Oil-Retention and Oil-Bearing Tribological Properties of Nanoporous Copper Prepared Using a Chemical Dealloying Method
Abstract
1. Introduction
2. Experiment
2.1. Preparation of Precursor Alloys and Bulk Nanoporous Copper
2.2. Material Characterizations
3. Results and Discussion
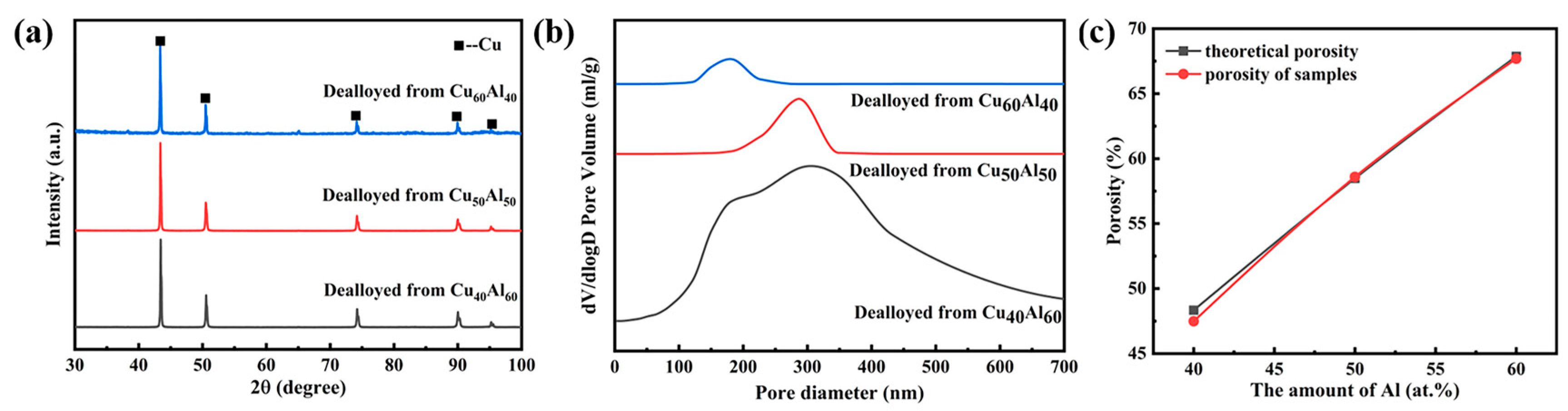
3.1. Preparation of Nanoporous Copper by Dealloying
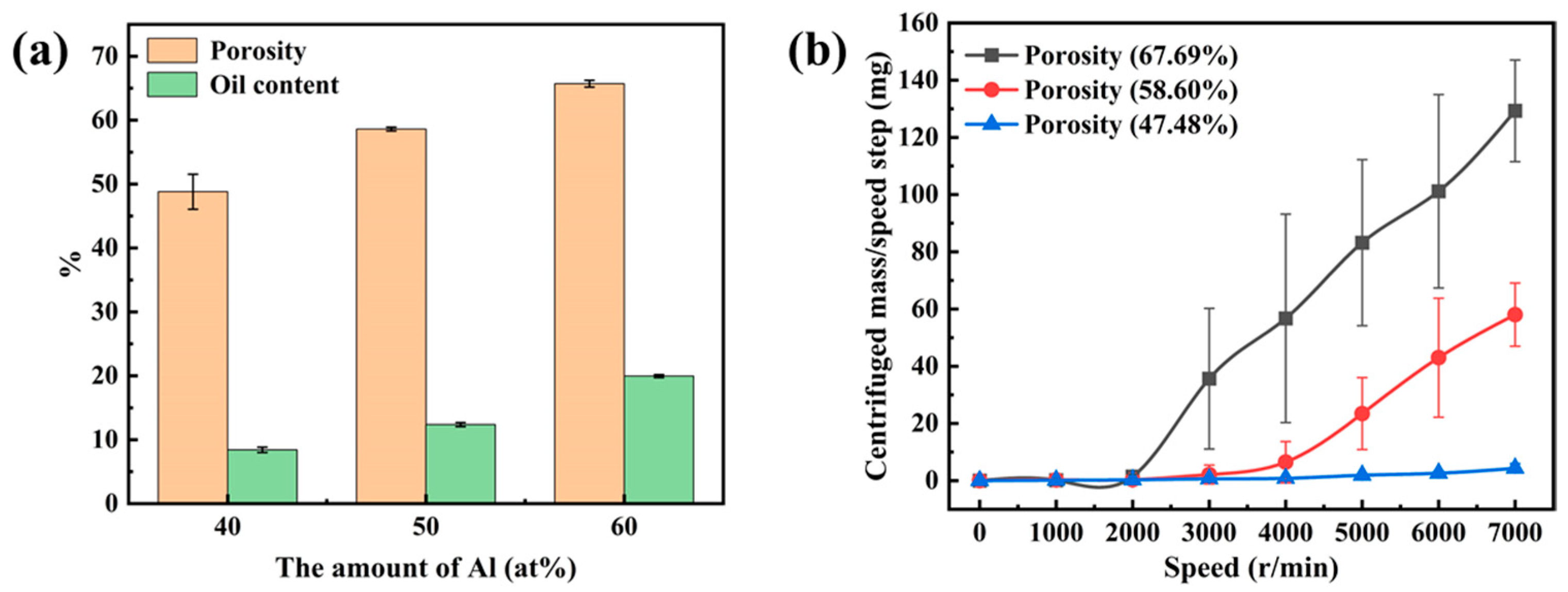
3.2. Oil-Retaining Capacity of Nanoporous Cu
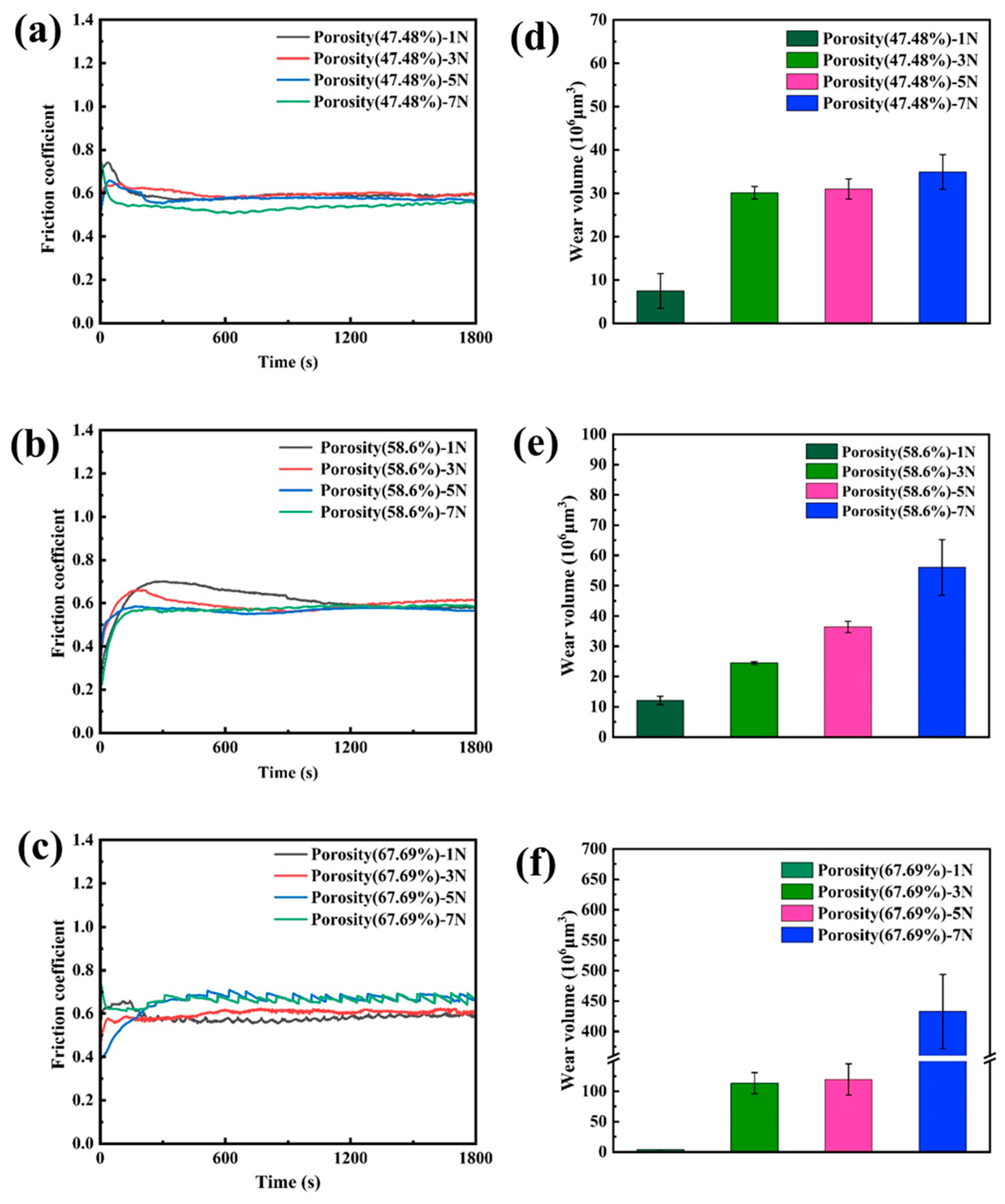
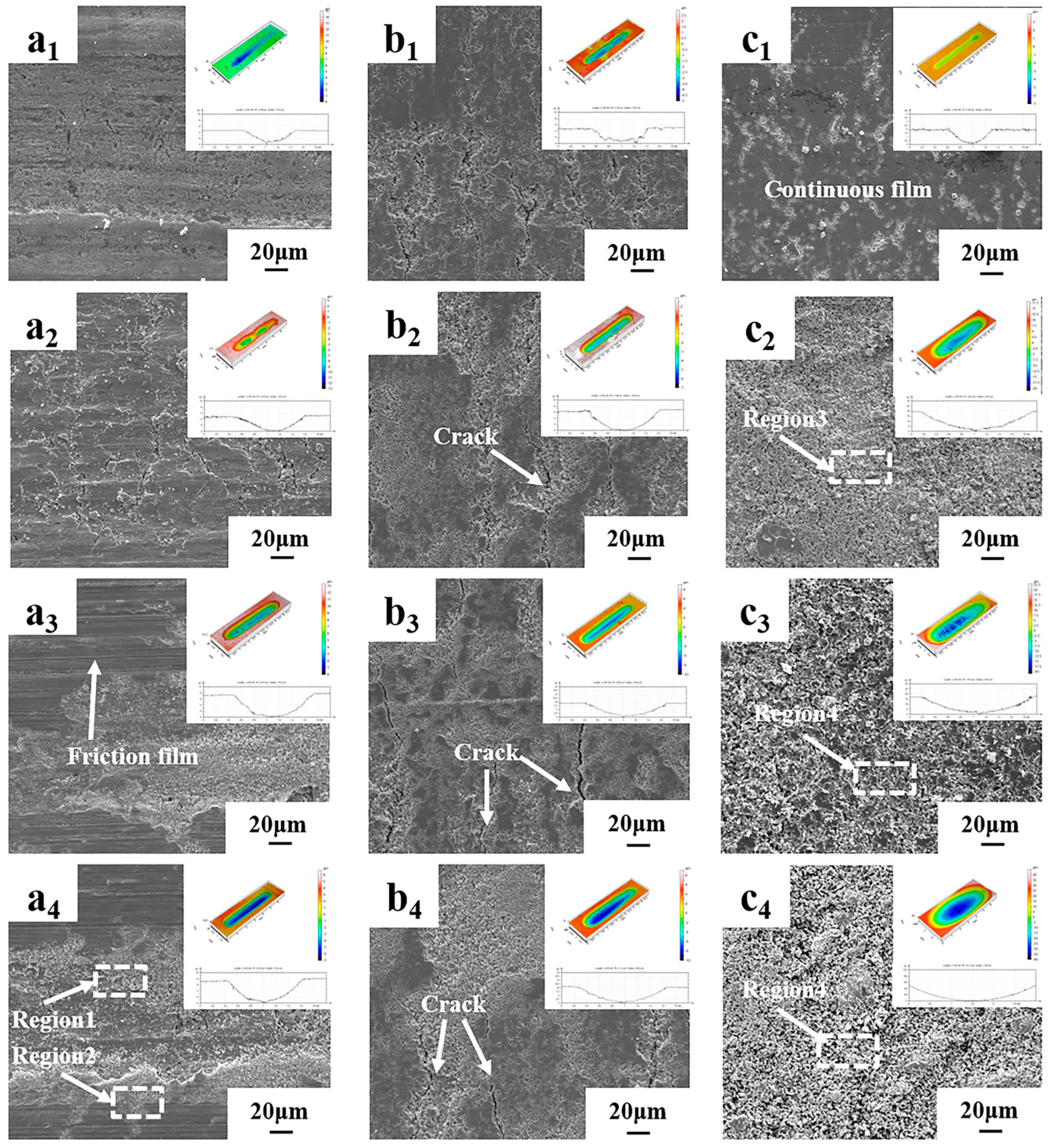
