The Influence of the Tantalum Content on the Main Properties of the TixTa9Nb8Zr2Ag Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphology and Structural Analysis
2.2. The Young’s Modulus
2.3. The Electrochemical Characterization
3. Results and Discussions
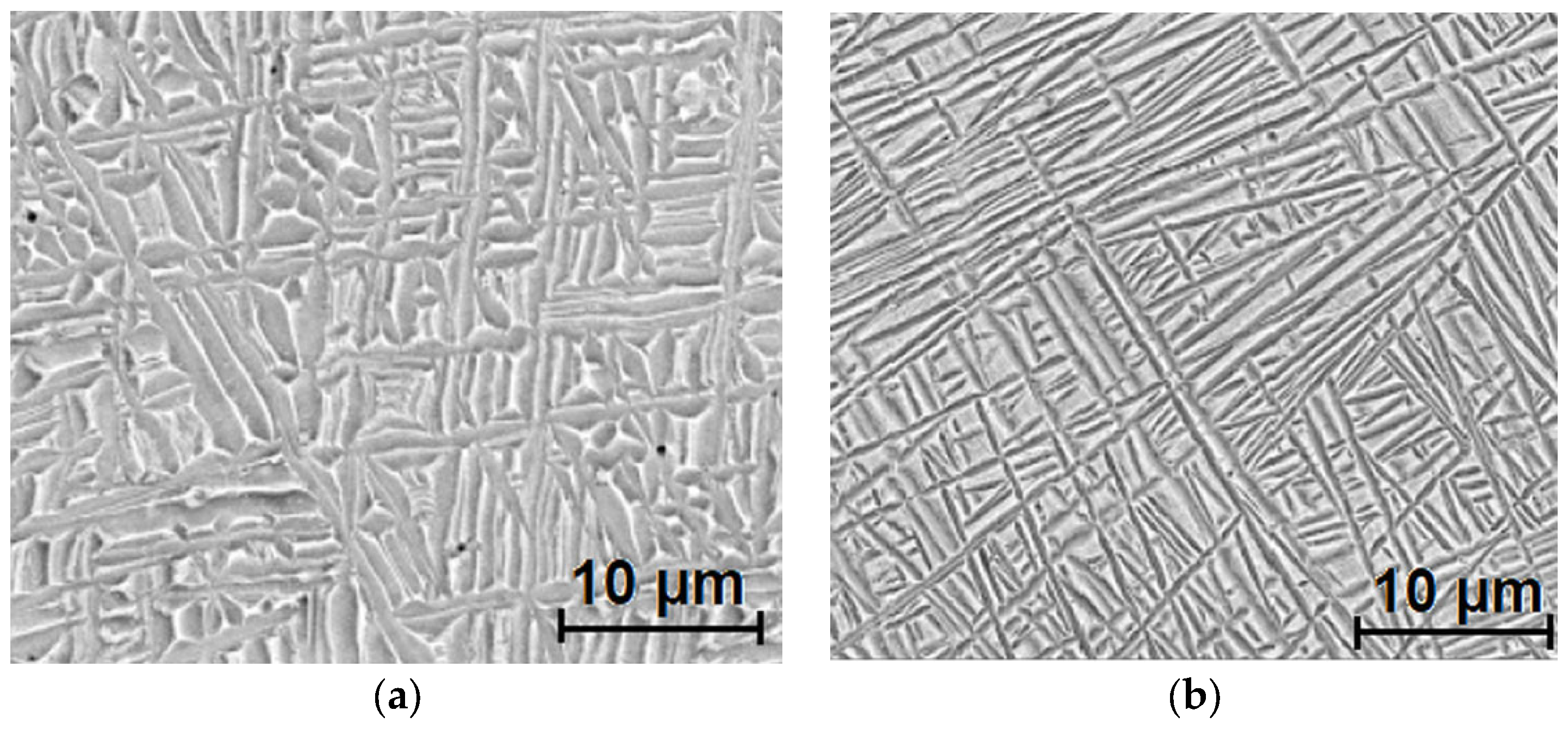
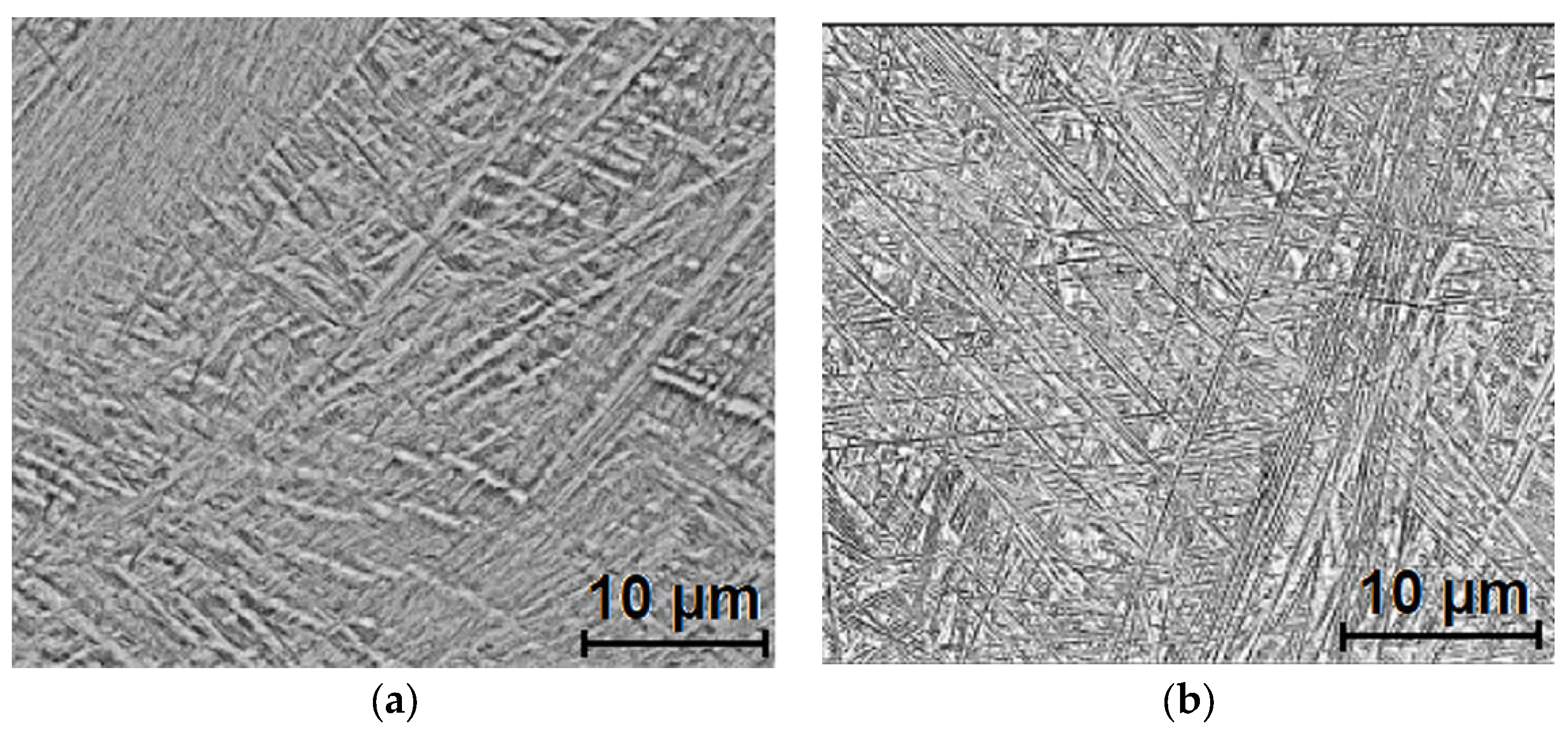
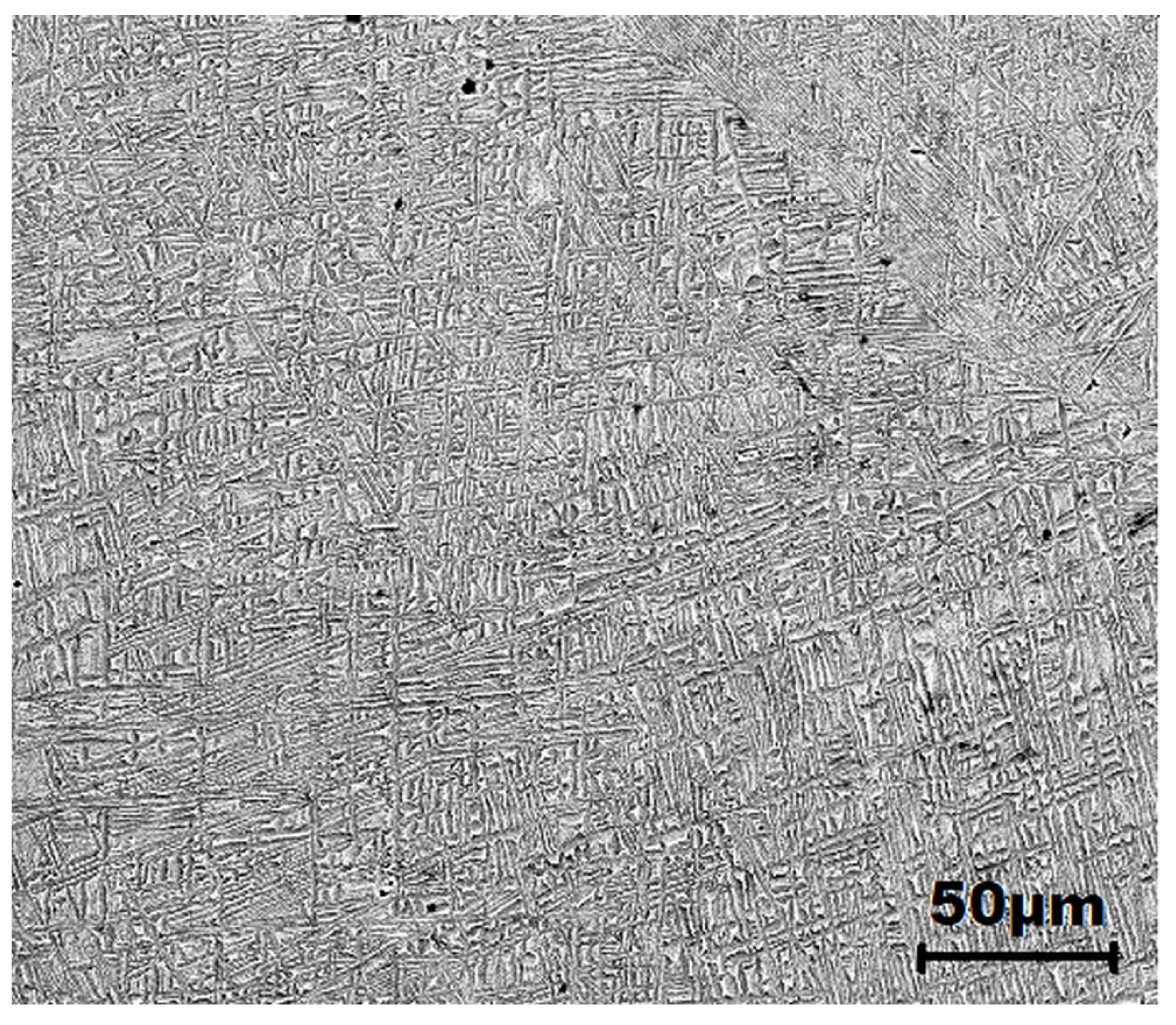
3.1. Microstructure and Morphology Analysis
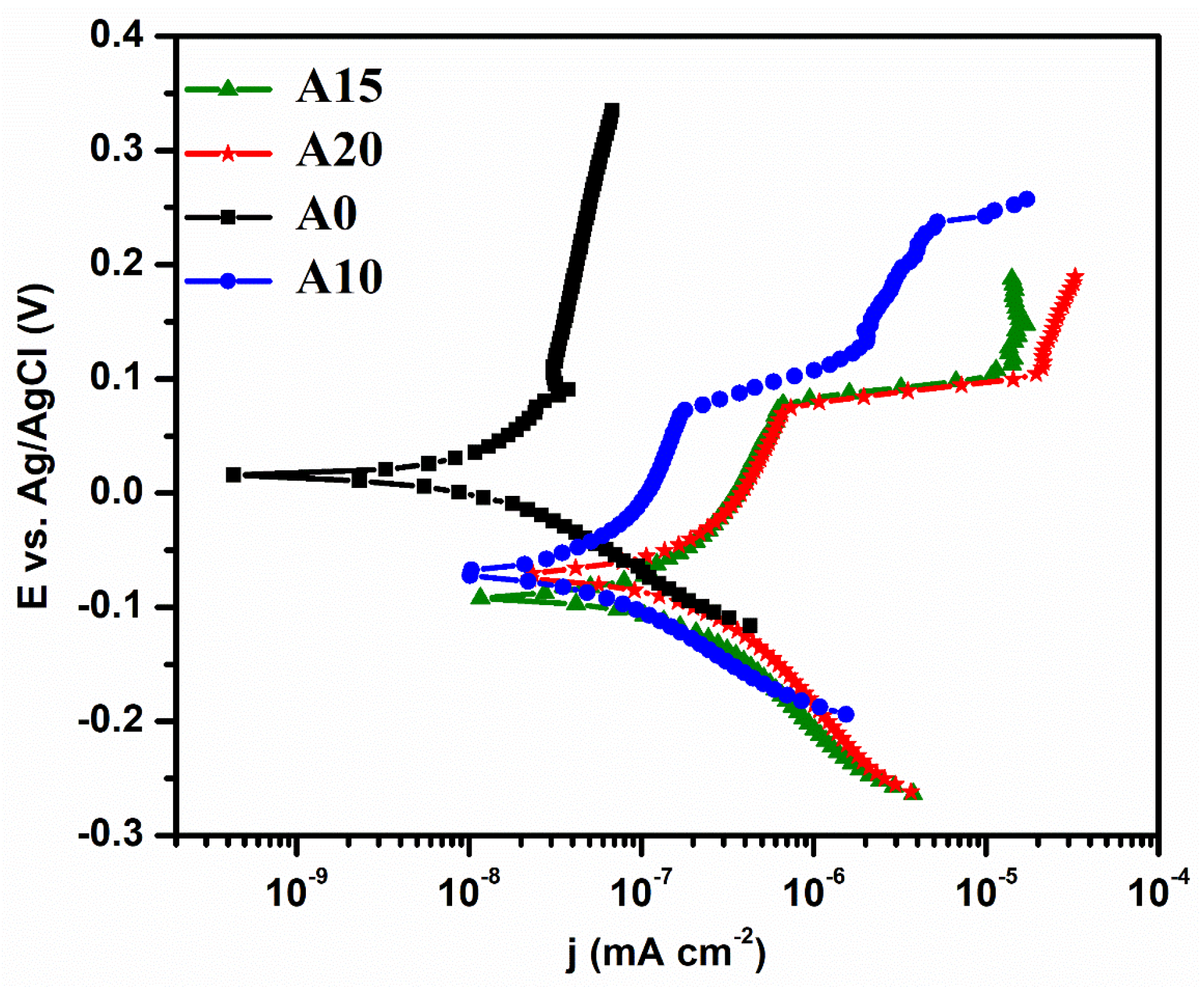
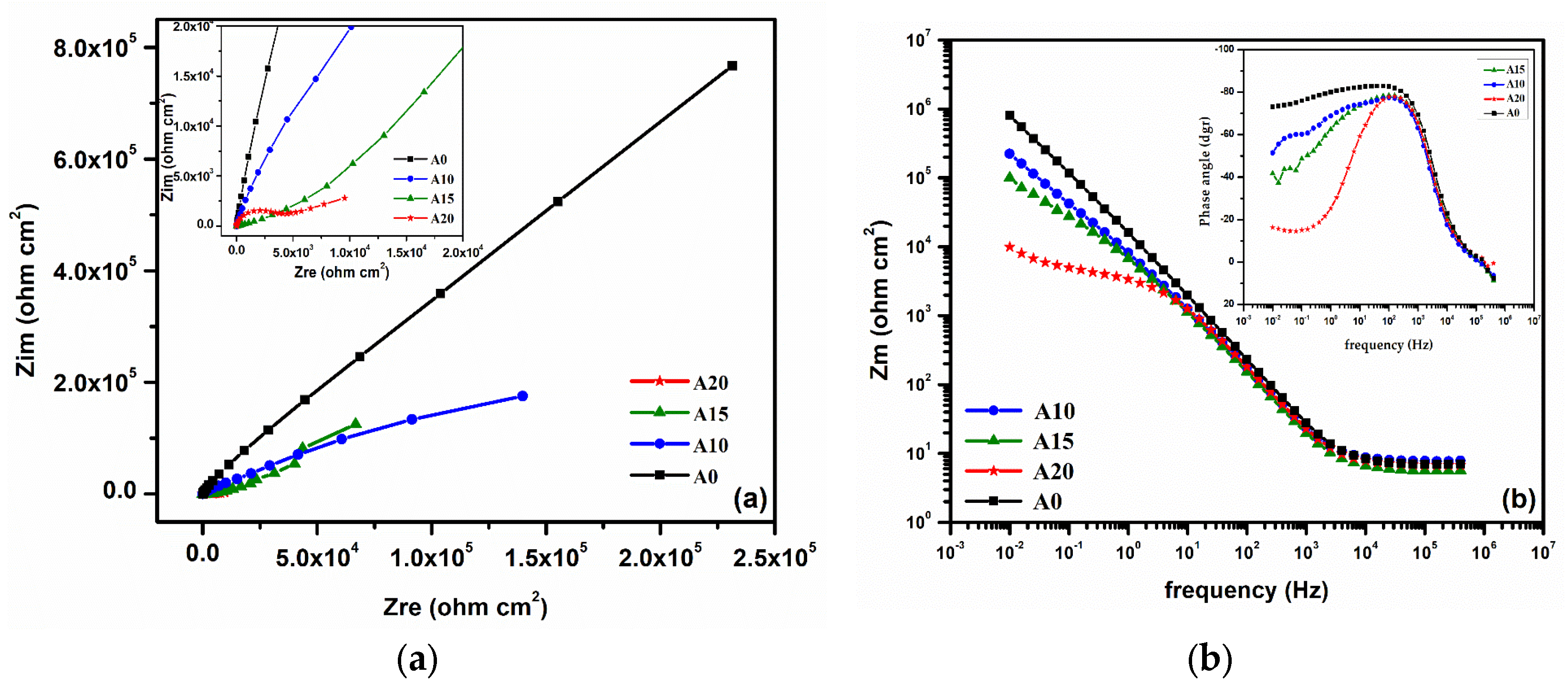
3.2. Electrochemical Behavior of Alloys
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, J.R. Handbook of Materials for Medical Devices, Overview of Biomaterials and Their Use in Medical Devices; Davis, J.R., Ed.; ASM International: Detroit, MI, USA, 2003. [Google Scholar]
- Banu, A.; Radovici, O.; Marcu, M. The Alloying Influence on Corrosion Behaviour of Chromium Surgical Alloys. Rev. Roum. Chim. 2008, 53, 947–953. [Google Scholar]
- Ijaz, M.F.; Vasilescu, C.; Drob, S.I.; Osiceanu, P.; Marcu, M.; Kim, H.Y.; Miyazaki, S. Electrochemical characterization of the superelastic (Ti-Zr)-Mo-Sn biomedical alloy displaying a large recovery strain. Mater. Corros. 2017, 68, 1220–1227. [Google Scholar] [CrossRef]
- Li, Z.; Lai, W.; Wang, B.; Tong, X.; You, D.; Li, W.; Wang, X. A novel Ti42.5Zr42.5Nb5Ta10 multi-principal element alloy with excellent properties for biomedical application. Intermetallics 2022, 151, 107731. [Google Scholar] [CrossRef]
