Cu Evaporation from Liquid Iron Alloy in Stream
Abstract
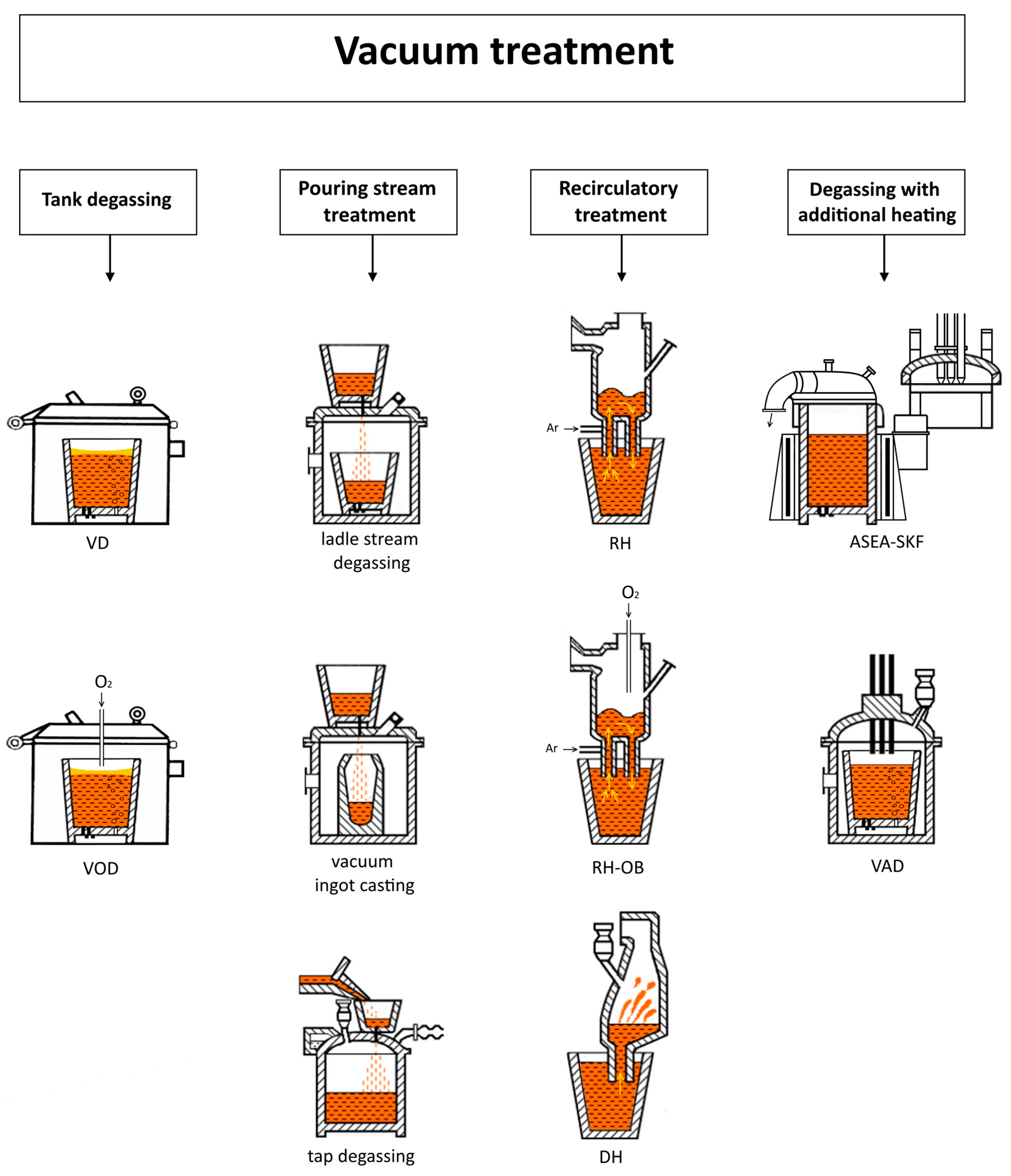
1. Introduction
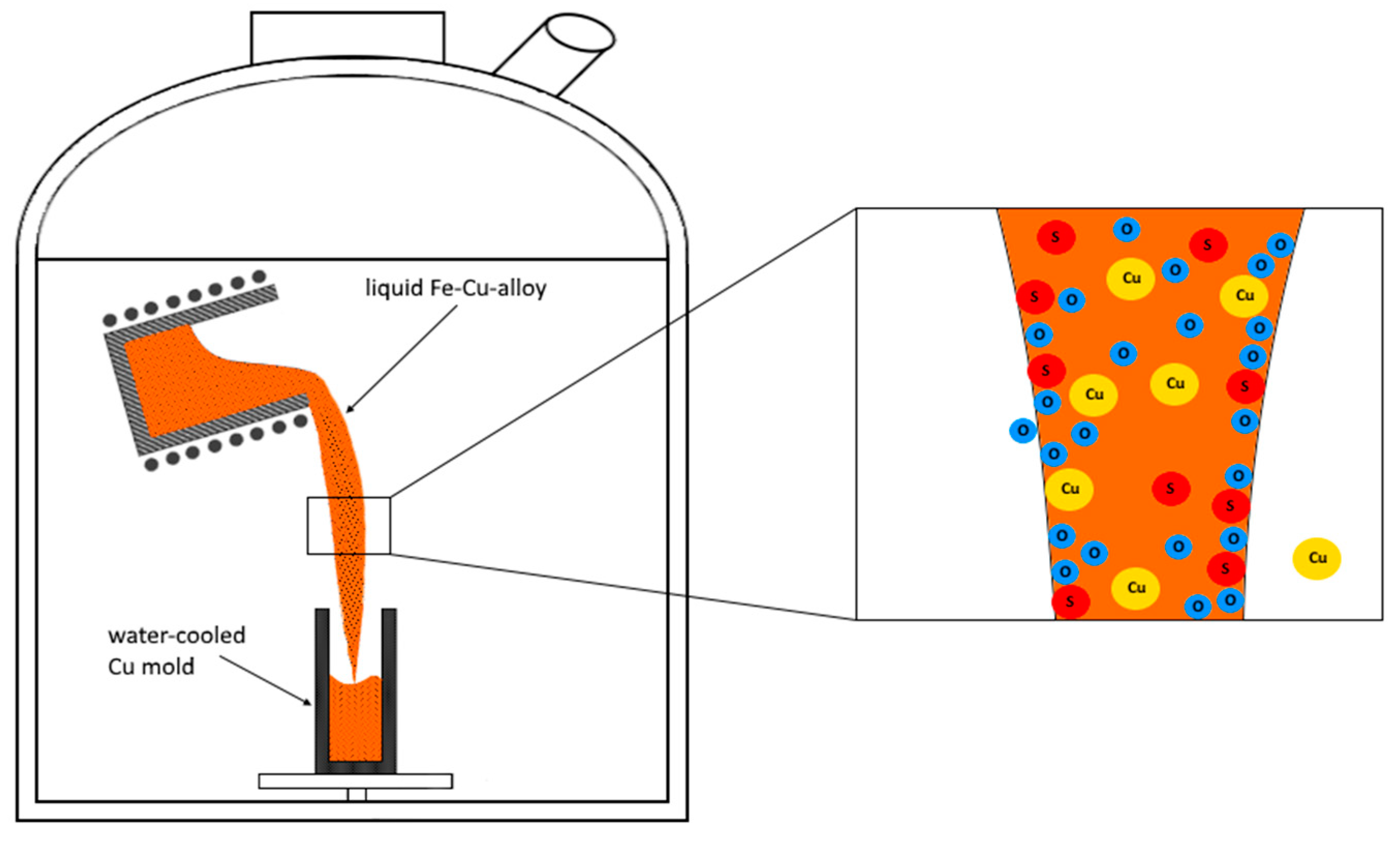
2. Materials and Methods
- Alloy 1—high oxygen and low sulfur;
- Alloy 2—high in both oxygen and sulfur;
- Alloy 3—low oxygen and low sulfur;
- Alloy 4—low oxygen content and high sulfur.
