Integrating Process Simulation and Life Cycle Assessment for Enhanced Process Efficiency and Reduced Environmental Impact in Ferromanganese Production
Abstract
1. Introduction
2. Methods
2.1. Production Process Description
2.2. Model Architecture
2.3. Cases
3. Results and Discussion
3.1. Manganese Ferroalloy Production in Closed Furnace
3.2. Manganese Ferroalloy Production in Open Furnace
3.3. Life Cycle Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- PreMa—Energy Efficient, Primary Production of Manganese Ferroalloys Through the Application of Novel Energy Systems in the Drying and Pre-Heating of Furnace Feed Materials. Available online: https://www.aspire2050.eu/prema (accessed on 11 March 2024).
- Tangstad, M.; Schanche, T.; de Preez, F. Use of H2 in Mn-Ferroalloy Production. In TMS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Hockaday, L. Solar Thermal Applications in Minerals Processing in South Africa. In Proceedings of the 6th South African Solar Energy Converence, East London, South Africa, 25–27 November 2019. [Google Scholar]
- Hockaday, L.; Reynolds, Q.; McGregor, C.; Dinter, F. A Comparison of Direct Concentrating Solar Thermal Treatment of Manganese Ores to Fossil Fuel Based Thermal Treatments. In Proceedings of the INFACON XVI, Trondheim, Norway, 27–29 September 2021. [Google Scholar]
- Schanche, T.L. Pretreatment of Manganese Ores in CO/CO2/H2 Atmospheres. Doctoral Thesis, NTNU, Trondheim, Norway, 2022. [Google Scholar]
- Larssen, T.A. Prereduction of Comilog- and Nchwaning Ore. Doctoral Thesis, NTNU, Trondheim, Norway, 2020. [Google Scholar]
- Kim, P. Reduction Rates of SiMn Slags from Various Raw Materials. Doctoral Thesis, NTNU, Trondheim, Norway, 2018. [Google Scholar]
- Letout, S.R.; Ratvik, A.P.; Tangstad, M.; Johansen, S.T.; Olsen, J.E. A Multiscale Numerical Approach of the Dripping Slag in the Coke Bed Zone of a Pilot Scale Si-Mn Furnace; SINTEF: Trondheim, Norway, 2017. [Google Scholar]
