A Study on the Aging Behavior of Nitrided W18Cr4V Steel in High-Temperature Sodium
Abstract
1. Introduction
2. Methods
2.1. Materials
2.2. Experiment
3. Results and Discussions
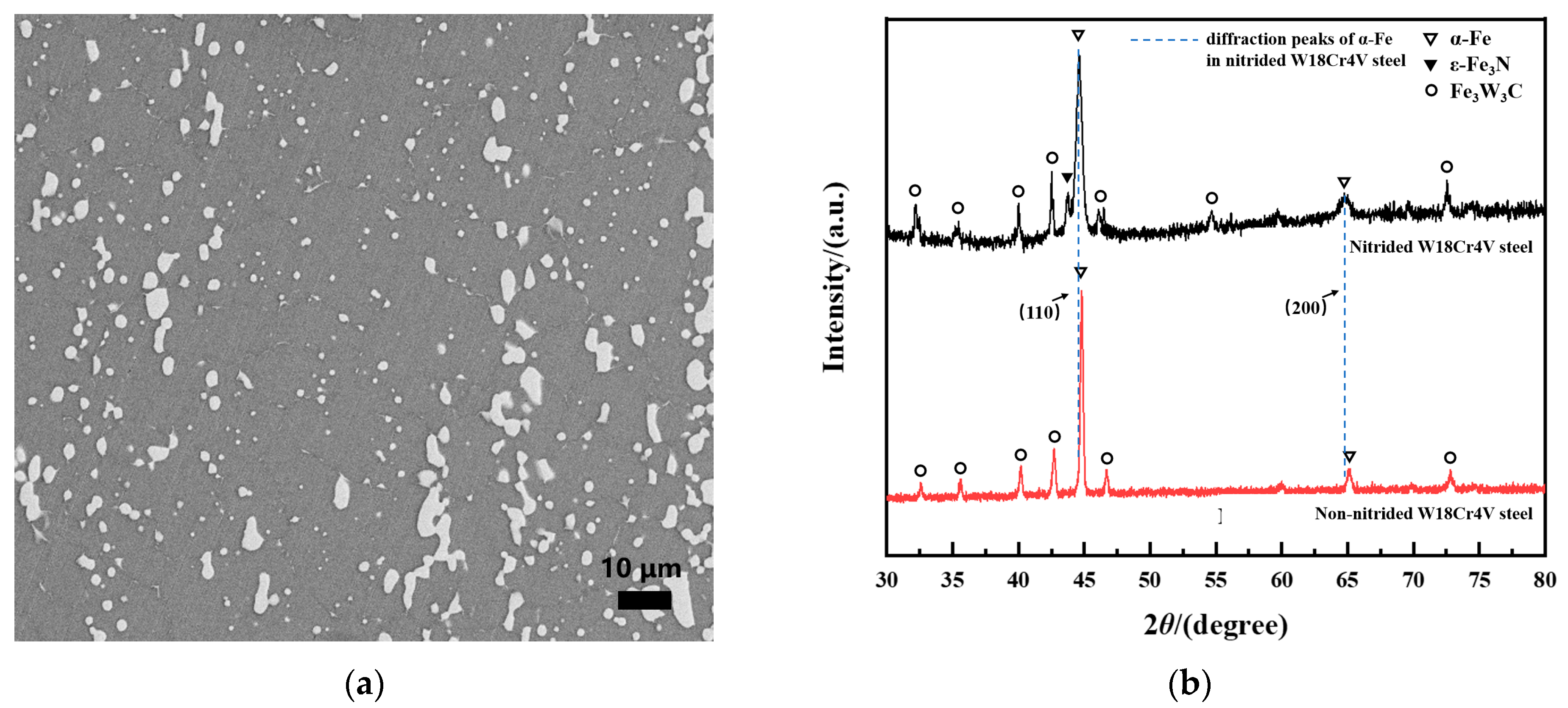
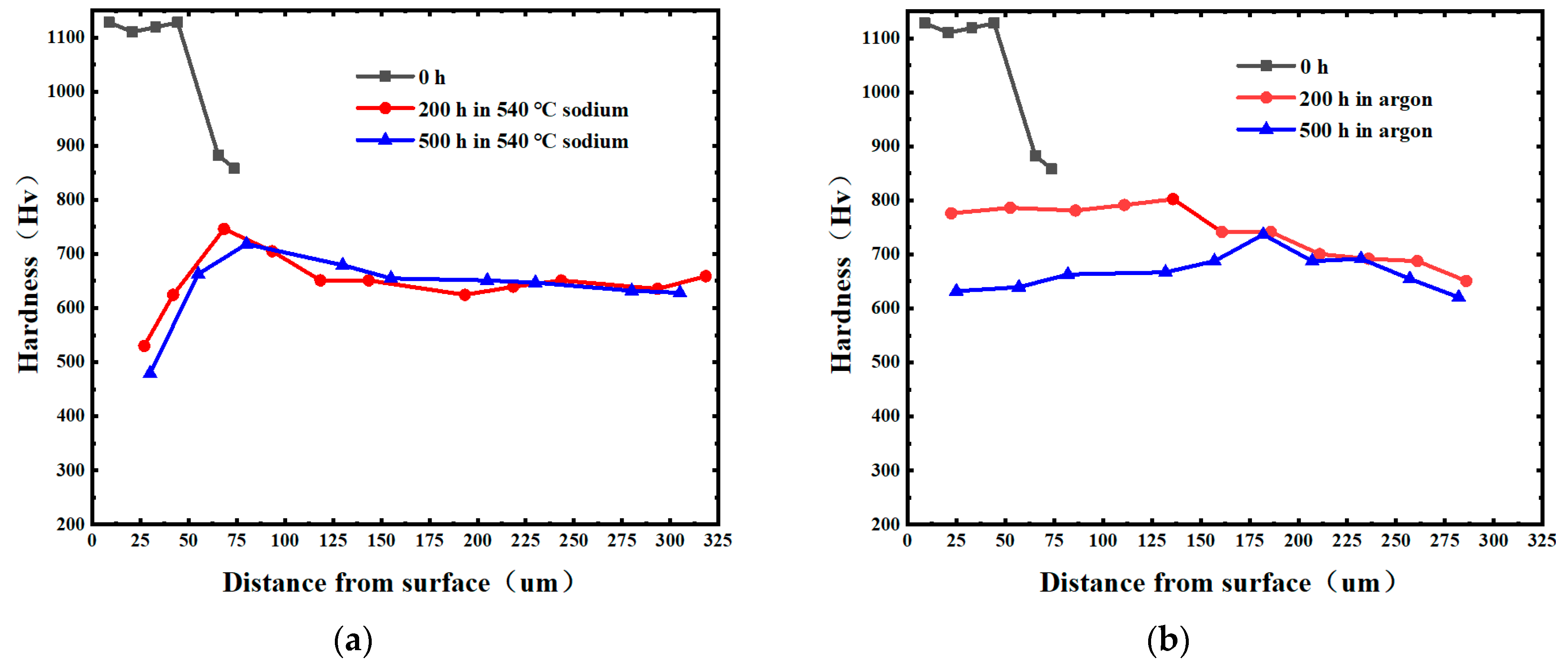
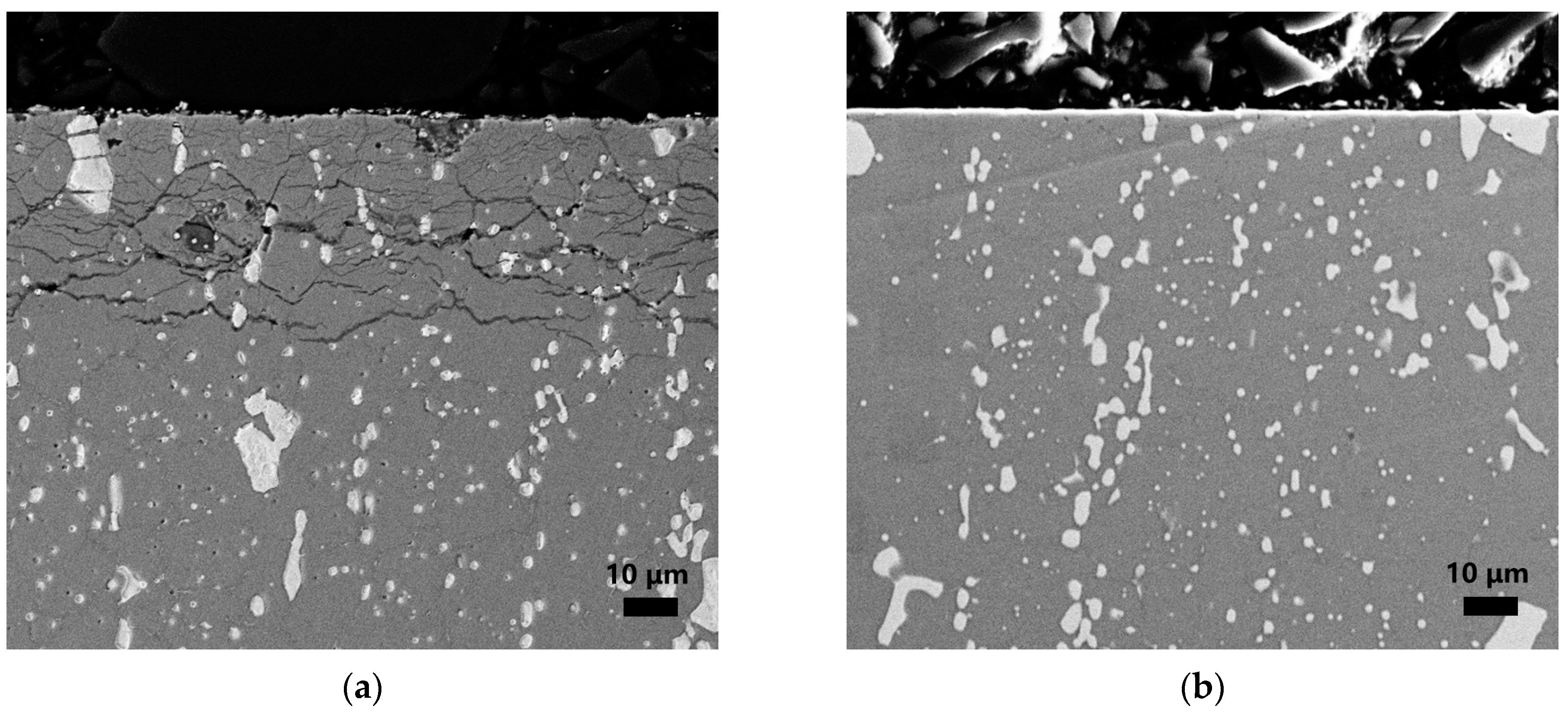
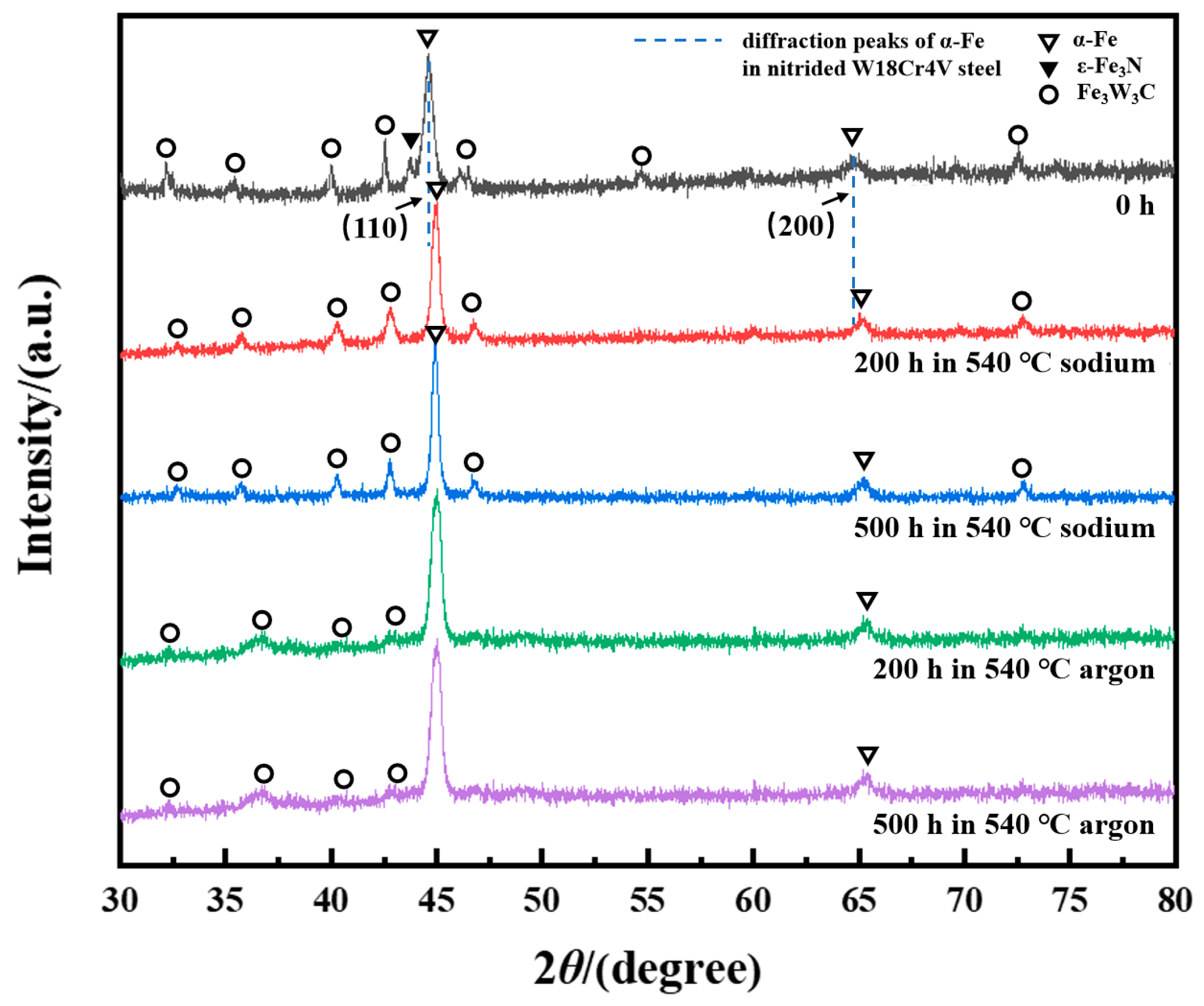
3.1. Aging Behavior of Nitrided W18Cr4V Steel in High-Temperature Sodium and Argon
3.2. Accelerated Aging Behavior of Nitrided W18Cr4V Steel in High-Temperature Sodium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abram, T.; Ion, S. Generation-IV nuclear power: A review of the state of the science. Energy Policy 2008, 36, 4323–4330. [Google Scholar] [CrossRef]
- Aoto, K.; Dufour, P. A summary of sodium-cooled fast reactor development. Prog. Nucl. Energy 2014, 77, 247–265. [Google Scholar] [CrossRef]
- Xu, M.; Yang, H.Y. Safety properties of sodium-cooled fast reactors. Physics 2016, 45, 561–568. [Google Scholar]
- Ichimiya, M. The status of generation IV Sodium-Cooled Fast Reactor technology development and its future Project. Energy Procedia 2011, 7, 79–87. [Google Scholar] [CrossRef][Green Version]
- Rodina, E.A.; Egorov, A.V.; Rachkov, V.I.; Khomiakov, I.S. Modeling and comparative analysis of changeover of homogeneous and heterogeneous core of BN-1200 reactor to equilibrium mode. Nucl. Eng. Des. 2022, 386, 111549. [Google Scholar] [CrossRef]