- Banu, A.; Marcu, M.; Juganaru, C.; Osiceanu, P.; Anastasescu, M.; Capra, L. Corrosion behavior of CoCrMoW cast alloy in lactic environment. Arab. J. Chem. 2019, 12, 2007–2016. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium-based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Yang, W.; Pang, S.; Liu, Y.; Wang, Q.; Liaw, P.K.; Zhang, T. Design and properties of novel Ti–Zr–Hf–Nb–Ta high-entropy alloys for biomedical applications. Intermetallics 2022, 141, 107421. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Chen, L.-Y.; Chu, Y.-H.; Zhang, L.; Li, R.; Lu, S.; Wang, L.; Zhang, L.-C. Metastable pitting corrosion behavior and characteristics of passive film of laser powder bed fusion produced Ti–6Al–4V in NaCl solutions with different concentrations. Corros. Sci. 2023, 215, 111017. [Google Scholar] [CrossRef]
- Guo, L.; Naghavi, S.A.; Wang, Z.; Varma, S.N.; Han, Z.; Yao, Z.; Wang, L.; Wang, L.; Liu, C. On the design evolution of hip implants: A review. Mater. Des. 2022, 216, 110552. [Google Scholar] [CrossRef]
- Thampi, A.; Ramanathan, S. Corrosion behavior of anodized Ti-Ta binary surface alloys in various physiological fluids for implant applications. Corros. Sci. 2023, 219, 111233. [Google Scholar] [CrossRef]
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical Ti-Zr-Nb-Ta-Mo high entropy alloys. J. Alloys Compd. 2021, 861, 157997. [Google Scholar] [CrossRef]
