Leaching Kinetics of Iron Collector Containing PGMs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Technique
2.3. Analysis Methods
2.4. Calculation Method
3. Results and Discussion
3.1. Characteristics of Cakes
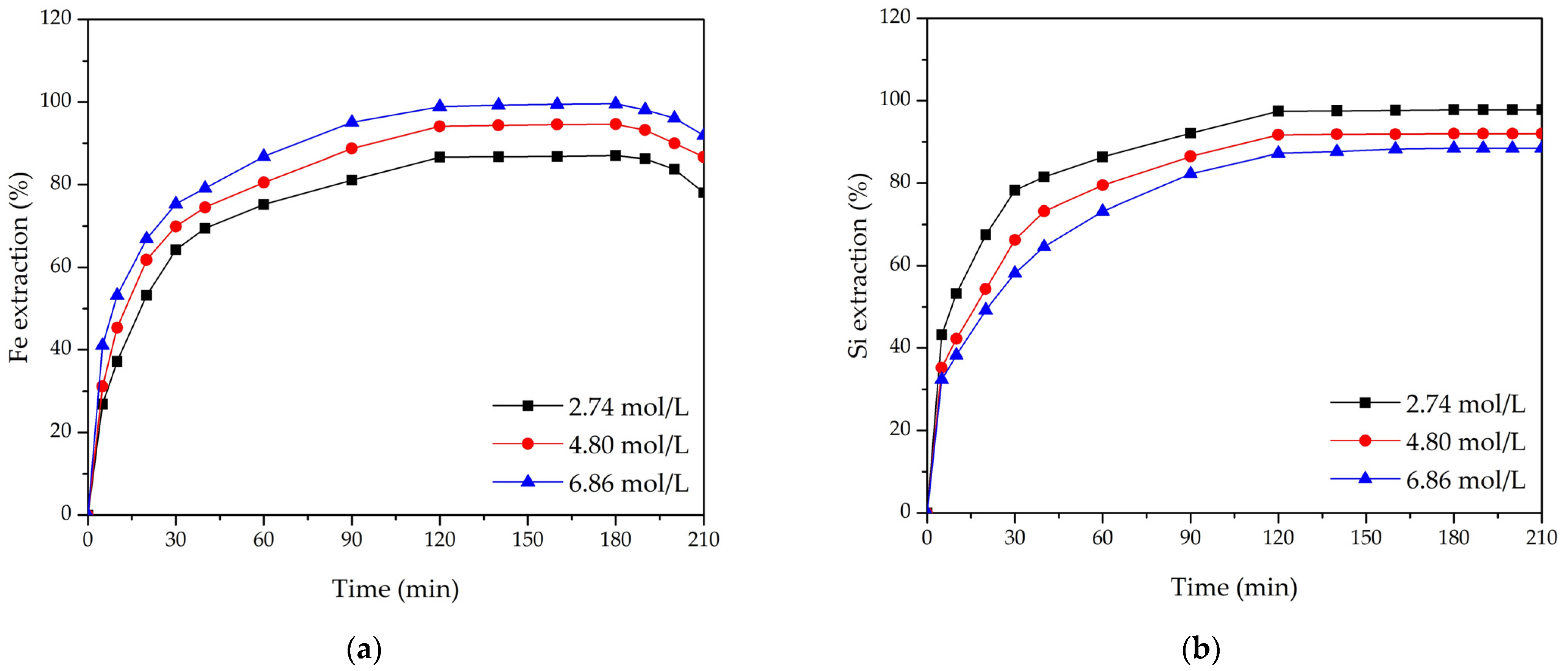
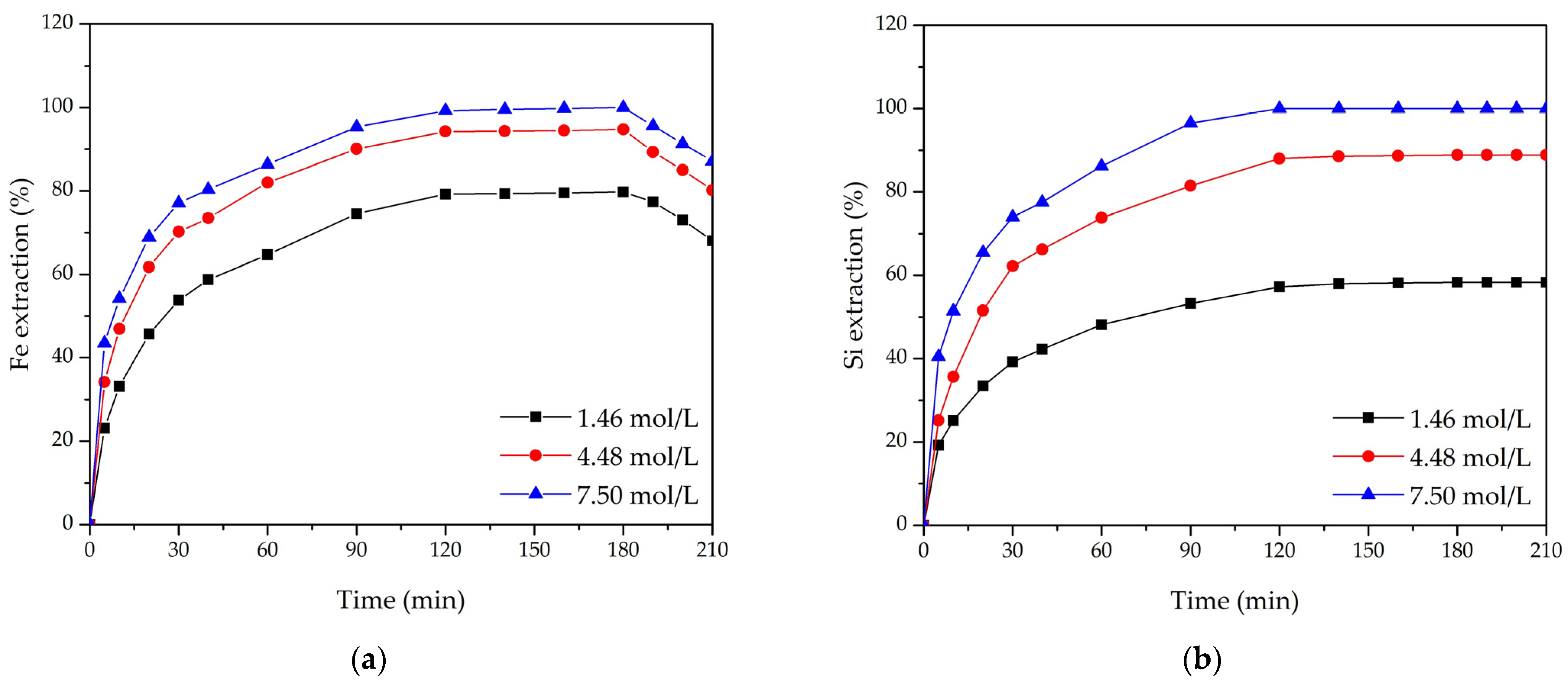
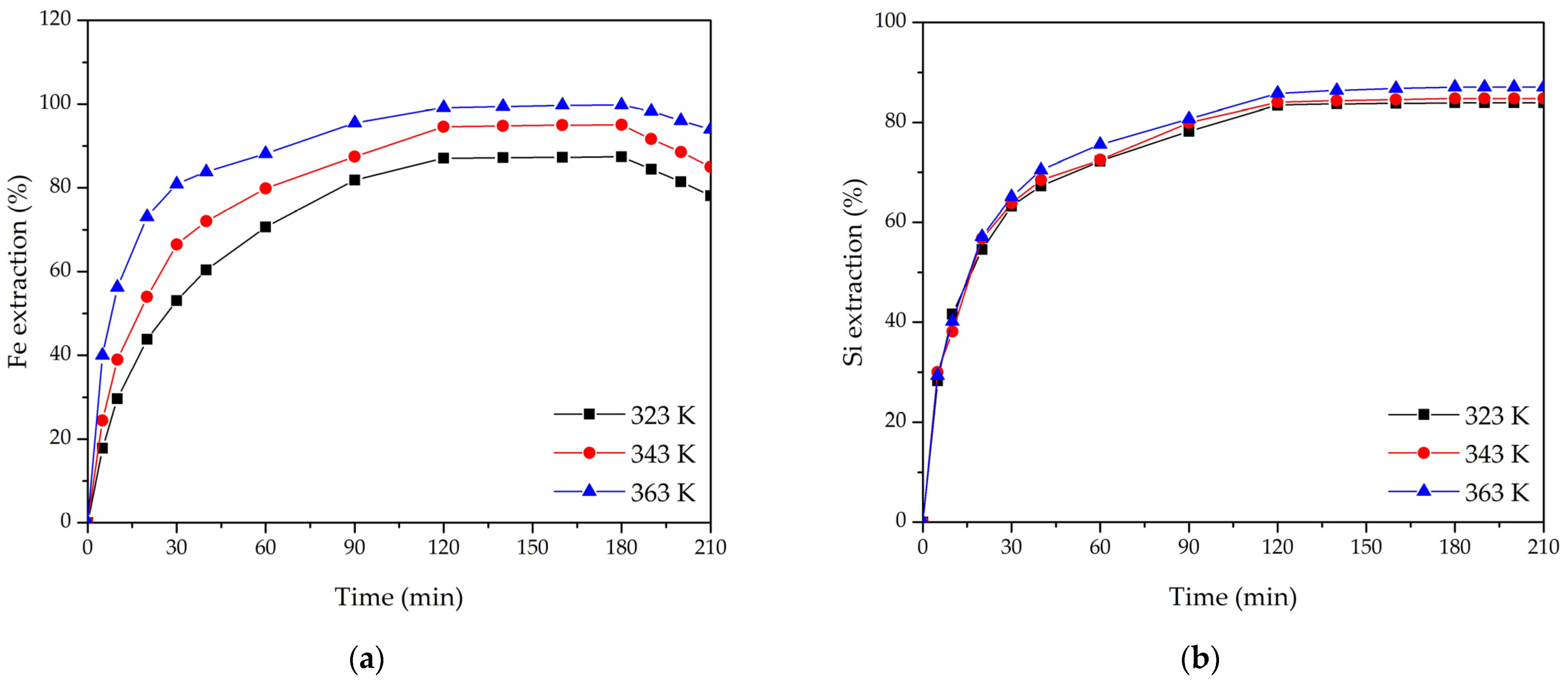
3.2. Effect of Leaching Parameters on Fe and Si Extraction
4. Modeling
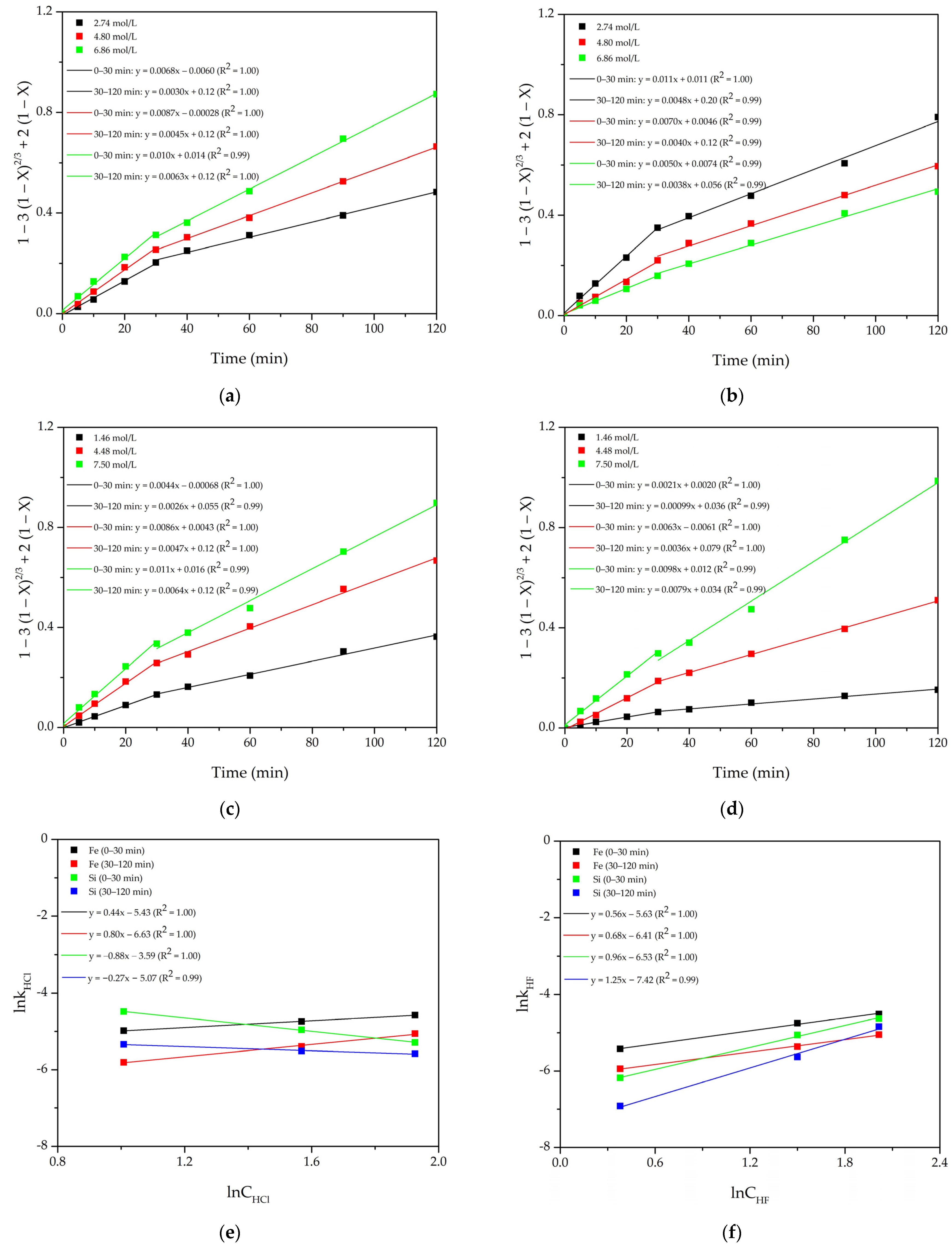
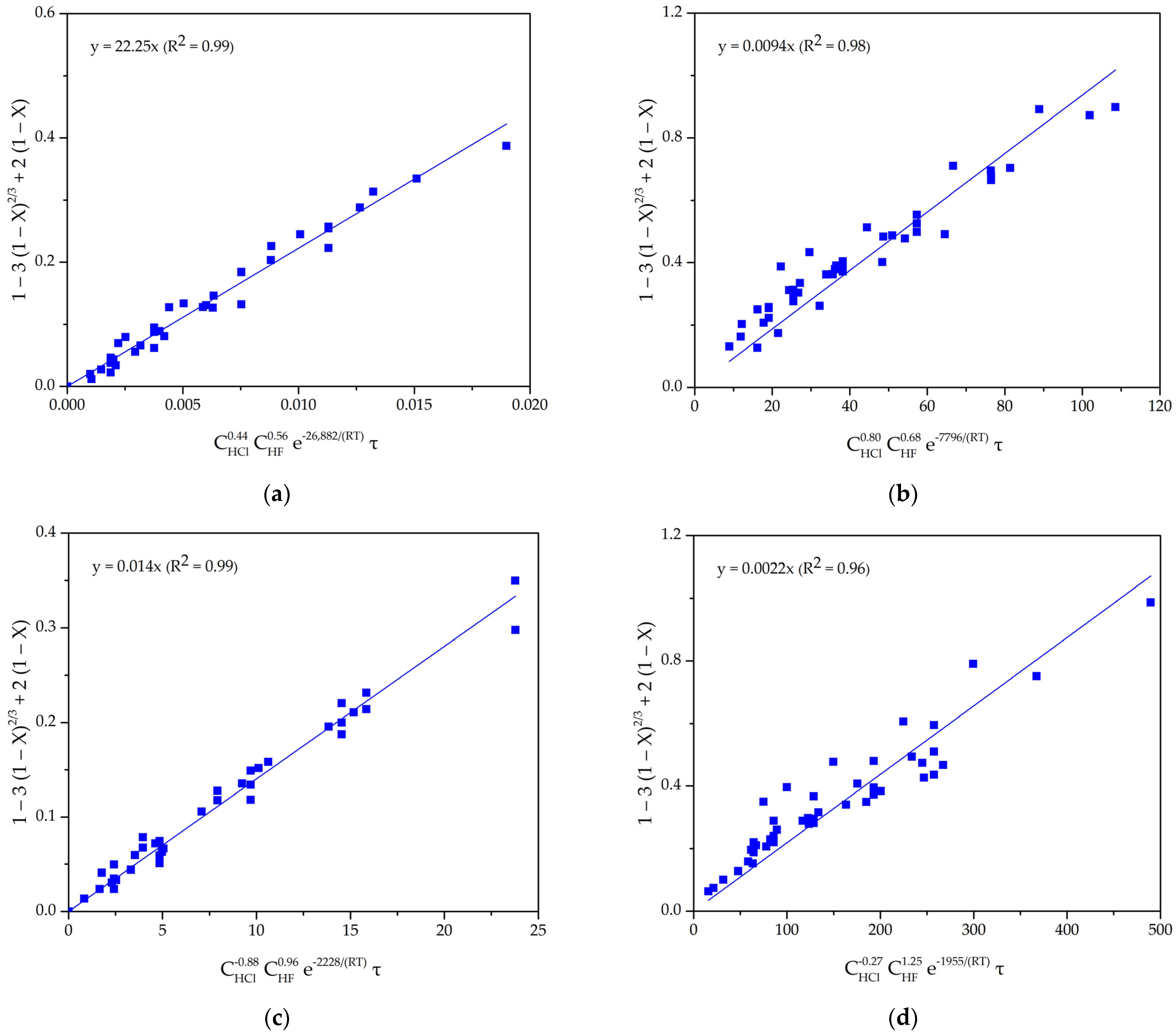
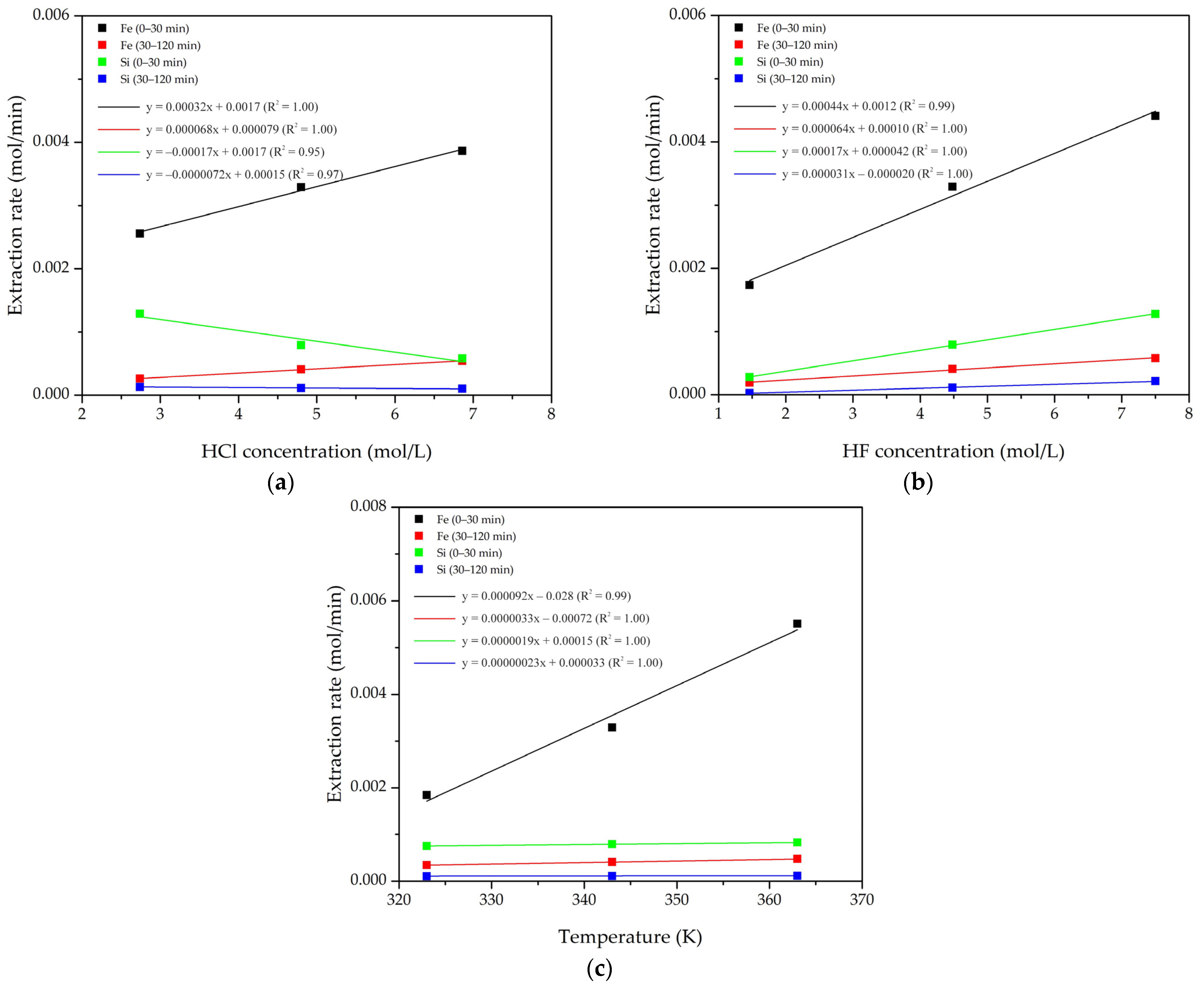
4.1. Shrinking Core Model
4.2. Full Factorial Experiment Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Liu, C.; Cao, H.; Ning, P.; Jin, W.; Yang, Z.; Wang, H.; Sun, Z. Understanding the features of PGMs in spent ternary automobile catalysts for development of cleaner recovery technology. J. Clean. Prod. 2019, 239, 118031. [Google Scholar] [CrossRef]
