Electrochemical Behavior of Niobium Oxide and Titanium Oxide in NaF–Na3AlF6 Molten Salt
Abstract
1. Introduction
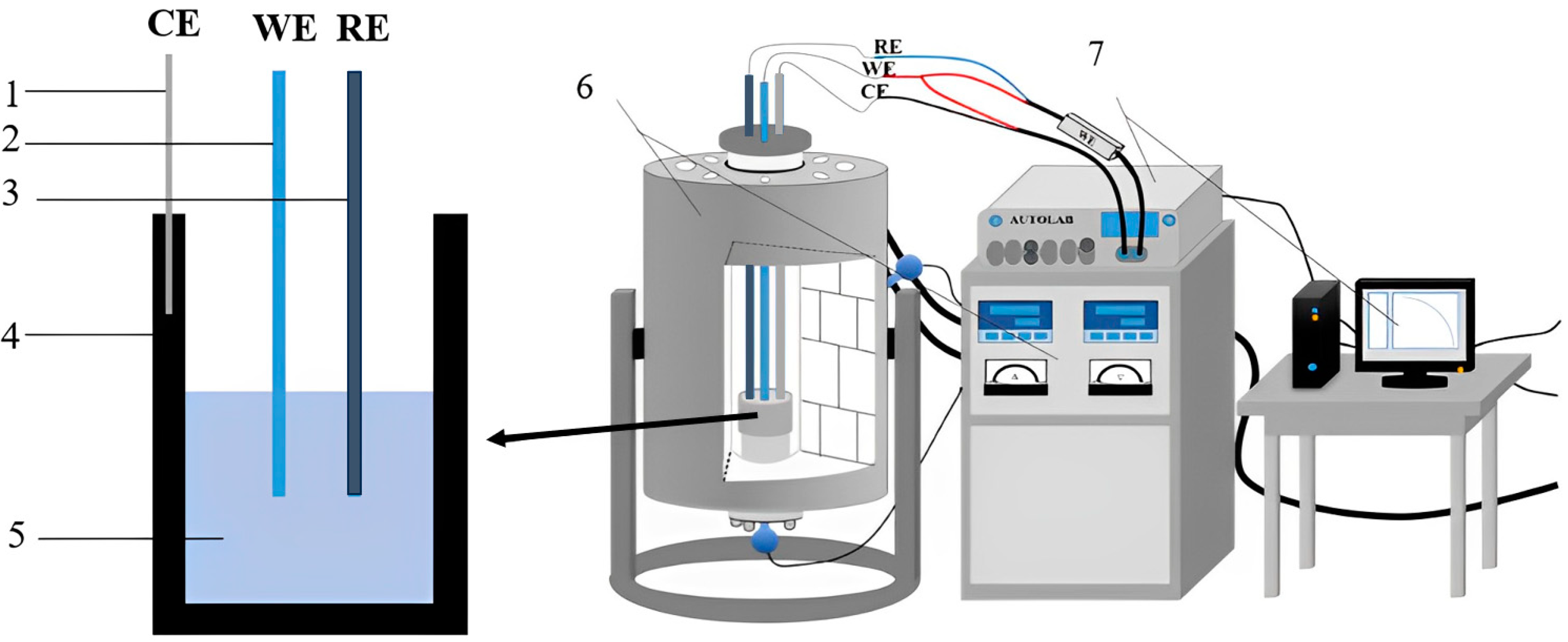
2. Experimental Methods
3. Results and Discussion
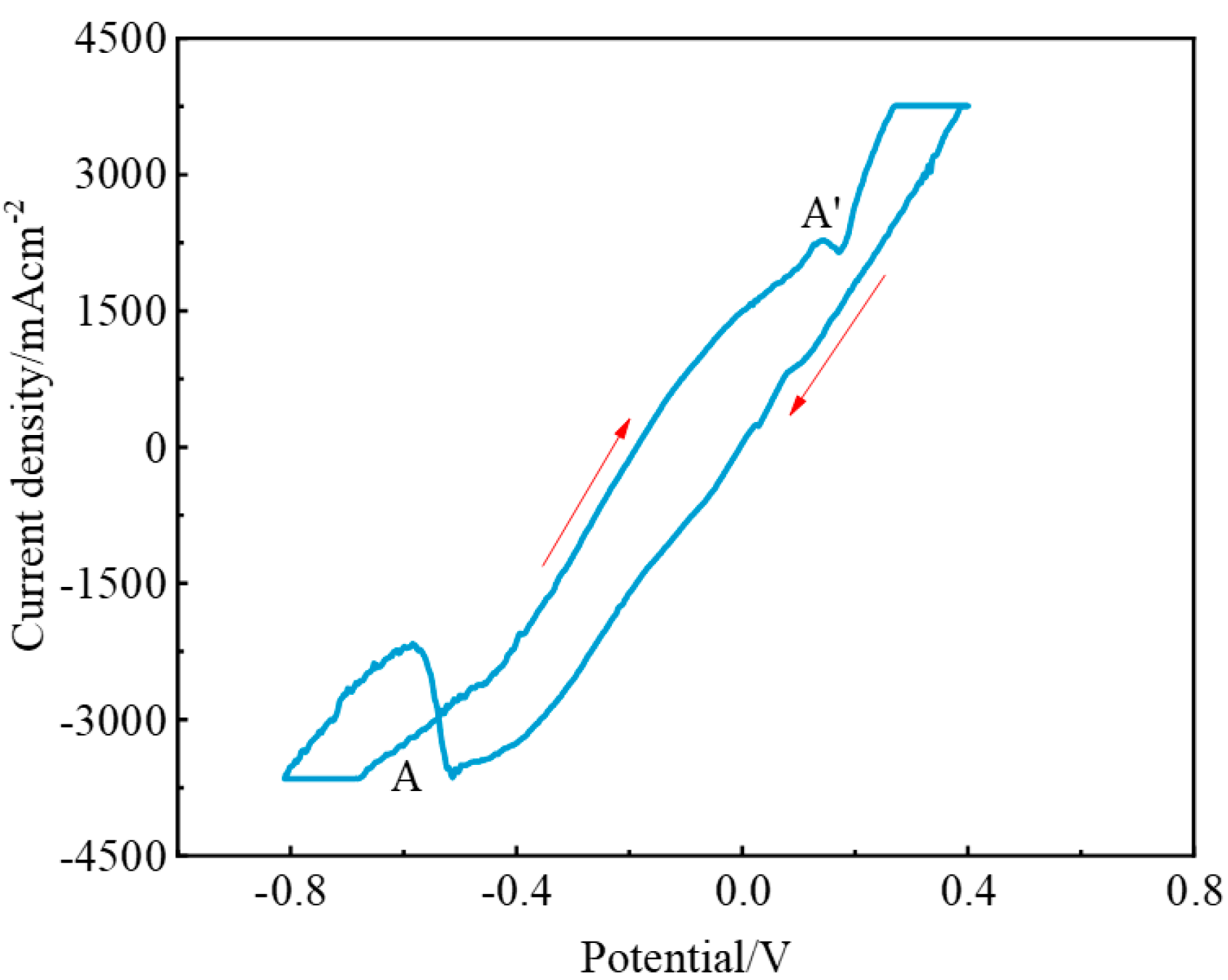
3.1. Electrochemical Behavior of NaF–Na3AlF6 Molten Salt
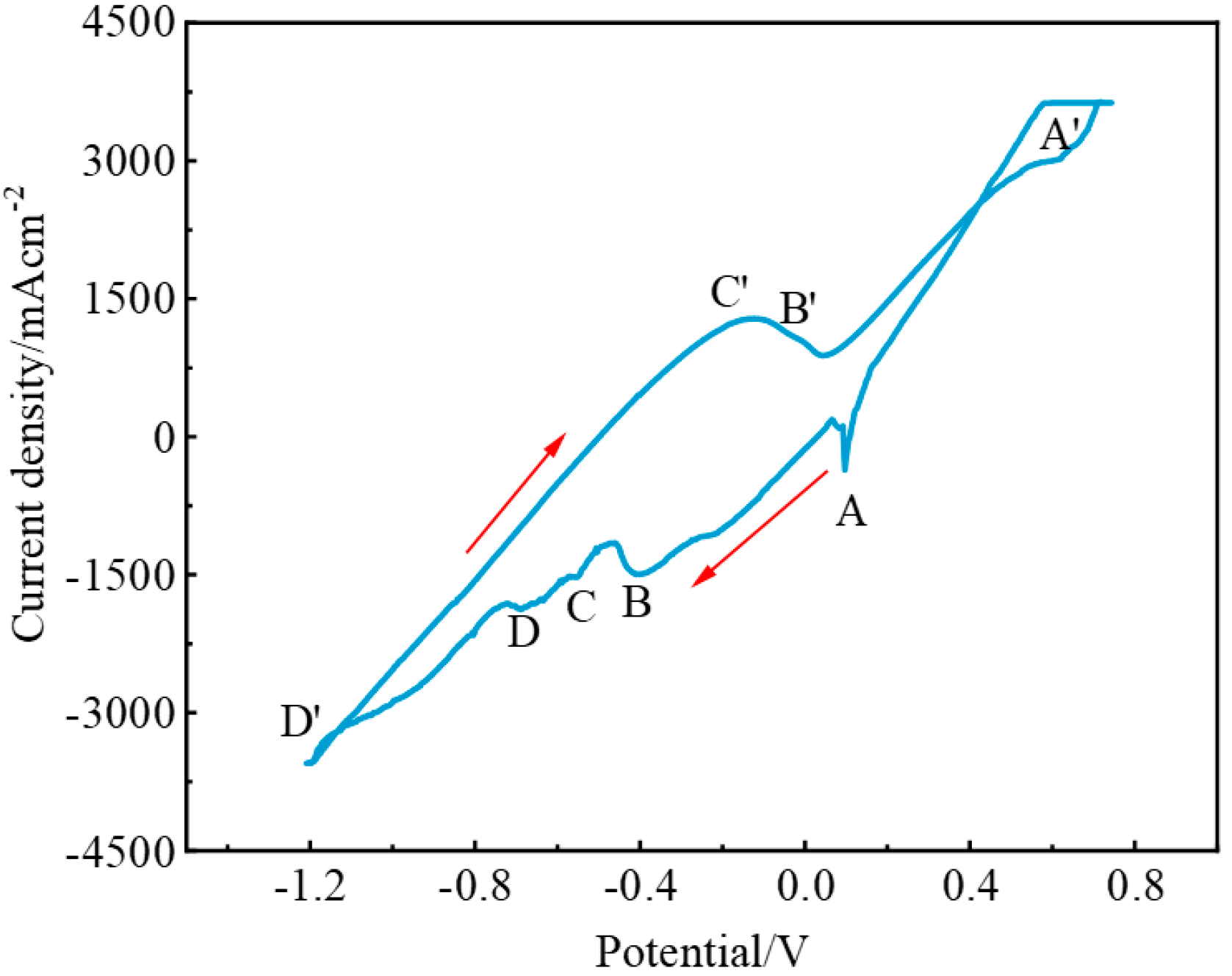
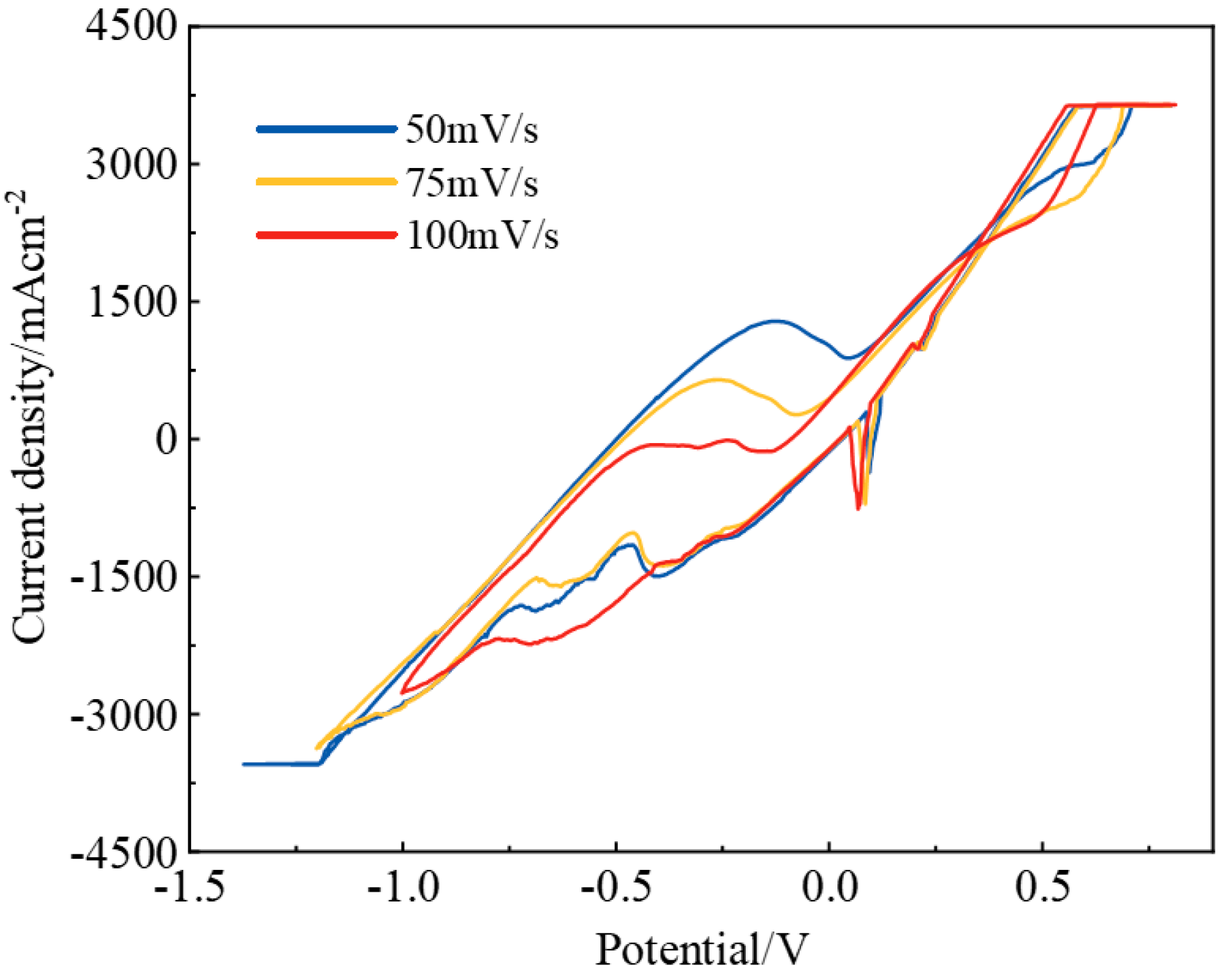
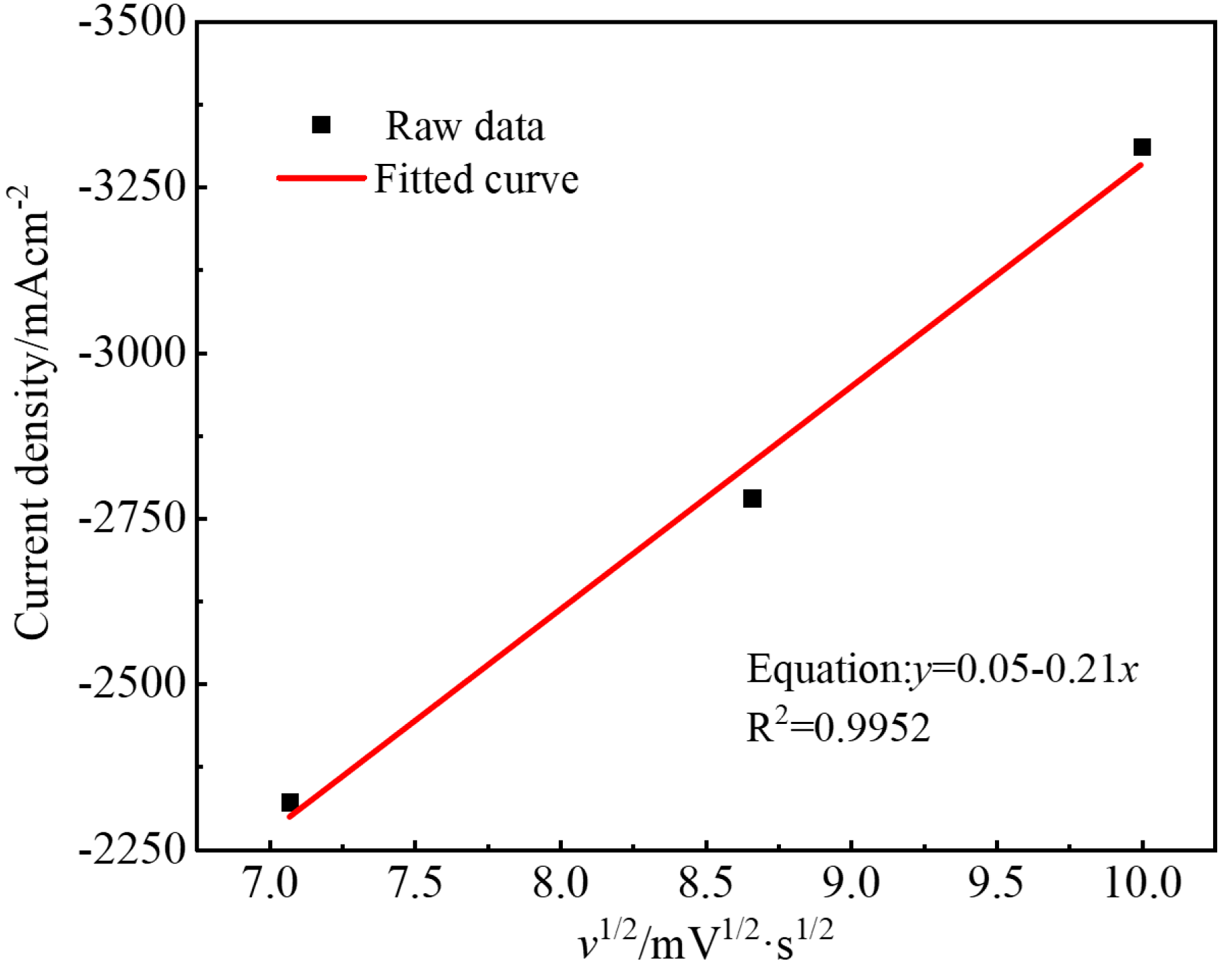
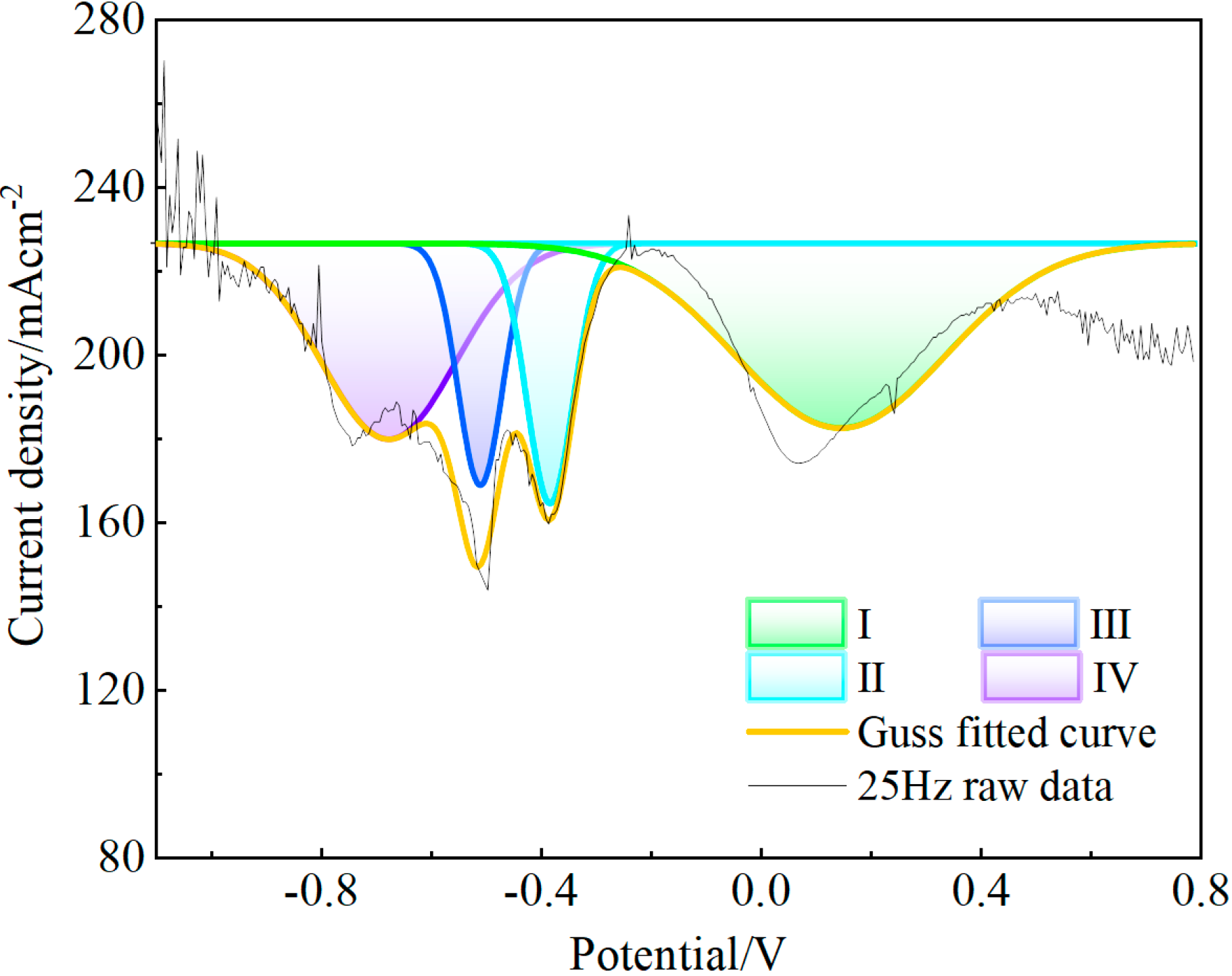
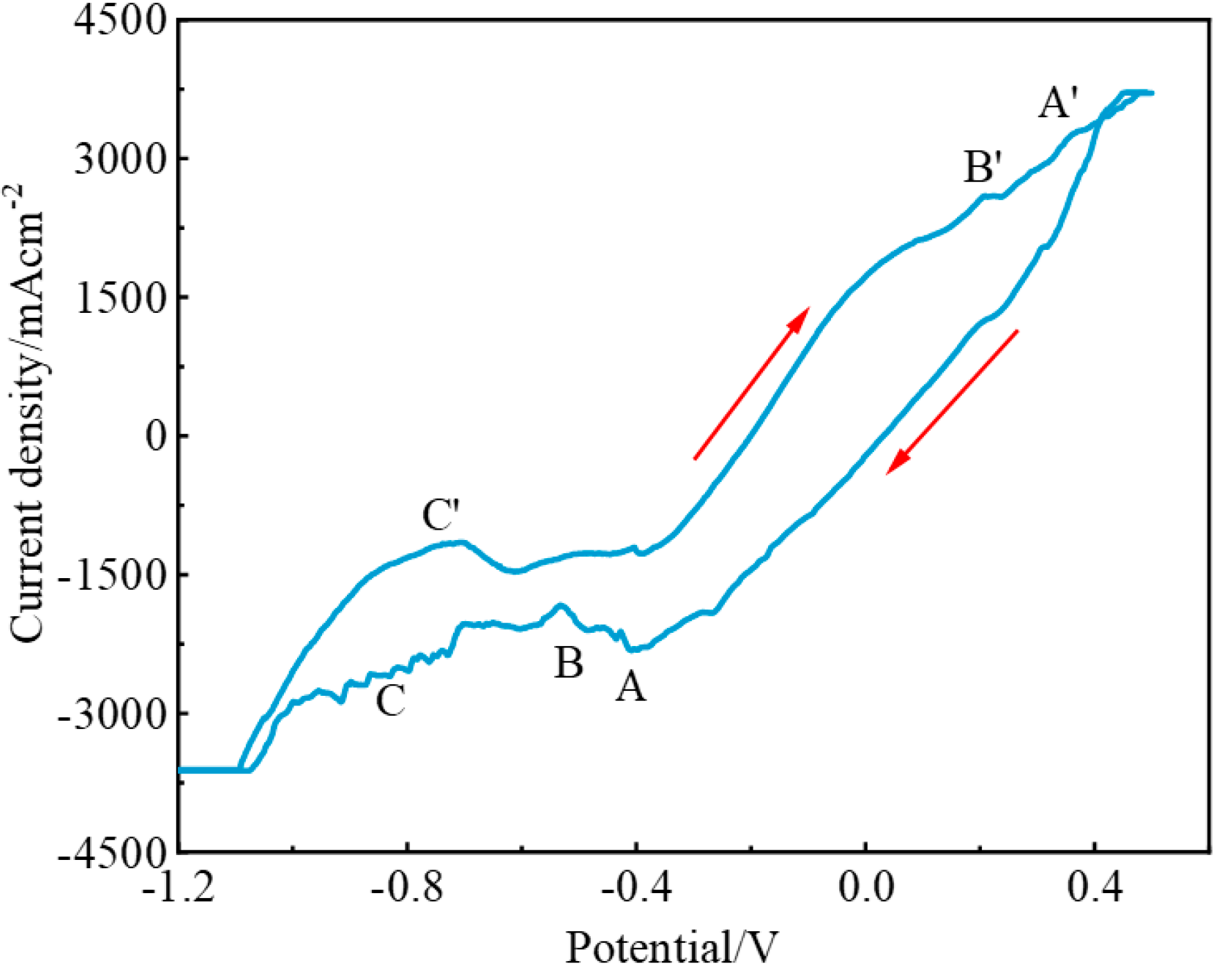
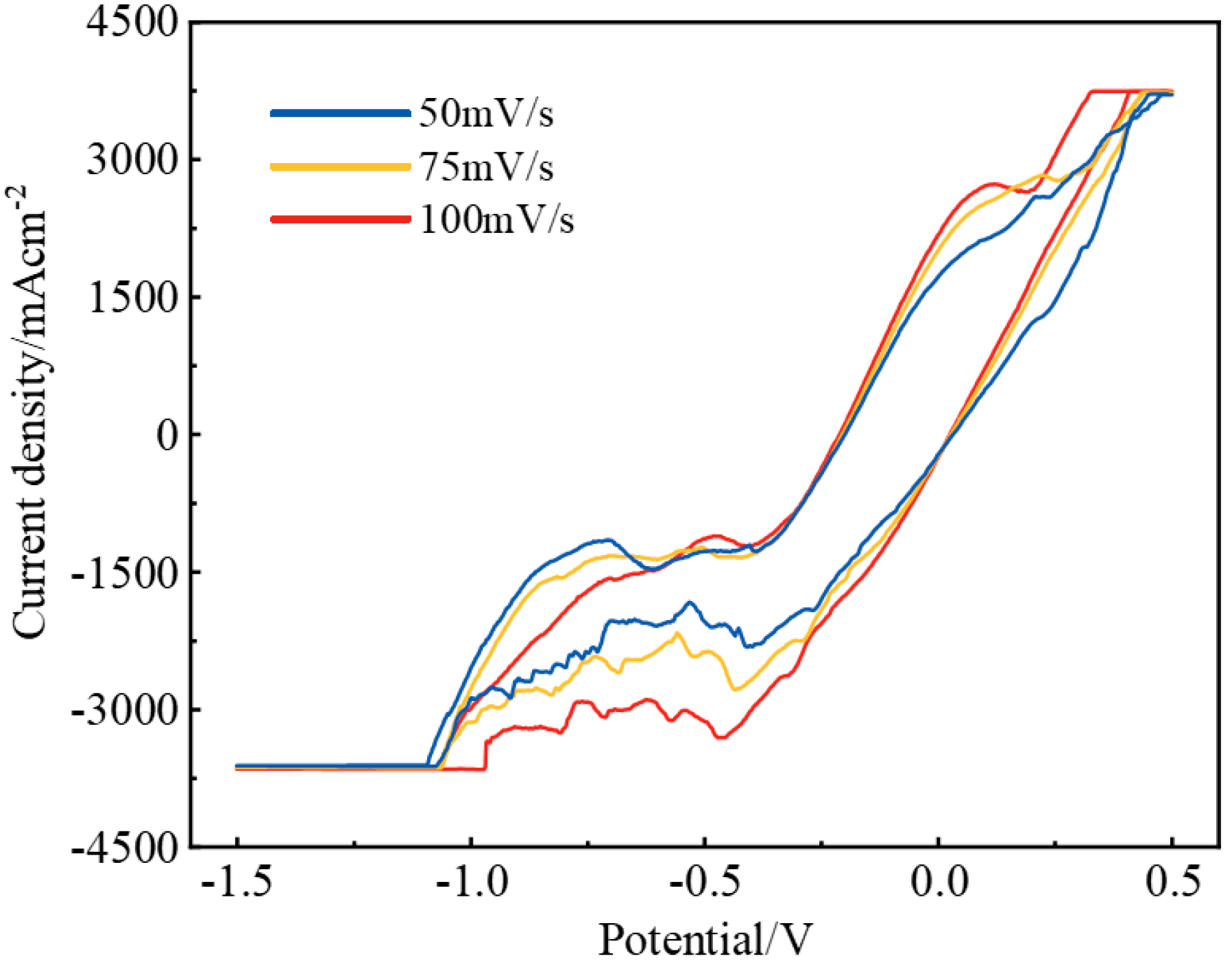
3.2. Electrochemical Behavior of the NaF–Na3AlF6–2 wt% Nb2O5 Molten Salt System
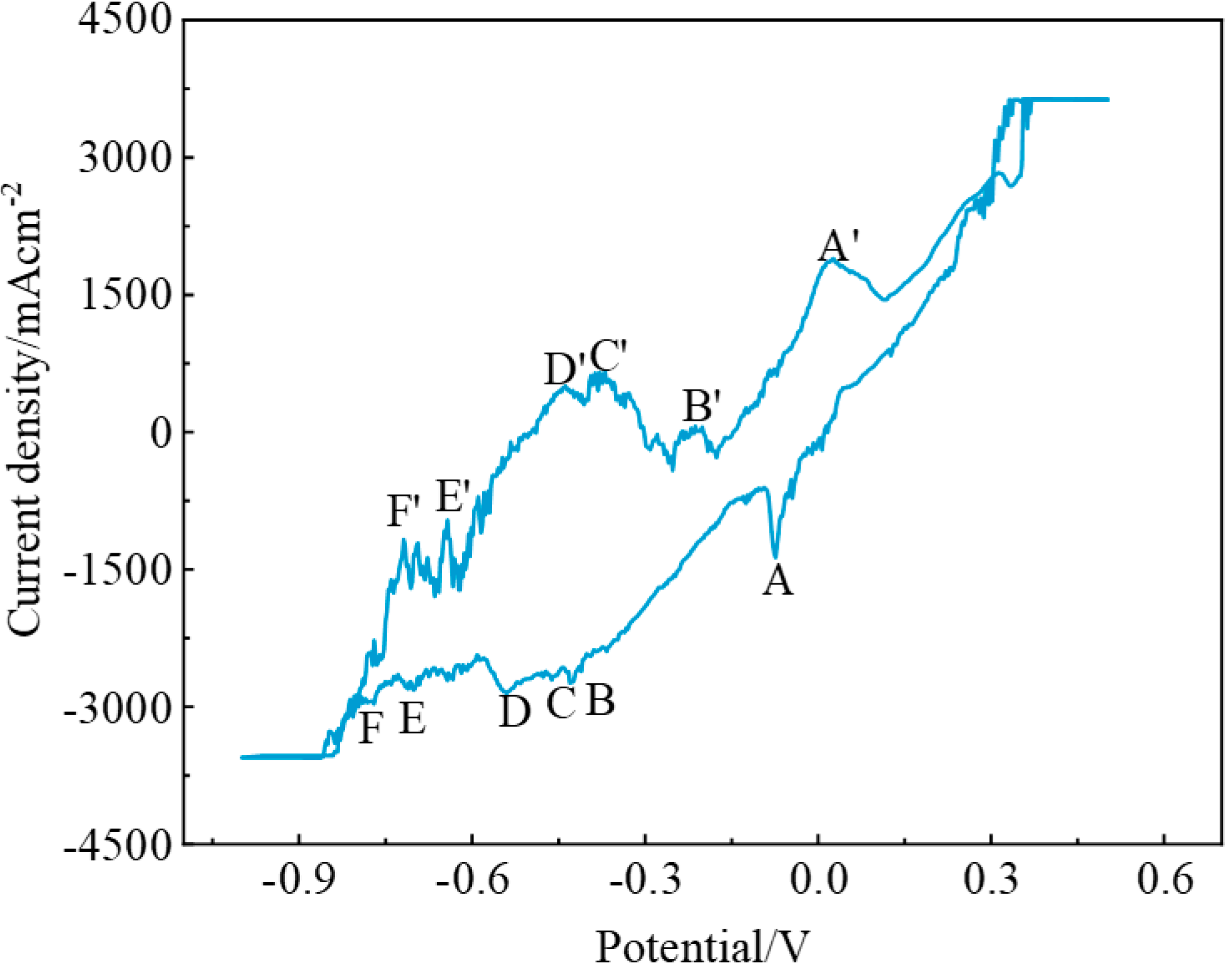
3.3. Electrochemical Behavior of the NaF–Na3AlF6–2 wt% TiO2 Molten Salt System
3.4. Electrochemical Behavior of the NaF–Na3AlF6–2 wt% Nb2O5–2 wt% TiO2 Molten Salt System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- She, H.D.; Fan, H.R.; Yang, K.F.; Li, X.C.; Yang, Z.F.; Wang, Q.W.; Zhang, L.F.; Wang, Z.J. Complex, multi-stage mineralization processes in the giant Bayan Obo REE-Nb-Fe deposit. Ore Geol. Rev. 2021, 139, 104461. