Abstract
Cermets are composites of a TiCN hard phase and a metal binder, typically a mixture of Co and Ni. They offer excellent combinations of hardness and fracture toughness as well as bending strength. Due to the current classification of both Co and Ni as CRM as well as CMR there is interest that their use shall be limited and their amount within cermets reduced. Within this study, a novel high entropy alloy-based metal binder system, comprising only elements that are not strong oxide or carbide formers is investigated with regard to their mechanical and microstructural properties they offer in TiCN-based cermets. Within the investigated equimolar MnFeCoNiCu composition, small Cu precipitations are found after sintering. Due to this, the Cu content was systematically reduced, and the maximal solubility estimated at which still a full solid solution occurs. With an optimized Cu content, dense cermets with a single FCC binder phase and with hardness values of up to 1213 HV30 and fracture toughness values of 14.8 MPa·m1/2 could be achieved.
1. Introduction
Cermets are composite materials consisting of a hard ceramic titanium carbide (TiC) or carbonitride (TiCN) hard phase and tough metal binder. They are mainly used in different tooling operations such as metal cutting, sawing of cement or wood cutting. Cermets can be differentiated from hardmetals (often also called cemented carbides) which contain WC as the main hard phase [1]. Typically the metal binder of choice for nearly all commercially available cermets are mixtures of cobalt (Co) and nickel (Ni) with different ratios [2,3]. Due to recent classifications of Co as carcinogenic, mutagenic, reprotoxic (CMR) [4] and Ni as carcinogenic [5] substances there is a need for alternative metal binder compositions. Since Co is also listed by the EU as a Critical Raw Material (CRM) [6] and used in high-capacity Li-ion batteries, possible price increases are a further reason to reduce the amount of both elements in binders of cermets and hardmetals alike.
Current developments include investigations of iron (Fe)-based binders [7,8,9,10,11,12,13,14] as well as more complex medium-to-high entropy alloys as binder metals. Medium entropy alloy (MEA) binders are mostly based on CoNiFe alloys [15,16,17] that were investigated already more than thirty years ago and are still relevant today and used to some extent within the industry (e.g., grade HE40 from Ceratizit [18]). High entropy alloys (HEAs) emerged more than 15 years ago and are defined as alloys which are “composed of five or more principal elements in equimolar ratio” [19] and which form a single solid solution phase. Such alloys are of high interest due to their high hardness and strength as well as high wear and corrosion resistance [20]. The use of such alloys has been studied for hardmetals and cermets as well [21,22]; however, most investigations are based on HEA compositions which were developed previously for metal applications. Since these compositions often include Ti, Al, Cr or refractory elements, reactions with the hard phase in cermets or hardmetals often occur. Examples are WC-based compositions with AlCoCr(Cu)FeNi [23,24], AlFeCoNiCrTi [25], CoNiFeCr [26,27] or NiCoCrTiAl [28], or in the case of TiC- or TiCN-based cermets with FeCoNiCrMnAl [29], FeCrAlTiMoNi [30] or FeCoCrNiMn [31] as binders. However, as reported in some of these investigations, reactions of the hard phase WC, TiC or TiCN with Al, Ti or Cr lead to partial decomposition of the binder phase or, due to the oxygen affinity of Al, to the formation of small alumina precipitations [32]. To avoid any significant reaction with the hard phase of cermets, a novel HEA based only on elements from the fourth period of the periodic table: Mn, Fe, Co, Ni and Cu has been investigated [33]. Here, none of these elements are known to be soluble within TiC or TiCN, thus a similar two-phase microstructure as in conventional TiCN-Co/Ni cermets can be expected. However, since the solubilities of these elements, and especially Cu, in such an HEA are unknown, a deeper understanding of such HEA binders is necessary. Thus, the focus of this study is on the solid solution formation and the attainable properties. Different HEA binder volumes and two industrial relevant C/N ratios of commercial TiCN of 50/50 and 70/30 were used. For studying the influence of Cu, a partial replacement by Ni was performed. Ni was chosen because it has full solubility with Cu in contrast to Fe and Co [34].
2. Materials and Methods
TiCN-based cermets with HEA binder volumes of both 16 and 30 vol-% were prepared with conventional powder metallurgy processing. For the hard phase, two TiCN powders from HCST Laufenburg (now Höganäs) and for the HEA binder phase elemental metal powders from BASF, Freeport Co, Umicore, CNPC powder and Good Fellow were used. Details on powder properties can be found in Table 1.

Table 1.
Details on starting powders used.
Sample designations and corresponding compositions with regard to the C/N ratio of TiCN, binder volume and binder composition are given in Table 2. Here, three cases are studied: equimolar HEA compositions of all five metal elements, equimolar MEA compositions of four metal elements (without Cu) and HEA compositions with a reduced Cu but increased Ni content. For the latter, the following adjustment of Cu content was used:
Mn0.2Fe0.2Co0.2Ni0.2+xCu0.2−x, x = 0; 0.05; 0.10; 0.15

Table 2.
Sample designations and compositions of studied cermets. Changes of composition from equimolar compositions are highlighted by their Ni and Cu content being written in bold.
In addition, one reference cermet with a 50/50 ratio of Co/Ni binder and a TiCN with a C/N ratio of 70/30 was produced for comparison reasons as well. The used sample IDs are derived from the C/N ratio of either 5/5 or 7/3, the binder volume of either 16 or 30 and the composition of either being equimolar with five elements (HEA), equimolar with four elements (MEA, Cu-free) or as HEA with a by x at-% reduced Cu and likewise increased Ni content.
Cermet powder mixtures were produced by dry-mixing the starting powders, ball milling in n-heptane for 48 h and subsequent vacuum-drying and sieve granulation to particles sizes below 315 µm. Cylindrical green parts with a diameter of 12.4 mm and a height of 6 mm were produced by uniaxial pressing at 150 MPa. Sintering was performed at 1500 °C under a partial pressure of 100 mbar Argon and a subsequent HIP treatment with 100 bar (SinterHIP furnace, FCT Anlagenbau, Sonnenberg, Germany).
Produced samples were characterized by measuring density according to ISO 3369 (Archimede’s method), magnetic properties according to ISO 3326 and porosity according to ISO 4499-4. The theoretical density was calculated using the rule of mixture and known densities of each element. The microstructure was investigated by taking images using an optical microscope Axioscope 7 KMAT (Carl Zeiss Microscopy, Oberkochen, Germany) as well as a field emission scanning electron microscope (FESEM) ULTRA 55 (Carl Zeiss Microscopy, Oberkochen, Germany). For chemical and phase analysis, energy dispersive X-ray spectroscopy (EDS) and EDS mapping as well as X-ray diffraction (XRD) using a D8 Advance (Bruker AXS, Karlsruhe, Germany; CuKα-radiation) were used. Lattice parameters were determined using a Pawley fit algorithm of the software TOPAS (Bruker AXS, Karlsruhe, Germany). For mechanical characterization, Vickers hardness HV30 according to ISO 3878 and indentation fracture toughness according to ISO 28079 was measured.
The software FactSage (version 8.0, database SGTE 2014, Aachen, Germany) was used for thermodynamic calculations of solid solution phase formation.
3. Results
3.1. Thermodynamic Calculations
Thermodynamic calculations using the software FactSage were determined for the prediction of solid solution formation of the studied MnFeCoNiCu HEA composition.
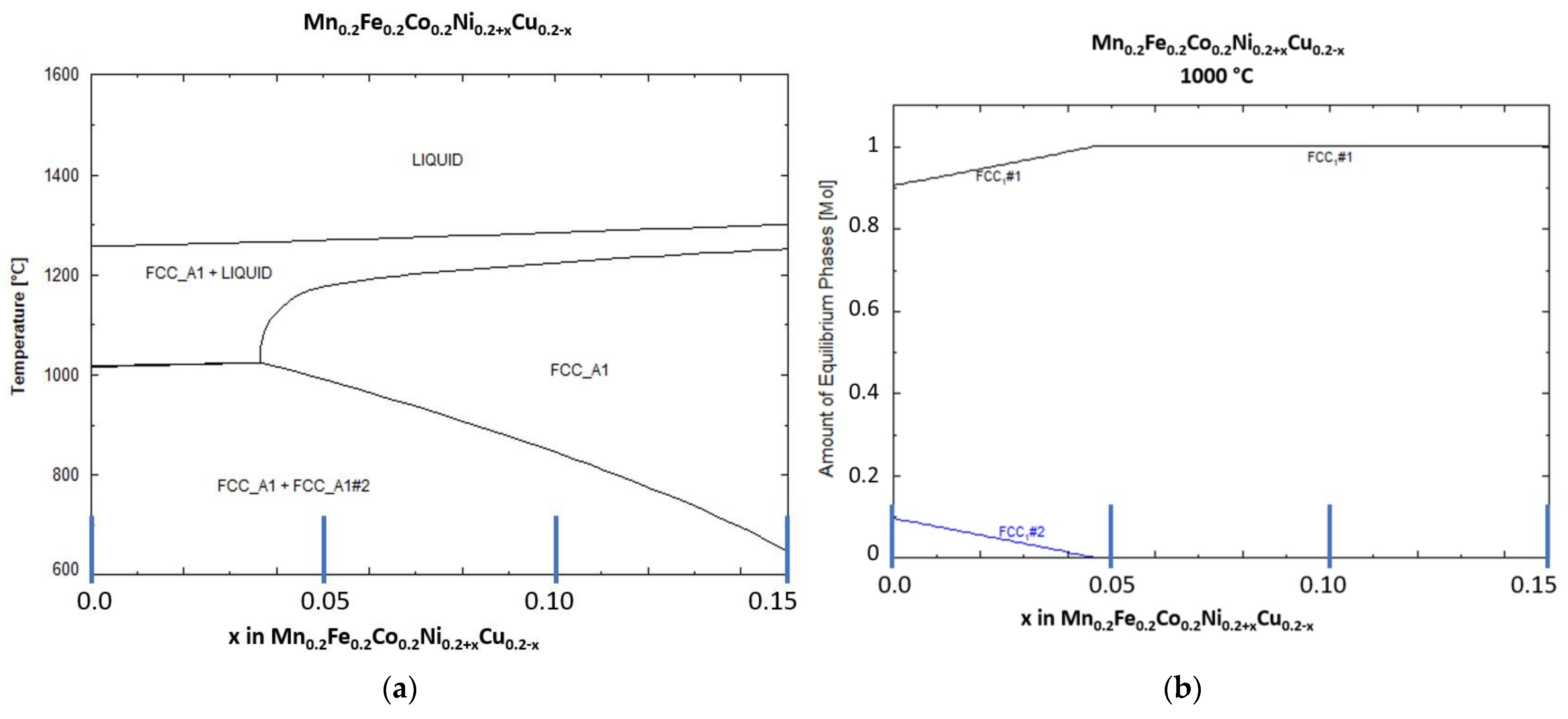
In Figure 1a, the predicted phases for the equimolar composition (x = 0) and the phases predicted with an increase in x (decrease in Cu content and increase in Ni content) in the HEA are shown. As visible for the equimolar composition (x = 0), two solid phases are predicted below the liquidus temperature. Only for x > 0.37 a single solid FCC phase is predicted. With further increase in x, the solid solution stability is extended to lower temperatures. The liquidus temperature without consideration of Ti or C was predicted to be in the range of 1250 to 1300 °C.
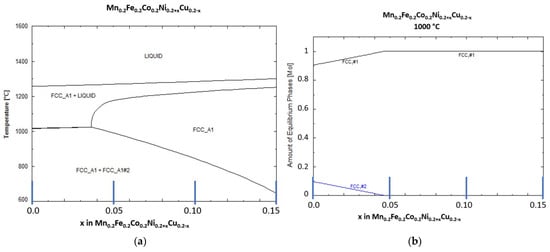
Figure 1.