- Jimenez de Aberasturi, D.; Pinedo, R.; Ruiz de Larramendi, I.; de Larramendi, J.R.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Angelidis, T. Development of a Laboratory Scale Hydrometallurgical Procedure for the Recovery of Pt and Rh from Spent Automotive Catalysts. Top. Catal. 2001, 16, 419–423. [Google Scholar] [CrossRef]
- Ghodrat, M.; Sharafi, P.; Samali, B. Recovery of Platinum Group Metals Out of Automotive Catalytic Converters Scrap: A Review on Australian Trends and Challenges; Lambotte, G., Lee, J., Allanore, A., Wagstaff, S., Eds.; Materials Processing Fundamentals 2018; TMS 2018; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; pp. 149–161. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recycl. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Trinh, B.; Lee, J.; Suh, Y.; Lee, J. A review on the recycling processes of spent auto-catalysts: Towards the development of sustainable metallurgy. Waste Manag. 2020, 114, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, Z.; Lin, X.; Tang, H.; Ye, L.; Ma, Y.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical Recovery of Platinum Group Metals from Spent Catalysts. J. Miner. Met. Mater. Soc. 2017, 69, 1553–1562. [Google Scholar] [CrossRef]
- Morcali, M.H. A new approach to recover platinum-group metals from spent catalytic converters via iron matte. Resour. Conserv. Recycl. 2020, 159, 104891. [Google Scholar] [CrossRef]