- Takano, K.; Ohki, S.; Ozawa, T.; Yamano, H.; Kubo, S.; Ogura, M.; Yamada, Y.; Koyama, K.; Kurita, K.; Costes, L.; et al. Core and safety design for France–Japan common concept on sodium-cooled fast reactor. EPJ Nucl. Sci. Technol. 2022, 8, 35. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, Y.H. Inherent safety enhancement by design optimization of a floating absorber for safety at transient (FAST) in an advanced burner reactor. Ann. Nucl. Energy 2022, 172, 109026. [Google Scholar] [CrossRef]
- Tashlykov, O.L.; Dolgii, Y.F.; Sesekin, A.N. Optimization of refueling times in fast neutron reactors. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021. [Google Scholar] [CrossRef]
- Dushev, S.A.; Timofeev, A.V.; Lyubimov, M.A.; Belinskii, D.L. Refueling system for fast reactors: Creation and development. At. Energy 2020, 129, 87–92. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.B.; Park, C.G. Conceptual designs and characteristic of the fuel handling and transfer system for 150 MWe PGSFR and 1400 MWe SFR burner reactor. Nucl. Eng. Technol. 2022, 54, 4125–4133. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H.Y.; Yang, C. Refueling system reliability research about sodium-cooled fast reactor. At. Energy Sci. Technol. 2021, 55, 672–677. [Google Scholar]