Thermodynamic calculation of (a) the liquid and solid phases present in the studied HEA and (b) amount of each of the two solid phases as function of x (0 to 0.15) in Mn0.2Fe0.2Co0.2Ni0.2+xCu0.2-x, marked blue lines represent studied HEA compositions.
Figure 1b shows the distribution of the two predicted FCC solid phases at 1000 °C. As can be seen, the content of FCC phase 2 is at round 10 at-% for the equimolar HEA composition and decreases to 0% at x = 0.045.
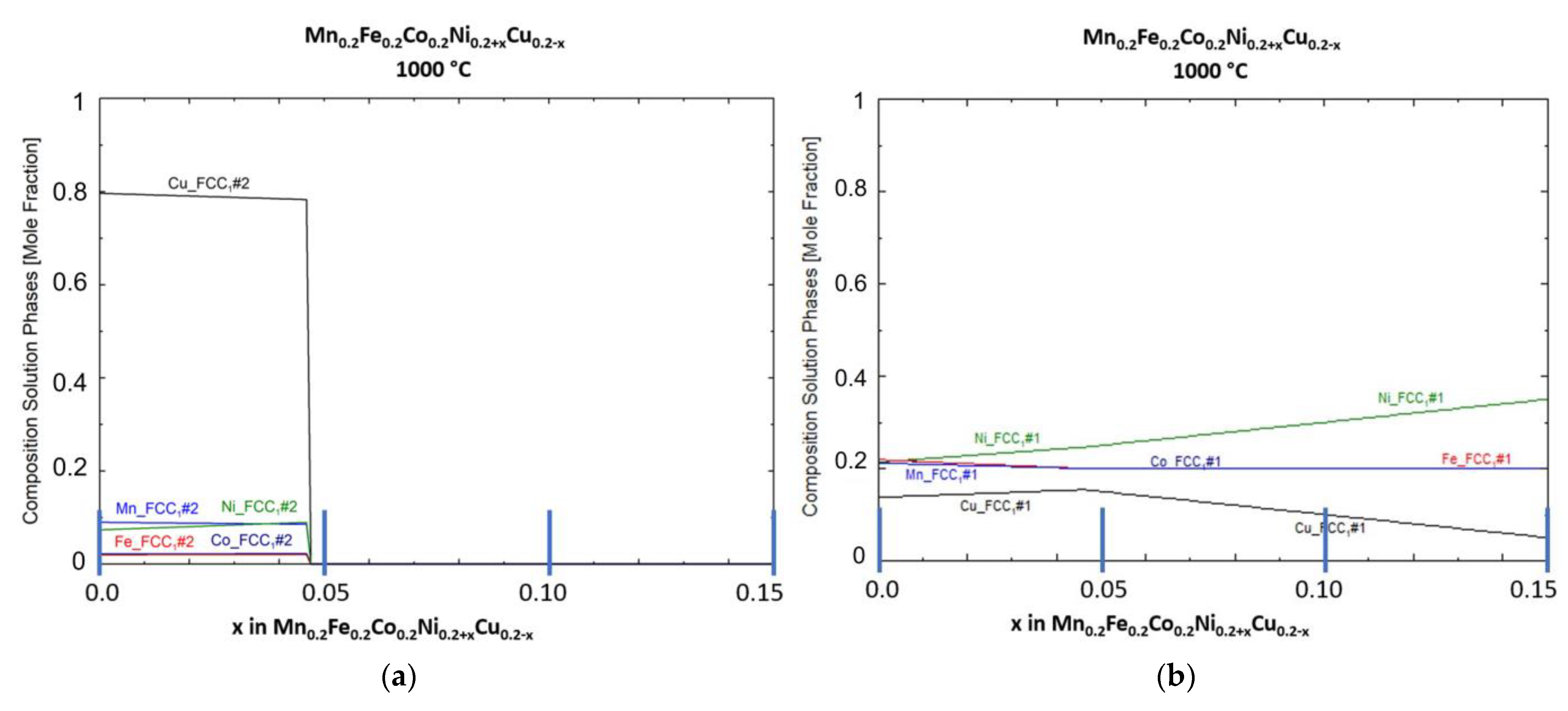
Figure 2 shows the phase compositions of both FCC phases at a temperature of 1000 °C, just below the solidus temperature for the equimolar HEA composition.
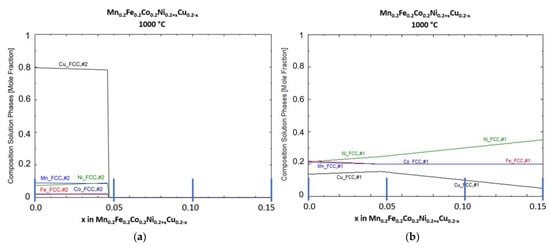
Figure 2.
Predicted phase composition for solid FCC phases at 1000 °C: (a) FCC phase #2, Cu rich; (b) FCC phase #1, HEA phase.
FCC phase #2 is primarily a Cu-rich phase with up to 20 at-% dissolved Mn, Ni, Co and Fe. For Mn and Ni, the highest amount was predicted (each ca. 9 at-%). The content of Co and Fe is predicted to be just around 1 at-%. FCC phase #1, the main HEA phase, has accordingly a reduced amount of Cu at equimolar composition (at x = 0, ca. 14 at-%) and a uniformly increased amount of the remaining HEA elements. With increase in x, the amount of dissolved Cu increases up to 16 mol % at x = 0.46. With higher x values, Cu content decreases and Ni content increases due to the change in composition.
3.2. Physical Properties
Results on the produced samples are presented by looking at the influence of HEA binder volume for equimolar Mn, Fe, Co, Ni and Cu compositions first, followed by investigations with adjusted Cu and Ni ratios and subsequently, the investigation on the influence of two C/N ratios of the TiCN hard phase.
In Table 3, density, relative density, magnetic properties, hardness HV30 and fracture toughness K1C for all produced cermets as well as a second reference cermet with a composition of TiC-20TiN-16Ni-2.87Mo2C (wt-%), designation 913 [35] are presented.

Table 3.
Sample designations and compositions of studied cermets.
All samples show a relative density above 97.7%. Magnetic properties show as expected an increase in saturation polarization mS as well as coercive strength Hc with increased amount of ferromagnetic binder elements (Fe, Co, Ni). This is the case for samples with an increased binder volume of HEA as well as for samples with an increased amount of Ni (x = 0 to x = 0.15). Comparison of similar binder compositions (5/5-16-X0.10 vs. 7/3-16-X0.10 and 5/5-16-MEA vs. 7/3-16-MEA) show in both cases with an increased C ratio in TiCN a decrease in saturation polarization. Coercive strength in general is quite low. Hardness values for all 16 vol-% binder samples are in the range of 940 to 1261 HV30 units. Here, a trend of increasing hardness with decreasing Cu content can be seen as well as an increasing hardness for samples having a TiCN hard phase with a 70/30 C/N ratio as compared to a 50/50 C/N ratio. Fracture toughness by indentation method shows values in the range of 13 to 15 MPa*m1/2 for all 16 vol-% HEA/MEA binders. For the 30 vol-% equimolar HEA sample 5/5-30-HEA as well as for the CoNi-based reference sample 7/3-16-CoNi, a higher fracture toughness but lower hardness value are measured.
3.3. Investigation of Microstructures Using Optical Imaging
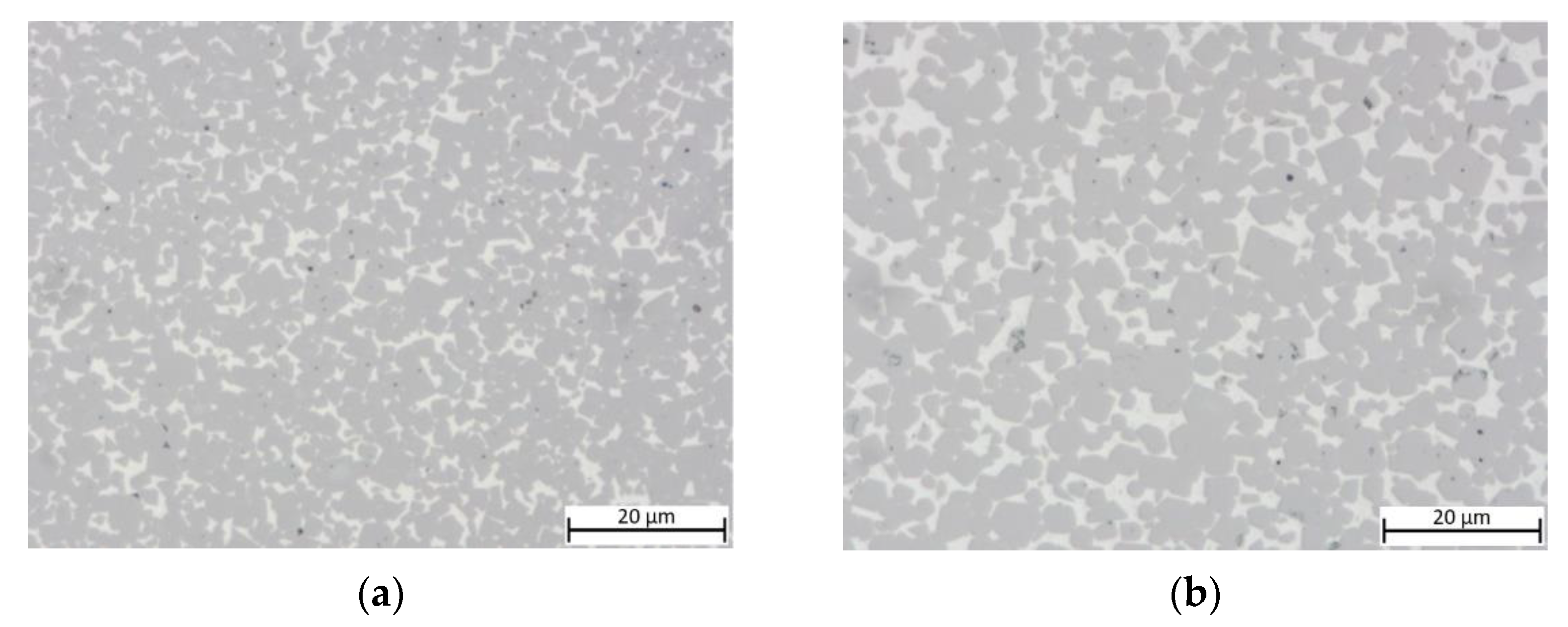
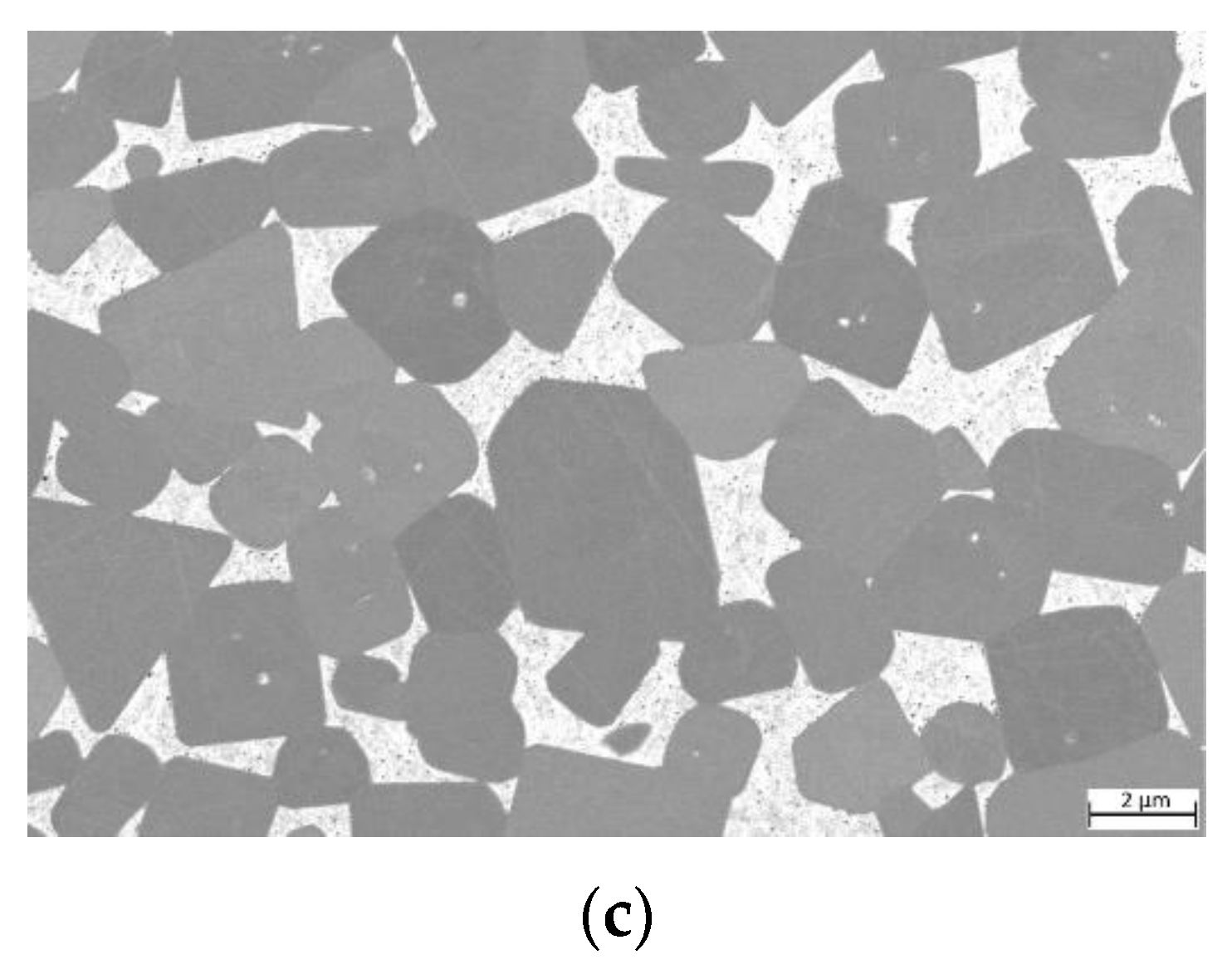
The equimolar HEA-bonded cermets with both binder volumes of 16 and 30% show a dense microstructure (Figure 3) after sintering. A slight porosity is visible and larger defects were found in areas closer to the surface of both samples (not shown).