- Mukono, T. Ferromanganese Production from Pretreated Manganese Ores: From Laboratory Scale to Pilot Scale. Doctoral Thesis, NTNU, Trondheim, Norway, 2023. [Google Scholar]
- ISO 14040:2006; Environmental Management Life Cycle Assessment Principles and Framework. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 15 October 2024).
- ISO 14044:2006; Environmental Management Life Cycle Assessment Requirements and Guidelines. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 15 October 2024).
- ISO 14067:2018; Greenhouse Gases—Carbon Footprint of Products—Requirements and Guidelines for Quantification. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/71206.html (accessed on 15 October 2024).
- Norgate, N.H.O.T. Estimation of greenhouse gas emissions from ferroalloy production using life cycle assessment with particular reference to Australia. J. Clean. Prod. 2013, 39, 220–230. [Google Scholar]
- Westfall, L.A.; Davourie, J.; Ali, M.; McGough, D. Cradle-to-gate life cycle assessment of global manganese alloy production. Int. J. Life Cycle Assess. 2016, 21, 1573–1579. [Google Scholar] [CrossRef]
- Elomaa, H.; Sinisalo, P.; Rintala, L.; Aromaa, J.; Lundstöm, M. Process simulation and gate-to-gate life cycle assessment of hydrometallurgical refractory gold concentrate processing. Int. J. Life Cycle Assess. 2020, 25, 456–477. [Google Scholar] [CrossRef]
- Hamuyuni, J. Simulation-based life cycle assessment of ferrochrome smelting technologies to determine environmental impacts. J. Clean. Prod. 2021, 295, 126503. [Google Scholar] [CrossRef]
- Ma, Y.; Preveniou, A.; Kladis, A.; Pettersen, J.B. Circular economy and life cycle assessment of alumina production: Simulation-based comparison of Pedersen and Bayer processes. J. Clean. Prod. 2022, 366, 132807. [Google Scholar] [CrossRef]