3. Results
3.1. The Evaporation Mechanism of Copper from the Melt
- 1.
- Convective transport of copper from the bulk of the liquid alloy to the diffusion boundary layer in the melt,
- 2.
- Diffusion of copper through the liquid phase interface,
- 3.
- Evaporation of copper at the gas–metal interface,
- 4.
- Diffusion of copper vapor through the gas phase interface,
- 5.
- Transport of the vapor through the bulk to the site of condensation,
- 6.
- Condensation.
3.2. Results of Experiments
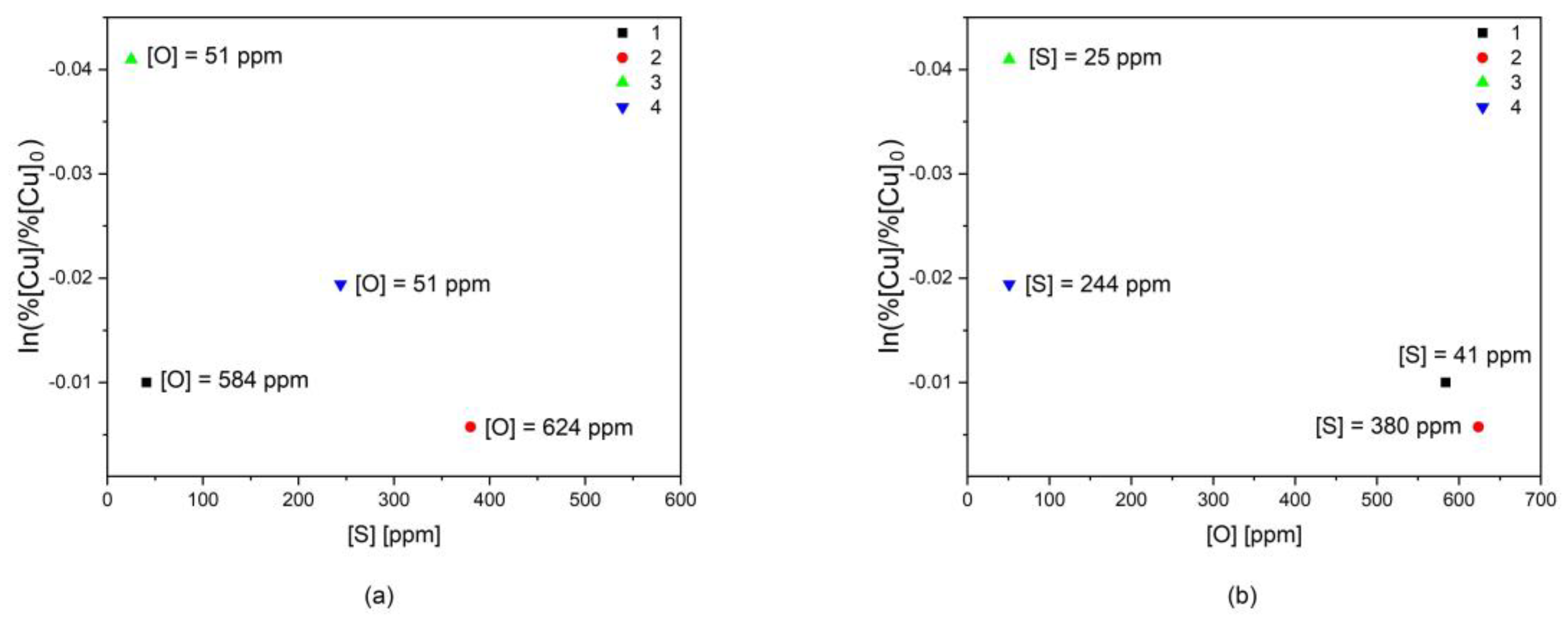
3.3. Effect of Sulfur and Oxygen on the Vaporization
3.4. Evaporation of Other Component from the Liquid Melt
4. Discussion of Results
- The increased surface layer of Fe-Cu alloy presented for copper evaporation, benefiting the rate coefficient of the vaporization reaction;
- The decreased height of the liquid melt benefits the liquid phase mass transfer;
- An appropriate pressure of the system for operational conditions in steelmaking,
- The absence of a time-consuming treatment.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global CO2 Emissions by Year 1940–2023. Available online: https://www.statista.com/statistics/276629/global-co2-emissions/ (accessed on 19 March 2024).