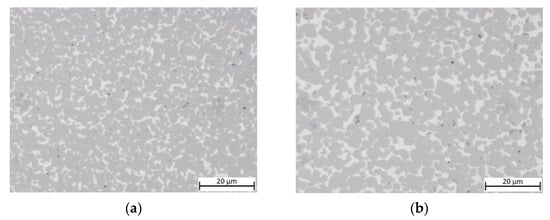
Figure 3.
Light optical images of cermets: (a) sample 5/5-16-HEA with 16 vol-% and equimolar HEA binder; (b) sample 5/5-30-HEA with 30 vol-% and equimolar HEA binder.
3.3.1. Variation of Cu Content
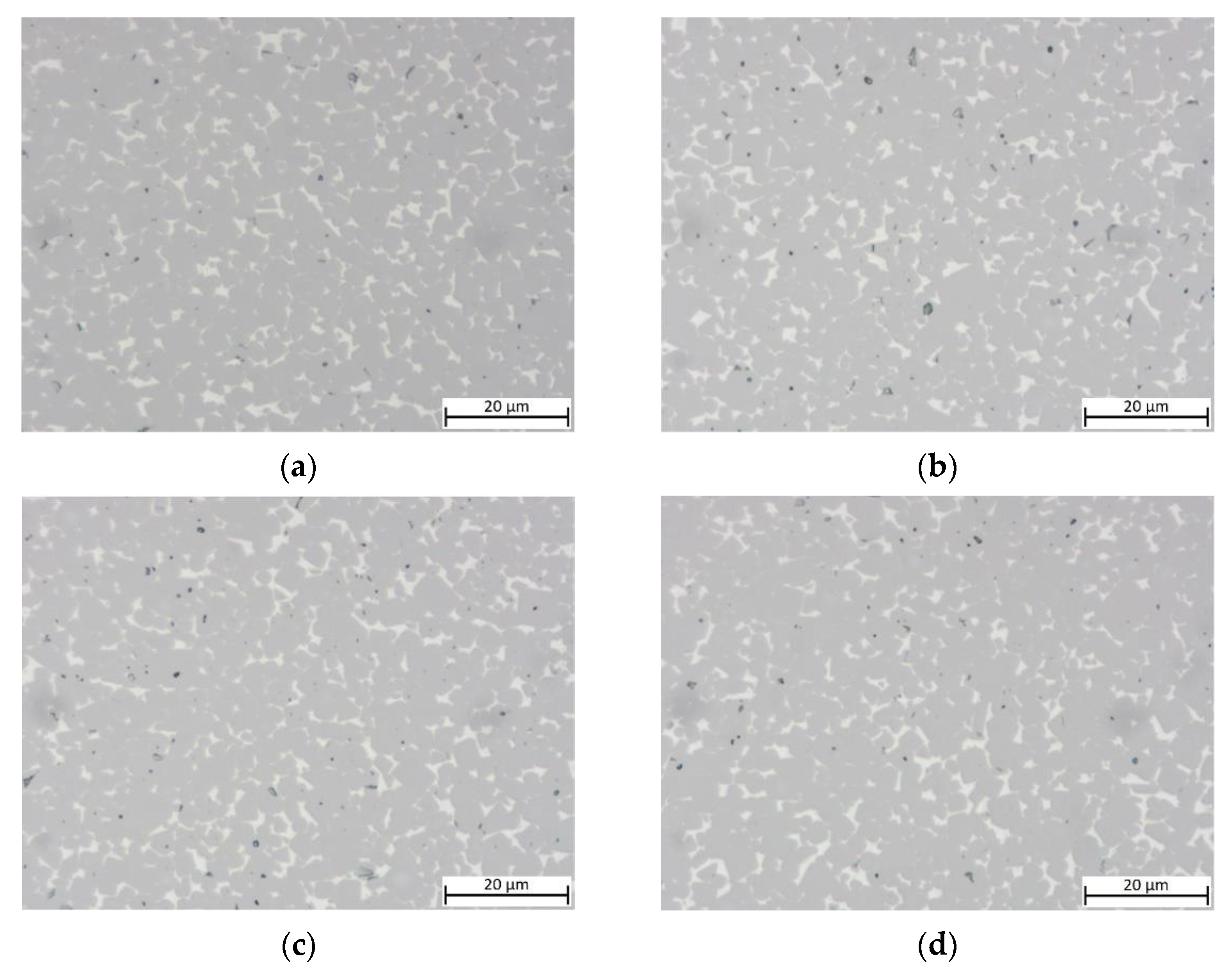
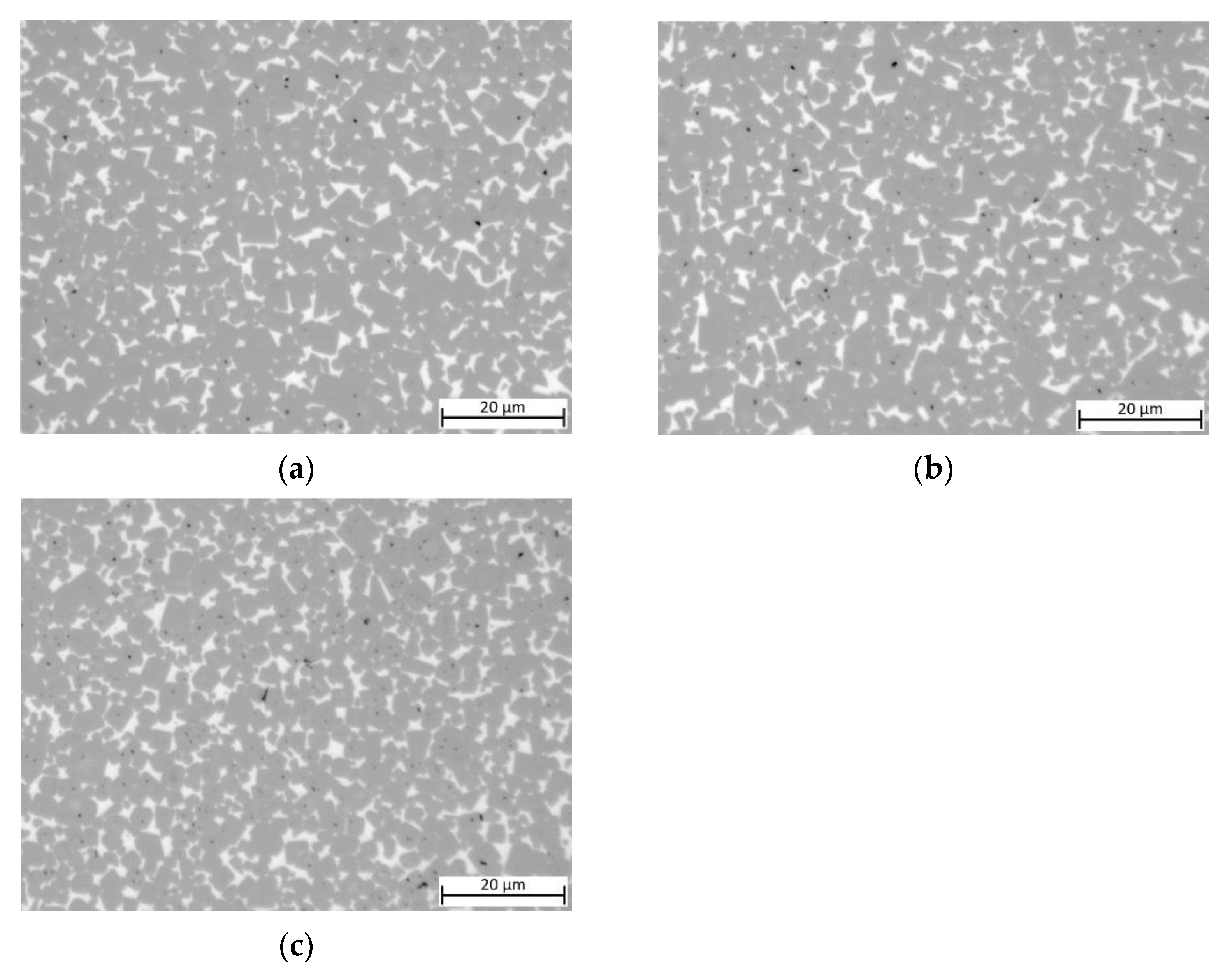
To study the influence of Cu content, samples with decreased Cu content (substituted with Ni) are shown in Figure 4. No clear difference between the samples with x = 0.05/0.10/0.15 as well as for the equimolar Cu-free MEA binder can be observed. All samples show an ISO porosity of A06 to A08, with no visible B porosity (pores > 10 µm) visible.
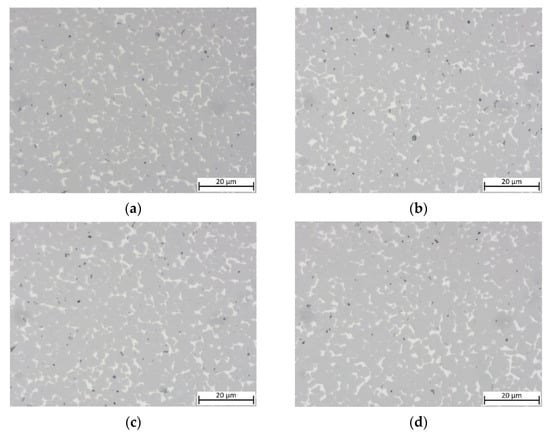
Figure 4.