- Meskers, C.; Worrell, E.; Reuter, M.A. Handbook of Recycling State-of-the-Art for Practitioners, Analysts, and Scientists, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Tangstad, M.; Calvert, P.; Brun, H.; Lindseth, A.G. Use of Comilog ore in ferromanganese production. In Proceedings of the Tenth International Ferroalloys Congress, Cape Town, South Africa, 1–4 February 2004. [Google Scholar]
- Tangstad, M. Handbook of Ferroalloys: Chapter 7. Manganese Ferroalloys Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Tangstad, M.; Ishak, R. Degree of prereduction without coke consumption in industrial furnaces. In Proceedings of the INFACON XI, New Delhi, India, 18–21 February 2007; Volume 1, pp. 268–279. [Google Scholar]
- Olsen, S.E.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Academic Press: Trondheim, Norway, 2007. [Google Scholar]
- Tangstad, M.; Olsen, S.E. The Importance of Slag Basicity in the Production of High Carbon Ferro Manganese; SINTEF: Trondheim, Norway, 1995. [Google Scholar]
- Ringdalen, E.; Gjøvik, J.E.; Larssen, T.A.; Tangstad, M. Pretreatment of manganese ores in different gas-atmospheres-a method to reduce energy consumption and CO2 emissions in Mn-alloy production. In Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI), Trondheim, Norway, 27–29 September 2021. [Google Scholar]
- Reiersen, H.S. Behavior of Manganese Ores during Pre-Reduction in Small Scale Furnaces. Ph.D. Thesis, University of Science and Technology, Trondheim, Norway, 2020. [Google Scholar]
- Canaguier, V.; Schanche, T.L.; Mukono, T.; Ringdalen, E. Kinetic Modelling of FeMn Pilot Experiments: Investigating the Effect of Charge Type and Pre-treatment. In Proceedings of the 62nd Conference of Metallurgists, COM 2023, Toronto, ON, Canada, 21–24 August 2023. [Google Scholar]
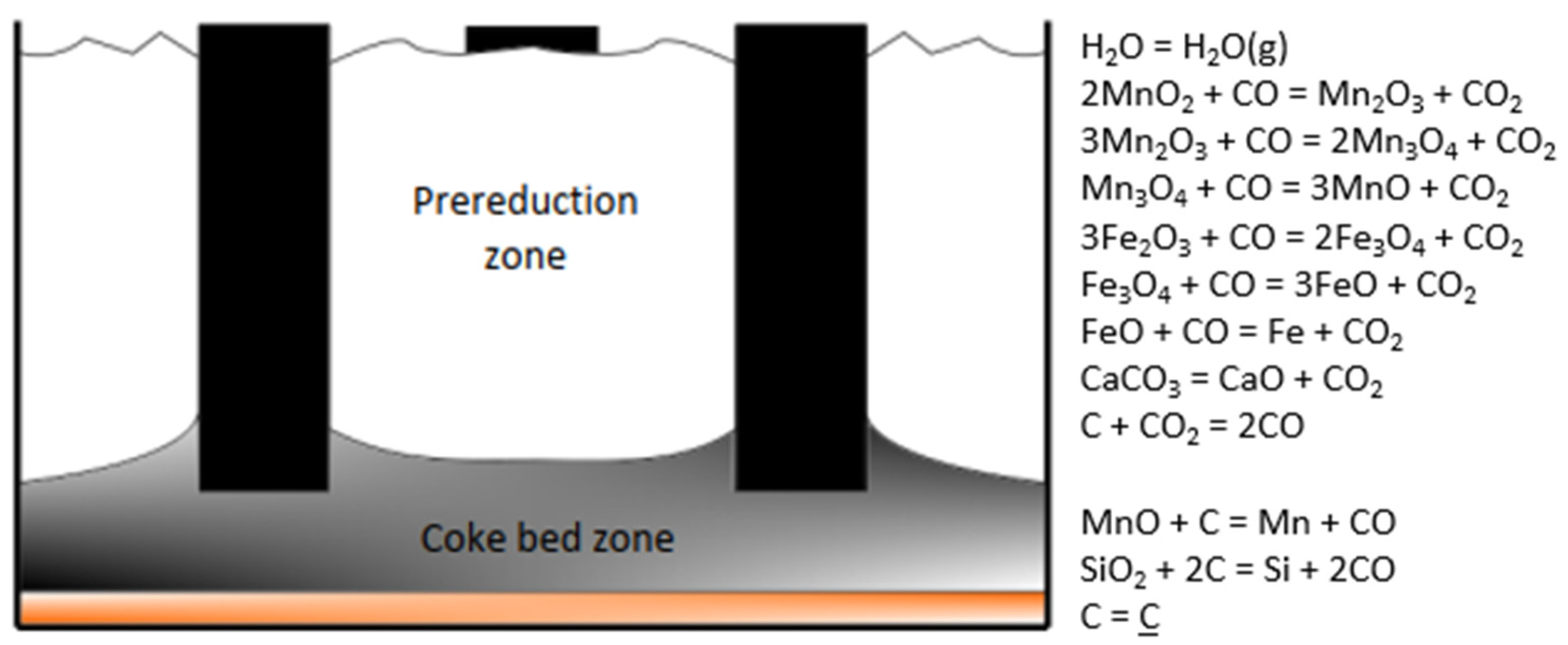

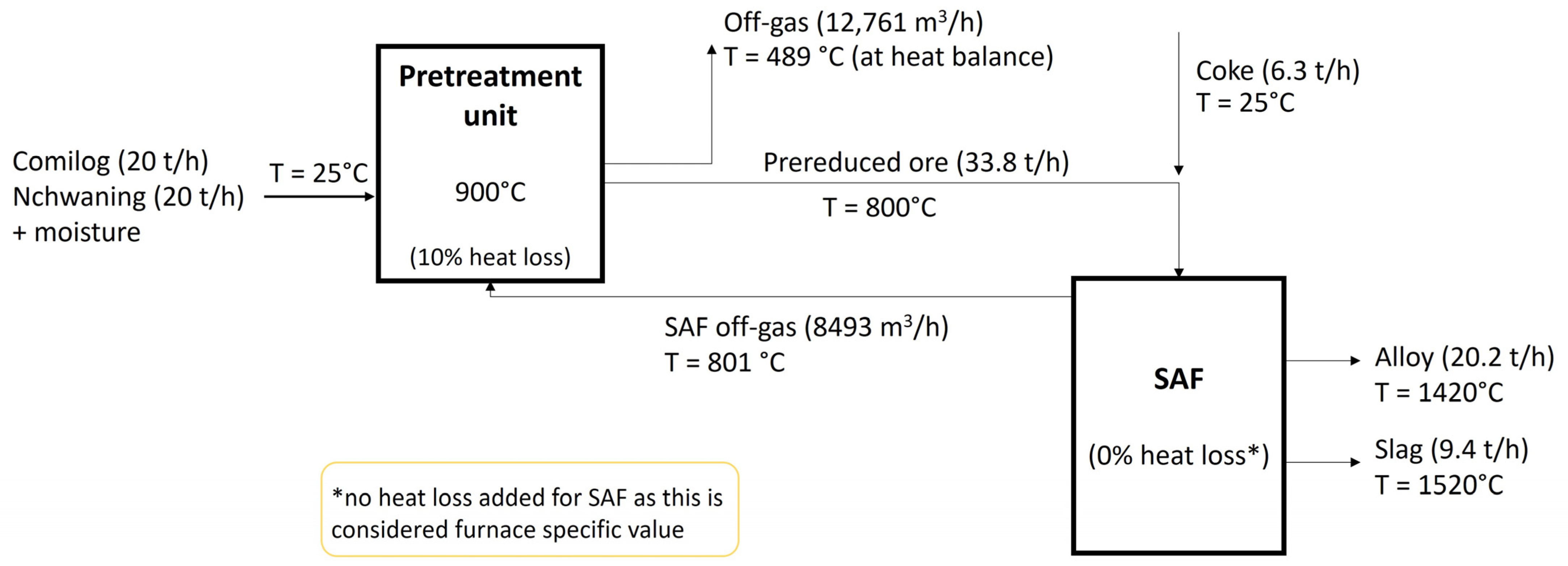
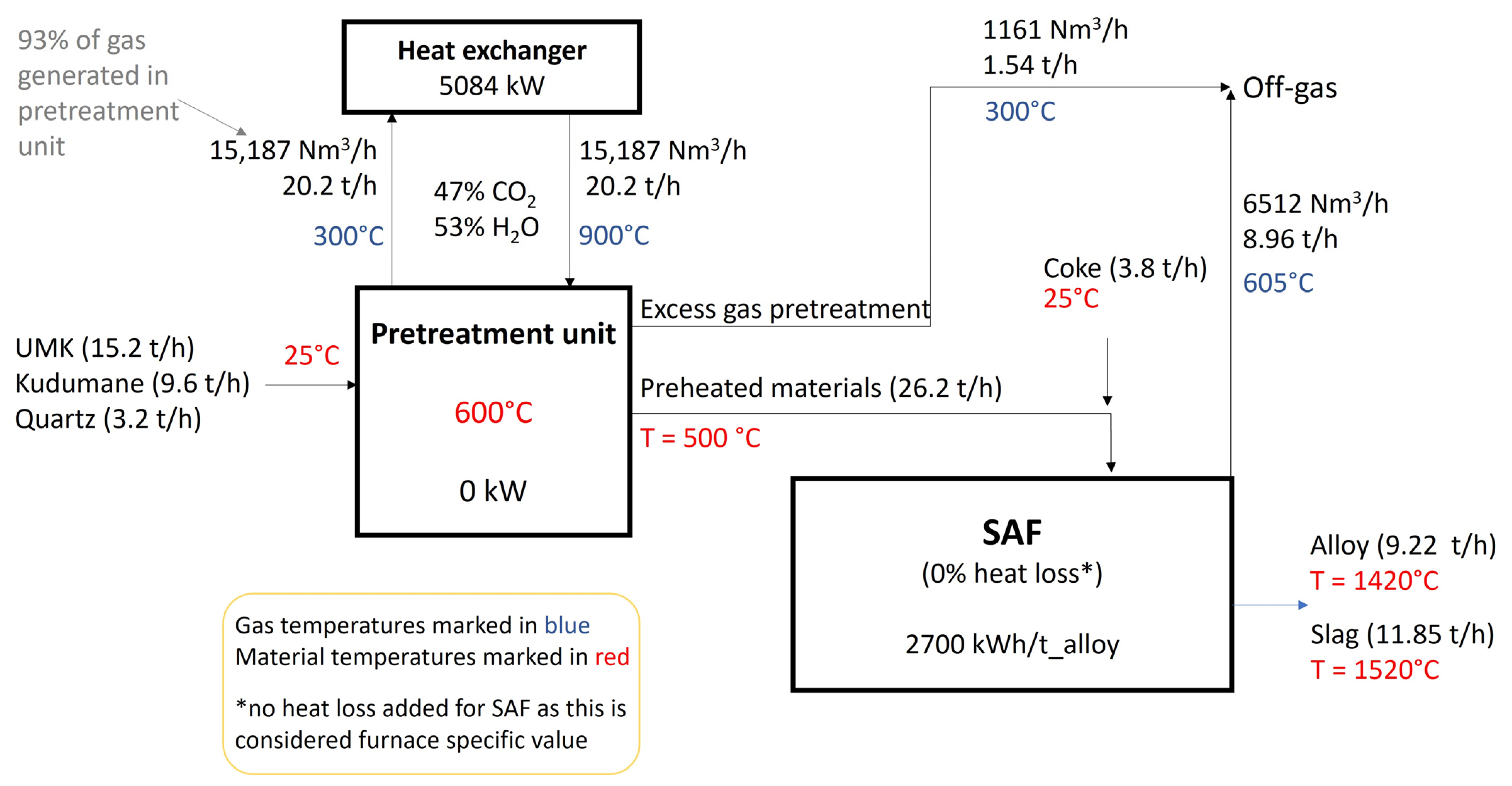


