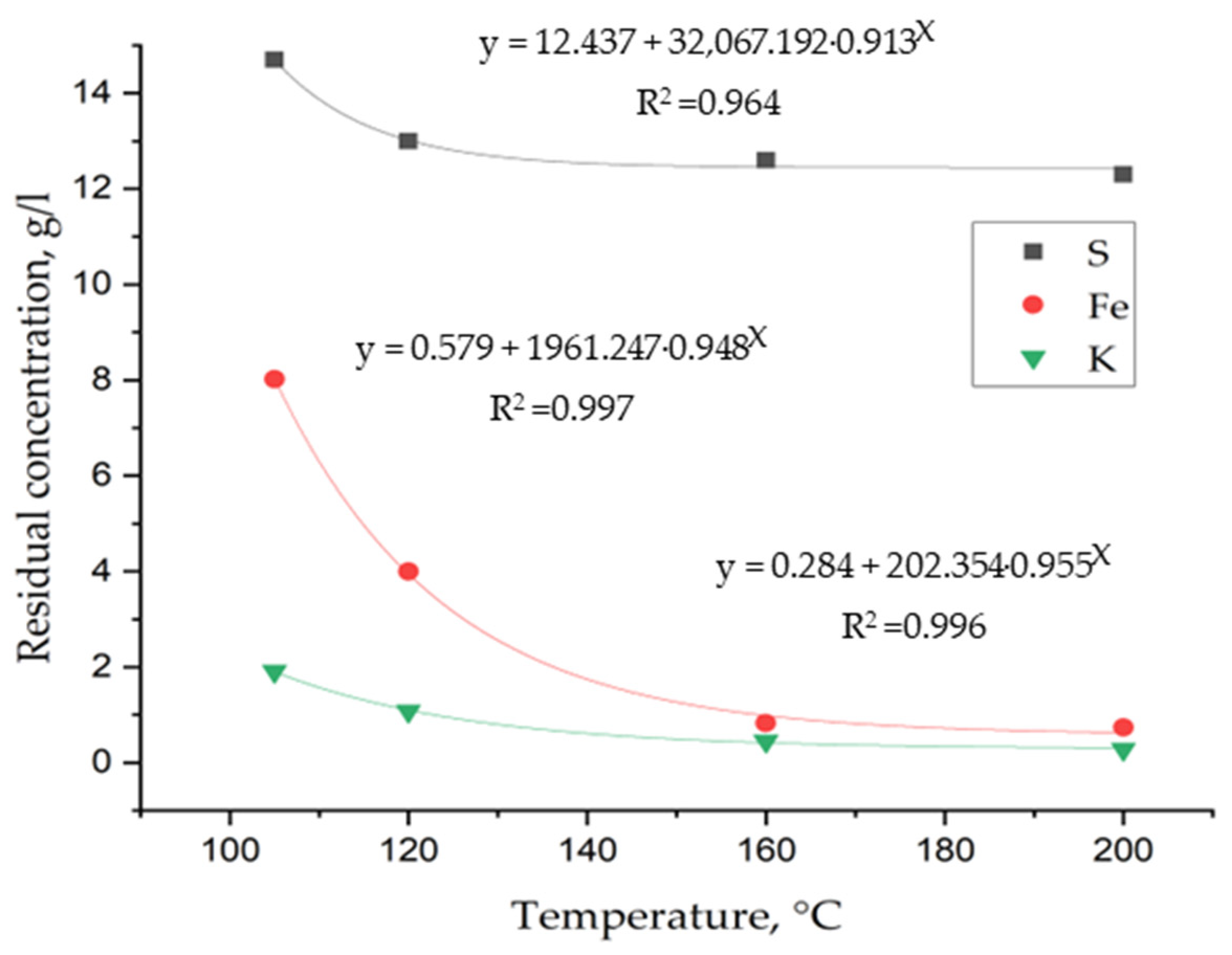
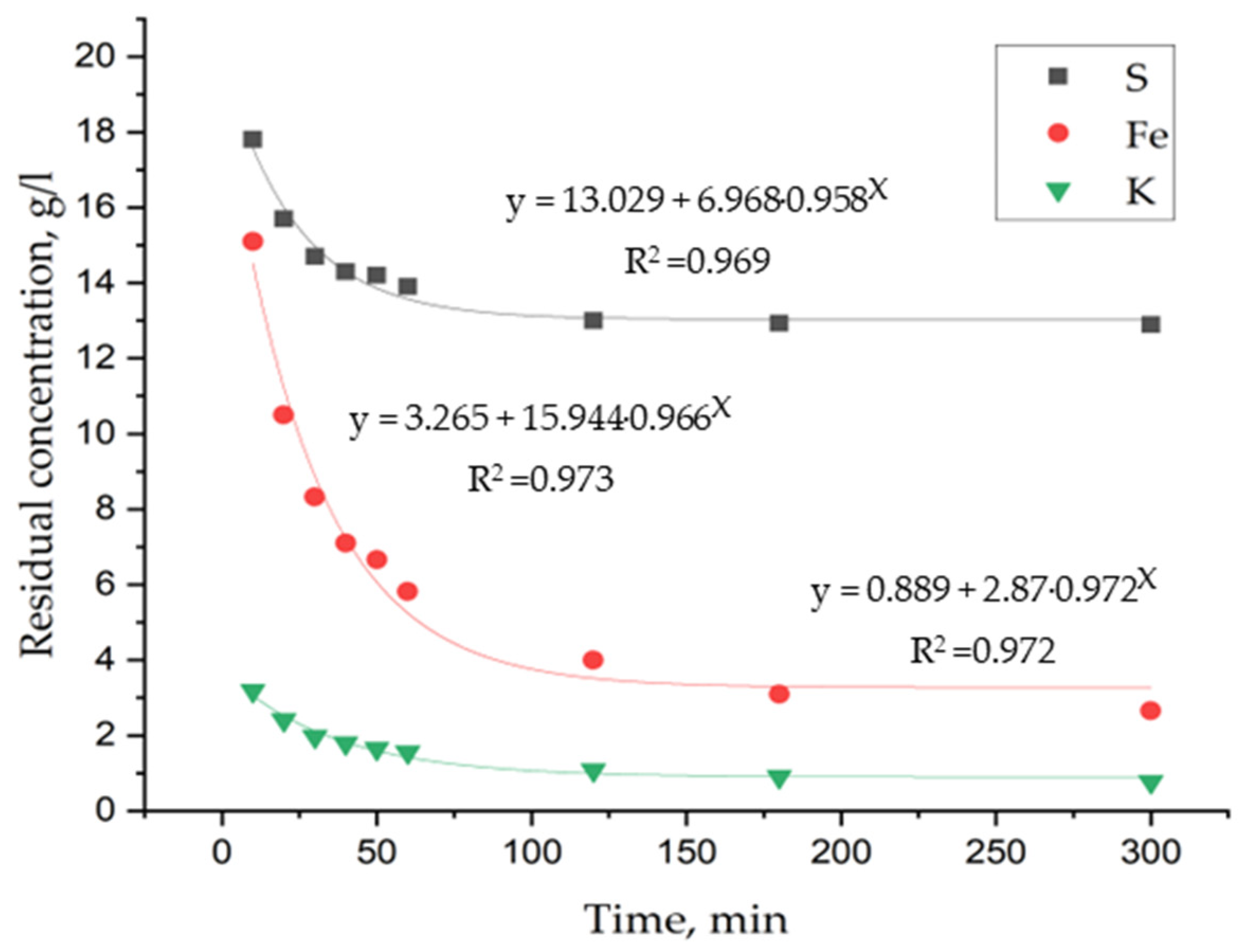
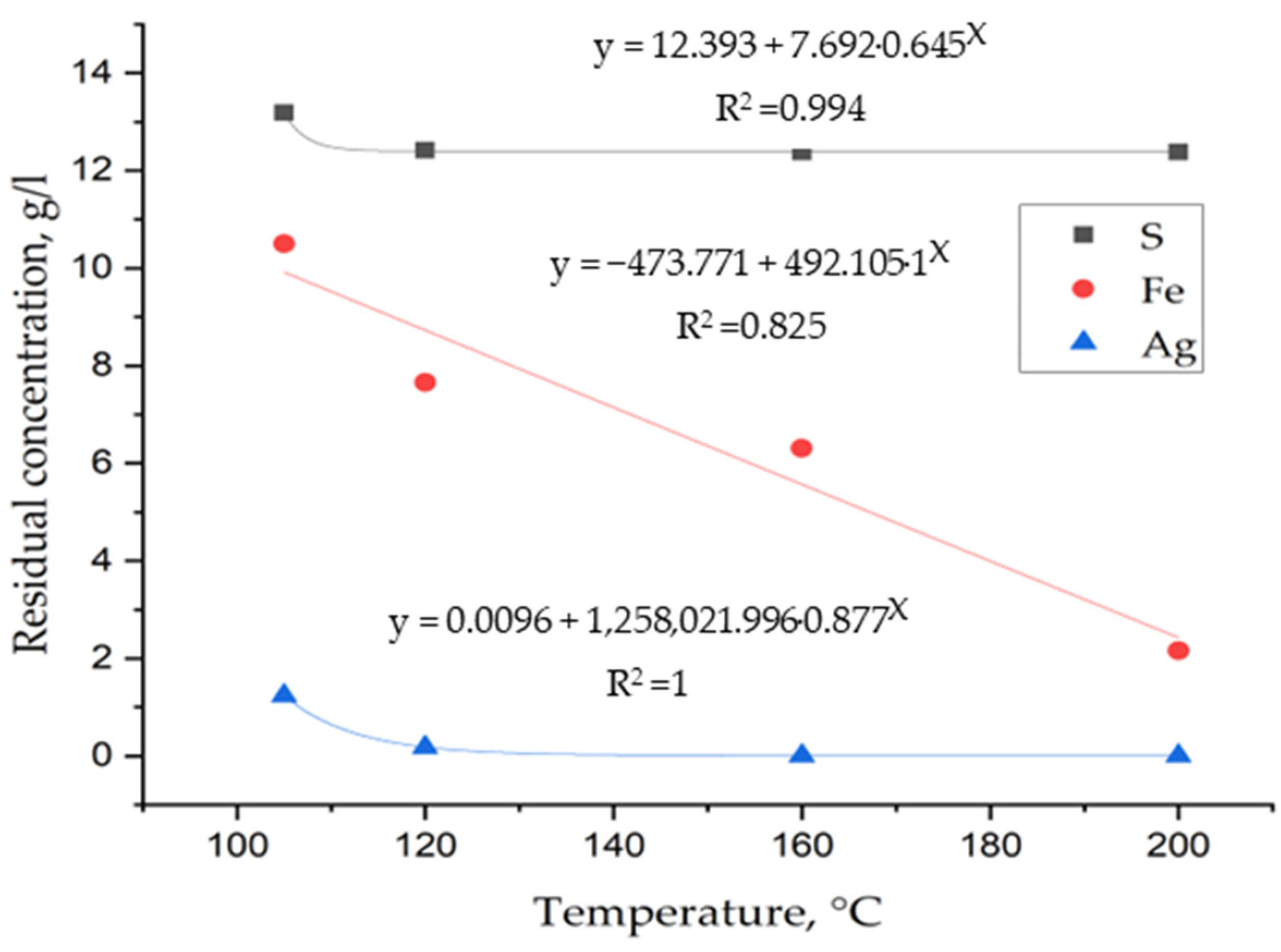
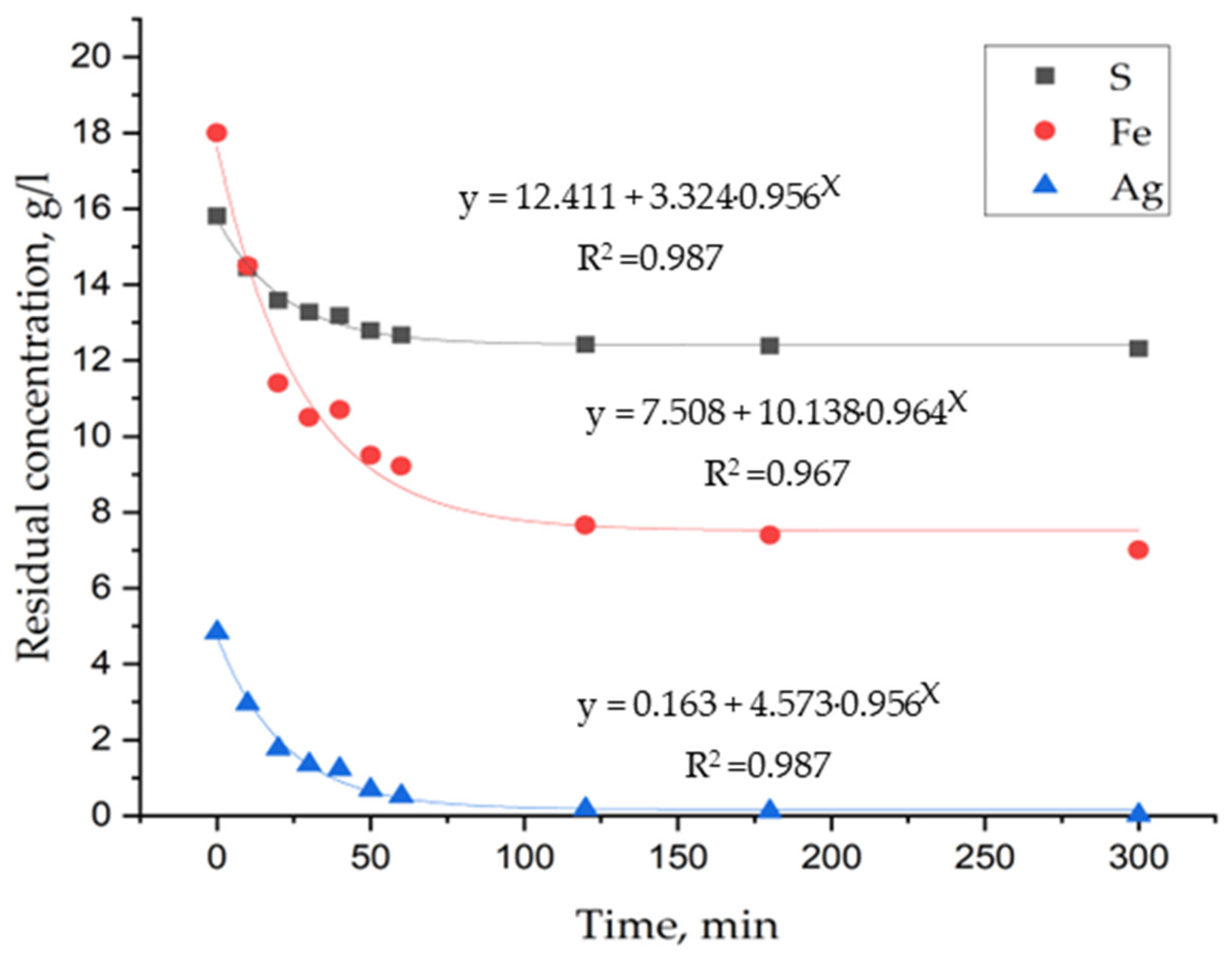

| Mineral | Chemical Formula | Gibbs Energy Value Calculated from Reference Data, kJ/mol |
|---|---|---|
| Jarosite | KFe3(SO4)2(OH)6 | −3314 |
| Natrojarosite | NaFe3(SO4)2(OH)6 | −3271 |
| Ammoniojarosite | NH4Fe3(SO4)2(OH)6 | −3095 |
| Argentojarosite | AgFe3(SO4)2(OH)6 | −2949 |
| Hydroniumjarosite | (H3O)Fe3(SO4)2(OH)6 | −3247 |
| Series No. | Element Content in the Initial Solution, g/L | |||||
|---|---|---|---|---|---|---|
| K | Ag | Fe | S | SO42− | pH | |