- Wang, M.Z.; Dong, S.G.; Song, Q.; Yang, K.L.; Gu, J.P.; Yan, H.; Wang, C.L.; Yu, T.J.; Jin, F.L.; Ma, H.S.; et al. The verifying test of refueling system of the China Experimental Fast Reactor. Chin. J. Nucl. Sci. Eng. 2008, 28, 210–217. [Google Scholar]
- Natesan, K. Influence of nonmetallic elements on the compatibility of structural materials with liquid alkali metals. J. Nucl. Mater. 1983, 115, 251–262. [Google Scholar] [CrossRef]
- Dai, Y.N.; Zheng, X.T.; Ding, P.S. Review on sodium corrosion evolution of nuclear-grade 316 stainless steel for sodium-cooled fast reactor applications. Nucl. Eng. Technol. 2021, 53, 3474–3490. [Google Scholar] [CrossRef]
- Fidler, R.S.; Collins, M.J. A review of corrosion and mass transport in liquid sodium and the effects on the mechanical properties. At. Energy Rev. 1965, 12, 3–50. [Google Scholar]
- Sivaibharasi, N.; Krishna, N.G.; Shankar, A.R.; Krishnakumar, S.; Chandramouli, S.; Karki, V.; Kannan, S.; Philip, J. Influence of long term sodium exposure on the corrosion and tensile properties of AISI type 316LN stainless steel and modified 9Cr-1Mo steel. J. Nucl. Mater. 2022, 567, 153830. [Google Scholar] [CrossRef]
- Emami, M.; Ghasemi, H.M.; Rassizadehghan, J. High temperature tribological behaviour of 31CrMoV9 gas nitrided steel. Surf. Eng. 2010, 26, 168–172. [Google Scholar] [CrossRef]