| Zone 1 | Zone 2 | Zone 3 | Zone 4 | ||
|---|---|---|---|---|---|
| 25–600 °C | 600–800 °C | 800–1250 °C | 1250–1420 °C | ||
| General | H2O = H2O(g) | 100 | 100 | 100 | 100 |
| H2O(g) + CO(g) = H2(g) + CO2(g) | 10 | 100 | 100 | 100 | |
| 2 FeO∙OH = Fe2O3 + H2O(g) | 100 | 100 | 100 | 100 | |
| 3Fe2O3 + CO = 2Fe3O4 + CO2 | |||||
| Fe3O4 + CO = 3 FeO + CO2 | |||||
| FeO + CO = Fe + CO2 | 0 | 0 | 50 | 100 | |
| CaMg(CO3)2 = CaCO3 + MgO + CO2 | 100 | 100 | 100 | 100 | |
| CaCO3 = CaO + CO2 | 0 | 0 | 100 | 100 | |
| MgCO3 = MgO + CO2 | 100 | 100 | 100 | 100 | |
| MnO + C = Mn + CO | 0 | 0 | 0 | x | |
| SiO2 + 2C = Si + 2 CO | 0 | 0 | 0 | x | |
| C = C | 0 | 0 | 0 | x | |
| Comilog | 2 MnO2 + CO = Mn2O3 + CO2 | 100 | 100 | 100 | 100 |
| 3 Mn2O3 + CO = 2 Mn3O4 + CO2 | 100 | 100 | 100 | 100 | |
| Mn3O4 + CO = 3 MnO + CO | 42.9 | 76.4 | 100 | 100 | |
| Nchwaning | 3 Mn2O3 + CO = 2 Mn3O4 + CO2 | 4 | 100 | 100 | 100 |
| Mn3O4 + CO = 3 MnO + CO | 0 | 14.6 | 100 | 100 | |
| Kudumane | 3 Mn2O3 + CO = 2 Mn3O4 + CO2 | 4 | 100 | 100 | 100 |
| Mn3O4 + CO = 3 MnO + CO | 0 | 14.6 | 100 | 100 | |
| UMK | 3 Mn2O3 + CO = 2 Mn3O4 + CO2 | 4 | 100 | 100 | 100 |
| Mn3O4 + CO = 3 MnO + CO | 0 | 14.6 | 100 | 100 |
| Comilog | Nchwaning | UMK | Kudumane | Quartz | Metallurgical Coke | |
|---|---|---|---|---|---|---|
| C | 88.0 | |||||
| MnO2 | 76.8 | |||||
| Mn2O3 | 1.6 | 62.3 | 30.7 | 28.9 | ||
| Mn3O4 | 3.0 | 21.6 | 24.1 | |||
| MnO | 2.7 | |||||
| CaMg(CO3)2 | 17.8 | 17.8 | ||||
| SiO2 | 3.3 | 3.9 | 5.4 | 5.4 | 94.3 | 5.1 |
| Al2O3 | 5.8 | 0.4 | 0.5 | 0.4 | 2.1 | 3.3 |
| Fe2O3 | 14.9 | 6.7 | 6.4 | |||
| FeO∙OH | 4.4 | 1.5 | ||||
| BaO | 0.2 | |||||
| P | 0.1 | |||||
| K2O | 0.9 | |||||
| CaO | 0.6 | 2.0 | 1.9 | |||
| CaCO3 | 7.9 | 11.7 | 11.7 | |||
| MgO | 0.2 | 2.9 | 2.2 | |||
| MgCO3 | 2.9 | |||||
| H2O(bound moisture) * | 4.6 | 0.0 | 0.0 | 0.0 |
| Input | Output | ||||||
