Enhancing the Strength and Toughness of A356.2-0.15Fe Aluminum Alloy by Trace Mn and Mg Co-Addition
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
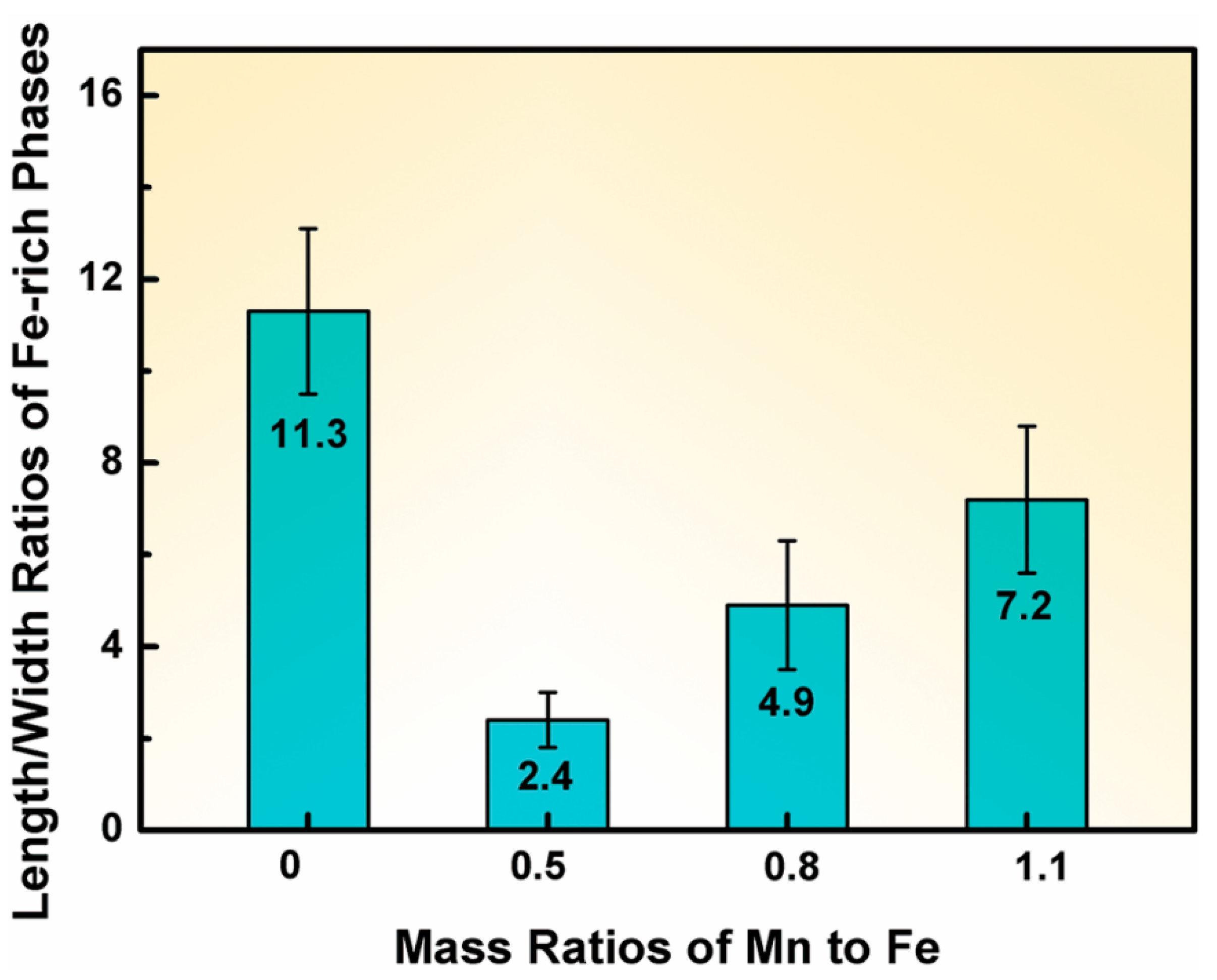
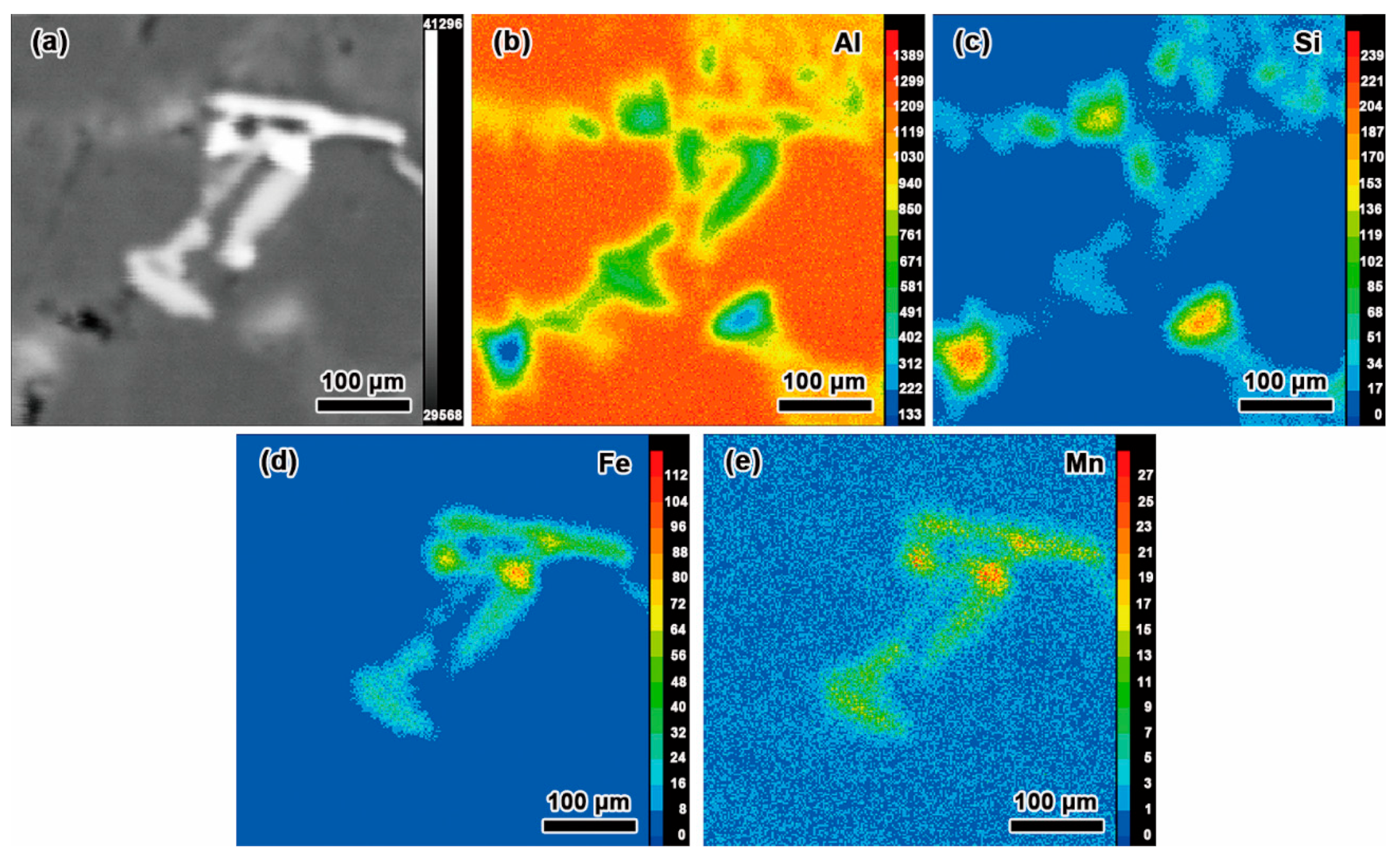
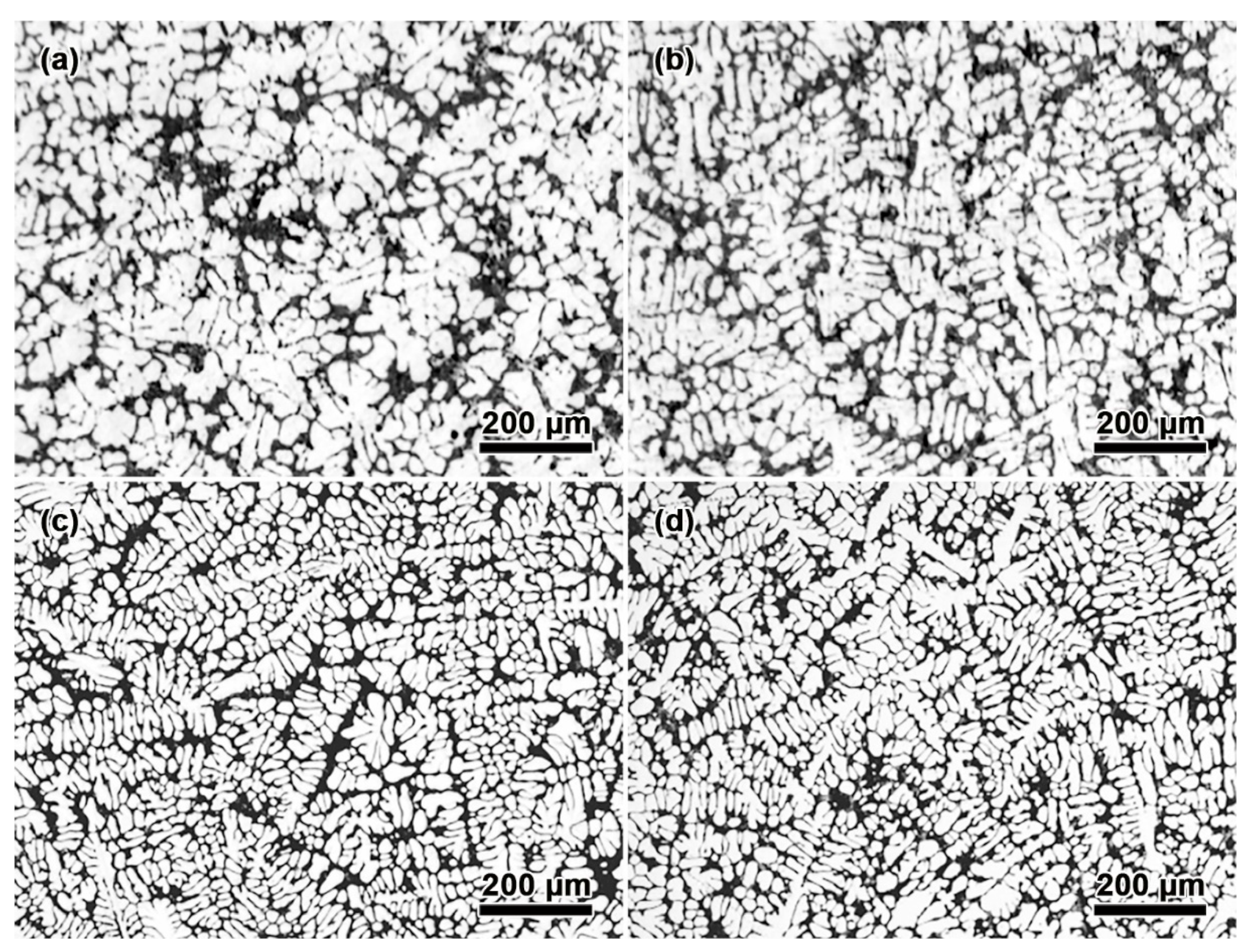
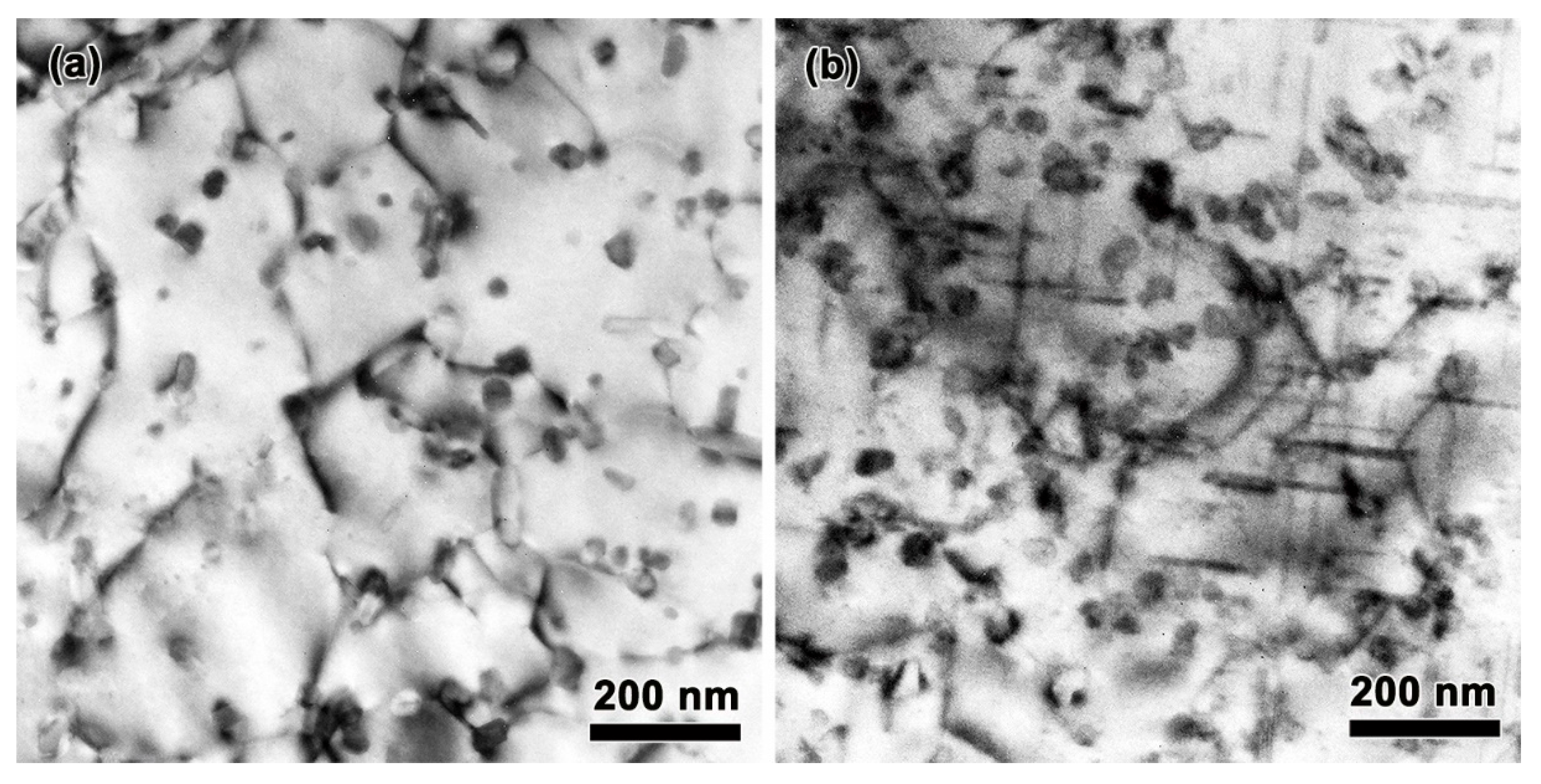
3.1. Effect of Mn and Mg Co-Addition on Microstructure of A356.2-0.15Fe Alloys
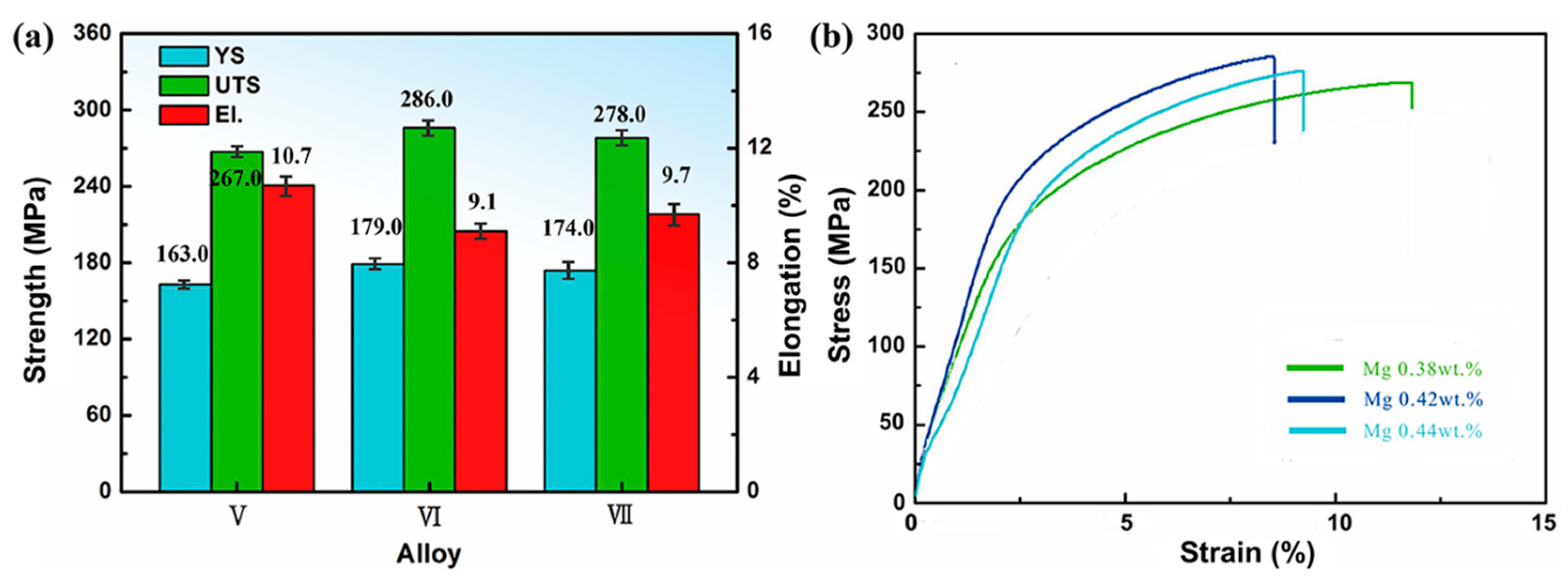
3.2. Effect of Mn and Mg Co-Addition on Tensile Properties of T6 Treated A356.2-0.15Fe Alloys
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, H.H.; Xu, J.J.; Yuan, X.; Ming, W.Q.; Zhang, Z.; Chen, J.H. The ill-defined π(AlFeMgSi) phase intermetallics formed in an automotive Al-Si-Mg alloy. Mater. Charact. 2023, 199, 112839. [Google Scholar] [CrossRef]
- Wazeer, A.; Das, A.; Abeykoon, C.; Sinha, A.; Karmakar, A. Composites for electric vehicles and automotive sector: A review. Green Energy Intell. Transp. 2023, 2, 100043. [Google Scholar] [CrossRef]
- Niu, G.; Wang, J.; Ye, J.; Mao, J. Enhancing Fe content tolerance in A356 alloys for achieving low carbon footprint aluminum structure castings. J. Mater. Sci. Technol. 2023, 161, 180–191. [Google Scholar] [CrossRef]
- Tuncay, T.; Bayoglu, S. The effect of iron content on microstructure and mechanical properties of A356 cast alloy. Met. Mater. Trans. B 2017, 48, 794–804. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, D.; Wang, H.; Jia, Y.; Lin, B.; Tang, Y.; Tang, Y.; Shu, D.; Sun, Z.; Fu, Y.; et al. Revealing the influence of Fe on Fe-rich phases formation and mechanical properties of cast Al-Mg-Mn-Fe alloys. J. Alloys Compd. 2022, 901, 163666. [Google Scholar] [CrossRef]