- Gerasimov, S.A.; Zhikharev, A.V.; Berezina, E.V.; Zubarev, G.I.; Pryanichnikov, V.A. New ideas on the mechanism of structure formation in nitrided steels. Met. Sci. Heat Treat. 2004, 46, 13–17. [Google Scholar] [CrossRef]
- Sun, D.S.; Li, F.Z.; Dai, J.Y.; Li, D.X. Crystal defects and interface structure of ion-nitrided layers. Acta Metall. Sin. 1993, 29, 203–207. [Google Scholar]
- Xi, Y.T.; Liu, D.X.; Han, D.; Han, Z.F. Improvement of mechanical properties of martensitic stainless steel by plasma nitriding at low temperature. Acta Metall. Sin. (Engl. Lett.) 2008, 21, 21–29. [Google Scholar] [CrossRef]
- Karami, M.B.; Gercekcioglu, E. Wear behaviour of plasma nitrided steels at ambient and elevated temperatures. Wear 2000, 243, 76–84. [Google Scholar] [CrossRef]
- Chen, X.D.; Feng, S.; Wang, L.W.; Tang, R.; Zhang, F.; Ming, S.L.; Cai, Z.B. Effect of QPQ on the fretting wear behavior of TP316H steel at varying temperatures in liquid sodium. J. Nucl. Mater. 2022, 562, 153583. [Google Scholar] [CrossRef]
- Chen, X.D.; Feng, S.; Wang, L.W.; Zhang, F.; Shi, Z.Y.; Ming, S.L.; Li, Y.; Liu, B.; Cai, Z.B. Effect of salt bath temperature on microstructure and fretting wear of nitrided 2.25Cr–1Mo steel in liquid sodium. Appl. Surf. Sci. 2022, 606, 154988. [Google Scholar] [CrossRef]
- Johnson, R.N.; Schrock, S.L.; Whitlow, G.A. Wear resistant coatings for reactor components in liquid sodium environments. J. Vac. Sci. Technol. 1974, 11, 759–764. [Google Scholar] [CrossRef]
