Frequency–Time Domain Analysis Based on Electrochemical Noise of Dual-Phase (DP) and Ferrite–Bainite (FB) Steels in Chloride Solutions for Automotive Applications
Abstract
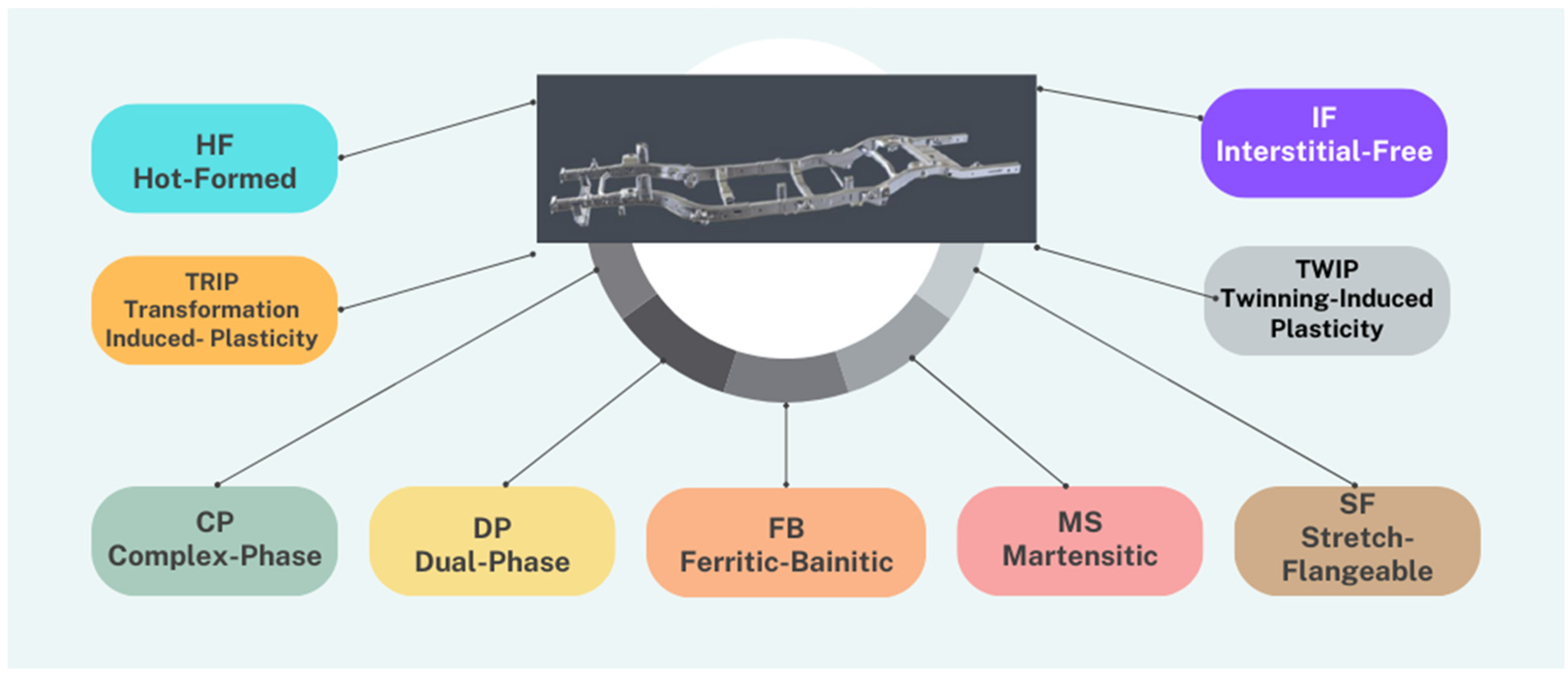
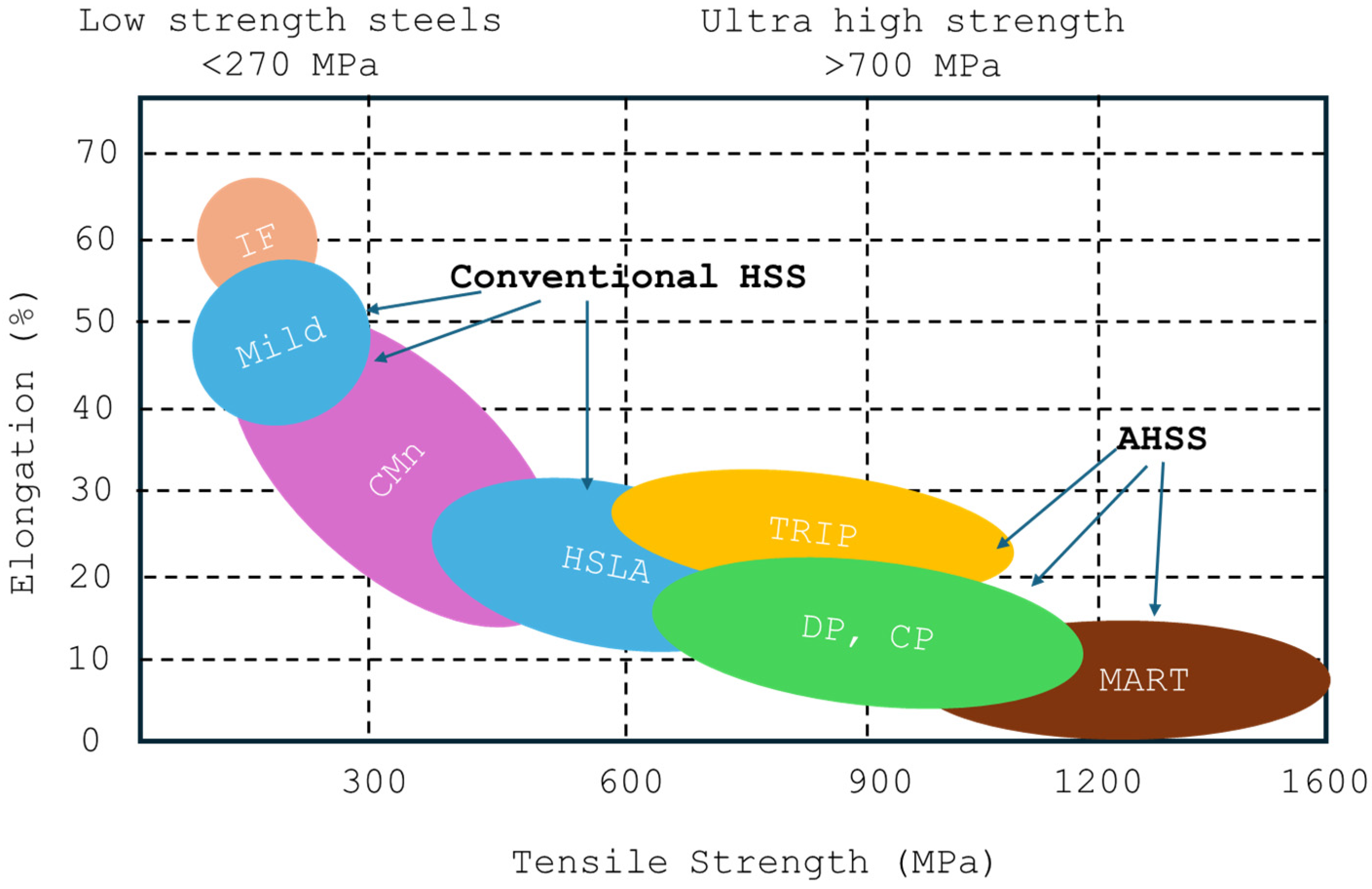
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructural Characterization