- Wang, Q.G. Microstructural effects on the tensile and fracture behavior of aluminum casting alloys A356/357. Metall Mater. Trans. A. 2003, 34A, 2887–2899. [Google Scholar] [CrossRef]
- Rathod, N.; Menghani, J. Detrimental effect of Fe as impurity on A356/TiB2+RE+Sn alloy fabricate via in situ route. Mater. Today Proc. 2021, 44, 1326–1330. [Google Scholar] [CrossRef]
- Liu, C.; Jiao, X.; Nishat, H.; Akhtar, S.; Wiesner, S.; Guo, Z.; Xiong, S. Characteristics of Fe-rich intermetallics compounds and their influence on the cracking behavior of a newly developed high-pressure die cast Al–4Mg–2Fe alloy. J. Alloys Compd. 2021, 854, 157121. [Google Scholar] [CrossRef]
- Kucharikova, L.; Medvecka, D.; Tillova, E.; Belan, J.; Kritikos, M.; Chalupova, M.; Uhricik, M. The effect of the beta-Al5FeSi phases on microstructure, mechanical and fatigue properties in A356.0 cast alloys with higher Fe content without additional alloying of Mn. Materials 2021, 14, 1943. [Google Scholar] [CrossRef]
- Knap, V.; Švecová, I.; Tillová, E.; Kuchariková, L. Influence of iron content on SDAS factor, Al5FeSi intermetallic phases and porosity of the secondary aluminum alloy AlSi7Mg0.6 used in the automotive industry. Transp. Res. Procedia 2021, 55, 814–820. [Google Scholar] [CrossRef]