- Johnson, R.N. Coatings for fast breeder reactor components. Thin Solid Film 1984, 118, 31–47. [Google Scholar] [CrossRef]
- Hemant, K.; Ramakrishnan, V.; Albert, S.K.; Bhaduri, A.K.; Ray, K.K. Friction and wear behaviour of Ni-Cr-B hardface coating on 316LN stainless steel in liquid sodium at elevated temperature. J. Nucl. Mater. 2017, 495, 431–437. [Google Scholar]
- Zhou, S.L.; Zhang, Z.; Zhang, J.; Wang, J.M.; Zhang, Z.G. Microstructure and wear resistance of TiAlZrCr/(Ti,Al,Zr,Cr)N gradient films deposited by multi-arc ion plating. Acta Metall. Sin. 2016, 52, 747–754. [Google Scholar]
- Junho, C.; Koji, S.; Takahisa, K.; Masahiro, K.; Wonsik, L. Nitriding of high speed steel by bipolar PBII for improvement in adhesion strength of DLC films. Nucl. Instrum. Methods Phys. Res. B 2012, 272, 357–360. [Google Scholar]
- He, J.L.; Chen, K.C.; Davison, A. Improvements in the understanding and application of duplex coating systems using arc plasma technology. Surf. Coat. Technol. 2005, 200, 1464–1471. [Google Scholar] [CrossRef]
- Sharma, A.; Swami, K.C. A Study of Plasma Nitriding Process on the AISI 4140 Steel. J. Mater. Sci. Surf. Eng. 2014, 1, 81–83. [Google Scholar]
- Wang, D.; Zhou, Y.W.; Zhang, K.C.; Su, Z.W.; Du, F.; Wu, J.S.; Guo, C. Study on diffusion behavior of nitrogen in typical steel by ionic nitriding. Mater. Rep. 2022, 36, 1–6. [Google Scholar]