- Banu, A.; Preda, L.; Marcu, M.; Dinca, L.L.; Maxim, M.E.; Dobri, G. Electrochemical behavior of SLM Ti-6Al-4V alloy after long time of immersion in lactic acid environment. Met. Mater. Trans. A 2022, 53, 2060–2070. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Abdel-Hady Gepreel, M.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Zareidoost, A.; Yousefpour, M. A study on the mechanical properties and corrosion behavior of the new ascast TZNT alloys for biomedical applications. Mater. Sci. Eng. C 2020, 110, 110725. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-K.; Hsu, H.-C.; Wu, S.-C.; Hung, T.-L.; Ho, W.-F. Structure, Properties, and Corrosion Behavior of Ti-Rich TiZrNbTa Medium-Entropy Alloys with β+α″+α′ for Biomedical Application. Materials 2022, 15, 7953. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.C.; Ventura, B.N.; Milián, L.; Escuder, A.V.; Borrás, V.A. Study of Electrochemical and Biological Characteristics of As-Cast Ti-Nb-Zr-Ta System Based on Its Microstructure. Metals 2022, 12, 476. [Google Scholar] [CrossRef]
- Feng, J.; Wei, D.; Zhang, P.; Yu, Z.; Lui, C.; Lu, W.; Wang, K.; Yan, H.; Zhang, L.; Wang, L. Preparation of TiNbTaZrMo high-entropy alloy with tunable Young’s modulus by selective laser melting. J. Manuf. Process. 2023, 85, 160–165. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, S.I.; Osiceanu, P.; Calderon Moreno, J.M.; Prodana, M.; Ionita, D.; Demetrescu, I.; Marcu, M.; Popovici, I.A.; Vasilescu, E. Microstructure, Surface characterization and electrochemical behavior of new Ti-Zr-Ta-Ag alloy in simulated human electrolyte. Metall. Mater. Trans A 2017, 48, 513–523. [Google Scholar] [CrossRef]
- Gurel, S.; Yagci, M.B.; Bal, B.; Canadinc, D. Corrosion behavior of novel Titanium-based high entropy alloys designed for medical implants. Mater. Chem. Phys. 2020, 254, 123377. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chuang, W.S.; Huanga, J.C.; Wang, X.; Chou, H.S.; Lai, Y.J.; Lin, P.H. On the bio-corrosion and biocompatibility of TiTaNb medium entropy alloy films. Appl. Surf. Sci. 2020, 508, 145307. [Google Scholar] [CrossRef]
- ISO 6892-1; Metallic materials—Tensile Testing—Part 1: Method of Test at Room Temperature. ISO: Geneva, Switzerland, 2019.
- ISO 6507-1; Metallic Materials—Vickers Hardness Test—Part 1: Test Method. ISO: Geneva, Switzerland, 2018.
- Dobri, G.; Berbecaru, A.; Paraschiv, A.; Geanta, V.; Ciuca, S.; Banu, A. The effect of tantalum content on microstructure and vickers hardness of TiNbZrTaAg alloy. U.P.B. Sci. Bull. Ser. B 2023, 85, 167–178. [Google Scholar]
- Preisler, D.; Strasky, J.; Harcuba, P.; Kosutova, T.; Dopita, M.; Janovska, M.; Hajek, M.; Janecek, M. Phase transformations in Ti-Nb-Zr-Ta-O β titanium alloys with high oxygen and reduced Nb and Ta content. Acta Crystallogr. A 2021, 77, C1251. [Google Scholar] [CrossRef]