- Dhinakar, A.; Lu, P.Y.; Chen, J.K.; Tang, N.K. Iron Reduction in 356 Secondary Aluminum Alloy by Mn and Cr Addition for Sediment Separation. Int. J. Metalcast. 2021, 15, 182–192. [Google Scholar] [CrossRef]
- Gao, T.; Li, Z.; Zhang, Y.; Qin, J.; Liu, X. Evolution of Fe–rich phases in Mg melt and a novel method for separating Al and Fe from Al–Si–Fe alloys. Mater. Des. 2017, 134, 71–80. [Google Scholar] [CrossRef]
- Luo, Q.; Cong, M.; Li, H.; Zhu, L.; Chen, H.; Li, Q. Mechanism of Fe removal by Sn addition in Al-7Si-1Fe alloy. J. Alloys Compd. 2023, 948, 169724. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; Xu, R.; Xiao, H.; Zhang, W.; Li, S. Effects of vanadium on modification of iron-rich intermetallics and mechanical properties in A356 cast alloys with 1.5 wt.% Fe. J. Mater. Eng. Perform. 2019, 28, 475–484. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Tezuka, H.; Kobayashi, E.; Sato, T. Effects of the Mn/Fe ratio and cooling rate on the modification of Fe intermetallic compounds in cast A356 based alloy with different Fe contents. Mater. Trans. 2013, 54, 1484–1490. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Arribas, M.; Galarraga, H.; Garcia de Cortazar, M.; Ellero, M.; Girot, F. Effects of Mn addittion, cooling rate and holding temperature on the modification and purification of iron-rich compounds in AlSi10MnMg(Fe) alloy. Heliyon 2023, 9, e13005. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhao, Y.; Jia, Y.; Huang, G.; Zhang, Z.; Zhou, N.; Li, X.; Zheng, K.; Fu, Y.; Zhang, W. Effect of B addition on the formation of Fe-rich phases in Al-Si-Fe alloys. J. Alloys Compd. 