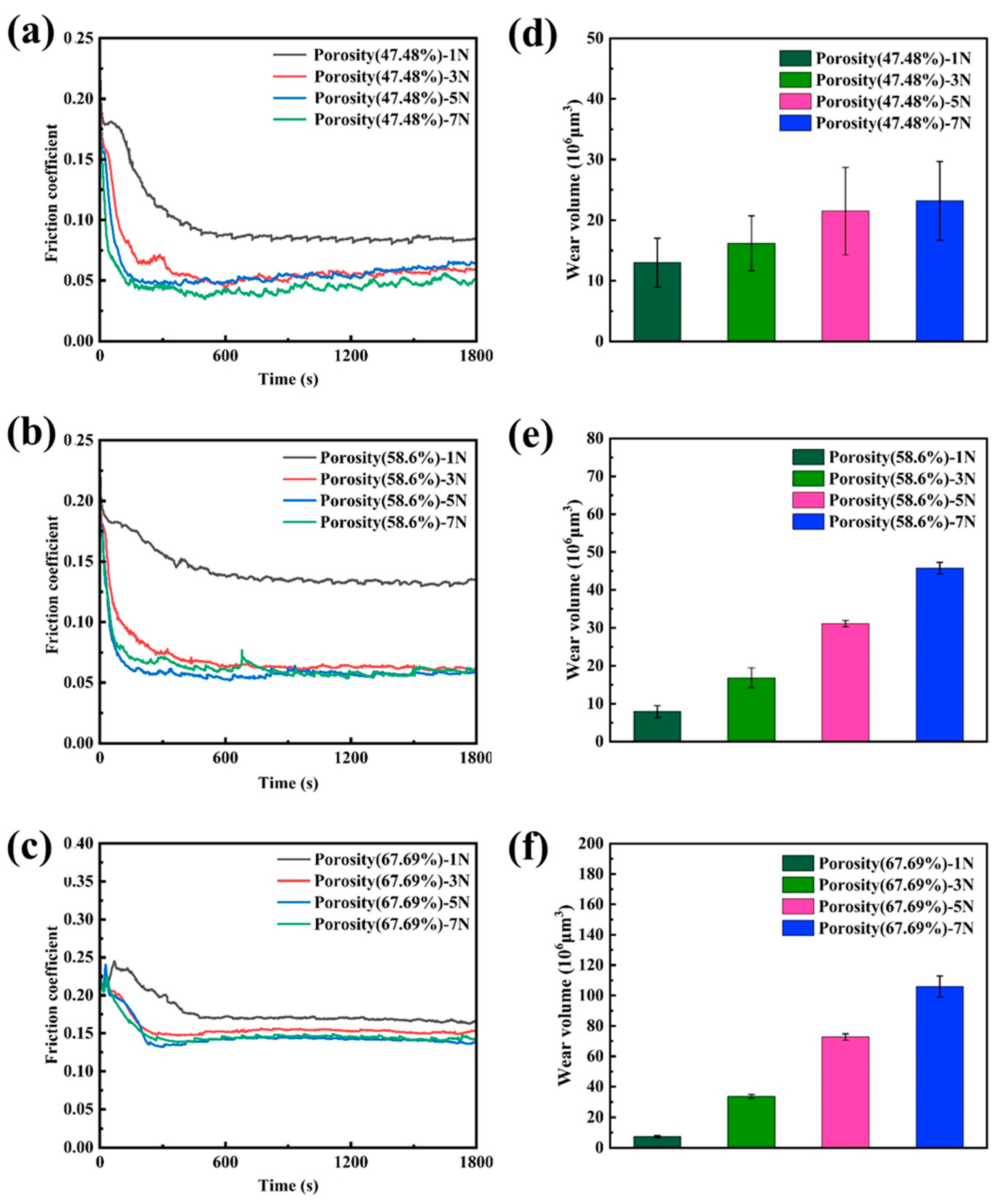
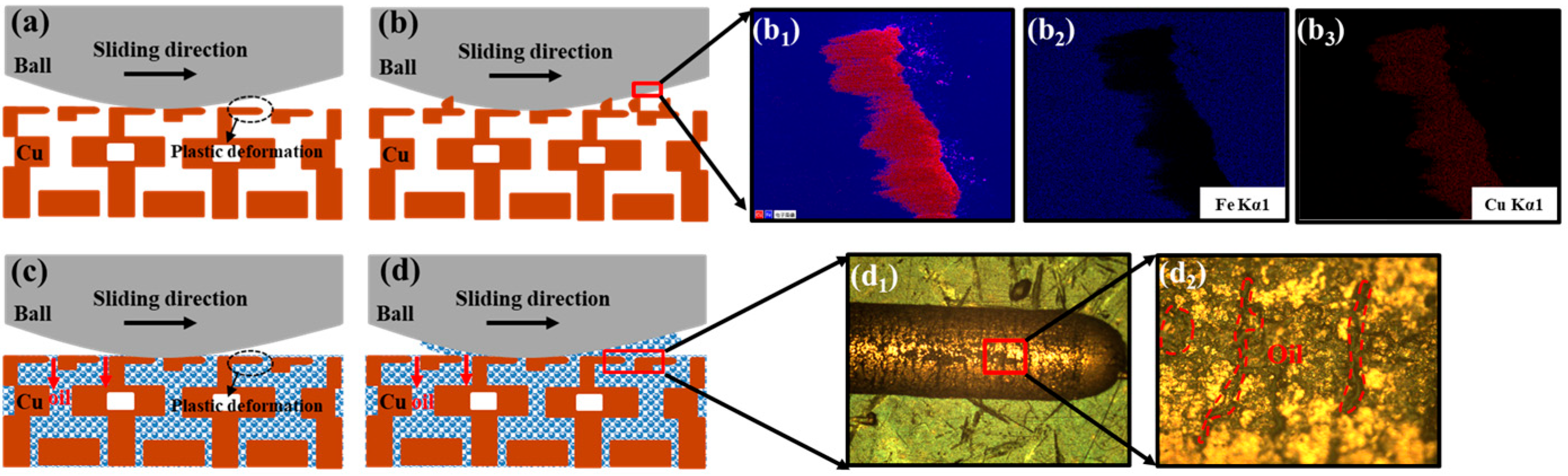
3.3. Friction Behavior of Nanoporous Copper
4. Conclusions
- By chemically dealloying Cu60Al40, Cu50Al50 and Cu40Al60 alloys in 10 wt.% H2SO4 solution at a temperature of 90 °C, bulk nanoporous copper (Փ15 × 2 mm) with consistent pore morphology was successfully produced. The pore structure increased from 204.35 nm to 309.06 nm, when the porosity increased from 47.48% to 67.69%.
- The oil content of porous copper increased with increasing porosity, reaching 20.18% on the specimen with the highest porosity of 67.69%. On the other hand, the oil-retention capacity of porous copper decreased with the increase in porosity. The nanoporous copper with a porosity about 47.48% can achieve a high oil retention of 97.12% at a centrifugal speed of 7000 (r/min).