- Yang, Y.; Songxiao, H.; Wenjun, Y.; Baiking, X. Mechanical properties and microstructure of an α + β titanium alloy with high strength and fracture toughness. Rare Met. 2009, 28, 346. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, J.; Zhao, T.; Schopphoven, T.; Fu, J.; Gasser, A.; Schleifenbaum, J.H. Laser Metal Deposition of Ti6Al4V—A Brief Review. Appl. Sci. 2020, 10, 764. [Google Scholar] [CrossRef]
- Yang, W.; Pang, S.-J.; Wang, G.; Liu, Y.; Liaw, P.K.; Zhang, T. Ti–Zr–Hf–Nb–Ta–Sn high-entropy alloys with good properties as potential biomaterials. Rare Met. 2022, 41, 2305–2315. [Google Scholar] [CrossRef]
- Hansen, A.W.; Fuhr, L.T.; Antonini, L.M.; Villarinho, D.J.; Marino, C.E.B.; Malfatti, C.D.F. The Electrochemical Behavior of the NiTi Alloy in Different Simulated Body Fluids. Mat. Res. 2015, 18, 184–190. [Google Scholar] [CrossRef]
- Vasilescu, C.; Osiceanu, P.; Calderon Moreno, J.M.; Drob, S.I.; Preda, S.; Popa, M.; Dan, I.; Marcu, M.; Prodana, M.; Popovici, I.A.; et al. Microstructure, surface characterization and long-term stability of new quaternary Ti-Zr-Ta-Ag alloy for implant use. Mater. Sci. Eng. C 2017, 71, 322–334. [Google Scholar] [CrossRef]