2023, 930, 167426. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, J.; Wang, T.; Chen, J.; He, K. The effects of neodymium addition on the intermetallic microstructure and mechanical properties of Al-7Si-0.3Mg-0.3Fe alloys. J. Alloys Compd. 2018, 741, 161–173. [Google Scholar] [CrossRef]
- Wan, B.; Chen, W.; Liu, L.; Cao, X.; Zhou, L.; Fu, Z. Effect of trace yttrium addition on the microstructure and tensile properties of recycled Al–7Si–0.3Mg–1.0Fe casting alloys. Mater. Sci. Eng. A Struct. 2016, 666, 165–175. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Wang, J.; Li, Q.; Liu, K.; Zhang, M. Uncovering the effects of Ce and superheat temperature on Fe-rich intermetallic and microporosity formation in aluminum alloy. Mater. Charact. 2022, 193, 112226. [Google Scholar] [CrossRef]
- Kim, H.Y.; Han, S.W.; Lee, H.M. The influence of Mn and Cr on the tensile properties of A356–0.20Fe alloy. Mater. Lett. 2006, 60, 1880–1883. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, H.; Yu, C.; Wu, Z.; Guo, C.; Nagaumi, H.; Zhu, K.; Li, B.; Cui, J. Investigating the influences of Fe, Mn and Mo additions on the evolution of microstructure and mechanical performances of Al–Si–Mg cast alloys. J. Mater. Res. Technol. 2023, 25, 319–332. [Google Scholar] [CrossRef]
- Song, D.; Zhao, Y.; Jia, Y.; Li, X.; Fu, Y.; Zhang, W. Synergistic effects of Mn and B on iron-rich intermetallic modification of recycled Al alloy. J. Mater. Res. Technol. 2023, 24, 527–541. [Google Scholar] [CrossRef]