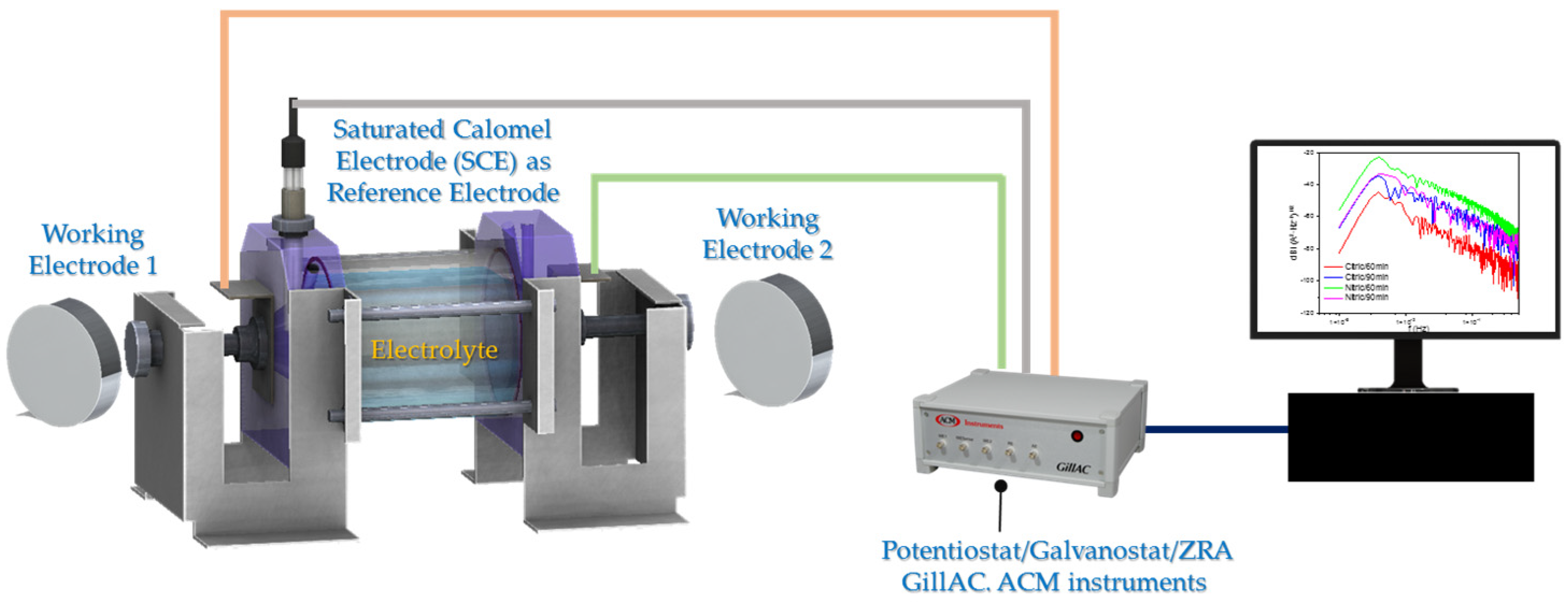
2.3. Electrochemical Technique
3. Results and Discussion
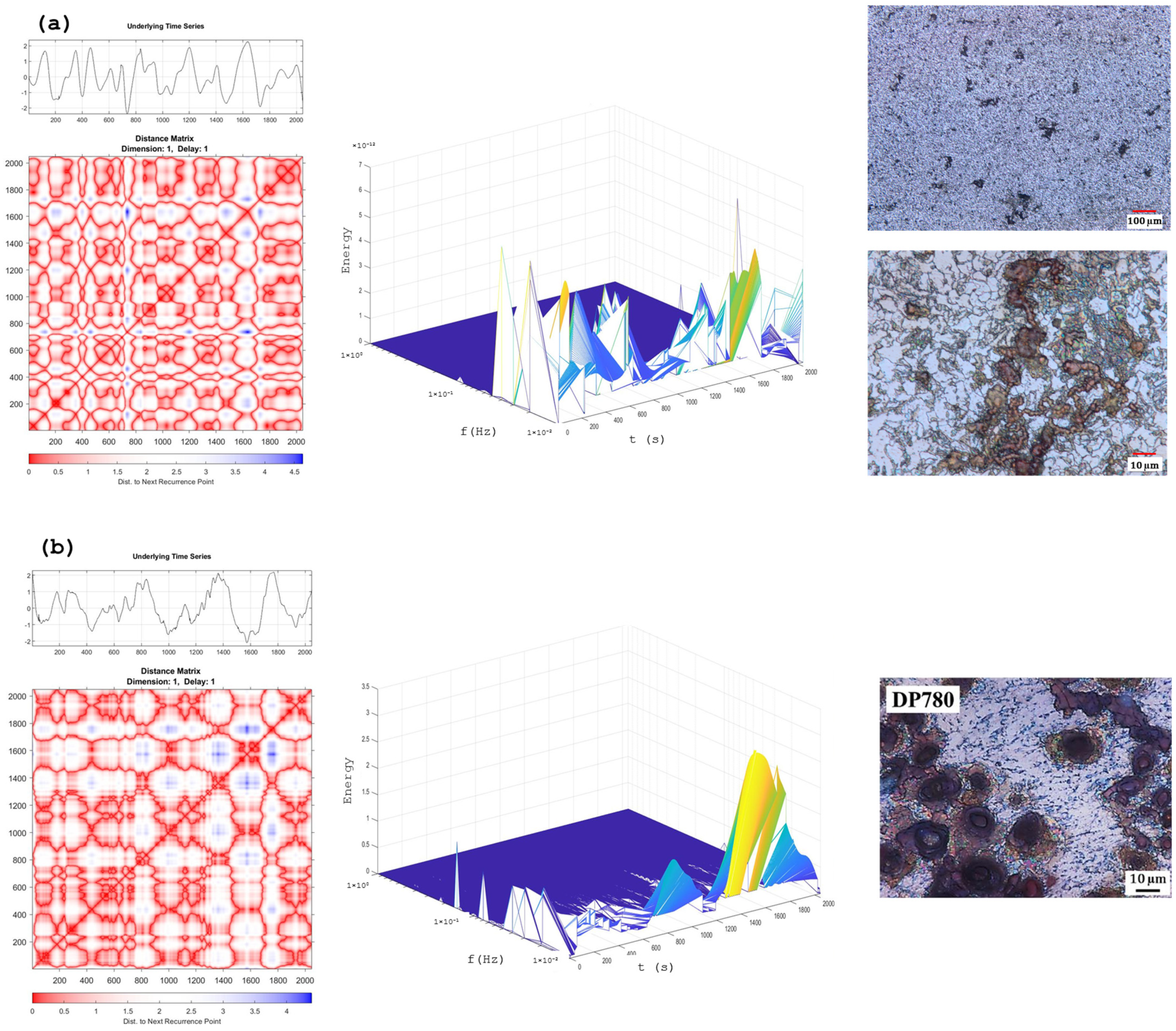
3.1. Microstructure
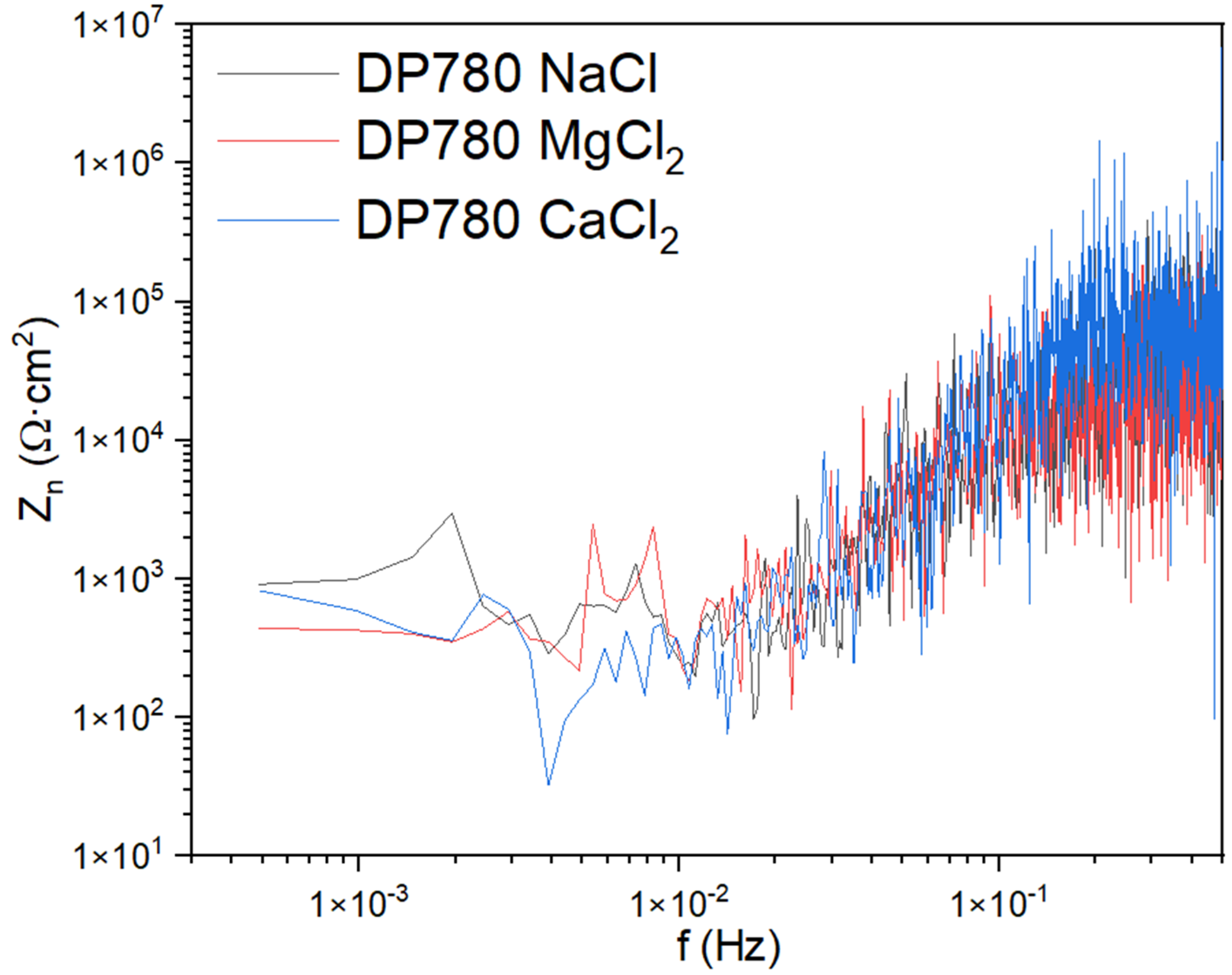
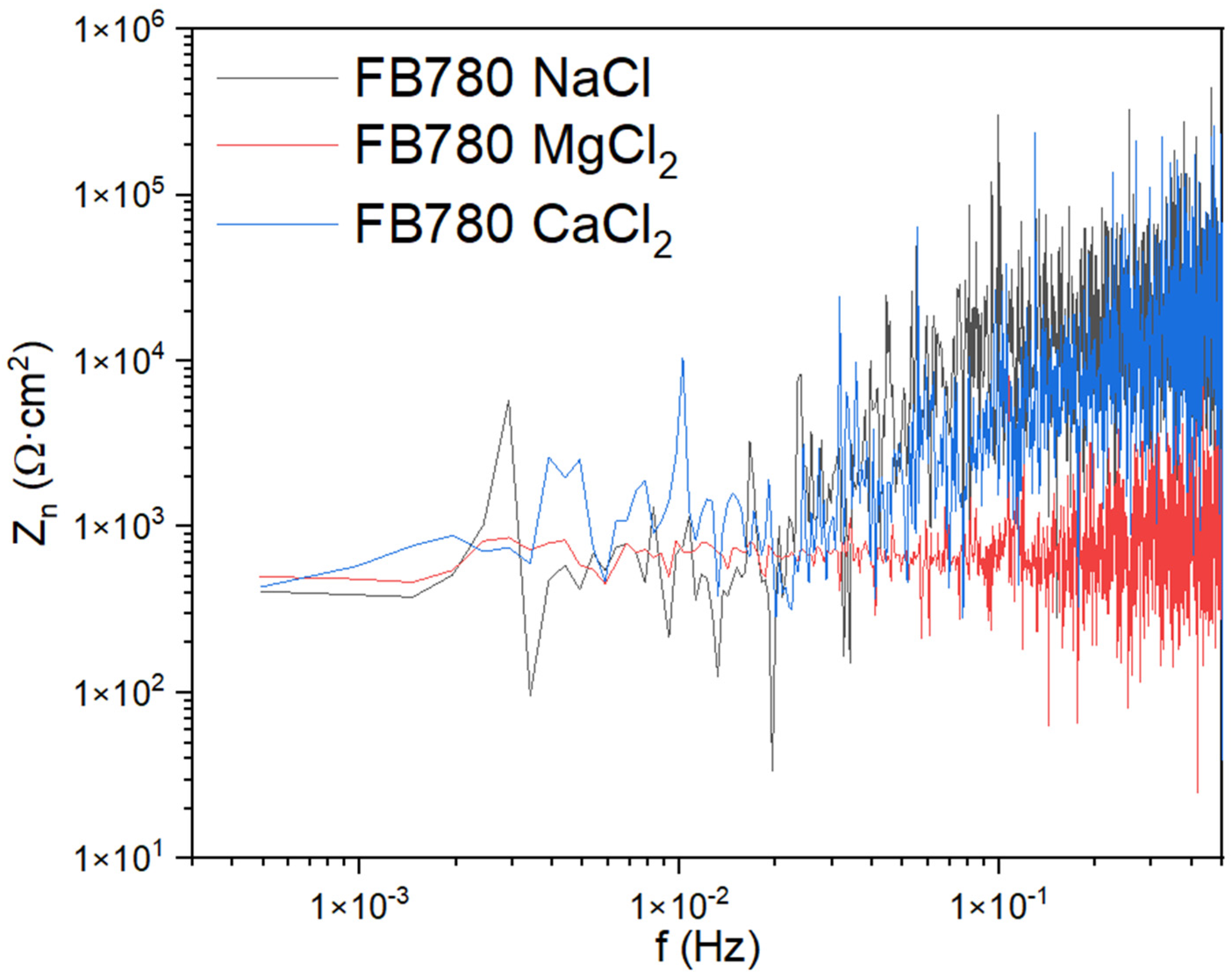
3.1.1. Noise Impedance
3.1.2. Time-Domain Analysis
Wavelets
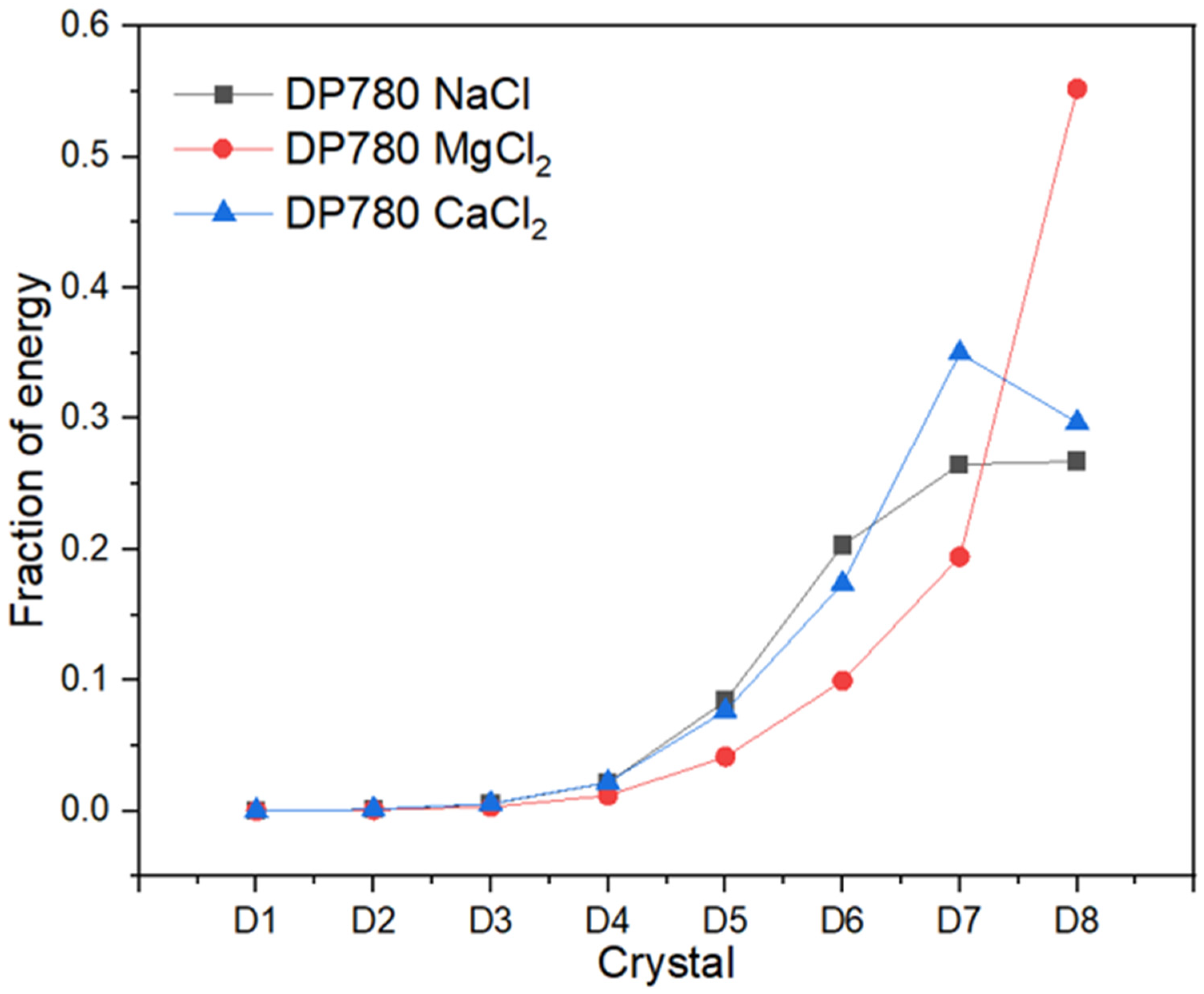