Light optical images of cermets with different Cu contents: (a) sample 5/5-16-X0.05: Cu content reduced to 15 at-%; (b) sample 5/5-16-X0.10:Cu content reduced to 10 at-%; (c) sample 5/5-16-X0.15: Cu content reduced to 5 at-%; (d) sample 55/5-16-MEA: —equimolar four component Mn0.25Fe0.25Co0.25Ni0.25 HEA binder, composition free of Cu.
3.3.2. Increase in C/N Ratio and Reference Cermet
Cermets produced with TiCN with a C/N ratio of 70/30 (see Figure 5) show a similar microstructure as compared to the previously used TiCN with a C/N ratio of 50/50. Additionally, the CoNi-bonded cermet shows a slight porosity, and its microstructure cannot be differentiated from the HEA- or MEA-based ones.
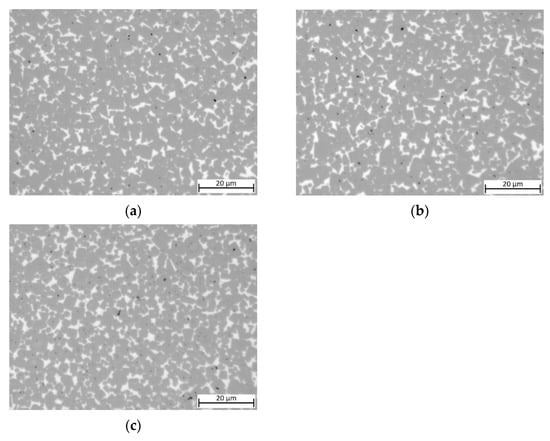
Figure 5.
Light optical images of cermets with a C/N ratio within TiCN of 70/30: (a) 7/3-16-X0.10— Cu content reduced to 10 at-%; (b) sample 7/3-16-MEA—equimolar four component Fe0.25Ni0.25Co0.25Mn0.25 HEA binder, free of Cu; (c) reference cermet sample Ref. 5/5-16 CoNi—with a two component Co-Ni binder.
3.4. Investigations Using FESEM
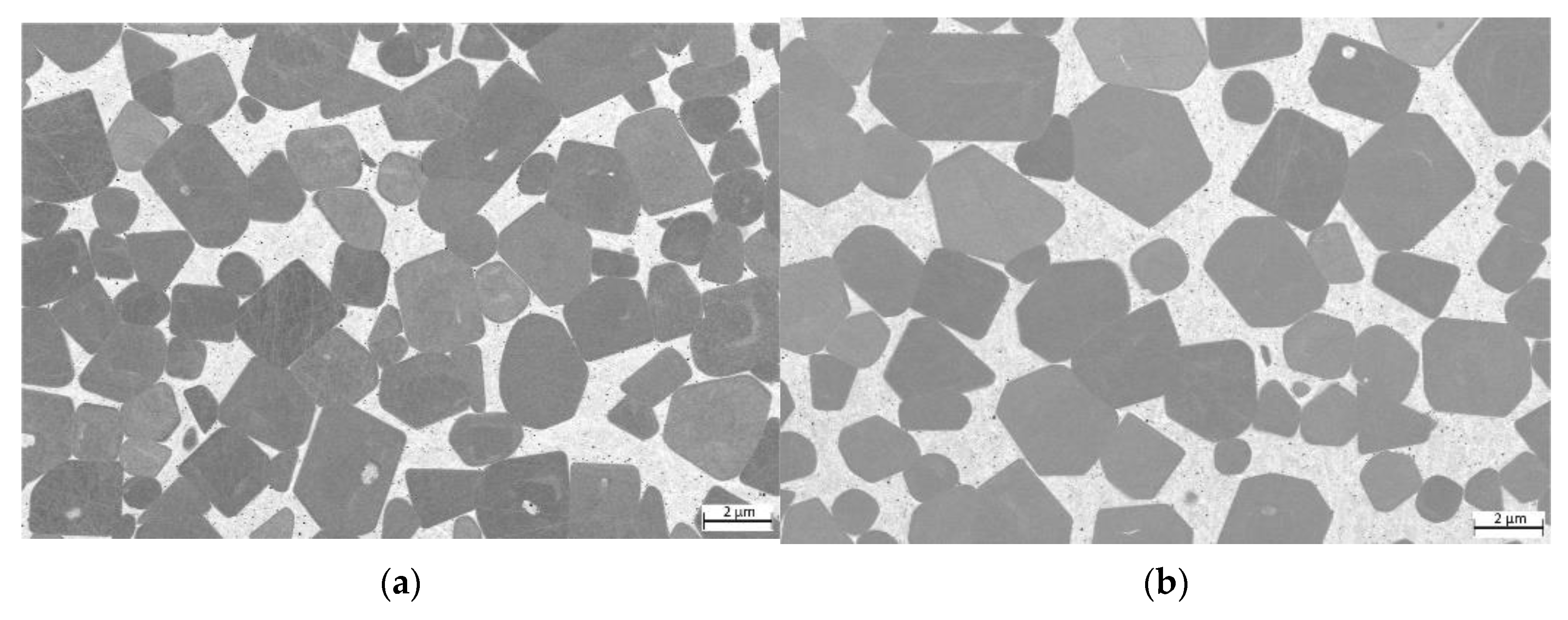
Further investigations were carried out using a field emission scanning microscope. The equimolar HEA-bonded cermets with binder volumes of 16 and 30%, respectively, are shown in Figure 6. As can be seen, just a two-phase microstructure with TiCN hard phase grains (dark grey) and a seemingly homogenous binder phase (light grey) can be observed. In some cases, a core–shell structure of the TiCN hard phase grains is visible, due to the dissolution of N out of the TiCN grains.
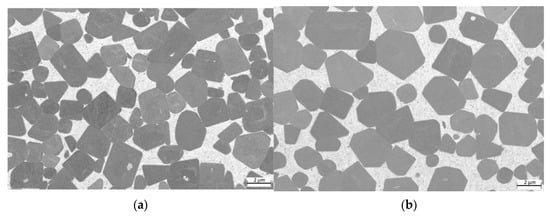
Figure 6.
ESB images of (a) sample 5/5-16-HEA with 16 vol-% of HEA binder; (b) sample 5/5-30-HEA with 30 vol-% of HEA binder.
3.4.1. Variation of Cu Content
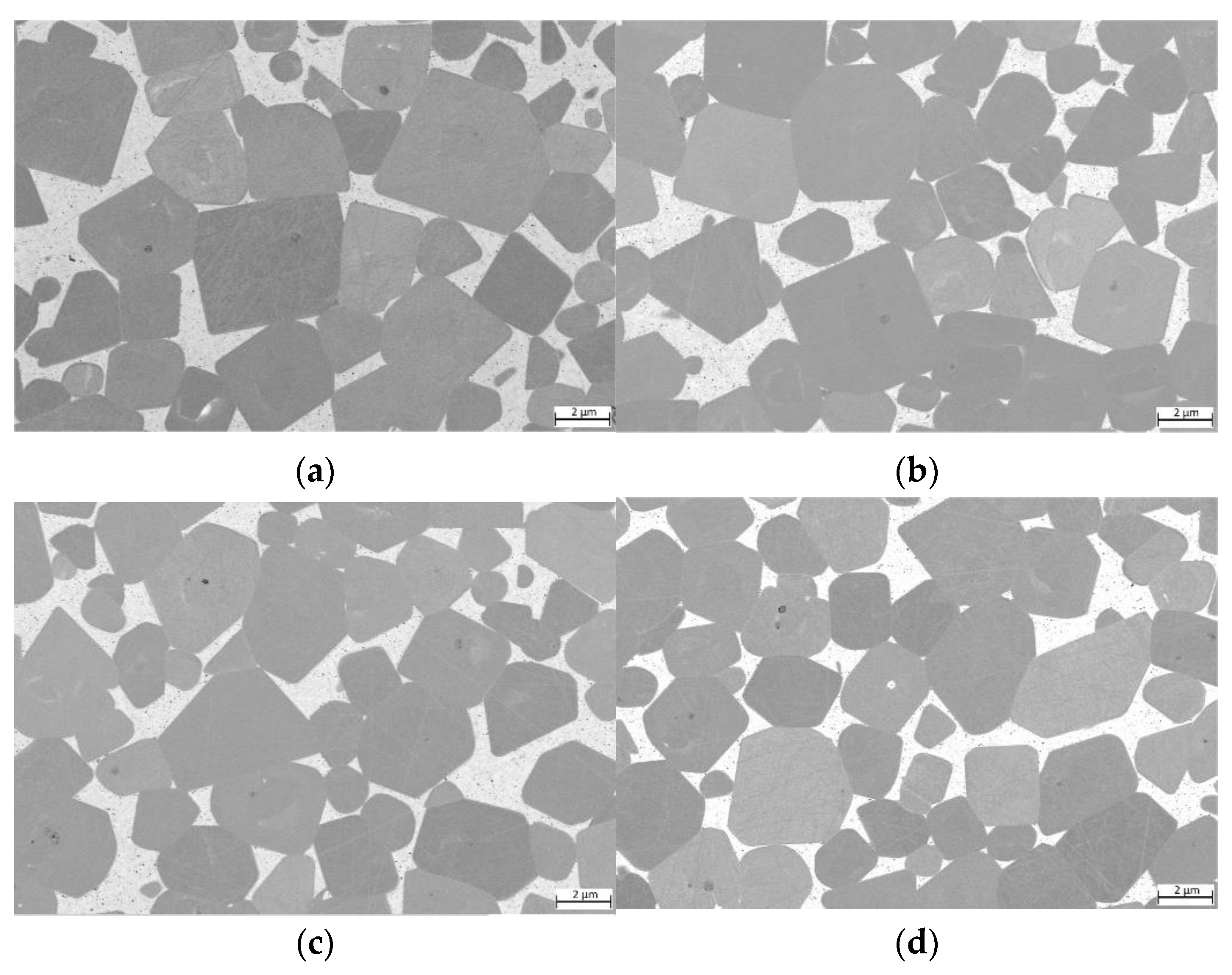
For samples with decreasing Cu content (Figure 7), no distinct differences can be observed. TiCN grain size is in the same range of 2 to 5 µm, similar to the pure HEA-based cermet shown in Figure 6a. Wetting of TiCN is in all cases complete and no areas with unwetted TiCN surfaces can be observed.
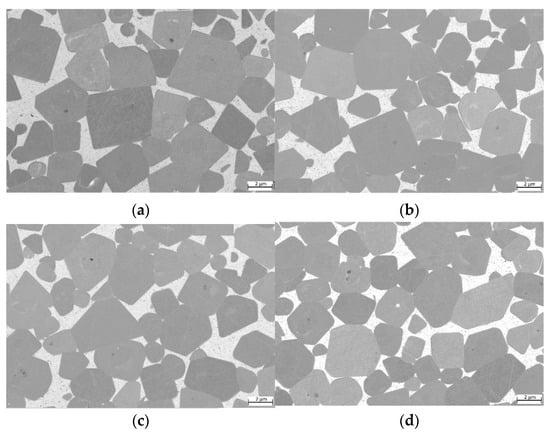
Figure 7.
ESB images of cermets with different Cu contents: (a) sample 5/5-16-X0.05—Cu content reduced to 15 at-%; (b) sample 5/5-16-X0.10—Cu content reduced to 10 at-%; (c) sample 5/5-16-X0.15—Cu content reduced to 5 at-%; (d) sample 5/5-5/5-16-MEA—equimolar four component Fe0.25Ni0.25Co0.25Mn0.25 HEA binder free of Cu.