- Holappa, L. A General Vision for Reduction of Energy Consumption and CO2 Emissions from the Steel Industry. Metals 2020, 10, 1117. [Google Scholar] [CrossRef]
- World Steel in Figures 2022. Available online: https://worldsteel.org/data/world-steel-in-figures-2022/ (accessed on 8 May 2024).
- Villa, A. Roadmap of Europe’s Low-Carbon Steel Production Projects. Available online: https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/metals/031424-roadmap-of-europes-low-carbon-steel-production-projects (accessed on 19 March 2024).
- Morfeldt, J.; Nijs, W.; Silveira, S. The Impact of Climate Targets on Future Steel Production—An Analysis Based on a Global Energy System Model. J. Clean. Prod. 2015, 103, 469–482. [Google Scholar] [CrossRef]
- Souza Filho, I.R.; da Silva, A.K.; Büyükuslu, Ö.K.; Raabe, D.; Springer, H. Sustainable Ironmaking Toward a Future Circular Steel Economy: Exploiting a Critical Oxygen Concentration for Metallurgical Cu Removal from Scrap-Based Melts. Steel Res. Int. 2024, 95, 2300785. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Sun, X.; Hao, W.; Geng, G.; Ma, T.; Li, Y. Solidification Microstructure Evolution of Undercooled Cu-15 Wt.% Fe Alloy Melt. Adv. Mater. Sci. Eng. 2018, 2018, 6304518. [Google Scholar] [CrossRef]
- Yeşiltepe, S.; Şeşen, M.K. Hot Shortness Mechanism and Heat Treatment of Cu Containing Carbon Steel. J. Innov. Eng. Nat. Sci. 2021, 1, 15–24. [Google Scholar] [CrossRef]
- Hasegawa, M. Ellingham Diagram. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 507–516. ISBN 978-0-08-096986-2. [Google Scholar]
- Langenberg, F.C.; Lindsay, R.W. Contributions to the Metallurgy of Steel; no. 51.; American Iron and Steel Institute: Washington, DC, USA, 1957. [Google Scholar]
- Yamaguchi, K.; Takeda, Y. Impurity Removal from Carbon Saturated Liquid Iron Using Lead Solvent. Mater. Trans. 2003, 44, 2452–2455. [Google Scholar]
- Parameswaran, K.; Metz, K.; Morris, A. Phase Equilibria for Iron-Rich Fe-Cu-C Alloys: 1500 to 950 °C. Metall. Trans. A 1979, 10, 1929–1939. [Google Scholar] [CrossRef]
- Wang, C.; Nagasaka, T.; Hino, M.; Ban-Ya, S. Copper Distribution between Molten FeS-NaS0.5 Flux and Carbon Saturated Iron Melt. ISIJ Int. 1991, 31, 1300–1308. [Google Scholar] [CrossRef]
- Savov, L.; Janke, D. Evaporation of Cu and Sn from Induction-Stirred Iron-Based Melts Treated at Reduced Pressure. ISIJ Int. 2000, 40, 95–104. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kang, Y.-B. Simultaneous Evaporation of Cu and Sn from Liquid Steel. Metall. Mater. Trans. B. 2016, 47, 2564–2570. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kang, Y.-B. Evaporation Mechanism of Cu from Liquid Fe Containing C and S. Metall. Mater. Trans. B 2016, 47, 2164–2176. [Google Scholar] [CrossRef]
- Chen, X.; Ito, N.; Nakashima, K.; Mori, K. Evaporation Rate of Copper in High Carbon Iron Melt under Reduced Pressure. Tetsu-to-Hagané 1995, 81, 959–964. [Google Scholar] [CrossRef][Green Version]