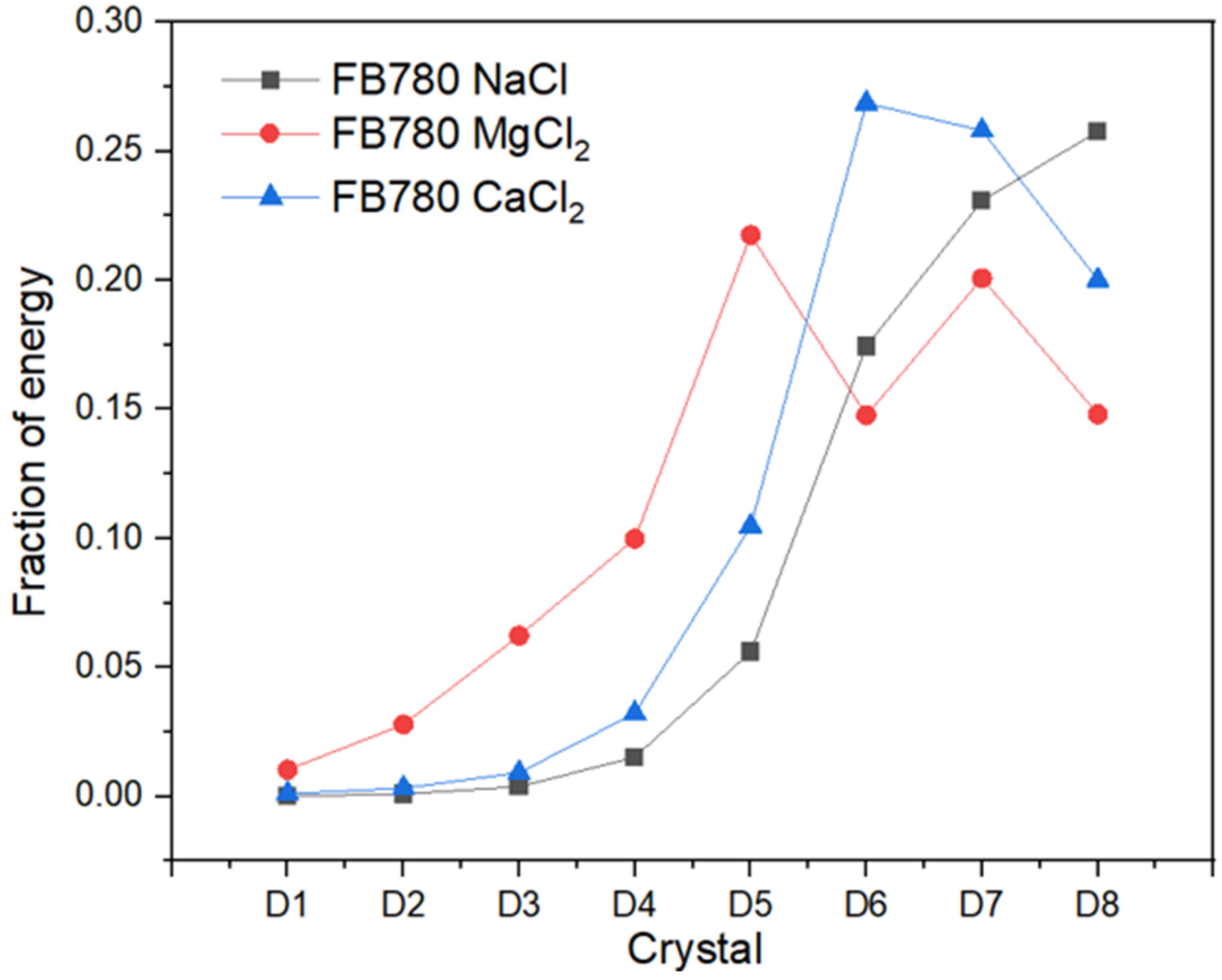
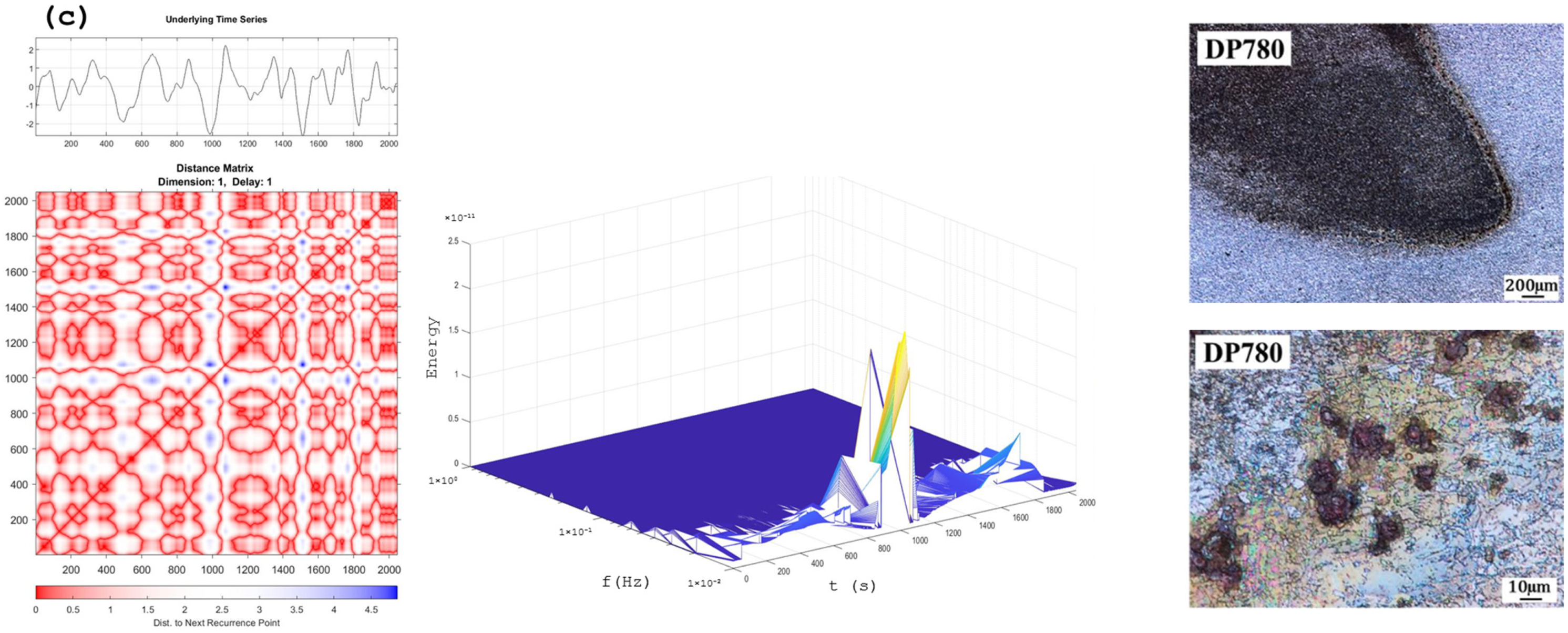
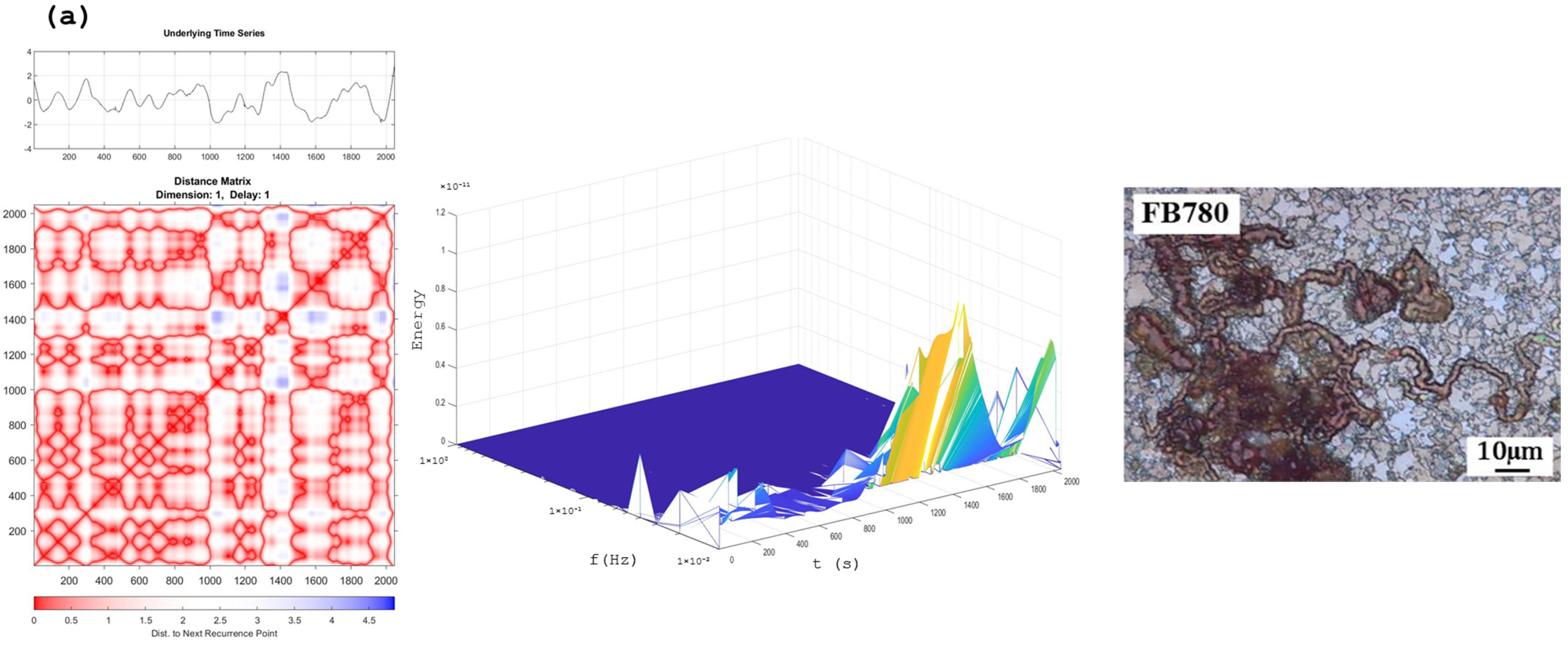
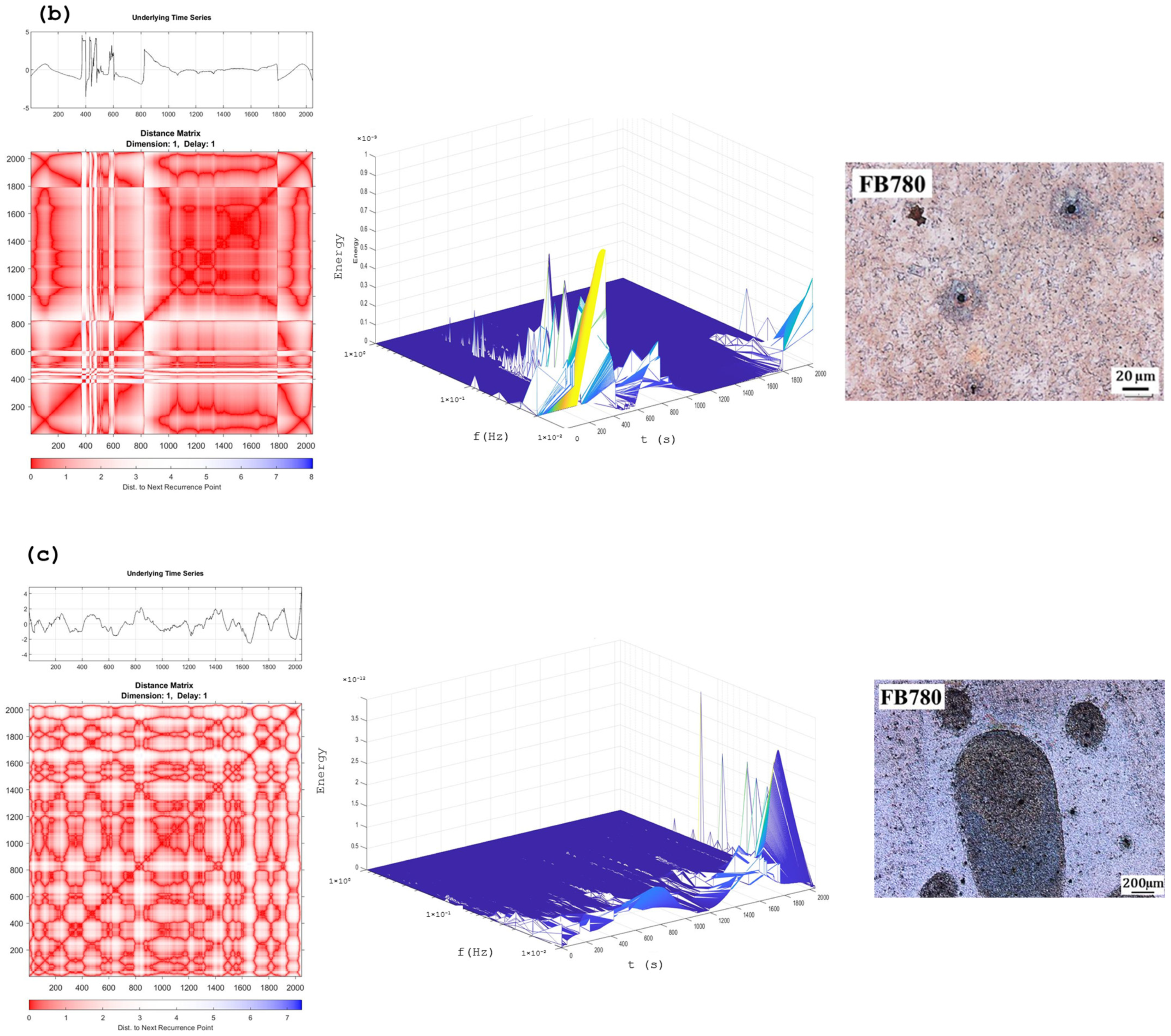
Recurrence Plots and Hilbert–Huang Transform
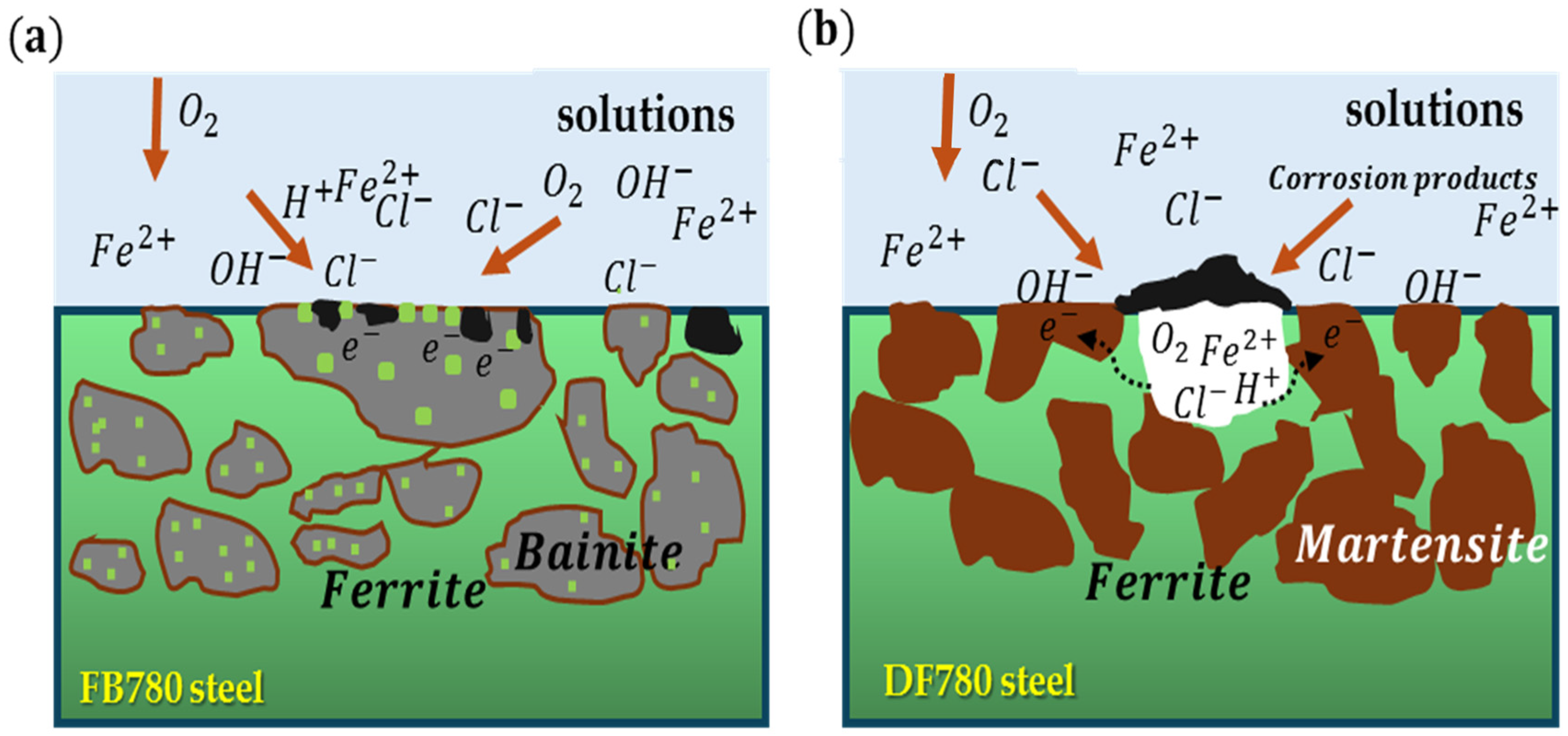
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamarelli, C.M. AHSS 101: The evolving use of advanced high-strength steel for automotive applications. Steel Mark. Dev. Inst. 2011, 1, 42. [Google Scholar]
- Montoya-Rangel, M.; de Garza-Montes, O.N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical noise measurements of advanced high-strength steels in different solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Keeler, S.; Kimchi, M. Advanced High-Strength Steels Application Guidelines Version 5.0; WorldAutoSteel: Brussels, Belgium, 2014. [Google Scholar]
- Available online: https://www.energy.gov/eere/vehicles/articles/autosteel-partnership-ahss-stamping-strain-rate-characterization-sheet-steel (accessed on 15 October 2024).