- Carnogurska, M.; Prihoda, M.; Lazar, M.; Kurilla, P.; Dobakova, R. High-temperature processing and recovery of autocatalysts. Mater. Technol. 2018, 52, 335–340. [Google Scholar] [CrossRef]
- Ding, Y.; Zheng, H.; Zhang, S.; Liu, B.; Wu, B.; Jian, Z. Highly efficient recovery of platinum, palladium, and rhodium from spent automotive catalysts via iron melting collection. Resour. Conserv. Recycl. 2020, 155, 104644. [Google Scholar] [CrossRef]
- Liu, C.; Sun, S.; Zhu, X.; Tu, G. Metals smelting-collection method for recycling of platinum group metals from waste catalysts: A mini review. Waste Manag. Res. J. A Sustain. Circ. Econ. 2021, 39, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Ding, Y.; Zheng, H.; Liu, B.; Zhang, S. Process and mechanism of electrolytic enrichment of PGMs from Fe-PGMs alloy. J. Clean. Prod. 2020, 271, 122829. [Google Scholar] [CrossRef]
- Kuzas, E.; Sandalov, I.; Karimov, K.; Kritskii, A.; Fomenko, I.; Zhidkov, I.; Abramov, A. Kinetics of Iron Collector Leaching in HCl and HF Media. Metals 2024, 14, 1077. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; Environmental Progress & Sustainable Energy 32; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Kuzas, E.; Rogozhnikov, D.; Dizer, O.; Karimov, K.; Shoppert, A.; Suntsov, A.; Zhidkov, I. Kinetic study on arsenopyrite dissolution in nitric acid media by the rotating disk method. Miner. Eng. 2022, 187, 107770. [Google Scholar] [CrossRef]
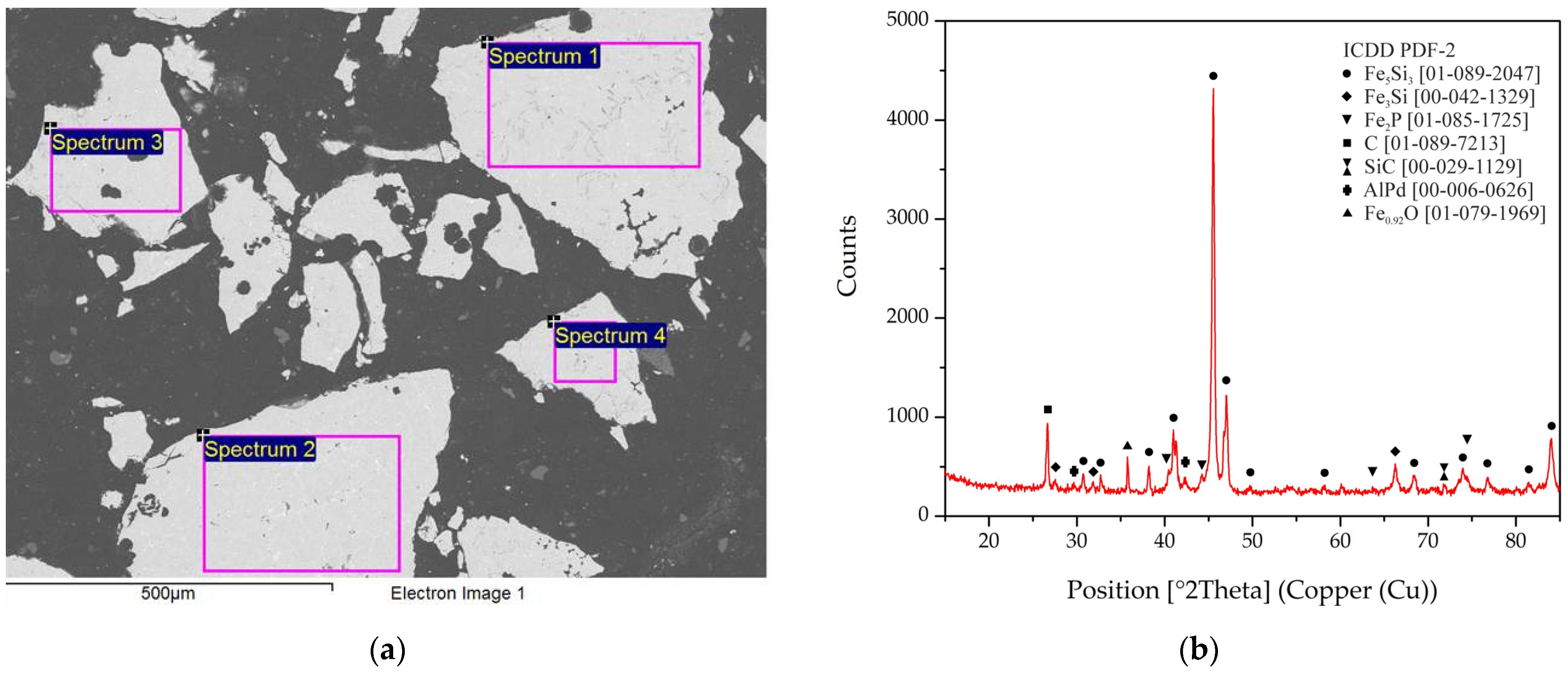


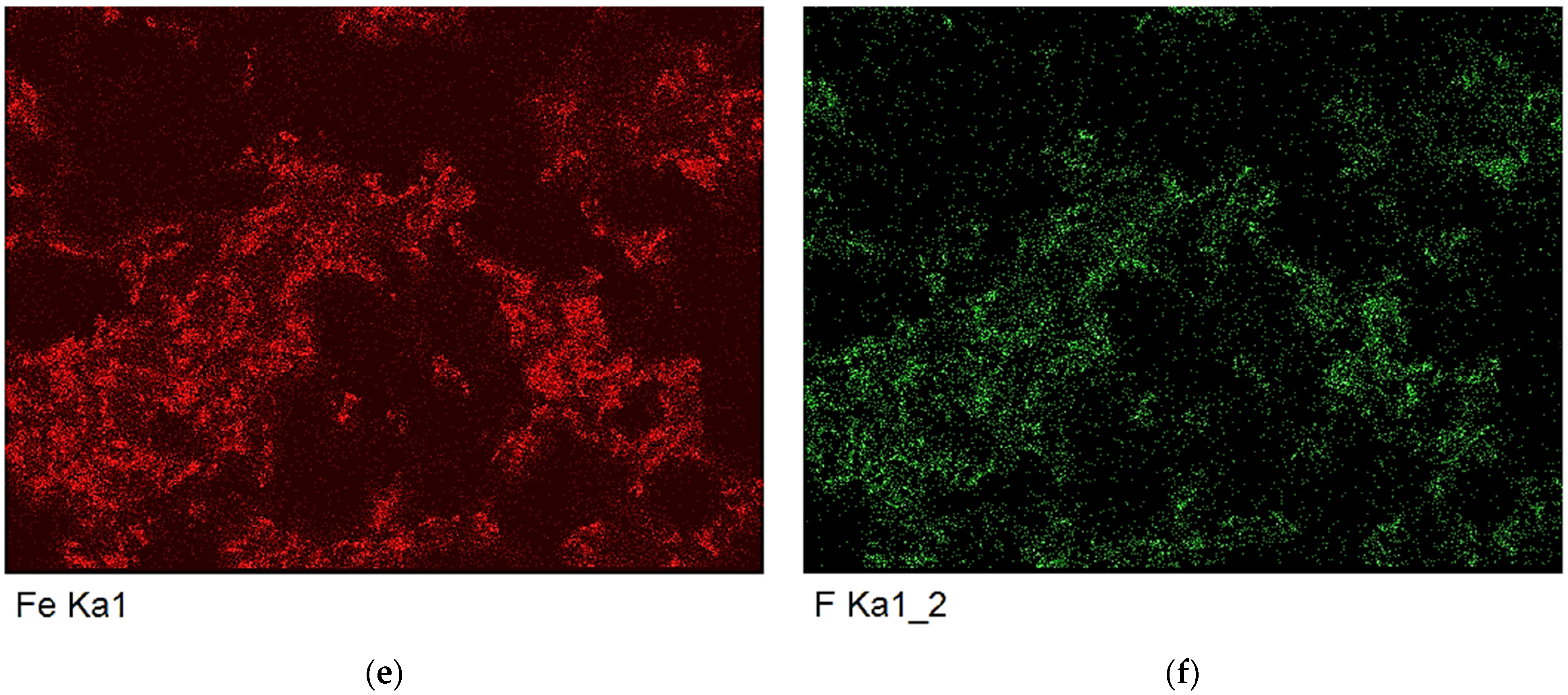

| Analysis | Area | Fe | Si | C | Pd | Pt | Rh |
|---|---|---|---|---|---|---|---|
| XRF | – | 76.8 | 11.9 | 4.9 | 1.0 | 0.3 | 0.1 |