- Ono-Nakazato, H.; Taguchi, K.; Usui, T. Estimation of the Evaporation Rate of Copper and Tin from Molten Iron-Silicon Alloy. ISIJ Int. 2003, 43, 1105–1107. [Google Scholar] [CrossRef][Green Version]
- Łabaj, J. Kinetics of Coper Evaporation from the Fe-Cu Alloys Under Reduced Pressure. Arch. Metall. Mater. 2012, 57, 165–172. [Google Scholar] [CrossRef]
- Ohno, R. Kinetics of Evaporation of Manganese, Copper and Sulfur from Iron Alloys in Vacuum Induction Melting. ISIJ Int. 1977, 17, 732–741. [Google Scholar] [CrossRef]
- Wei, X.; Dudczig, S.; Chebykin, D.; Aneziris, C.G.; Volkova, O. Verification of Possibility of Molten Steels Decopperization with ZnAl2O4. Metals 2021, 11, 2030. [Google Scholar] [CrossRef]
- Schenck, H.; Steinmetz, E. Wirkungsparameter von Begleitelement Eisenlegierungen und Ihre Gegenseitigen Beziehungen; Verlag Stahleisen: Essen, Germany, 1968. [Google Scholar]
- Kreschel, T.; Volkova, O. Steel. In Handbook of Recycling; Elsevier: Amsterdam, The Netherlands, 2024; pp. 301–318. ISBN 978-0-323-85514-3. [Google Scholar]
- Burghardt, H.; Neuhof, G. Stahlerzeugung; Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1983. [Google Scholar]
- Fischer, W.A.; Janke, D.; Stahlschmidt, K. Die Verdampfung von Kupfer, Mangan Und Chrom Aus Schmelzen Des Stahles X 5 CrNi 18 9 Unter Vermindertem Druck. Arch. Eisenhüttenwes. 1974, 45, 361–365. [Google Scholar] [CrossRef]
- Salomon-de-Friedberg, H.; Davenport, W.G. Vacuum Removal of Copper from Melted Steel Scrap. Can. Metall. Q. 1977, 16, 225–231. [Google Scholar] [CrossRef]
- Morohoshi, K.; Uchikoshi, M.; Isshiki, M.; Fukuyama, H. Surface Tension of Liquid Iron as Functions of Oxygen Activity and Temperature. ISIJ Int. 2011, 51, 1580–1586. [Google Scholar] [CrossRef]
- Fischer, W.A.; Janke, D.; Stahlschmidt, K. Vergleich experimenteller Ergebnisse und theoretischer Ansätze zur Verdampfung von Begleitelementen aus Stahlschmelzen. Arch. Eisenhüttenwes. 1974, 45, 509–515. [Google Scholar] [CrossRef]
- Ohno, R. Rates of Evaporation of Silver, Lead, and Bismuth from Copper Alloys in Vacuum Induction Melting. Metall. Trans. B 1976, 7, 647–653. [Google Scholar] [CrossRef]
- Richardson, F.D. Physical Chemistry of Melts in Metallurgy. Volume 1; Academic Press: London, UK, 1974; ISBN 978-0-12-587901-9. [Google Scholar]
- Lee, J.; Morita, K. Evaluation of Surface Tension and Adsorption for Liquid Fe-S Alloys. ISIJ Int. 2002, 42, 588–594. [Google Scholar] [CrossRef]
- Sain, D.R.; Belton, G.R. The Influence of Sulfur on Interfacial Reaction Kinetics in the Decarburization of Liquid Iron by Carbon Dioxide. Metall. Trans. B 1978, 9, 403–407. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kang, Y.-B.; Seo, J.-D.; Park, J.-K.; Choi, J. Evaporation Mechanism of Sn and SnS from Liquid Fe: Part III. Effect of C on Sn Removal. Metall. Mater. Trans. B 2015, 46, 267–277. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, W.-B.; Park, S.-C.; Kang, Y.-B. Evaporation of As and Sn from Liquid Iron: Experiments and a Kinetic Model during Top-Blown Oxygen Steelmaking Process. Materials 2022, 15, 4771. [Google Scholar] [CrossRef]