| 1 | 4.50 | 0.00 | 19.40 | 19.30 | 57.90 | 1.26 |
| 2 | 0.00 | 6.18 | 19.40 | 16.71 | 50.05 | 1.26 |
| 3 | 4.50 | 6.18 | 19.40 | 19.30 | 57.90 | 1.26 |
| Series No. | Content of Solid Phase Elements, % | Compound Formula Based on Mineralogy Results | |||
|---|---|---|---|---|---|
| K | Ag | Fe | S | ||
| 1 | 6.52 | 0.00 | 30.20 | 12.00 | K0.92(H3O)0.08Fe3(SO4)2(OH)6 |
| 2 | 0.00 | 15.11 | 30.20 | 10.99 | Ag0.80(H3O)0.2Fe3(SO4)2(OH)6 |
| 3 | 4.61 | 4.80 | 29.00 | 11.42 | K0.6Ag0.27(H3O)0.13Fe3(SO4)2(OH)6 |
| Equations | I mol/L | h (K3·kg/mol)0.5 | B m−1·mol−0.5·kg·K0.5 | C L/mol | log(γ) | γ | Keq. | ΔGT kJ/mol |
|---|---|---|---|---|---|---|---|---|
| 12 | 1.12 | 0.23 | 2.52 × 109 | −0.0206 | −0.158 | 0.695 | 3.38 × 107 | −56.66 |
| 13 | 1.51 | 0.23 | 2.52 × 109 | −0.0194 | −0.175 | 0.668 | 3.05 × 106 | −48.81 |
| 14 | 1.08 | 0.23 | 2.52 × 109 | −0.0204 | −0.156 | 0.699 | 1.31 × 1013 | −98.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheremisina, O.; Vasiliev, R.; Fedorov, A. Effect of Potassium Salt Addition on Silver Precipitation During Hydrothermal Synthesis of Argentojarosites. Metals 2025, 15, 24. https://doi.org/10.3390/met15010024
Cheremisina O, Vasiliev R, Fedorov A. Effect of Potassium Salt Addition on Silver Precipitation During Hydrothermal Synthesis of Argentojarosites. Metals. 2025; 15(1):24. https://doi.org/10.3390/met15010024
Chicago/Turabian StyleCheremisina, Olga, Roman Vasiliev, and Aleksei Fedorov. 2025. "Effect of Potassium Salt Addition on Silver Precipitation During Hydrothermal Synthesis of Argentojarosites" Metals 15, no. 1: 24. https://doi.org/10.3390/met15010024
APA StyleCheremisina, O., Vasiliev, R., & Fedorov, A. (2025). Effect of Potassium Salt Addition on Silver Precipitation During Hydrothermal Synthesis of Argentojarosites. Metals, 15(1), 24. https://doi.org/10.3390/met15010024