- Galán, J.; Samek, L.; Verleysen, P.; Verbeken, K.; Houbaert, Y. Advanced high strength steels for automotive industry. Rev. Metal. 2012, 48, 118–131. [Google Scholar] [CrossRef]
- Maffei, B.; Salvatore, W.; Valentini, R. Dual-phase steel rebars for high-ductile r.c. structures, Part 1: Microstructural and mechanical characterization of steel rebars. Eng. Struct. 2007, 29, 3325–3332. [Google Scholar] [CrossRef]
- Khan, A.S.; Baig, M.; Choi, S.H.; Yang, H.S.; Sun, X. Quasi-static and dynamic responses of advanced high strength steels: Experiments and modeling. Int. J. Plast. 2012, 30–31, 1–17. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.B.; Ray, K.K. Influence of bainite/martensite-content on the tensile properties of low carbon dual-phase steels. Mater. Sci. Eng. A 2008, 474, 270–282. [Google Scholar] [CrossRef]
- Qu, S.; Zhang, Y.; Pang, X.; Gao, K. Influence of temperature field on the microstructure of low carbon microalloyed ferrite-bainite dual-phase steel during heat treatment. Mater. Sci. Eng. A 2012, 536, 136–142. [Google Scholar] [CrossRef]
- Lesch, C.; Kwiaton, N.; Klose, F.B. Advanced High Strength Steels (AHSS) for Automotive Applications −Tailored Properties by Smart Microstructural Adjustments. Steel Res. Int. 2017, 88, 1700210. [Google Scholar] [CrossRef]
- Mintz, B. Hot dip galvanising of transformation induced plasticity and other intercritically annealed steels. Int. Mater. Rev. 2001, 46, 169–197. [Google Scholar] [CrossRef]
- Uzun, H.; Önal, E. Mechanical Properties and Corrosion Behaviors in 3.5% NaCl Solution of Grade-A and Dual-Phase Steels Welded by FCAW. Period. Eng. Nat. Sci. 2013, 1, 25–32. [Google Scholar] [CrossRef]
- Abedini, O.; Behroozi, M.; Marashi, P.; Ranjbarnodeh, E.; Pouranvari, M. Intercritical heat treatment temperature dependence of mechanical properties and corrosion resistance of dual phase steel. Mater. Res. 2019, 22, e20170969. [Google Scholar] [CrossRef]
- Schmitta, J.H.; Iungb, T. New developments of advanced high-strength steels for automotive applications. Comptes Rendus. Phys. 2018, 19, 641–656. [Google Scholar] [CrossRef]
- Keleştemur, O.; Yildiz, S. Effect of various dual-phase heat treatments on the corrosion behavior of reinforcing steel used in the reinforced concrete structures. Constr. Build. Mater. 2009, 23, 78–84. [Google Scholar] [CrossRef]
- Billur, E.; Karabulut, S.; Yılmaz, İ.Ö.; Erzincanlıoğlu, S.; Çelik, H.; Altınok, E.; Başer, T. Mechanical Properties of Trip Aided Bainitic Ferrite TBF Steels in Production and Service Conditions. Hittite J. Sci. Eng. 2018, 5, 231–237. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; Garza-Montes-de-Oca, N.F.; Gaona-Tiburcio, C.; Almeraya-Calderón, F. Corrosion mechanism of advanced high strength dual-phase steels by electrochemical noise analysis in chloride solutions. Mater. Today Commun. 2023, 35, 105663. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, X.; Li, J. Corrosion Resistance of the Welded Joint of Submarine Pipeline Steel with Ferrite Plus Bainite Dual-Phase Microstructure. Steel Res. Int. 2015, 86, 1260–1270. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, P.H.; Guan, Y.; Chen, Q.F.; Pu, S.K. The corrosion resistance of ultra-low carbon bainitic steel. Corros. Sci. 2009, 51, 954–961. [Google Scholar] [CrossRef]
- Mehdipour, M.; Naderi, R.; Markhali, B.P. Electrochemical study of effect of the concentration of azole derivatives on corrosion behavior of stainless steel in H2SO4. Prog. Org. Coat. 2014, 77, 1761–1767. [Google Scholar] [CrossRef]
- Xia, D.; Song, S.; Wang, J.; Shi, J.; Bi, H.; Gao, Z. Determination of corrosion types from electrochemical noise by phase space reconstruction theory. Electrochem. Commun. 2012, 15, 88–92. [Google Scholar] [CrossRef]
- Monticelli, C. Evaluation of Corrosion Inhibitors by Electrochemical Noise Analysis. J. Electrochem. Soc. 1992, 139, 706. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Jáquez-Muñoz, J.M.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olgui-Coca, J.; Lopez-Leon, L.D.; Estupiñán-López, F.; Lira-Martínez, A.; Gaona Tiburcio, C. Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions. Metals 2023, 13, 1510. [Google Scholar] [CrossRef]
- Suresh, G.U.; Kamachi, M.S. Electrochemical Noise Analysis of Pitting Corrosion of Type 304L Stainless Steel. Corrosion 2014, 70, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Homborg, A.M.; Cottis, R.A.; Mol, J.M.C. An integrated approach in the time, frequency and time-frequency domain for the identification of corrosion using electrochemical noise. Electrochim. Acta 2016, 222, 627–640. [Google Scholar] [CrossRef]
- Mansfeld, F.; Sun, Z. Technical Note: Localization Index Obtained from Electrochemical Noise Analysis. Corrosion 1999, 55, 915–918. [Google Scholar] [CrossRef]
- Nagiub, A.M. Electrochemical Noise Analysis for Different Green Corrosion Inhibitors for Copper Exposed to Chloride Media. Corros. Sci. 2017, 35, 201–210. [Google Scholar] [CrossRef]
- Ikpeseni, S.C.; Ameh, E.S. Effect of Temperature and Microstructure on the Corrosion Behaviour of a low Carbon Dual Phase Steel. Am. J. Eng. Res. 2017, 6, 01–07. [Google Scholar]
- Si, Y. Investigation of Galvanic Corrosion Behavior of Dual Phase Steel. ECS Trans. 2016, 72, 13. [Google Scholar] [CrossRef]
- Fushimi, K.; Yanagisawa, K.; Nakanishi, T.; Hasegawa, Y.; Kawano, T.; Kimura, M. Microelectrochemistry of dual-phase steel corroding in 0.1 M sulfuric acid. Electrochim. Acta 2013, 114, 83–87. [Google Scholar] [CrossRef]
- Gerengi, H.; Sen, N.; Uygur, I.; Kaya, E. Corrosion behavior of dual phase 600 and 800 steels in 3.5 wt.% NaCl environment. J. Adhes. Sci. Technol. 2020, 34, 903–915. [Google Scholar] [CrossRef]
- Park, I.J.; Kim, S.T.; Lee, I.S.; Park, Y.S.; Moon, M.B. A study on corrosion behavior of DP-type and TRIP-type cold rolled steel sheet. Mater. Trans. 2009, 50, 1440–1447. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, S.M.; Kang, M.; Lee, S.; Lee, Y.K. Pitting corrosion behavior in advanced high strength steels. J. Alloys Compd. 2015, 619, 205–210. [Google Scholar] [CrossRef]
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM G199-09; Standard Guide for Electrochemical Noise Measurement. ASTM International: West Conshohocken, PA, USA, 2014.