- Sargeant, A.; Goswami, T. Hip implants: Paper V. Physiological effects. Mater. Des. 2006, 27, 287–307. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Bahri, Y.A.; Alharbi, H.F.; Ijaz, M.F.; Alnaser, I.A. Influence of Tantalum Addition on the Corrosion Passivation of Titanium-Zirconium Alloy in Simulated Body Fluid. Materials 2022, 15, 8812. [Google Scholar] [CrossRef]
- Yen, S.K.; Huang, T.Y. Characterization of the electrolytic ZrO2 coating on Ti-6A1-4V. Mater. Chem. Phys. 1998, 56, 214–221. [Google Scholar] [CrossRef]
- Oh, K.-T.; Joo, U.-H.; Park, G.-H.; Hwang, C.-J.; Kim, K.-N. Effect of Silver Addition on the Properties of Nickel–Titanium Alloys for Dental Application. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 306–314. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Murugaraj, S.R.; Rajendran, N. Electrochemical impedance spectroscopic studies of titanium and its alloys in saline medium. Mater. Corros. 2007, 58, 113–120. [Google Scholar] [CrossRef]
- Preda, L.; Negrila, C.; Lazarescu, M.F.; Enache, M.; Anastasescu, M.; Toader, A.M.; Ionescu, S.; Lazarescu, V. Ga and As Competition for Thiolate Formation at p-GaAs(111) Surfaces. Electrochim. Acta 2013, 104, 1–11. [Google Scholar] [CrossRef]
- Chelariu, R.; Bolat, G.; Izquierdo, J.; Mareci, D.; Gordin, D.M.; Gloriant, T.; Souto, R.M. Metastable beta Ti-Nb-Mo alloys with improved corrosion resistance in saline solution. Electrochim. Acta 2014, 137, 280–289. [Google Scholar] [CrossRef]