| EDS | Spectrum 1 | 74.1 | 11.8 | – | 1.1 | – | – |
| Spectrum 2 | 73.4 | 11.1 | – | 1.4 | – | – | |
| Spectrum 3 | 73.0 | 11.5 | – | 1.5 | – | – | |
| Spectrum 4 | 73.4 | 11.4 | – | 1.4 | – | – |
| Fe5Si3 | Fe3Si | Fe2P | C | SiC | AlPd | Fe0.92O |
|---|---|---|---|---|---|---|
| 38.2 | 35.9 | 9.7 | 8.0 | 5.0 | 2.1 | 1.2 |
| Material | Analysis | Fe | Si | C | F | Pd | Pt | Rh |
|---|---|---|---|---|---|---|---|---|
| Cake 1 | XRF | 52.3 | 10.0 | 5.6 | – | 3.7 | 1.0 | 0.2 |
| EDS | 73.6 | 17.7 | – | 2.9 | 1.4 | – | – | |
| Cake 2 | XRF | 49.1 | 7.1 | 6.2 | – | 5.3 | 2.0 | 0.5 |
| EDS | 40.6 | 6.9 | – | 2.4 | 6.7 | – | – | |
| Cake 3 | XRF | 43.6 | 8.3 | 9.9 | 1.7 | 9.5 | 3.1 | 0.6 |
| EDS | 30.0 | 11.7 | – | 3.6 | 11.2 | – | – |