- Kuang, W.W.; Wang, H.F.; Li, X.; Zhang, J.B.; Zhou, Q.; Zhao, Y.H. Application of the thermodynamic extremal principle to diffusion-controlled phase transformations in Fe-C-X alloys: Modeling and applications. Acta Mater. 2018, 159, 16–30. [Google Scholar] [CrossRef]
- Tian, X.L.; Zhao, Y.H.; Gu, T.; Guo, Y.L.; Xu, F.Q.; Hou, H. Cooperative effect of strength and ductility processed by thermomechanical treatment for Cu-Al-Ni alloy. Mater. Sci. Eng. A Struct. 2022, 849, 11. [Google Scholar] [CrossRef]
- Wang, L.W.; Wu, T.; Wang, D.L.; Liang, Z.M.; Yang, X.; Peng, Z.Z.; Liu, Y.; Liang, Y.M.; Zeng, Z.; Oliveira, J.P. A novel heterogeneous multi-wire indirect arc directed energy deposition for in-situ synthesis Al-Zn-Mg-Cu alloy: Process, microstructure and mechanical properties. Addit. Manuf. 2023, 72, 16. [Google Scholar] [CrossRef]
- Ma, G.; Li, R.; Li, R. Effect of Mg2Si particles on low-temperature fracture behavior of A356 alloy. Mater. Sci. Eng. A Struct. 2016, 674, 666–671. [Google Scholar] [CrossRef]
- Salleh, M.S.; Omar, M.Z.; Syarif, J. The effects of Mg addition on the microstructure and mechanical properties of thixoformed Al–5%Si–Cu alloys. J. Alloys Compd. 2015, 621, 121–130. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takahashi, M.; Kamikubo, Y.; Sugiura, Y.; Iwasawa, S.; Nakata, T.; Kamado, S. Effect of Mg content on age-hardening response, tensile properties, and microstructures of a T5-treated thixo-cast hypoeutectic Al–Si alloy. Mater. Sci. Eng. A 2020, 798, 140089. [Google Scholar] [CrossRef]