- Shilov, A.L.; Shornikov, S.I.; Archakov, I.Y.; Ivanov, G.G.; Stolyarova, V.L.; Shultz, M.M. A Mass Spectrometric Study of the Vaporization and Thermodynamic Properties of Melts in the SnO–ZnO–P2O5 System. Glass Phys. Chem. 1998, 24, 400–405. [Google Scholar]
- Zaitsev, A.I.; Zaitseva, N.E.; Shakhpazov, E.K.; Mogutnov, B.M. Evaporation of Copper from Iron Melts. ISIJ Int. 2004, 44, 639–646. [Google Scholar] [CrossRef]
- Nadif, M.; Suero, J.; Rodhesly, C.; Salvadori, D.; Schadow, F.; Schutz, R.; Perrin, E.; Peeters, L. Desulfurization Practices in ArcelorMittal Flat Carbon Western Europe. Metall. Res. Technol. 2009, 106, 270–279. [Google Scholar] [CrossRef]

| C | Mn | P | S | Cu | N | Sn | Si | Al | Cr | Mo | Ni | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 70 | 450 | 30 | 30 | 90 | 46 | 20 | 30 | 30 | 260 | 30 | 210 | 30 |
| Alloy | Cuinitial | Sinitial | Oinitial |
|---|---|---|---|
| 1 | 5030 | 41 | 584 |
| 2 | 5250 | 380 | 624 |
| 3 | 5230 | 25 | 51 |
| 4 | 5160 | 244 | 51 |
| Alloy | Cuend | Send | Oend | ΔCu | ΔS | ΔO |
|---|---|---|---|---|---|---|
| 1 | 4980 | 38 | 572 | 50 (0.99) * | 3 | 12 |
| 2 | 5220 | 306 | 593 | 30 (0.53) | 74 | 31 |
| 3 | 5020 | 23 | 22 | 210 (4.02) | 2 | 29 |
| 4 | 5100 | 234 | 38 | 60 (1.16) | 10 | 13 |
| Alloy | Pinitial/Pend | ΔP, wt% | Mninitial/Mnend | ΔMn, wt% | Sninitial/Snend | ΔSn, wt% |
|---|---|---|---|---|---|---|
| 1 | 127/92 | 27.56 | 171/153 | 10.53 | 41/32 | 21.95 |
| 2 | 156/120 | 23.08 | 132/118 | 10.61 | 49/41 | 16.33 |
| 3 | 132/120 | 9.09 | 146/104 | 28.77 | 45/30 | 33.33 |
| 4 | 141/130 | 7.80 | 133/121 | 9.02 | 43/38 | 11.63 |
| Unkilled/ Desulfurized | Unkilled/ Non-Desulfurized | Killed/ Desulfurized | Killed/ Non-Desulfurized | |
|---|---|---|---|---|
| Cu | − * | − | + ** | + *** |
| P | + | + | − | − |
| Mn | − | − | + | − |
| Sn | − | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalimova, G.; Levchenko, M.; Markus, H.-P.; Sosin, D.; Kreschel, T.; Volkova, O. Cu Evaporation from Liquid Iron Alloy in Stream. Metals 2024, 14, 1233. https://doi.org/10.3390/met14111233
Khalimova G, Levchenko M, Markus H-P, Sosin D, Kreschel T, Volkova O. Cu Evaporation from Liquid Iron Alloy in Stream. Metals. 2024; 14(11):1233. https://doi.org/10.3390/met14111233
Chicago/Turabian StyleKhalimova, Galiia, Mykyta Levchenko, Hans-Peter Markus, Dariusz Sosin, Thilo Kreschel, and Olena Volkova. 2024. "Cu Evaporation from Liquid Iron Alloy in Stream" Metals 14, no. 11: 1233. https://doi.org/10.3390/met14111233
APA StyleKhalimova, G., Levchenko, M., Markus, H.-P., Sosin, D., Kreschel, T., & Volkova, O. (2024). Cu Evaporation from Liquid Iron Alloy in Stream. Metals, 14(11), 1233. https://doi.org/10.3390/met14111233