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Baltazar-Zamora, M.Á.; Estupinán-López, F.; Bautista-Margulis, R.G.; Cuevas-Rodríguez, J.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Corrosion of Titanium Alloys Anodized Using Electrochemical Techniques. Metals 2023, 13, 476. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Martínez-Ramos, C.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Estupinan-Lopez, F.; Landa-Ruiz, L.; Nieves-Mendoza, D.; Almeraya-Calderon, F. Electrochemical Noise Analysis: An Approach to the Effectivity of Each Method in Different Materials. Materials 2024, 17, 4013. [Google Scholar] [CrossRef] [PubMed]
- Almeraya-Calderon, F.; Villegas-Tovar, M.; Maldonado-Bandala, E.; Lara-Banda, M.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Nieves-Mendoza, D.; Lopez-Leon, L.D.; Jaquez-Muñoz, J.M.; Estupiñán-López, F.; et al. Use of Electrochemical Noise for the Study of Corrosion by Passivated CUSTOM 450 and AM 350 Stainless Steels. Metals 2024, 14, 341. [Google Scholar] [CrossRef]
- Thewlis, G. Classification and quantification of microstructures in steels. J. Mater. Sci. Technol. 2004, 20, 143–160. [Google Scholar] [CrossRef]
- Kang, Y.; Han, Q.; Zhao, X.; Cai, M. Influence of nanoparticle reinforcements on the strengthening mechanisms of an ultrafine-grained dual phase steel containing titanium. Mater. Des. 2013, 44, 331–339. [Google Scholar] [CrossRef]
- Martínez-Villafañe, A.; Almeraya-Calderón, F.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.; Porcayo-Calderón, J. High-Temperature Degradation and Protection of Ferritic and Austenitic Steels in Steam Generators. J. Mater. Eng. Perform. 1998, 7, 108–113. [Google Scholar] [CrossRef]
- Martínez-Aparicio, B.; Gaona-Tiburcio, C.; Almeraya-Calderon, F.; Goldsberry, R.; Castaneda, H. Evaluation of Passive Films on 17-7PH and 410 Stainless Steel Exposed to NaCl Solution. Materials 2024, 17, 4060. [Google Scholar] [CrossRef]
- Kwon, O.; Lee, K.; Kim, G.; Chin, K.-G. New Trends in Advanced High Strength Steel Developments For Automotive Application. Mat. Sci. Forum. 2010, 638–642, 136–141. [Google Scholar] [CrossRef]
- Aydin, K.; Essadiqi, E.; Yue, S. Development of 3rd generation AHSS with medium Mn content alloying compositions. Mater. Sci. Eng. A. 2013, 564, 501–508. [Google Scholar] [CrossRef]
- Saeidi, N.; Ekrami, A. Comparison of mechanical properties of martensite/ferrite and bainite/ferrite dual phase 4340 steels. Mater. Sci. Eng. A 2009, 523, 125–129. [Google Scholar] [CrossRef]
- Homborg, A.M.; Oonincx, P.J.; Mol, J.M.C. Wavelet Transform Modulus Maxima and Holder Exponents Combined with Transient Detection for the Differentiation of Pitting Corrosion Using Electrochemical Noise. Corrosion 2018, 74, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Brockwell, P.J.; Davis, R.A. Introduction to Time Series and Forecasting; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Cao, F.; Gao, Z.; Zhang, J.; Cao, C. Analysis of Pitting Corrosion Behavior of Pure Al in Sodium Chloride Solution with the Wavelet Technique. J. Electroanal. Chem. 2005, 578, 143–150. [Google Scholar] [CrossRef]
- Homborg, A.M.; van Westing, E.P.M.; Tinga, T.; Zhang, X.; Oonincx, P.J.; Ferrari, G.M.; de Wit, J.H.W.; Mol, J.M.C. Novel Time–Frequency Characterization of Electrochemical Noise Data in Corrosion Studies Using Hilbert Spectra. Corros. Sci. 2013, 66, 97–110. [Google Scholar] [CrossRef]
- Legat, A.; Dolecek, V. Corrosion Monitoring System Based on Measurement and Analysis of Electrochemical Noise. Corrosion 1995, 51, 295–300. [Google Scholar] [CrossRef]
- Coakley, J.; Vorontsov, V.A.; Littrell, K.C.; Heenan, R.K.; Ohnuma, M.; Jones, N.G.; Dye, D. Nanoprecipitation in a Beta-Titanium Alloy. J. Alloys Compd. 2015, 623, 146–156. [Google Scholar] [CrossRef]
- Botona Pedemonte, F.J.; Aballe Villero, A.; Marcos Bárcena, M. Ruido Electroquímico. Métodos de Análisis; Septem Ediciones, S.L.: Oviedo, Spain, 2002; ISBN 84-95687-33-X. [Google Scholar]
- Martínez-Ramos, C.; Olguin-Coca, J.; Lopez-Leon, L.D.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Castañeda-Robles, I.; Jaquez-Muñoz, J.M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals 2023, 13, 1850. [Google Scholar] [CrossRef]
- Cappeln, F.; Bjerrum, N.J.; Petrushina, I.M. Electrochemical Noise Measurements of Steel Corrosion in the Molten NaCl-K[Sub 2]SO[Sub 4] System. J. Electrochem. Soc. 2005, 152, B228. [Google Scholar] [CrossRef][Green Version]
- Calabrese, L.; Galeano, M.; Proverbio, E. Identifying Corrosion Forms on Synthetic Electrochemical Noise Signals by the Hilbert–Huang Transform Method. Corros. Eng. Sci. Technol. 2018, 53, 492–501. [Google Scholar] [CrossRef]
- Bertocci, U.; Huet, F.; Nogueira, R.P.; Rousseau, P. Drift Removal Procedures in the Analysis of Electrochemical Noise. Corrosion 2002, 58, 337–347. [Google Scholar] [CrossRef]
- Homborg, A.M.; Tinga, T.; Van Westing, E.P.M.; Zhang, X.; Ferrari, G.M.; De Wit, J.H.W.; Mol, J.M.C. A Critical Appraisal of the Interpretation of Electrochemical Noise for Corrosion Studies. Corrosion 2014, 70, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Enestam, S.; Bankiewicz, D.; Tuiremo, J.; Mäkelä, K.; Hupa, M. Are NaCl and KCl equally corrosive on superheater materials of steam boilers? Fuel 2013, 104, 294–306. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Shibaeva, T.V.; Laurinavichyute, V.K.; Tsirlina, G.A.; Arsenkin, A.M.; Grigorovich, K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Zhang, Z.; Cao, F.H.; Zhang, J.Q. Dimensional analysis applied to pitting corrosion measurements. Electrochim. Acta 2008, 53, 2688–2698. [Google Scholar] [CrossRef]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Processing Route Effects on the Mechanical and Corrosion Properties of Dual Phase Steel. Met. Mater. Int. 2020, 26, 882–890. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Mendez-Ramirez, C.T.; Carrera-Ramirez, M.G.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Lara-Banda, M.; Estupiñan-Lopez, F.; Nieves-Mendoza, D.; Almeraya-Calderon, F. Corrosion of Anodized Titanium Alloys. Coatings 2024, 14, 809. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Vinaya; Madhusudhan, R.; Sah, R.; Manjini, S. Mechanical and Electrochemical Behavior of Dual-Phase Steels Having Varying Ferrite Martensite Volume Fractions. J. Mater. Eng. Perform. 2019, 28, 3600–3613. [Google Scholar] [CrossRef]
- Haisch, T.; Mittemeijer, E.J.; Schultze, J.W. On the influence of microstructure and carbide content of steels on the electrochemical dissolution process in aqueous NaCl electrolytes. Mater. Corros. 2002, 53, 740–755. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Yang, F.; Chen, H.; Liang, N.; Wu, Y.; Zhang, J.; Ma, H.; Klu, E.E.; Gao, B.; et al. Corrosion Behavior and Mechanism of Cr–Mo Alloyed Steel: Role of Ferrite/Bainite Duplex Microstructure. J. Alloys Compd. 2019, 809, 151787. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Zhao, X.; Dong, C.; Kang, S. Effects of Nucleation Site and Morphology of Carbide-Free Bainite on Microstructures and Properties of Bainite/Martensite Multi-Phase Steels. Mater. Sci. Eng. A 2019, 744, 86–93. [Google Scholar] [CrossRef]
- Qu, S.; Pang, X.; Wang, Y.; Gao, K. Corrosion Behavior of Each Phase in Low Carbon Microalloyed Ferrite–Bainite Dual-Phase Steel: Experiments and Modeling. Corros. Sci. 2013, 75, 67–77. [Google Scholar] [CrossRef]
- Neetu; Katiyar, P.K.; Sangal, S.; Mondal, K. Effect of Various Phase Fraction of Bainite, Intercritical Ferrite, Retained Austenite and Pearlite on the Corrosion Behavior of Multiphase Steels. Corros. Sci. 2021, 178, 109043. [Google Scholar] [CrossRef]
- Xi, Y.; Xie, Z. Corrosion Effects of Magnesium Chloride and Sodium Chloride on Automobile Components; Colorado Department of Transportation: Denver, CO, USA, 2002; pp. 1–91.