- Kang, J.; Su, R.; Wu, D.Y.; Liu, C.H.; Li, T.; Wang, L.S.; Narayanaswamy, B. Synergistic effects of Ce and Mg on the microstructure and tensile properties of Al-7Si-0.3Mg-0.2Fe alloy. J. Alloys Compd. 2019, 796, 267–278. [Google Scholar] [CrossRef]
- Alhawari, K.S.; Omar, M.Z.; Ghazali, M.J.; Salleh, M.S.; Mohammed, M.N. Microstructural evolution during semisolid processing of Al–Si–Cu alloy with different Mg contents. Trans. Nonferr. Metal. Soc. 2017, 27, 1483–1497. [Google Scholar] [CrossRef]
- Vandersluis, E.; Ravindran, C. Comparison of measurement methods for secondary dendrite arm spacing. Metallogr. Microstruc. 2017, 6, 89–94. [Google Scholar] [CrossRef]
- ASTM E1245-03; Standard Practice for Determining the Inclusion or Second-Phase Constituent Content of Metals by Automatic Image Analysis. ASTM: West Conshohocken, PA, USA, 2016.
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. SAC: Beijing, China, 2021.
- Murali, S.; Row, T.N.G.; Sastry, D.H.; Raman, K.S.; Murthy, K.S.S. Crystal-structure of Beta-FeSiAl5 and (Be-Fe)-BeSiFe2Al8 phases. Scr. Met. Mater. 1994, 31, 267–271. [Google Scholar] [CrossRef]
- Terzi, S.; Taylor, J.A.; Cho, Y.H.; Salvo, L.; Suéry, M.; Boller, E.; Dahle, A.K. In situ study of nucleation and growth of the irregular α-Al/β-Al5FeSi eutectic by 3-D synchrotron X-ray microtomography. Acta Mater. 2010, 58, 5370–5380. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, T.Y.; Han, S.W.; Lee, H.M. Effects of Mn on the crystal structure of α-Al(Mn,Fe)Si particles in A356 alloys. J. Cryst. Growth 2006, 291, 207–211. [Google Scholar] [CrossRef]