- Larson, F.R.; Miller, J. A Time-Temperature Relationship for Rupture and Creep Stresses. Trans. Am. Soc. Mech. Eng. 1952, 74, 765–771. [Google Scholar] [CrossRef]


| Elements | C | Mn | Si | Mo | Cr | W | V | Fe |
|---|---|---|---|---|---|---|---|---|
| wt.% | 0.7–0.8 | ≤0.4 | ≤0.4 | ≤0.3 | 3.8–4.4 | 17.5–19.0 | 1.0–1.4 | Bal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Liang, N.; Zhang, W.; Tao, L.; Qin, B.; Ruan, Z.; Long, B.; Lv, S. A Study on the Aging Behavior of Nitrided W18Cr4V Steel in High-Temperature Sodium. Metals 2024, 14, 357. https://doi.org/10.3390/met14030357
Fu X, Liang N, Zhang W, Tao L, Qin B, Ruan Z, Long B, Lv S. A Study on the Aging Behavior of Nitrided W18Cr4V Steel in High-Temperature Sodium. Metals. 2024; 14(3):357. https://doi.org/10.3390/met14030357
Chicago/Turabian StyleFu, Xiaogang, Na Liang, Wei Zhang, Liu Tao, Bo Qin, Zhangshun Ruan, Bin Long, and Shasha Lv. 2024. "A Study on the Aging Behavior of Nitrided W18Cr4V Steel in High-Temperature Sodium" Metals 14, no. 3: 357. https://doi.org/10.3390/met14030357
APA StyleFu, X., Liang, N., Zhang, W., Tao, L., Qin, B., Ruan, Z., Long, B., & Lv, S. (2024). A Study on the Aging Behavior of Nitrided W18Cr4V Steel in High-Temperature Sodium. Metals, 14(3), 357. https://doi.org/10.3390/met14030357