- The dry friction effect of nanoporous copper can be ascribed to the formation of a transfer film, which makes the friction proceed steadily. The self-lubricating effect of oil-containing nanoporous copper can be attributed to the formation of a thick oil film on the friction surface, which reduced the friction coefficient with the lowest value about 0.047 and reduced the wear rate.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fusaro, R.L. Lubrication of Space Systems; NASA/TM-1994-106392; National Aeronautics and Space Administration: Washington, DC, USA, 1994. [Google Scholar]
- Wang, Y.; Liu, Q.; Yan, J. Characteristic Study on the Porous Reservoir of Liquid Lubricant in Space. Chin. J. Space Sci. 2008, 28, 592–596. [Google Scholar] [CrossRef]
- Neville, A.; Morina, A.; Haque, T.; Voong, M. Compatibility between tribological surfaces and lubricant additives—How friction and wear reduction can be controlled by surface/lube synergies. Tribol. Int. 2007, 40, 1680–1695. [Google Scholar]
- Yang, C.; Jiang, P.; Qin, H.; Wang, X.; Wang, Q. 3D printing of porous polyimide for high-performance oil impregnated self-lubricating. Tribol. Int. 2021, 160, 107009. [Google Scholar] [CrossRef]
- Gao, S.; Han, Q.; Zhou, N.; Pennacchi, P.; Chu, F. Stability and skidding behavior of spacecraft porous oil-containing polyimide cages based on high-speed photography technology. Tribol. Int. 2022, 165, 107294. [Google Scholar] [CrossRef]
- Shao, M.; Li, S.; Duan, C.; Yang, Z.; Qu, C.; Zhang, Y.; Zhang, D.; Wang, C.; Wang, T.; Wang, Q. Cobweb-like structural stimuli-responsive composite with oil warehouse and transportation system for oil storage and recyclable smart-lubrication. ACS Appl. Mater. Interfaces 2018, 10, 41699–41706. [Google Scholar] [CrossRef]
- Kong, Q.; Lian, L.; Liu, Y.; Zhang, J. Fabrication and compression properties of bulk hierarchical nanoporous copper with fine ligament. Mater. Lett. 2014, 127, 59–62. [Google Scholar] [CrossRef]
- Körner, C.; Singer, R.F. Processing of metal foams—Challenges and opportunities. Adv. Eng. Mater. 2000, 2, 159–165. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Dan, Z.; Qin, F.; Wada, T.; Yamaura, S.-I.; Xie, G.; Sugawara, Y.; Muto, I.; Makino, A.; Hara, N. Nanoporous palladium fabricated from an amorphous Pd42.5Cu30Ni7.5P20 precursor and its ethanol electro-oxidation performance. Electrochim. Acta 2013, 108, 512–519. [Google Scholar] [CrossRef]
- Zhao, C. Review on thermal transport in high porosity cellular metal foams with open cells. Int. J. Heat Mass Transf. 2012, 55, 3618–3632. [Google Scholar] [CrossRef]
- Du, H.; Qi, J.; Lao, Y.; Xiong, T. Oil retaining capability and sliding friction behaviour of porous copper with elongated cylindrical pores. J. Mater. Process. Technol. 2012, 212, 1796–1801. [Google Scholar] [CrossRef]
- Du, H.; Qi, J.; Du, S.; Xiong, T.; Li, T.; Lee, S.W. Structure and oil retaining capacity of gasar copper fabricated by radial solidification with a combined crystallizer. J. Mater. Process. Technol. 2010, 210, 1523–1528. [Google Scholar] [CrossRef]
- Zhao, H.; Prieto-López, L.O.; Zhou, X.; Deng, X.; Cui, J. Multistimuli Responsive Liquid-Release in Dynamic Polymer Coatings for Controlling Surface Slipperiness and Optical Performance. Adv. Mater. Interfaces 2019, 6, 1901028. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Zhang, C.; Luo, J. Preparation of self-lubricating NiTi alloy and its self-adaptive behavior. Tribol. Int. 2019, 130, 43–51. [Google Scholar] [CrossRef]