- Kristen, D.; Jungert, D.; Gibson, S. Brine Corrosion Research Study; Corrpro Canada Inc.: Calgary, AB, Canada, 2019. [Google Scholar]
- Lee, C.C.; Mansfeld, F. Analysis of Electrochemical Noise Data for a Passive System in the Frequency Domain. Corros. Sci. 1998, 40, 959–962. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.Z.; Behnamian, Y. Detection of Corrosion Degradation Using Electrochemical Noise (EN): Review of Signal Processing Methods for Identifying Corrosion Forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Xia, D.H.; Qin, Z.; Song, S.; Macdonald, D.; Luo, J.L. Combating Marine Corrosion on Engineered Oxide Surface by Repelling, Blocking and Capturing Cl−: A Mini Review. Corros. Commun. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Pan, C.; Wang, X.; Behnamian, Y.; Wu, Z.; Qin, Z.; Xia, D.H.; Hu, W. Monododecyl Phosphate Film on LY12 Aluminum Alloy: PH-Controlled Self-Assembly and Corrosion Resistance. J. Electrochem. Soc. 2020, 167, 161510. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, M.K.; Jung, G.C.; Vang, M.S.; Park, Y.J. The Effects of Spark Anodizing Treatment of Pure Titanium Metals and Titanium Alloys on Corrosion Characteristics. Surf. Coatings Technol. 2007, 201, 8738–8745. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Jáquez-Muñoz, J.M.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Lara-Banda, M.; Lira-Martinez, M.A.; Reyes-Blas, H.; Baltazar-Zamora, M.Á.; Landa-Ruiz, L.; Lopez-Leon, L.D.; et al. Corrosion Behavior of Titanium Alloys (Ti CP2, Ti-6Al-2Sn-4Zr-2Mo, Ti-6Al-4V and Ti Beta-C) with Anodized and Exposed in NaCl and H2SO4 Solutions. Metals 2024, 14, 160. [Google Scholar] [CrossRef]
- MacDonald, D.D. The History of the Point Defect Model for the Passive State: A Brief Review of Film Growth Aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Macdonald, D.D. The Role of Determinism in the Prediction of Corrosion Damage. Corros. Mater. Degrad. 2023, 4, 212–273. [Google Scholar] [CrossRef]
- Chen, A.; Cao, F.; Liao, X.; Liu, W.; Zheng, L.; Zhang, J.; Cao, C. Study of Pitting Corrosion on Mild Steel during Wet–Dry Cycles by Electrochemical Noise Analysis Based on Chaos Theory. Corros. Sci. 2013, 66, 183–195. [Google Scholar] [CrossRef]
- Lafront, A.M.; Safizadeh, F.; Ghali, E.; Houlachi, G. Study of the Copper Anode Passivation by Electrochemical Noise Analysis Using Spectral and Wavelet Transforms. Electrochim. Acta 2010, 55, 2505–2512. [Google Scholar] [CrossRef]
- Curioni, M.; Skeldon, P.; Koroleva, E.; Thompson, G.E.; Ferguson, J. Role of Tartaric Acid on the Anodizing and Corrosion Behavior of AA 2024 T3 Aluminum Alloy. J. Electrochem. Soc. 2009, 156, C147. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Liao, Y.; Chen, X.; Zhang, C.; Wu, H.; Wang, Z.; Huang, W. Effect of Anodizing Parameters on Film Morphology and Corrosion Resistance of AA2099 Aluminum-Lithium Alloy. J. Electrochem. Soc. 2016, 163, C369–C376. [Google Scholar] [CrossRef]
- Li, J.; Du, C.W.; Liu, Z.Y.; Li, X.G.; Liu, M. Effect of Microstructure on the Corrosion Resistance of 2205 Duplex Stainless Steel. Part 2: Electrochemical Noise Analysis of Corrosion Behaviors of Different Microstructures Based on Wavelet Transform. Constr. Build. Mater. 2018, 189, 1294–1302. [Google Scholar] [CrossRef]
- Jáquez-Munõz, J.M.; Gaona-Tiburcio, C.; Cabral, J.A.; Lara-Banda, M.; Estupinãn-López, F.H.; Zambrano, P.; Almeraya-Calderón, F. Corrosion Behavior of Titanium Alloys Using Electrochemical Noise. ECS Trans. 2021, 101, 167. [Google Scholar] [CrossRef]
- Arellano-Pérez, J.H.; Escobar-Jiménez, R.F.; Granados-Lieberman, D.; Gómez-Aguilar, J.F.; Uruchurtu-Chavarín, J.; Alvarado-Martínez, V.M. Electrochemical Noise Signals Evaluation to Classify the Type of Corrosion Using Synchrosqueezing Transform. J. Electroanal. Chem. 2019, 848, 113249. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; Estupinán-López, F.; López-León, L.D.; Chacón-Nava, J.; Almeraya-Calderón, F. Frequency Analysis of Transients in Electrochemical Noise of Superalloys Waspaloy and Ultimet. Metals 2021, 11, 702. [Google Scholar] [CrossRef]
- Xia, D.; Mao, Y.; Zhu, Y.; Yuan, Q.; Deng, C.; Hu, W. A novel approach used to study the corrosion susceptibility of metallic materials at a dynamic seawater/air interface. Corros. Commun. 2022, 6, 62–66. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, Y.; Zhang, B.; Behnamian, Y.; Xia, D.; Hu, W. A review of micro-scale and atomic-scale corrosion mechanisms of the second phases in aluminum alloys. Trans. Nonferrous Met. Soc. China 2021, 31, 3205–3227. [Google Scholar] [CrossRef]

| Element | DP780 | FB780 |
|---|---|---|
| Fe | Balance | Balance |
| C | 0.10 | 0.09 |
| Mn | 2.61 | 1.73 |
| Cr | 0.420 | 0.640 |
| Mo | - | 0.006 |
| Si | 0.510 | 0.300 |
| Ti | 0.080 | 0.021 |
| Alloy | Electrolyte | Zn0 (Ω·cm2) |
|---|---|---|
| DP780 | NaCl | 918 ± 24 |
| MgCl2 | 441 ± 16 | |
| CaCl2 | 825 ± 28 | |
| FB780 | NaCl | 409 ± 21 |
| MgCl2 | 502 ± 14 | |
| CaCl2 | 432 ± 17 |
| Alloy | Electrolyte | RR | DET | L | TT |
|---|---|---|---|---|---|
| DP780 | NaCl | 0.0562 ± 0.0002 | 0.9769 ± 0.001 | 5.06 ± 0.02 | 6.51 ± 0.05 |
| MgCl2 | 0.0592 ± 0.0001 | 0.9831 ± 0.0002 | 6.28 ± 0.09 | 8.16 ± 0.09 | |
| CaCl2 | 0.0589 ± 0.0004 | 0.9844 ± 0.0004 | 5.04 ± 0.005 | 6.48 ± 0.07 | |
| FB780 | NaCl | 0.0593 ± 0.0002 | 0.987 ± 0.0008 | 6.26 ± 0.008 | 8.12 ± 0.05 |
| MgCl2 | 0.0823 ± 0.0001 | 0.9391 ± 0.0001 | 10.07 ± 0.03 | 13.92 ± 0.2 | |
| CaCl2 | 0.06 ± 0.004 | 0.9142 ± 0.0001 | 4.17 ± 0.08 | 5.45 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeraya-Calderón, F.; Montoya-Rangel, M.; Nieves-Mendoza, D.; Jáquez-Muñoz, J.M.; Baltazar-Zamora, M.A.; Landa-Ruiz, L.; Lara-Banda, M.; Maldonado-Bandala, E.; Estupiñan-Lopez, F.; Gaona-Tiburcio, C. Frequency–Time Domain Analysis Based on Electrochemical Noise of Dual-Phase (DP) and Ferrite–Bainite (FB) Steels in Chloride Solutions for Automotive Applications. Metals 2024, 14, 1208. https://doi.org/10.3390/met14111208
Almeraya-Calderón F, Montoya-Rangel M, Nieves-Mendoza D, Jáquez-Muñoz JM, Baltazar-Zamora MA, Landa-Ruiz L, Lara-Banda M, Maldonado-Bandala E, Estupiñan-Lopez F, Gaona-Tiburcio C. Frequency–Time Domain Analysis Based on Electrochemical Noise of Dual-Phase (DP) and Ferrite–Bainite (FB) Steels in Chloride Solutions for Automotive Applications. Metals. 2024; 14(11):1208. https://doi.org/10.3390/met14111208
Chicago/Turabian StyleAlmeraya-Calderón, Facundo, Marvin Montoya-Rangel, Demetrio Nieves-Mendoza, Jesús Manuel Jáquez-Muñoz, Miguel Angel Baltazar-Zamora, Laura Landa-Ruiz, Maria Lara-Banda, Erick Maldonado-Bandala, Francisco Estupiñan-Lopez, and Citlalli Gaona-Tiburcio. 2024. "Frequency–Time Domain Analysis Based on Electrochemical Noise of Dual-Phase (DP) and Ferrite–Bainite (FB) Steels in Chloride Solutions for Automotive Applications" Metals 14, no. 11: 1208. https://doi.org/10.3390/met14111208
APA StyleAlmeraya-Calderón, F., Montoya-Rangel, M., Nieves-Mendoza, D., Jáquez-Muñoz, J. M., Baltazar-Zamora, M. A., Landa-Ruiz, L., Lara-Banda, M., Maldonado-Bandala, E., Estupiñan-Lopez, F., & Gaona-Tiburcio, C. (2024). Frequency–Time Domain Analysis Based on Electrochemical Noise of Dual-Phase (DP) and Ferrite–Bainite (FB) Steels in Chloride Solutions for Automotive Applications. Metals, 14(11), 1208. https://doi.org/10.3390/met14111208