3.4.2. Increase in C/N Ratio and Reference Cermet
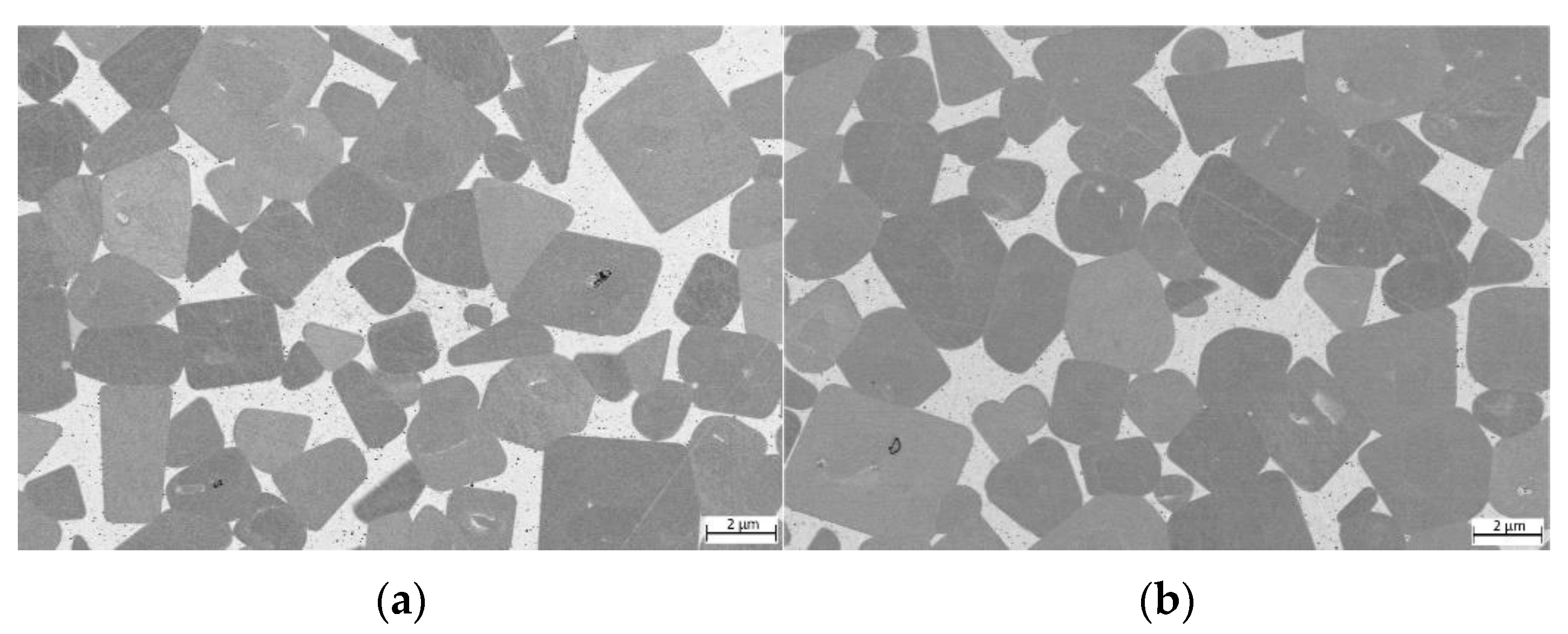
For samples where TiCN particles with a C/N ratio of 70/30 (see Figure 8) were used, the microstructure seems to be slightly finer. However, no grain size measurements according to ISO 4499 were performed.
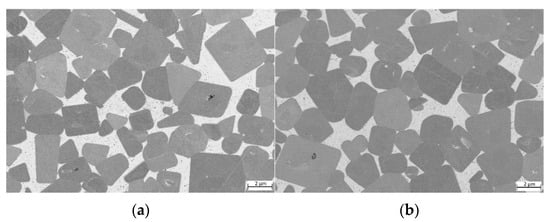
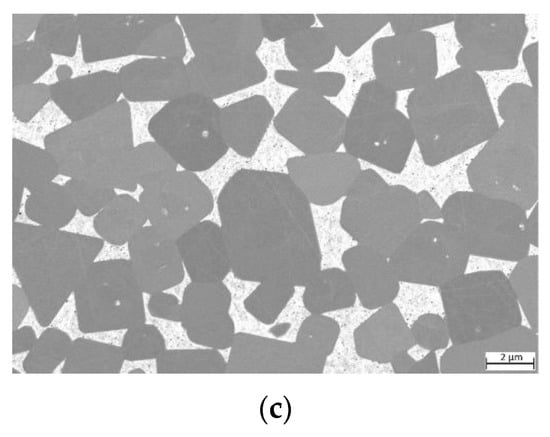
Figure 8.
ESB images of cermets with a C/N ratio within TiCN of 70/30: (a) sample 7/3-16-X0.10—Cu content reduced to 10 at-%; (b) sample 7/3-16-MEA—equimolar four component Fe0.25Ni0.25Co0.25Mn0.25 MEA binder free of Cu; (c) reference cermet sample Ref. 5/5-16 CoNi—with a two component CoNi binder.
3.5. Investigations Using EDS Mappings
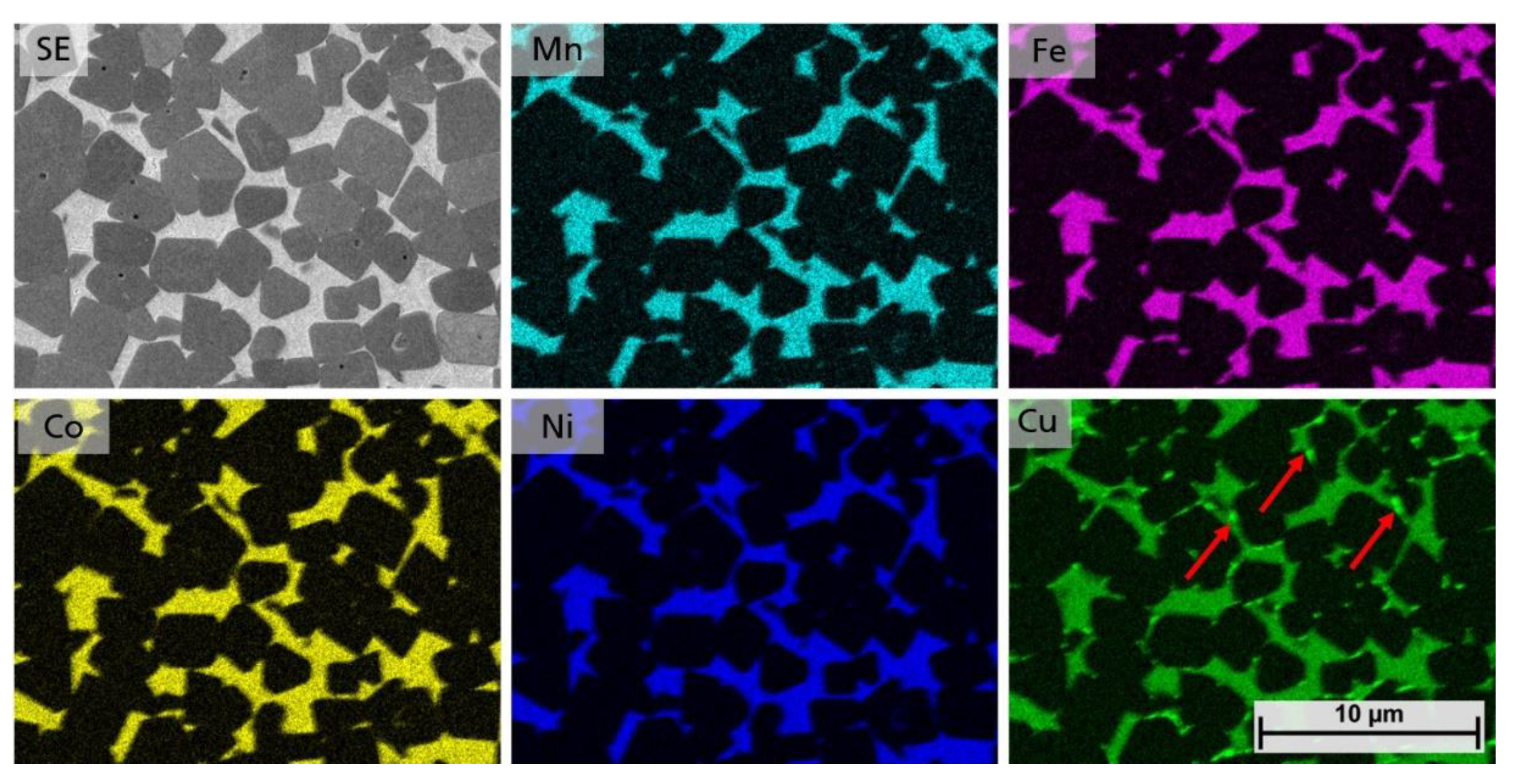
Since no distinct differences could be seen in the microstructures, EDS mappings were performed to investigate the element distribution within the binder phase and to answer at which Cu contents a single solid solution HEA phase occurs. In Figure 9 an EDS mapping of the equimolar sample 5/5-16-HEA is shown. The binder phase clearly consists of all HEA elements. However, for Cu small brighter spots indicate the precipitation of a secondary binder phase, as indicated by arrows.
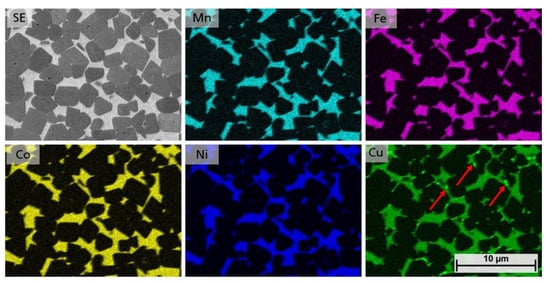
Figure 9.
EDS mapping of samples 5/5-16-HEA, showing the distribution of Mn, Fe, Co, Ni and Cu.