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, C.; Wang, R.; Liu, C.; Jiang, M. Selective Smelting Reduction of Metal Oxides in REE-Nb-Fe Deposit. JOM 2022, 74, 993–1001. [Google Scholar] [CrossRef]
- Zhang, S.; Rao, M.; Xiao RYou, J.; Li, G. Beneficiation of Nb and Ti carbides from pyrochlore ore via carbothermic reduction followed by magnetic separation. Miner. Eng. 2022, 180, 107492. [Google Scholar] [CrossRef]
- Zhang, S.H.; Rao, M.J.; Xiao, R.D.; You, J.X.; Li, G.H.; Jiang, T. Enrichment of Nb and Ti from carbonatite pyrochlore ore via calcining-slaking followed by gravity separation. Int. J. Min. Sci. Technol. 2022, 32, 615–626. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, X.; Yang, H. A novel process for recovery of scandium, rare earth and niobium from Bayan Obo tailings: NaCl-Ca(OH)2-coal roasting and acid leaching. Miner. Eng. 2022, 178, 107401. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.J.; Li, C.L.; Jiang, M.F. A novel approach for recovery of rare earths and niobium from Bayan Obo tailings. Miner. Eng. 2014, 65, 17–23. [Google Scholar] [CrossRef]
- Sun, L.; Yu, H.; Meng, F.; Qi, T.; Zheng, S.; Peng, Y.; Wang, L. A novel method for the separation of niobium and titanium from sulfuric acid-oxalate solutions using N235 and MIBK. Hydrometallurgy 2021, 205, 105748. [Google Scholar] [CrossRef]
- Sun, L.; Yu, H.; Meng, F.; Qi, T.; Wang, L.; Peng, Y. Recovery of niobium and titanium from ilmenorutile by NaOH roasting-H2SO4 leaching process. J. Mater. Res. Technol. 2021, 15, 2575–2583. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Wang, L. Separation and extraction of niobium from H2SO4 solution containing titanium and iron impurities. Sep. Purif. Technol. 2022, 295, 121207. [Google Scholar] [CrossRef]
- Li, H.E.; Lu, X.G.; Chen, C.Y.; Li, Q.; Li, C.H.; Zhong, Q.D. Metal niobium by solid oxygen-ion membrane. Chin. J. Nonferrous Met. 2008, 18, 1336–1341. [Google Scholar]