|---|---|---|---|---|---|---|---|
| Alloy (20.2 t/h) | Slag (9.4 t/h) | Off-Gas (12,761 Nm3/h) | |||||
| t/h | % | % | Mol% | ||||
| Comilog ore | 20 | Mn | 79.4 | MnO | 46.8 | CO | 30.9 |
| Nchwaning ore | 20 | Fe | 13.1 | CaO | 14.9 | CO2 | 53.9 |
| Coke | 7.6 | Si | 0.2 | MgO | 3.3 | H2 + H2O | 15.2 |
| Moisture | 2.3 | C | 7.0 | Al2O3 | 15.1 | ||
| SiO2 | 17.7 | ||||||
| LB1 | 1.0 | ||||||
| Mn/Fe | 6.1 | Al2O3/SiO2 | 0.86 | CO2/(CO + CO2) | 0.64 | ||
| Submerged Arc Furnace | Pretreatment Unit | |
|---|---|---|
| CO, mol% | 100 | 9.9 |
| CO2, mol% | 0 | 50.4 |
| CO2/(CO + CO2) | - | 0.84 |
| Temperature [°C] | 800.7 | 488.5 |
| Stand-Alone SAF | SAF + Pretreatment | % Change | |
|---|---|---|---|
| (1) Energy consumption [kWh/tonne alloy] | 1803 | ||
| (2) Unutilized energy [kWh/tonne alloy] | 944 | ||
| (1) + (2): Total energy balance [kWh/tonne alloy] | 2748 | 2268 | −17.5 |
| Coke consumption [kg/tonne alloy] | 357 | 310 | −13.2 |
| Direct CO2 emissions [tonne CO2/tonne alloy] | 0.99 | 0.83 | −16.2 |
| Input | Output | ||||||
|---|---|---|---|---|---|---|---|
| Alloy (9.2 t/h) | Slag (11.9 t/h) | Off-Gas (7673 Nm3/h) | |||||
| t/h | % | % | Mol% | ||||
| Kudumane ore | 9.4 | Mn | 80.0 | MnO | 20.1 | CO | 66.7 |
| UMK ore | 14.9 | Fe | 12.7 | CaO | 25.6 | CO2 | 25.2 |
| Quartz | 3.1 | Si | 0.2 | MgO | 13.7 | H2 | 0.8 |
| Coke | C | 7.0 | Al2O3 | 2.6 | H2O | 7.3 | |
| Moisture | SiO2 | 38.1 | |||||
| LB1 | 1.03 | ||||||
| Mn/Fe | 6.3 | Al2O3/SiO2 | 0.07 | CO2/(CO + CO2) | 0.3 | ||
| Stand-Alone SAF | SAF + Pretreatment | % Change | |
|---|---|---|---|
| Energy consumption [kWh/tonne alloy] | 3040 | 2700 | −11.2 |
| Energy demand heat exchanger [kWh/tonne alloy] | 535 | ||
| Coke consumption [kg C/tonne alloy] | 422 | 422 | 0 |
| Direct CO2 emissions [tonne/tonne alloy] | 1.44 | 1.44 | 0 |
| * Indirect CO2-emissions [tonne CO2/tonne alloy] | 2.88 | 2.56 | −11.2 |