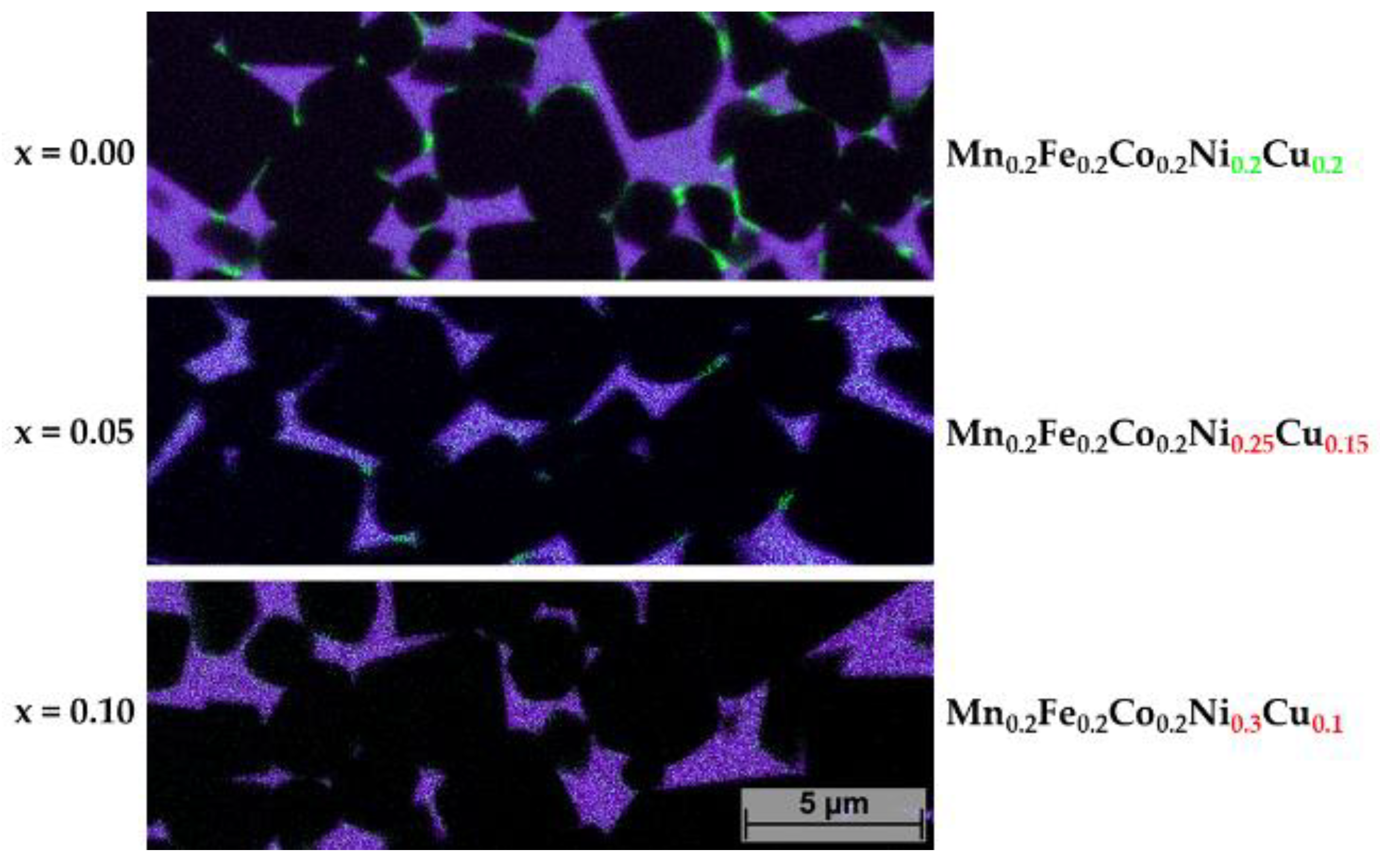
For further investigations, samples with decreasing Cu content were studied in a similar way. To investigate the Cu-rich precipitations more closely, EDS mapping images were prepared in which just Cu in comparison to all other HEA elements are presented. In Figure 10, the occurrence of Cu-rich precipitations as a function of decreasing Cu content is shown. Already at x = 0.05 (Cu reduced to 0.15 at-%), only a few Cu precipitations can be seen. As in the HEA (x = 0) composition, they all seem to segregate at TiCN grains. No precipitations within larger HEA binder areas were found. At x = 10, no more precipitations of Cu could be found. This is the same case for sample 5/5-16-X0.15 and 5/5-16-MEA in which the Cu contents were reduced further (not shown).
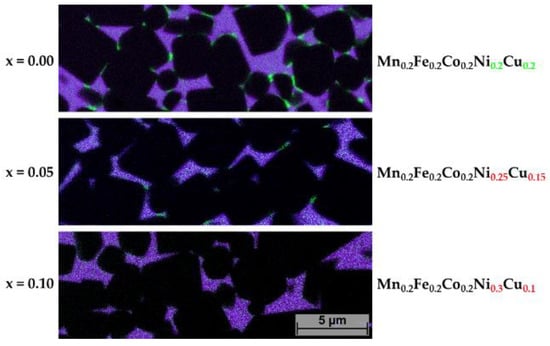
Figure 10.
EDS mapping of samples 5/5-16-HEA, 5/5-16-X0.05 and 5/5-16-X0.05, showing Cu distribution in green and all HEA elements in purple.
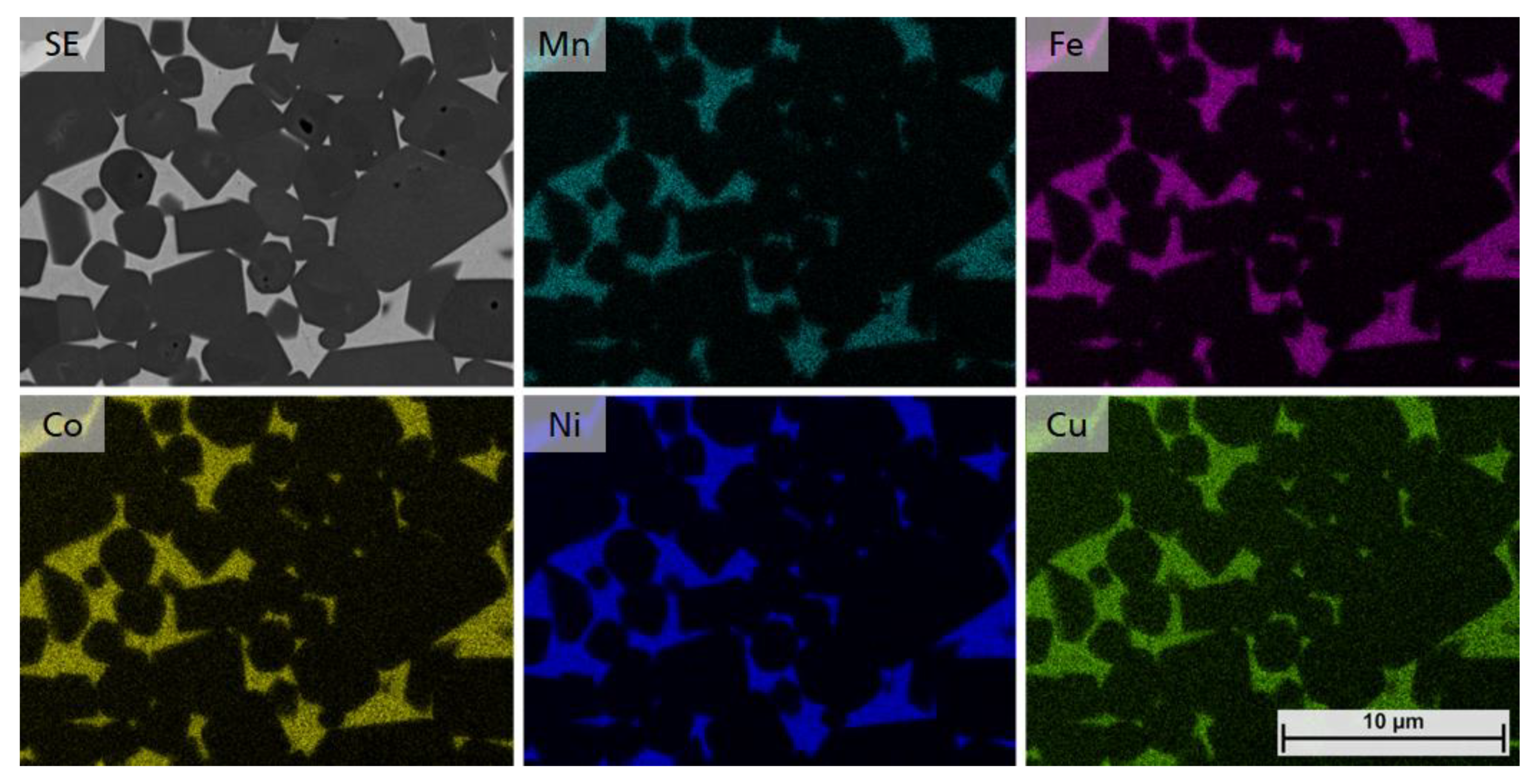
As shown in Figure 11, distribution of all HEA elements in the composition of samples 5/5-16-X0.10 (Mn0.2Fe0.2Co0.2Ni0.30Cu0.10) show that a single solid solution HEA binder phase can be achieved by adjusting the Cu content to values of x ≥ 0.10.
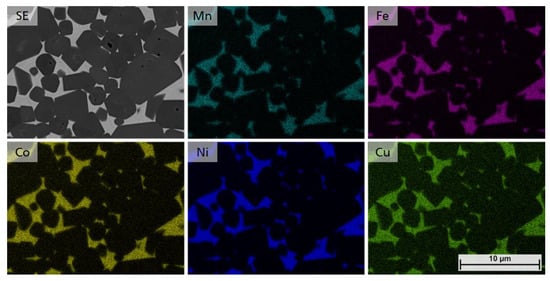
Figure 11.
EDS mapping of samples 5/5-16-X0.10, showing the even distribution of Mn, Fe, Co, Ni and Cu.
3.6. X-ray Analysis
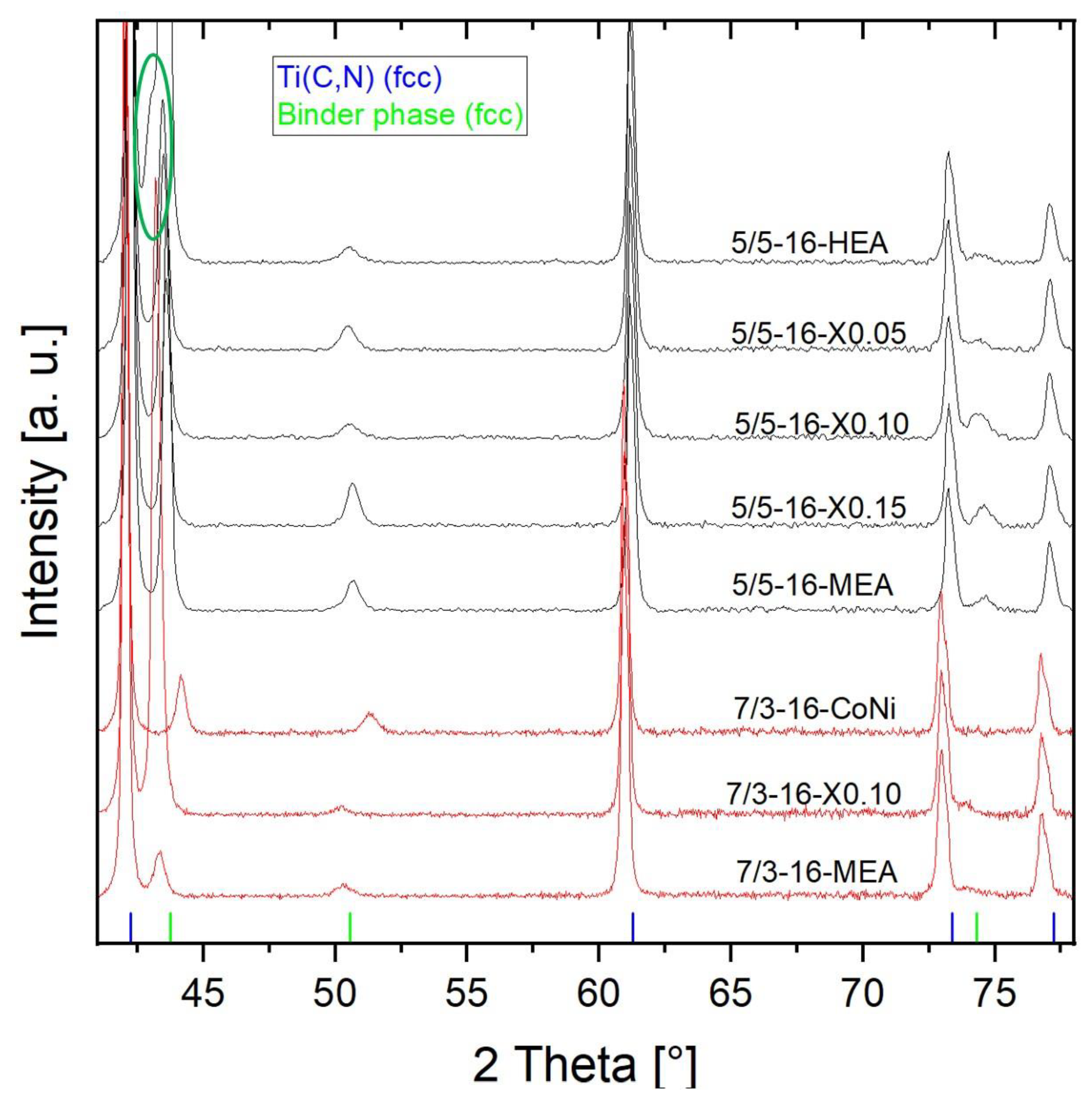
For phase analysis, all samples were characterized by XRD. As shown in Figure 12, only two FCC phases (both Fm-3m) can be clearly detected. These are TiCN hard phases, with slightly higher 2 Theta angles for the TiCN 50/50 (in black) than for the TiCN 70/30 (in red) and the binder phase. However, for the HEA sample 5/5-16-HEA with equimolar composition, a shoulder (encircled in green) next to the HEA binder reflex at around 43° 2 Theta is slightly visible. This indicates a second phase that can be identified as Cu-rich, because of the good alignment with the reflex for pure Cu (Powder Diffraction File 03-065-9026), which is clearly distinguishable from the HEA binder peak at this position. Further changes in the HEA/MEA FCC binder phase with decreased Cu content are visible by a slight change in binder peaks to higher 2 Theta angles. The reference cermet based on a 50/50 Co-Ni binder shows a significantly higher 2 Theta angle, due to a smaller lattice parameter of pure Co and Ni.