- Li, C.; Li, S.; Che, Y.; Li, J.; Shu, Y.; He, J.; Song, J. Electrochemical behavior of niobium ions in molten KCl-NaCl. J. Mater. Res. Technol. 2020, 9, 9341–9347. [Google Scholar] [CrossRef]
- Christensen, E.; Wang, X.D.; Barner JH, V.; Ostfold, T.; Bjerrum, N.J. The Influence of Oxide on the Electrodeposition of Niobium from Alkali Fluoride Melts. Cheminform 2010, 25, 141. [Google Scholar] [CrossRef][Green Version]
- Matthiessen, F.; Christensen, E. The Redox Chemistry of Niobium(V) Fluoro and Oxofluoro Complexes in LiF-NaF-KF Melts. J. Electrochem. Soc. 1996, 143, 1793–1799. [Google Scholar] [CrossRef][Green Version]
- Bailey, R.A.; Yoko, T. High-temperature electroplating of chromium from molten FLINAK. J. Appl. Electrochem. 1986, 16, 737–744. [Google Scholar] [CrossRef]
- Kuznetsov, S.A. Electrolytic production of niobium powder from chloride—Fluoride melts containing compounds of niobium and zirconium. Russ. J. Electrochem. 2000, 36, 509–515. [Google Scholar] [CrossRef]