- Ma, Z.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. A study of tensile properties in Al–Si–Cu and Al–Si–Mg alloys: Effect of β-iron intermetallics and porosity. Mater. Sci. Eng. A Struct. 2008, 490, 36–51. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, F.; Dong, B.; Yang, H.; Shu, S.; Zha, M.; Jiang, Q. Investigation of the influences of ternary Mg addition on the solidification microstructure and mechanical properties of as-cast Al–10Si alloys. Mater. Sci. Eng. A Struct. 2020, 798, 140247. [Google Scholar] [CrossRef]
- Eshaghi, A.; Ghasemi, H.M.; Rassizadehghani, J. Effect of heat treatment on microstructure and wear behavior of Al-Si alloys with various iron contents. Mater. Des. 2011, 32, 1520–1525. [Google Scholar] [CrossRef]
- Yang, M.; Orekhov, A.; Hu, Z.; Feng, M.; Jin, S.; Sha, G.; Li, K.; Samaee, V.; Song, M.; Du, Y.; et al. Shearing and rotation of β″ and β′ precipitates in an Al-Mg-Si alloy under tensile deformation: In-situ and ex-situ studies. Acta Mater. 2021, 220, 117310. [Google Scholar] [CrossRef]

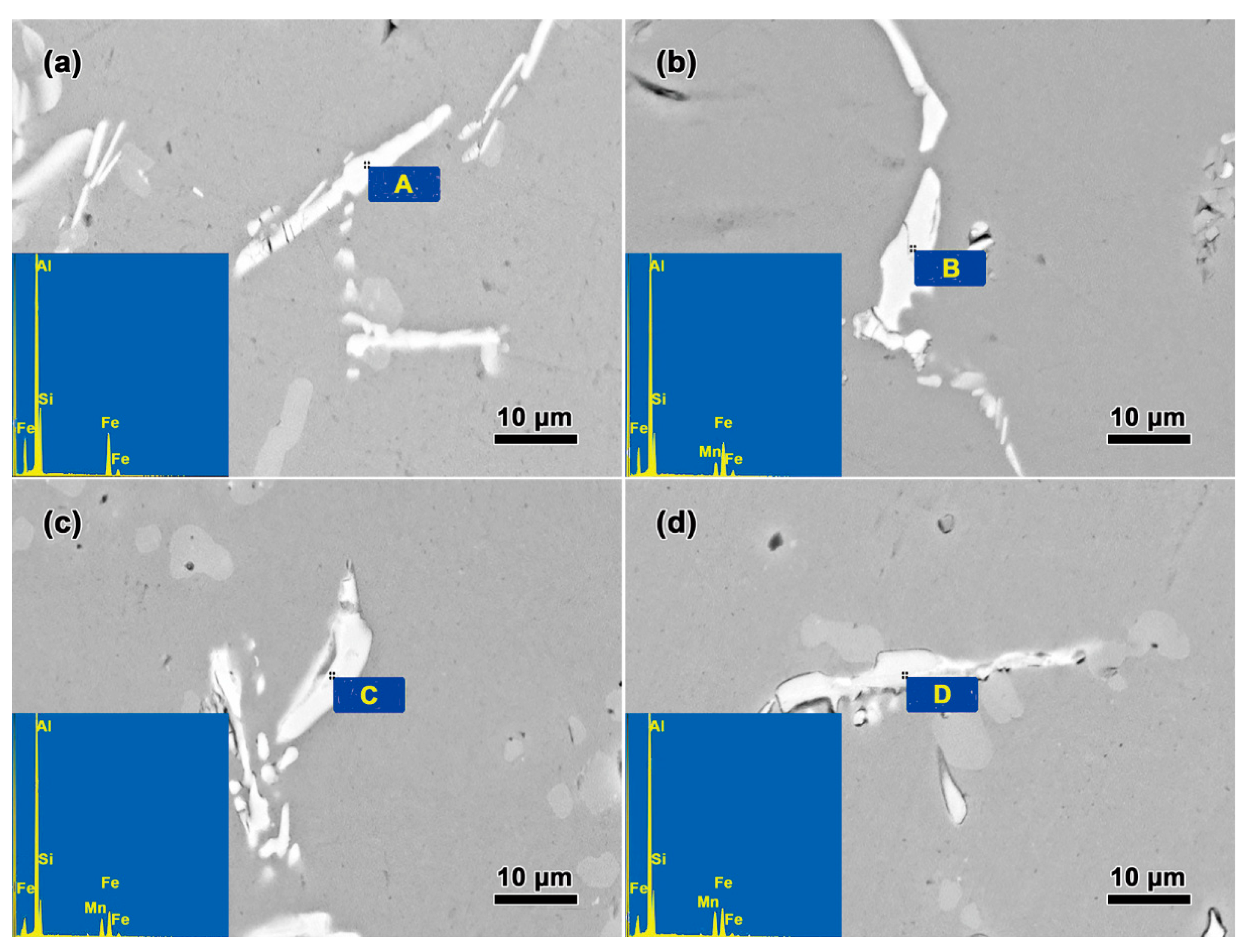
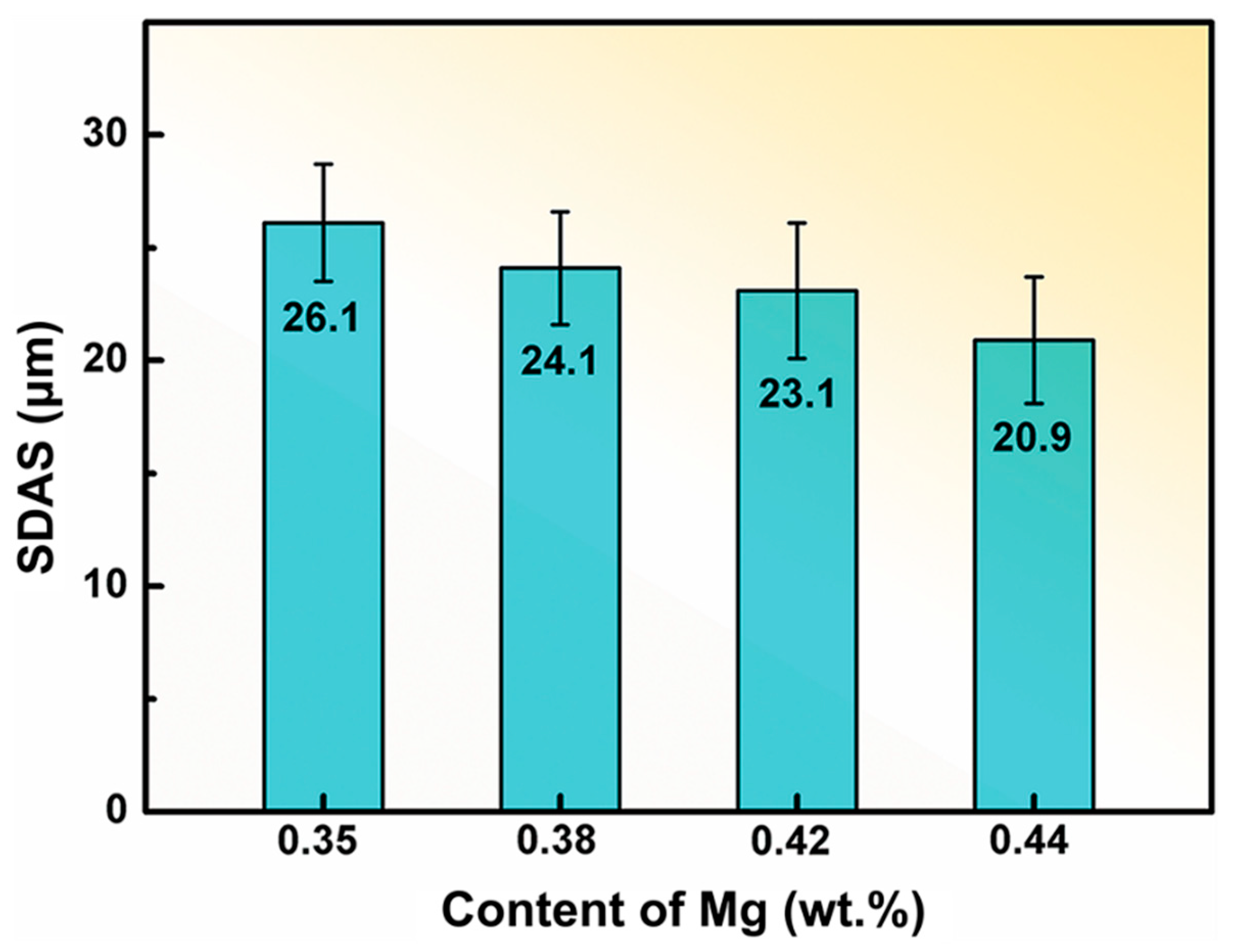
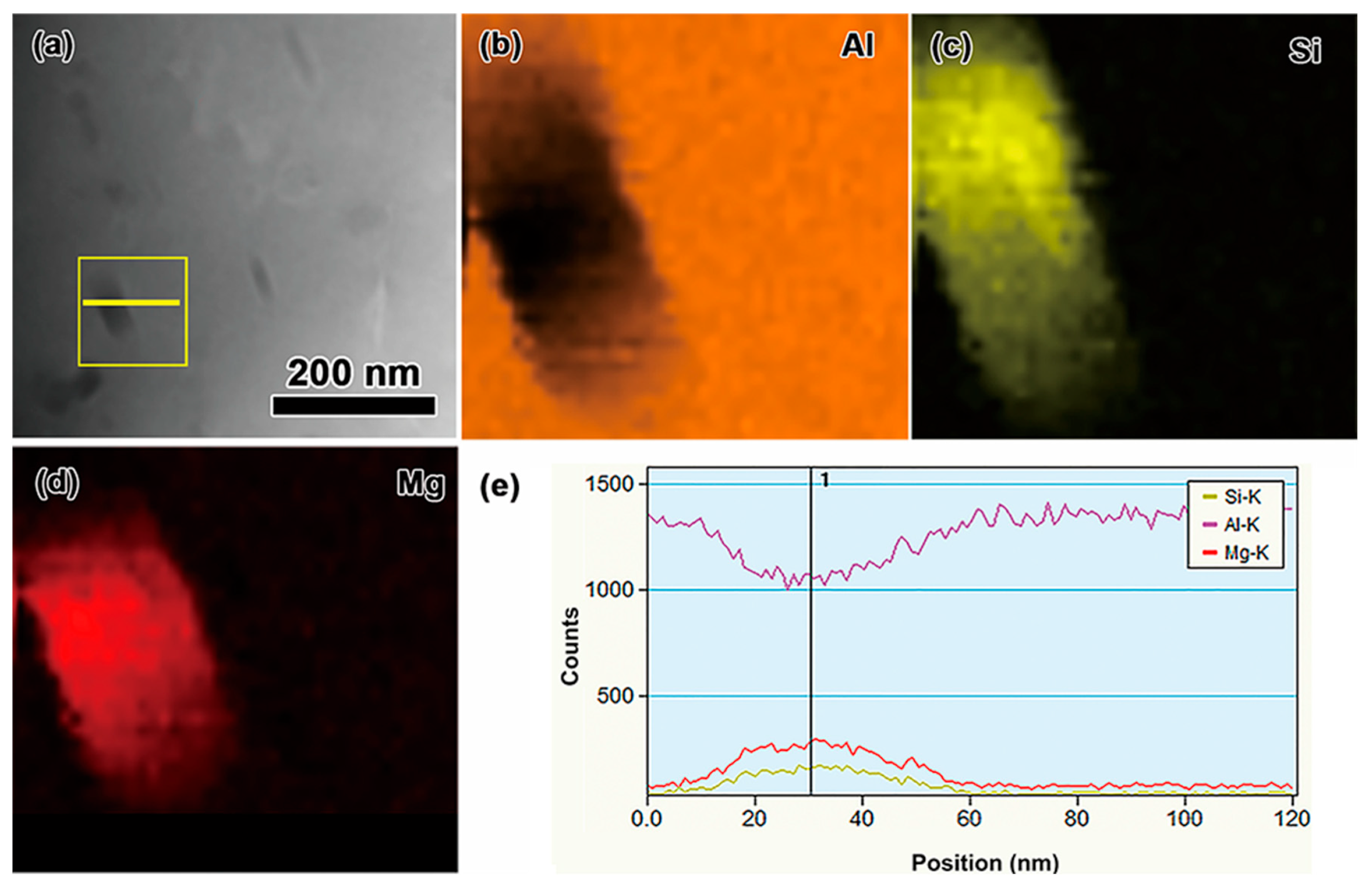
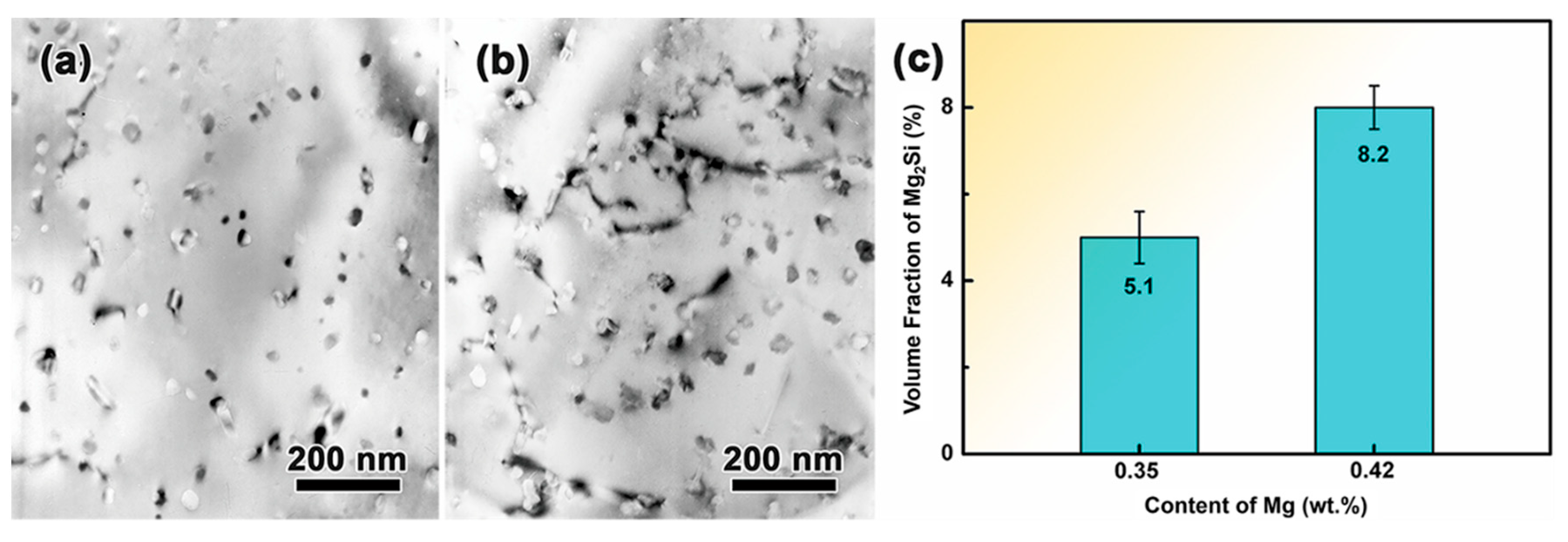
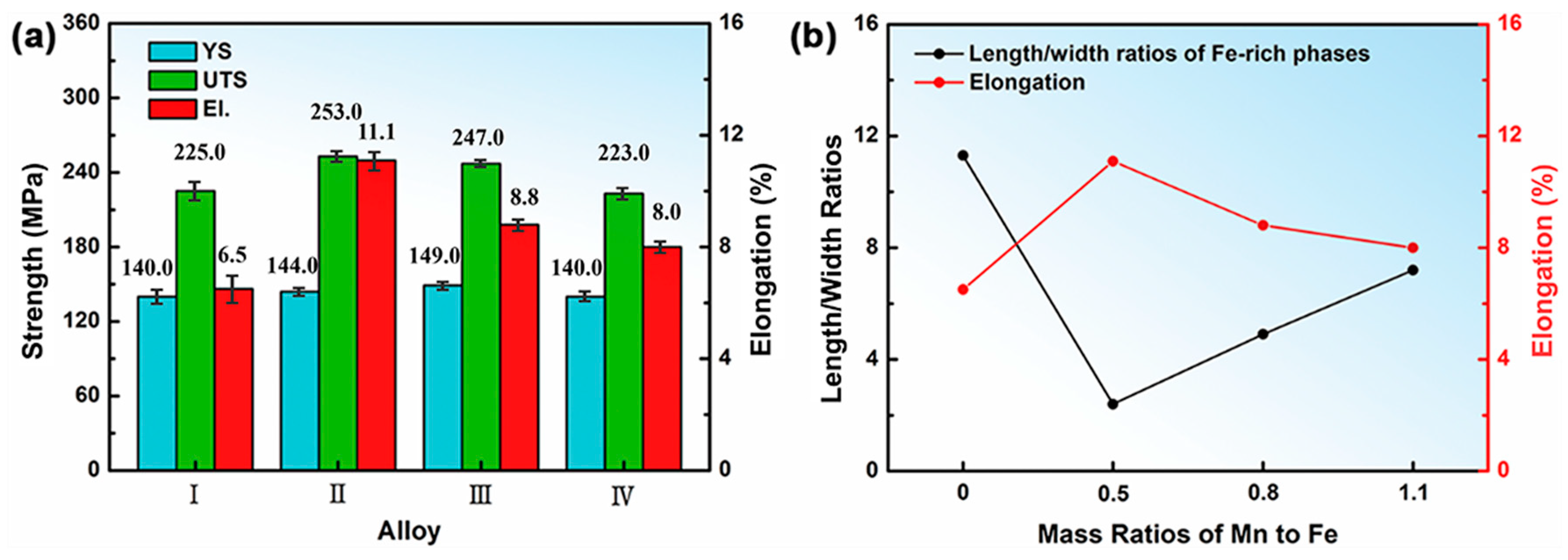
| Alloy | Si | Fe | Mn | Mg | Ti | Sr | Al |
|---|---|---|---|---|---|---|---|