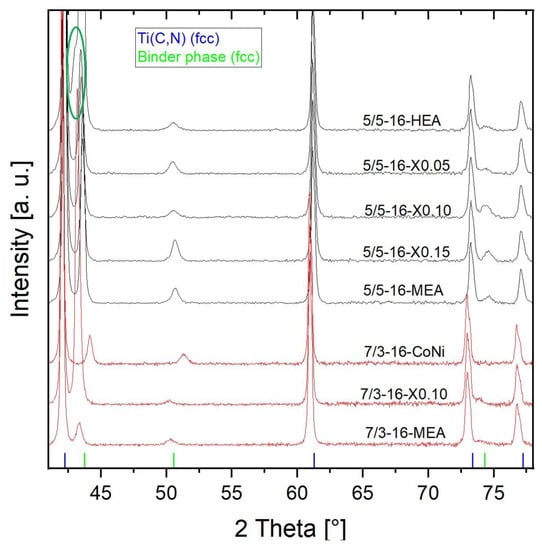
Figure 12.
X-ray diffraction pattern of all 16 vol-% binder based cermets.
For samples with continuously decreased Cu content, the lattice parameters calculated using Rietveld (Pawley fit algorithm) are shown in Table 4. Here, a clear trend of a reduced lattice parameter can be seen with increasing x, and accordingly, decreasing content of Cu and increasing content of Ni.

Table 4.
Lattice parameters with decreasing Cu content.
4. Discussion
High entropy alloys used in cermets so far mostly included strong oxide- and carbide-forming elements such as Al, Ti or Cr. To investigate HEA compositions that are free of such elements, novel HEA-based binder systems consisting of Co, Ni, Fe, Mn and Cu, which have not been prepared before, were studied. The thermodynamic calculations predicted at an equimolar composition the formation of a secondary Cu-rich binder phase. Since thermodynamic calculations with TiCN are difficult due to the large C and N solubility range, experimental investigations were performed to find out if the predicted Cu-rich binder phase forms in the presence of TiCN, and if in turn, other reactions take place that change the occurrence of hard or binder phases.
For the equimolar HEA samples, the presence of this secondary binder phase is only visible using FESEM investigations. Interestingly, the microstructure of all cermets with equivalent binder content and TiCN grade looks quite similar in light optical images. Even though no grain size measurements were determined, it seems that the investigated change in HEA composition has no major effect on grain growth during sintering. Cu precipitations where only found at TiCN–binder interfaces, which suggests that during cooling of samples the HEA phase gradually precipitates and due to limited solid solubility the remaining liquid enriches with Cu. The location of the Cu-rich areas indicates a temperature gradient with the highest temperatures close to the surface of the TiCN grains. Due to the lower thermal conductivity of TiCN compared to the liquid binder alloy, the hard phase grains cool more slowly. Additionally, during solidification of the HEA alloy, the enthalpy of crystallization is released into the surrounding liquid. Therefore, the remaining liquid is located at the surface of the TiCN grains before it solidifies and forms the Cu-rich segregations. However, since no thermal conductivity measurements of cermets, nor the pure HEA or MEA binder compositions and TiCN hard phases have been performed yet, no measurement can validate this assumption for now.
Within studied bulk properties (density, magnetic properties and mechanical properties) the biggest differences could be found for a decreasing Cu content with significant changes in magnetic saturation (increases with decreasing Cu content) and with a slightly increased hardness (with decreasing Cu content). Since the decrease in Cu content was compensated with a simultaneous increase in Ni, the increase in magnetic saturation can be explained by the increase in ferromagnetic elements (Co, Fe and Ni).
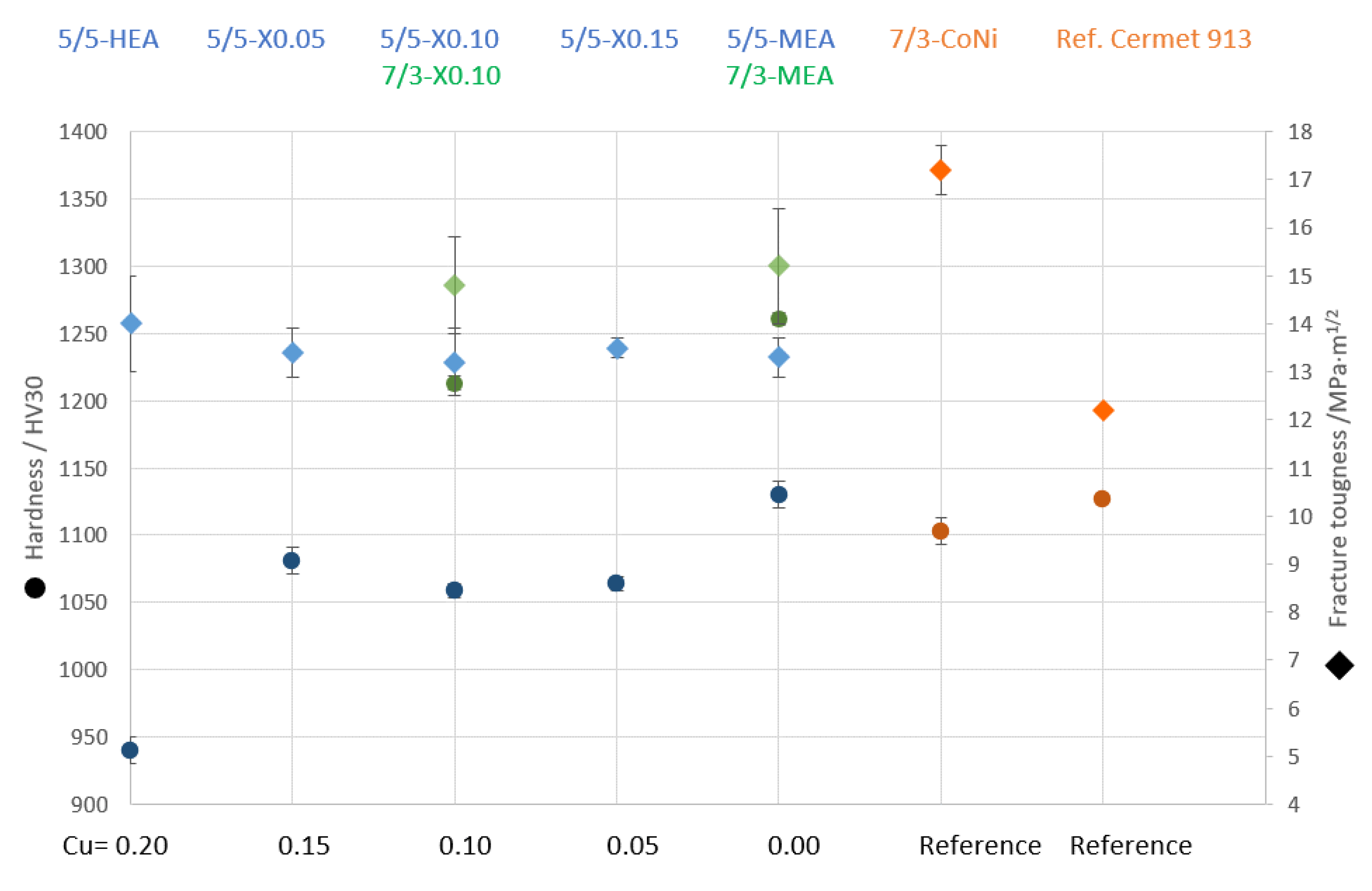
In Figure 13, hardness and fracture toughness values are shown as function of Cu content.
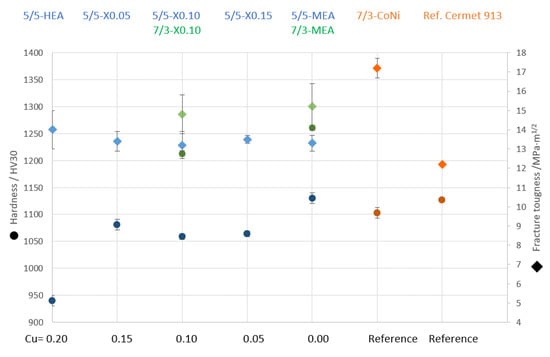
Figure 13.
Comparison of hardness and fracture toughness vs. Cu content within HEA and vs. C/N ratio of TiCN-based cermets. Blue indicates TiCN 50/50; green indicates TiCN 70/30; orange indicates reference samples.
It can be seen that the hardness increases by more than 100 HV30 units by decreasing the Cu content within the binder phase by just 5 at-%. This shows that the secondary Cu-rich phase significantly influences the hardness. It is expected that the hardness of these evenly distributed Cu-rich grains is significantly lower than the surrounding HEA binder phase. Interestingly, no significant difference in fracture toughness for HEA-bonded cermets with equimolar or adjusted composition can be seen. A much larger influence on mechanical properties is visible when cermets with C/N ratios of 50/50 and 70/30 are compared. With 70/30, both the hardness and the fracture toughness values are increased. This increase is most likely due a slightly finer TiCN grain size (increase in hardness) and increase in interphase bonding strength of the HEA–TiCN grain boundaries. Here, the higher C content within the TiCN hard phase is thought to increase the wettability of the binder [36] and a stronger interface which in turn is more crack resistant (higher fracture toughness). The highest hardness and fracture toughness of all studied HEA/MEA cermets was interestingly found with the Cu-free MEA composition consisting just of Co, Ni, Fe and Mn. Here, both the cermets with TiCN 50/50 and TiCN 70/30 showed significantly higher values as compared to an HEA cermet with a Cu content of 10 at-%. Both compositions are also favorable in comparison with the CoNi-based reference cermets with regard to a higher hardness and partially higher fracture toughness. However, the studied MEA binder composition is different to the HEA not just by the absence of Cu but also by the increase in all other elements to 25 at-%. Thus, any beneficial effects due to the higher amount of Co, Fe or Mn cannot be differentiated from the studied investigation of lower Cu and simultaneously increased Ni contents.
5. Conclusions
Cermets with novel MnFeCoNiCu HEA-based binder compositions with different binder values and two different C/N rations of the TiCN hard phase were studied for the first time. As predicted by thermodynamic calculations and validated by experimental investigations, equimolar compositions of MnFeCoNiCu HEA-based binder resulted in the precipitation of a secondary Cu-rich FCC binder phase. Only by reduction in Cu content by 50% and a simultaneous increase in Ni by 50%, a single solid solution binder phase could be obtained. The cermet with adjusted HEA binder phase shows similar two-phase microstructures as studied reference cermets with a conventional CoNi binder composition. Additionally, mechanical properties for a TiCN 70/30 cermet with 16 vol-% of a Mn0.2Fe0.2Co0.2Ni0.2Cu0.1 binder phase are comparable or even better than the conventional reference cermets, with values of hardness >1210 HV30 and a fracture toughness of 14.8 MPam1/2. TiCN cermets with a copper-free MEA binder composition consisting of only Co, Ni, Fe and Mn had a two-phase microstructure as well and higher hardness and fracture toughness than all studied HEA binders.