- Lantelme, F.; Salmi, A. Electrochemistry of Titanium in NaCl - KCl Mixtures and Influence of Dissolved Fluoride Ions. J. Electrochem. Soc. 1995, 142, 3451–3456. [Google Scholar] [CrossRef]
- Chen, G.S.; Masazumi, O.; Takeo, O. Electrochemical studies of titanium ions (Ti4+) in equimolar KCl-NaCl molten salts with 1 wt% K2TiF6. Electrochim. Acta 1987, 32, 1637–1642. [Google Scholar] [CrossRef]
- Robin, A.; Lepinay, J.D. Determination of the apparent standard potential of the Ti/Ti(III) system in the LiF-NaF-KF eutectic using voltammetry, chronopotentiometry and open-circuit potentiometry. Electrochim. Acta 1991, 36, 1009–1012. [Google Scholar] [CrossRef]
- Jun, L.; Bing, L. Electrochemical reduction and electrocrystallization process of B(III) in the LiF-NaF-KF-KBF4 molten salt. Rare Met. Mater. Eng. 2007, 36, 15–19. [Google Scholar] [CrossRef]
- Jiao, H.; Zhang, L.; Jiao, S. Electrochemical Behavior of Titanium Ions at Liquid Metal Cathodes in Molten Salts. In Proceedings of the 13th World Conference on Titanium; VenkateshVenkatesh, V., Pilchak, A.L., Allison, J.E., Eds.; John Wiley & Sons: San Diego, CA, USA, 2016; Volume 28, pp. 183–186. [Google Scholar]