| Material | Fe2P | Fe5Si3 | C | Fe3Si | SiC | AlPd | Cu2O | TiO2 | Cu3P | Fe0.92O | Cr0.2Pt0.8 | Pt2Si | Fe3Pt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cake 1 | 26.1 | 22.9 | 19.6 | 9.7 | 7.9 | 5.7 | 2.2 | 2.0 | 1.9 | 0.9 | 0.8 | 0.4 | – |
| Cake 2 | 42.9 | 6.4 | 25.9 | 1.6 | 4.6 | 9.1 | 2.5 | 2.2 | 1.0 | 2.1 | 1.4 | 0.5 | – |
| Cake 3 | 38.6 | 1.3 | 28.4 | – | 3.7 | 14.6 | 2.3 | 3.3 | 1.4 | 1.9 | 1.9 | 0.4 | 2.1 |
| No. | CHCl (mol/L) | CHF (mol/L) | T (K) | τ (min) | WFe (mol/min) | WSi (mol/min) | ||
|---|---|---|---|---|---|---|---|---|
| 0–30 min | 30–120 min | 0–30 min | 30–120 min | |||||
| 1 | 3.59 | 4.80 | 363 | 120 | 0.0044 | 0.00059 | 0.0012 | 0.00021 |
| 2 | 4.11 | 5.10 | 343 | 120 | 0.0033 | 0.00039 | 0.0010 | 0.00014 |
| 3 | 4.36 | 6.93 | 363 | 80 | 0.0068 | 0.00058 | 0.0013 | 0.00020 |
| 4 | 4.99 | 7.36 | 343 | 80 | 0.0050 | 0.00040 | 0.0011 | 0.00015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzas, E.; Sandalov, I.; Kritskii, A.; Karimov, K. Leaching Kinetics of Iron Collector Containing PGMs. Metals 2024, 14, 1416. https://doi.org/10.3390/met14121416
Kuzas E, Sandalov I, Kritskii A, Karimov K. Leaching Kinetics of Iron Collector Containing PGMs. Metals. 2024; 14(12):1416. https://doi.org/10.3390/met14121416
Chicago/Turabian StyleKuzas, Evgeniy, Ivan Sandalov, Aleksei Kritskii, and Kirill Karimov. 2024. "Leaching Kinetics of Iron Collector Containing PGMs" Metals 14, no. 12: 1416. https://doi.org/10.3390/met14121416
APA StyleKuzas, E., Sandalov, I., Kritskii, A., & Karimov, K. (2024). Leaching Kinetics of Iron Collector Containing PGMs. Metals, 14(12), 1416. https://doi.org/10.3390/met14121416