Further studies are planned to investigate the influence of substituting Cu not by Ni but by Fe or Co. Additionally, other hard phases such as WC, NbC, TiC or novel High Entropy Carbides (HECs) [37,38,39,40] should be investigated. With regard to properties, measurement of bending strength as well as thermo-physical properties (thermal conductivity, electric conductivity, etc.) are planned.
Author Contributions
Conceptualization, J.P. and A.V.; methodology, J.P.; software, M.v.S.; validation, M.v.S. and J.P.; formal analysis, M.v.S.; investigation, M.v.S. and J.P.; writing—original draft preparation, J.P.; writing—review and editing, J.P., M.v.S. and A.V.; supervision, J.P.; project administration, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, J.P., upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lengauer, W.; Scagnetto, F. Ti(C,N)-Based Cermets: Critical Review of Achievements and Recent Developments. Solid State Phenom. 2018, 274, 53–100. [Google Scholar] [CrossRef]
- Pötschke, J.; Höhn, S.; Richter, V.; Mayer, M. Microstructural evolution during sintering of cermets studied using interrupted sintering experiments and novel 2D and 3D FESEM based techniques. Int. J. Refract. Hard Mater. 2017, 63, 47–54. [Google Scholar] [CrossRef]
- Ettmayer, P.; Kolaska, H.; Lengauer, W.; Dreyer, D. Ti(C,N)Cermets—Metallurgy and Properties. Int. J. Refract. Hard Mater. 1995, 13, 343–351. [Google Scholar] [CrossRef]
- ECHA. Substance Infocard: Cobalt. Available online: https://echa.europa.eu/de/substance-information/-/substanceinfo/100.028.325 (accessed on 20 June 2023).
- ECHA. Substance Infocard: Nickel. Available online: https://echa.europa.eu/de/substance-information/-/substanceinfo/100.028.283 (accessed on 20 June 2023).
- European Commission. Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability. Available online: https://ec.europa.eu/docsroom/documents/42849 (accessed on 20 June 2023).
- Pittari, J.J.; Murdoch, H.A.; Kilczewski, S.M.; Hornbuckle, B.C.; Swab, J.J.; Darling, K.A.; Wright, J.C. Sintering of tungsten carbide cermets with an iron-based ternary alloy binder: Processing and thermodynamic considerations. Int. J. Refract. Hard Mater. 2018, 76, 1–11. [Google Scholar] [CrossRef]
- Gries, B.; Prakash, L. Cobalt free binder alloys for Hard Metals: Consolidation of ready-to-press powder and sintered properties. H C Stark. 2007. [Google Scholar]
- Humphry-Baker, S.A.; Marshall, J.M.; Smith, G.; Lee, W.E. Thermophysical properties of Co-free WC-FeCr hardmetals. In Proceedings of the 19th International Plansee Seminar; Plansee SE: Reutte, Austria, 2017; p. HM19, p. 1–13. [Google Scholar]
- Hinners, H.; Konyashin, I.; Ries, B.; Petrzhik, M.; Levashov, E.A.; Park, D.; Weirich, T.; Mayer, J.; Mazilkin, A.A. Novel hardmetals with nano-grain reinforced binder for hard-facings. Int. J. Refract. Hard Mater. 2017, 67, 98–104. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Tang, H.; Ma, X.; Zhao, W. Investigation on the mechanical properties of WC–Fe–Cu hard alloys. J. Alloys Compd. 2015, 632, 729–734. [Google Scholar] [CrossRef]
- Hanyaloglu, C.; Aksakal, B.; Bolton, J.D. Production and indentation analysis of WC/Fe-Mn as an alternative to cobalt-bonded hardmetals. Mater. Charact. 2001, 47, 315–322. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Vilhena, L.M.; Pinho, C.; Oliveira, F.J.; Soares, E.; Sacramento, J.; Senos, A. Mechanical characterization of WC–10 wt% AISI 304 cemented carbides. Mater. Sci. Eng. A 2014, 618, 629–636. [Google Scholar] [CrossRef]
- Tarraste, M.; Kübarsepp, J.; Juhani, K.; Mere, A.; Kolnes, M.; Viljus, M.; Maaten, B. Ferritic chromium steel as binder metal for WC cemented carbides. Int. J. Refract. Hard Mater. 2018, 73, 183–191. [Google Scholar] [CrossRef]
- Qian, C.; Li, K.; Guo, X.; Liu, B.; Long, Z.; Liu, Y. Effect of WC grain size on mechanical properties and microstructures of cemented carbide with medium entropy alloy Co-Ni-Fe binder. J. Cent. South Univ. 2020, 27, 1146–1157. [Google Scholar] [CrossRef]
- Soria-Biurrun, T.; Sánchez-Moreno, J.M.; Frisk, K. Experimental and theoretical study of WC-40Fe-20Co-40Ni. Int. J. Refract. Hard Mater. 2022, 102, 105719. [Google Scholar] [CrossRef]
- Prakash, L. A Review of the Properties of Tungsten Carbide Hardmetals with Alternative Binder Systems. In Proceedings of the 13th International Plansee Seminar, Plansee SE, Reutte, Austria, 24–28 May 2013; Bildstein, H., Eck, R., Eds.; Plansee Metall AG: Reutte, Austria, 1993; Volume 2. HM 9, 80–109. [Google Scholar]
- Ceratizit Catalog All Inserts. Available online: http://www.grasche.com/links/doc/Ceratizit-Catalog-All-Inserts.pdf (accessed on 20 June 2023).
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Ke, B.; Sun, Y.; Zhang, Y.; Wang, W.; Wang, W.; Ma, P.; Ji, W.; Fu, Z. Powder metallurgy of high-entropy alloys and related composites: A short review. Int. J. Min. Met. Mater. 2021, 28, 931–943. [Google Scholar] [CrossRef]
- Ruiz-Esparza-Rodriguez, M.A.; Garay-Reyes, C.G.; Estrada-Guel, I.; Garcia-Aguirre, K.A.; Guia-Tello, J.C.; Mendoza-Duarte, J.M.; Martinez-Sanchez, R. Effect of Sintering Temperature in Tungsten Carbides Bonded with High and Medium Entropy Alloys. Microsc. Microanal. 2022, 28, 2802–2804. [Google Scholar] [CrossRef]
- Straumal, B.; Konyashin, I. WC-Based Cemented Carbides with High Entropy Alloyed Binders: A Review. Metals 2023, 13, 171. [Google Scholar] [CrossRef]
- Fa, C.; Jungui, Z.; Shijia, L.; Keke, G.; Pinqiang, D.; Chao, L.; Xiaofeng, Z. The microstructure and high-temperature oxidation resistance of tungsten carbide with high entropy alloys as binder. J. Ceram. Soc. Jpn. 2022, 130, 477–486. [Google Scholar] [CrossRef]
- Luo, W.; Liu, Y.; Luo, Y.; Wu, M. Fabrication and characterization of WC-AlCoCrCuFeNi high-entropy alloy composites by spark plasma sintering. J. Alloys Compd. 2018, 754, 163–170. [Google Scholar] [CrossRef]
- Zhou, P.-F.; Xiao, D.-H.; Yuan, T.-C. Comparison between ultrafine-grained WC–Co and WC–HEA-cemented carbides. Powder Metall. 2017, 60, 1–6. [Google Scholar] [CrossRef]
- Li, X.; Wei, D.; Vitos, L.; Lizárraga, R. Micro-mechanical properties of new alternative binders for cemented carbides: CoCrFeNiW high-entropy alloys. J. Alloys Compd. 2020, 820, 153141. [Google Scholar] [CrossRef]
- Qian, C.; Liu, Y.; Cheng, H.; Li, K.; Liu, B. Effect of the carbon content on the morphology evolution of the η phase in cemented carbides with the CoNiFeCr high entropy alloy binder. Int. J. Refract. Met. Hard Mater. 2022, 102, 105731. [Google Scholar] [CrossRef]
- Soria-Biurrun, T.; Navarrete-Cuadrado, J.; Lozada-Cabezas, L.; Ibarreta-Lopez, F.; Martinez-Pampliega, R.; Sánchez-Moreno, J.M. Microstructure, mechanical properties and fracture behavior of NiCoCrTiAl and FeNiCoCr new alternative binders for WC based hardmetals. Int. J. Refract. Met. Hard Mater. 2022, 103, 105748. [Google Scholar] [CrossRef]
- Real, C.; Alcalá, M.D.; Trigo, I.; Fombella, I.; Córdoba, J.M. Fabrication and characterization of FeCoNiCrMn,(Al) high entropy alloy based (Ti,Ta,Nb)(C,N) cermet. Int. J. Refract. Met. Hard Mater. 2021, 101, 105694. [Google Scholar] [CrossRef]
- Prieto, E.; Vaz-Romero, A.; Gonzalez-Julian, J.; Guo, S.; Alvaredo, P. Novel high entropy alloys as binder in cermets: From design to sintering. Int. J. Refract. Met. Hard Mater. 2021, 99, 105592. [Google Scholar] [CrossRef]
- Wang, Z.; Du, J.; Su, G.; Sun, Y.; Zhang, C.; Kong, X. Microstructure, preparation and properties of TiC-Fe/ FeCoCrNiMn cermet with a core-rim structure. Vacuum 2022, 200, 110984. [Google Scholar] [CrossRef]
- Ezquerra, B.L.; Biurrun, T.S.; Cabezas, L.L.; Sánchez Moreno, J.M.; Lopez, F.I.; Pampliega, R.M. Sintering of WC hardmetals with Ni-Co-Cr-Ti-Al multi-component alloys. Int. J. Refract. Hard Mater. 2018, 77, 44–53. [Google Scholar] [CrossRef]
- Pötschke, J.; von Spalden, M.; Vornberger, A. Novel FeCoNiCuMn High Entropy Alloys as Binders for TiCN based Cermets. In Proceedings of the World PM 2022 Congress Proceedings, Lyon, France, 9–13 October 2022. [Google Scholar]
- Marković, B.; Živković, D.; Vřešt’ál, J.; Manasijević, D.; Minić, D.; Talijan, N.; Stajić-Trošić, J.; Todorović, R. Experimental study and thermodynamic remodeling of the Bi–Cu–Ni system. Calphad 2010, 34, 294–300. [Google Scholar] [CrossRef]
- Chychko, A.; García, J.; Collado Ciprés, V.; Holmström, E.; Blomqvist, A. HV-KIC property charts of cemented carbides: A comprehensive data collection. Int. J. Refract. Met. Hard Mater. 2022, 103, 105763. [Google Scholar] [CrossRef]
- Yasinskaya, G.A. The wetting refractory carbides borides and nitrides by molten metals. Powder Metall. Met. Ceram. 1966, 5, 557–559. [Google Scholar] [CrossRef]
- Pötschke, J.; Vornberger, A.; Gestrich, T.; Berger, L.-M.; Michaelis, A. Influence of different binder metals in high entropy carbide based hardmetals. Powder Metall. 2022, 65, 373–381. [Google Scholar] [CrossRef]
- Pötschke, J.; Dahal, M.; Herrmann, M.; Vornberger, A.; Matthey, B.; Michaelis, A. Preparation of high-entropy carbides by different sintering techniques. J. Mater. Sci. 2021, 56, 11237–11247. [Google Scholar] [CrossRef]
- Pötschke, J.; Dahal, M.; Vornberger, A.; Herrmann, M.; Michaelis, A. Production and Properties of High Entropy Carbide Based Hardmetals. Metals 2021, 11, 271. [Google Scholar] [CrossRef]
- Anwer, Z.; Vleugels, J.; Datye, A.; Zhang, S.; Huang, S. Influence of varying carbon content in (V,Nb,Ta,Ti,W)C high entropy carbide—Ni based cermets on densification, microstructure, mechanical properties and phase stability. Ceram. Int. 2023, 49, 4997–5012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).