- Yuan, T.C.; Weng, Q.G.; Zhou, Z.H.; Li, J.; He, Y.H. Preparation of High-Purity Titanium by Molten-Salt Electrolysis Process. Adv. Mater. Res. 2011, 284–286, 1477–1482. [Google Scholar] [CrossRef]
- Ma, M.; Wang, D.; Wang, W. Extraction of titanium from different titania precursors by the FFC Cambridge process. J. Alloys Compd. 2019, 25, 6107–6114. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Guo, H. Functional COFs for Electrochemical Sensing: From Design Principles to Analytical Applications. ChemistrySelect 2023, 8, 2365–6549. [Google Scholar] [CrossRef]
- Pogliano, U.; Durbiano, F.; Serazio, D. A system for controlling electrical and chemical parameters in the Faraday constant determination by dissolution of silver. Meas. Sci. Technol. 2011, 22, 055102. [Google Scholar] [CrossRef]
- Su, C.; An, M.; Yang, P.; Gu, H.; Guo, X. Electrochemical behavior of cobalt from 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. Appl. Surf. Sci. 2010, 256, 4888–4893. [Google Scholar] [CrossRef]
- He, X.; Hou, B.; Li, C.; Zhu, Q.; Jiang, Y.; Wu, L. Electrochemical mechanism of trivalent chromium reduction in 1-butyl-3-methylimidazolium bromide ionic liquid. Electrochem. Acta 2014, 130, 245–252. [Google Scholar] [CrossRef]
- Halford, G.C.; Personick, M.L. Bridging Colloidal and Electrochemical Nanoparticle Growth with In Situ Electrochemical Measurements. Acc. Chem. Res. 2023, 56, 1228–1238. [Google Scholar] [CrossRef] [PubMed]




| Electrolyte System | Test Method | Scan Rate |
|---|---|---|
| NaF–Na3AlF6 | CV | 50 mV/s |
| NaF–Na3AlF6–2 wt% Nb2O5 | CV, SWV | 50 mV/s, 75 mV/s, 100 mV/s |
| NaF–Na3AlF6–2 wt% TiO2 | CV, SWV | 50 mV/s, 75 mV/s, 100 mV/s |
| NaF–Na3AlF6–2 wt% Nb2O5–2 wt% TiO2 | CV | 50 mV/s |
| Reduction Process | Reduction Potential | ||
|---|---|---|---|
| NaF–Na3AlF6– 2 wt% Nb2O5 | NaF–Na3AlF6– 2 wt% TiO2 | NaF–Na3AlF6– 2 wt% Nb2O5–2 wt% TiO2 | |
| Nb (Ⅴ)→Nb (Ⅳ) | 0.09 V | – | −0.07 V |