| I | 6.95 | 0.148 | - | 0.35 | 0.12 | 0.02 | Bal. |
| II | 7.06 | 0.155 | 0.078 | 0.35 | 0.12 | 0.02 | Bal. |
| III | 6.95 | 0.149 | 0.115 | 0.34 | 0.13 | 0.02 | Bal. |
| IV | 7.03 | 0.149 | 0.158 | 0.35 | 0.11 | 0.02 | Bal. |
| V | 7.01 | 0.154 | 0.082 | 0.38 | 0.12 | 0.02 | Bal. |
| VI | 7.05 | 0.156 | 0.083 | 0.42 | 0.12 | 0.02 | Bal. |
| VII | 7.02 | 0.152 | 0.081 | 0.44 | 0.12 | 0.02 | Bal. |
| Alloy | Position | Al | Si | Fe | Mn |
|---|---|---|---|---|---|
| I | A | 76.95 | 13.58 | 12.47 | - |
| II | B | 73.98 | 9.88 | 11.84 | 4.30 |
| III | C | 76.12 | 9.31 | 8.60 | 5.57 |
| IV | D | 75.72 | 11.48 | 7.67 | 7.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Chen, J.; Li, Y.; Luo, T. Enhancing the Strength and Toughness of A356.2-0.15Fe Aluminum Alloy by Trace Mn and Mg Co-Addition. Metals 2023, 13, 1451. https://doi.org/10.3390/met13081451
Cui J, Chen J, Li Y, Luo T. Enhancing the Strength and Toughness of A356.2-0.15Fe Aluminum Alloy by Trace Mn and Mg Co-Addition. Metals. 2023; 13(8):1451. https://doi.org/10.3390/met13081451
Chicago/Turabian StyleCui, Jie, Jiayan Chen, Yongbo Li, and Tianjiao Luo. 2023. "Enhancing the Strength and Toughness of A356.2-0.15Fe Aluminum Alloy by Trace Mn and Mg Co-Addition" Metals 13, no. 8: 1451. https://doi.org/10.3390/met13081451
APA StyleCui, J., Chen, J., Li, Y., & Luo, T. (2023). Enhancing the Strength and Toughness of A356.2-0.15Fe Aluminum Alloy by Trace Mn and Mg Co-Addition. Metals, 13(8), 1451. https://doi.org/10.3390/met13081451