| Impact Category | Reference Unit | Ecoinvent | SAF | Pretreatment + SAF |
|---|---|---|---|---|
| Climate change—GWP100 | kg CO2-eq | 2.661 | 1.394 | 1.142 |
| Freshwater eutrophication—FEP | kg P-Eq | 0.001 | 0.0001 | 0.0000 |
| Photochemical oxidant formation—POFF | kg NMVOC | 0.015 | 0.0034 | 0.0030 |
| Water depletion—WDP | m3 | 0.013 | 0.0534 | 0.0520 |
| Impact Category | Reference Unit | Ecoinvent | SAF | Pretreatment + SAF |
|---|---|---|---|---|
| Climate change—GWP100 | kg CO2-eq | 2.661 | 1.922 | 1.915 |
| Freshwater eutrophication—FEP | kg P-Eq | 0.001 | 0.000 | 0.000 |
| Photochemical oxidant formation—POFF | kg NMVOC | 0.015 | 0.004 | 0.004 |
| Water depletion—WDP | m3 | 0.013 | 0.089 | 0.079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larssen, T.A.; Canaguier, V.; Reuter, M.A.; Ringdalen, E. Integrating Process Simulation and Life Cycle Assessment for Enhanced Process Efficiency and Reduced Environmental Impact in Ferromanganese Production. Metals 2024, 14, 1212. https://doi.org/10.3390/met14111212
Larssen TA, Canaguier V, Reuter MA, Ringdalen E. Integrating Process Simulation and Life Cycle Assessment for Enhanced Process Efficiency and Reduced Environmental Impact in Ferromanganese Production. Metals. 2024; 14(11):1212. https://doi.org/10.3390/met14111212
Chicago/Turabian StyleLarssen, Trine A., Vincent Canaguier, Markus A. Reuter, and Eli Ringdalen. 2024. "Integrating Process Simulation and Life Cycle Assessment for Enhanced Process Efficiency and Reduced Environmental Impact in Ferromanganese Production" Metals 14, no. 11: 1212. https://doi.org/10.3390/met14111212
APA StyleLarssen, T. A., Canaguier, V., Reuter, M. A., & Ringdalen, E. (2024). Integrating Process Simulation and Life Cycle Assessment for Enhanced Process Efficiency and Reduced Environmental Impact in Ferromanganese Production. Metals, 14(11), 1212. https://doi.org/10.3390/met14111212