- Xie, F.X.; He, X.B.; Cao, S.L.; Lu, X.; Qu, X.H. Structural characterization and electrochemical behavior of a laser-sintered porous Ti–10Mo alloy. Corros. Sci. 2013, 67, 217–224. [Google Scholar] [CrossRef]
- Assis, S.I.; Wolynek, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- ISO 8044; Corrosion of Metals and Alloys—Basic Terms and Definitions. ISO: Geneva, Switzerland, 2020.
- Yang, J.; Yang, H.; Yu, H.; Wang, Z.; Zeng, X.A. Corrosion Behavior of Additive Manufactured Ti6Al4V Alloy in NaCl Solution. Metall. Mater. Trans. A 2017, 48, 3583–3593. [Google Scholar] [CrossRef]



| Specimen | % Nb | % Zr | % Ag | % Ta | % Ti |
|---|---|---|---|---|---|
| A0 | 9.22 ± 0.41 | 8.28 ± 0.19 | 1.86 ± 0.16 | 0.0 | Balance |
| A10 | 10.26 ± 0.68 | 7.95 ± 0.28 | 1.97 ± 0.09 | 8.94 ± 0.36 | Balance |
| A15 | 9.23 ± 0.25 | 7.80 ± 0.12 | 1.87 ± 0.09 | 15.57 ± 0.60 | Balance |
| A20 | 9.01 ± 0.16 | 7.88 ± 0.17 | 1.92 ± 0.08 | 20.02 ± 0.64 | Balance |
| Specimen | 3% NaCl | Artificial Saliva | ||||||
|---|---|---|---|---|---|---|---|---|
| Ecorr, mV | icor, μA cm−2 | rcor, μm y−1 | Eoc, mV | Ecorr, mV | icor, μA cm−2 | rcor, μm y−1 | Eoc, mV | |
| A0 | 14.3 | 0.027 | 0.9 | 135 | 11.5 | 0.011 | 0.41 | −10.5 |
| A10 | −69.8 | 0.13 | 5 | 57 | 0.002 | 0.09 | 4.6 | 0.002 |
| A15 | −90.3 | 0.5 | 18 | −12 | 0.008 | 0.15 | 17.2 | 0.008 |
| A20 | −72.8 | 1.1 | 47 | −10 | 0.011 | 42.25 | 34 | 0.011 |