| Ti (Ⅳ)→Ti (Ⅰ) | – | −0.43 V | −0.43 V |
| Nb (Ⅳ)→Nb (Ⅰ) | −0.38 V | – | −0.46 V |
| Nb (Ⅰ)→Nb | −0.68 V | – | −0.64 V |
| Ti (Ⅰ)→Ti | – | −0.84 V | −0.77 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Yu, S.; Liang, Y.; Jiang, M. Electrochemical Behavior of Niobium Oxide and Titanium Oxide in NaF–Na3AlF6 Molten Salt. Metals 2024, 14, 297. https://doi.org/10.3390/met14030297
Zhang B, Yu S, Liang Y, Jiang M. Electrochemical Behavior of Niobium Oxide and Titanium Oxide in NaF–Na3AlF6 Molten Salt. Metals. 2024; 14(3):297. https://doi.org/10.3390/met14030297
Chicago/Turabian StyleZhang, Bo, Shuiqing Yu, Yudong Liang, and Maofa Jiang. 2024. "Electrochemical Behavior of Niobium Oxide and Titanium Oxide in NaF–Na3AlF6 Molten Salt" Metals 14, no. 3: 297. https://doi.org/10.3390/met14030297
APA StyleZhang, B., Yu, S., Liang, Y., & Jiang, M. (2024). Electrochemical Behavior of Niobium Oxide and Titanium Oxide in NaF–Na3AlF6 Molten Salt. Metals, 14(3), 297. https://doi.org/10.3390/met14030297