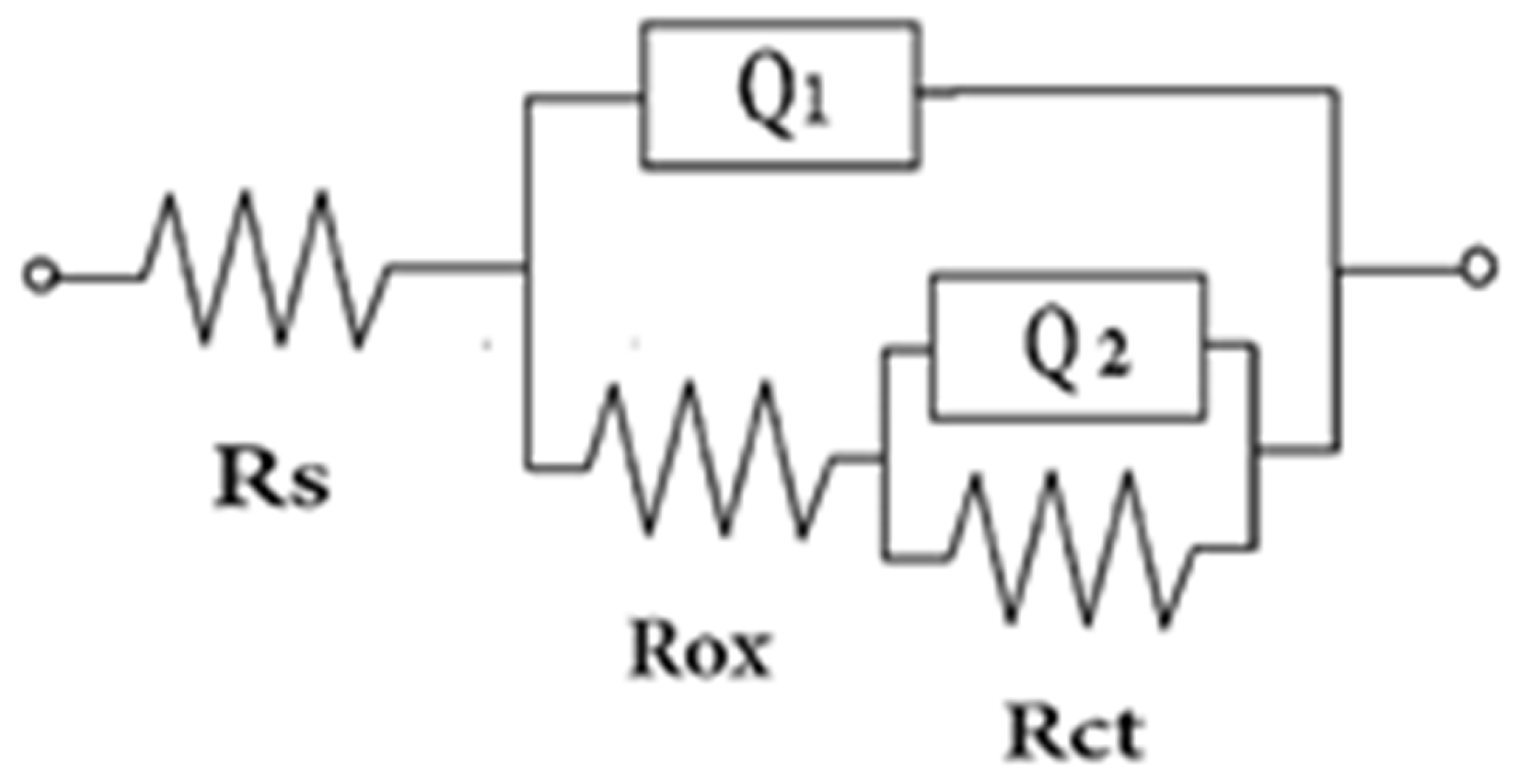
| Specimen | Rs (Ω cm2) | Rox (KΩ cm2) | Q1 (Ω−1 cm−2 sn) | n1 | Rct (KΩ cm2) | Q2 (Ω−1 cm−2 sn) | n2 | Chi |
|---|---|---|---|---|---|---|---|---|
| A0 | 3.09 | 37 | 6.6 × 10−6 | 0.62 | 1022 | 11.5 × 10−6 | 0.9 | 0.4 |
| A10 | 3.4 | 9.5 | 2.68 × 10−6 | 0.72 | 258.2 | 12.68 × 10−6 | 0.88 | 0.335 |
| A15 | 2.8 | 8.1 | 13.37 × 10−6 | 0.69 | 82.1 | 97.03 × 10−6 | 0.89 | 0.538 |
| A20 | 4.6 | 2.2 | 861 × 10−6 | 0.60 | 5.04 | 821.9 × 10−6 | 0.92 | 0.439 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobri, G.; Banu, A.; Donath, C.; Marcu, M. The Influence of the Tantalum Content on the Main Properties of the TixTa9Nb8Zr2Ag Alloy. Metals 2023, 13, 1294. https://doi.org/10.3390/met13071294
Dobri G, Banu A, Donath C, Marcu M. The Influence of the Tantalum Content on the Main Properties of the TixTa9Nb8Zr2Ag Alloy. Metals. 2023; 13(7):1294. https://doi.org/10.3390/met13071294
Chicago/Turabian StyleDobri, Gabriel, Alexandra Banu, Cristina Donath, and Maria Marcu. 2023. "The Influence of the Tantalum Content on the Main Properties of the TixTa9Nb8Zr2Ag Alloy" Metals 13, no. 7: 1294. https://doi.org/10.3390/met13071294
APA StyleDobri, G., Banu, A., Donath, C., & Marcu, M. (2023). The Influence of the Tantalum Content on the Main Properties of the TixTa9Nb8Zr2Ag Alloy. Metals, 13(7), 1294. https://doi.org/10.3390/met13071294