- Fukumasu, N.; Boidi, G.; Seriacopi, V.; Machado, G.; Souza, R.; Machado, I. Numerical analyses of stress induced damage during a reciprocating lubricated test of fecmo sps sintered alloy. Tribol. Int. 2017, 113, 443–447. [Google Scholar] [CrossRef]
- Carabajar, S.; Verdu, C.; Hamel, A.; Fougères, R. Fatigue behaviour of a nickel alloyed sintered steel. Mater. Sci. Eng. A 1998, 257, 225–234. [Google Scholar] [CrossRef]
- Carabajar, S.; Verdu, C.; Fougeres, R. Damage mechanisms of a nickel alloyed sintered steel during tensile tests. Mater. Sci. Eng. A 1997, 232, 80–87. [Google Scholar] [CrossRef]
- Straffelini, G.; Fontanari, V.; Molinari, A. True and apparent Young’s modulus in ferrous porous alloys. Mater. Sci. Eng. A 1999, 260, 197–202. [Google Scholar] [CrossRef]
- Zou, L.; Ge, M.; Zhao, C.; Meng, Q.; Wang, H.; Liu, X.; Lin, C.-H.; Xiao, X.; Lee, W.-K.; Shen, Q.; et al. Designing Multiscale Porous Metal by Simple Dealloying with 3D Morphological Evolution Mechanism Revealed via X-ray Nano-tomography. ACS Appl. Mater. Interfaces 2019, 12, 2793–2804. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef]
- Jia, W.; Yang, S.; Ren, S.; Ma, L.; Wang, J. Preparation and tribological behaviors of porous oil-containing polyimide/hollow mesoporous silica nanospheres composite films. Tribol. Int. 2020, 145, 106184. [Google Scholar] [CrossRef]
- Li, X.; Ling, Y.; Zhang, G.; Yin, Y.; Dai, Y.; Zhang, C.; Luo, J. Preparation and tribological properties of solid-liquid synergetic self-lubricating PTFE/SiO2/PAO6 composites. Compos. Part B Eng. 2020, 196, 108133. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Yan, L.; Li, M.; Wang, C.; Zhu, Y. A computed model for tribological properties of porous self-lubricating PPS composites: Numerical analysis and experimental verification. Wear 2014, 320, 94–102. [Google Scholar] [CrossRef]
- Qiu, Y.X.; Wang, Q.H.; Wang, C.; Wang, T. Preparation and properties of controllable porous films containing lubricant oil. Tribology 2012, 32, 480–485. [Google Scholar]
- Li, X.; Olofsson, U. A study on friction and wear reduction due to porosity in powder metallurgic gear materials. Tribol. Int. 2017, 110, 86–95. [Google Scholar] [CrossRef]
- Martin, F.; García, C.; Blanco, Y. Influence of residual porosity on the dry and lubricated sliding wear of a powder metallurgy austenitic stainless steel. Wear 2015, 328–329, 1–7. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, P.; Liang, H.; Wang, W. Molecular Dynamics Simulations of Lubricant Recycling in Porous Polyimide Retainers of Bearing. Langmuir 2021, 37, 2426–2435. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Qing, T.; Huang, H.; Zhou, N. Effects of surface pore size on the tribological properties of oil-impregnated porous polyimide material. Wear 2021, 484–485, 204042. [Google Scholar] [CrossRef]
- Xu, X.; Shu, X.; Pei, Q.; Qin, H.; Guo, R.; Wang, X.; Wang, Q. Effects of porosity on the tribological and mechanical properties of oil-impregnated polyimide. Tribol. Int. 2022, 170, 107502. [Google Scholar] [CrossRef]
- MacNeill, G.F. Porous Material Development for Instrument-Ball-Bearing Retainer Applications; MIT Charles Stark Draper Laboratory: Cambridge, MA, USA, 1973. [Google Scholar]
- Myant, C.; Fowell, M.; Spikes, H.A.; Stokes, J.R. An Investigation of Lubricant Film Thickness in Sliding Compliant Contacts. Tribol. Trans. 2010, 53, 684–694. [Google Scholar] [CrossRef]
- Spikes, H. The History and Mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]

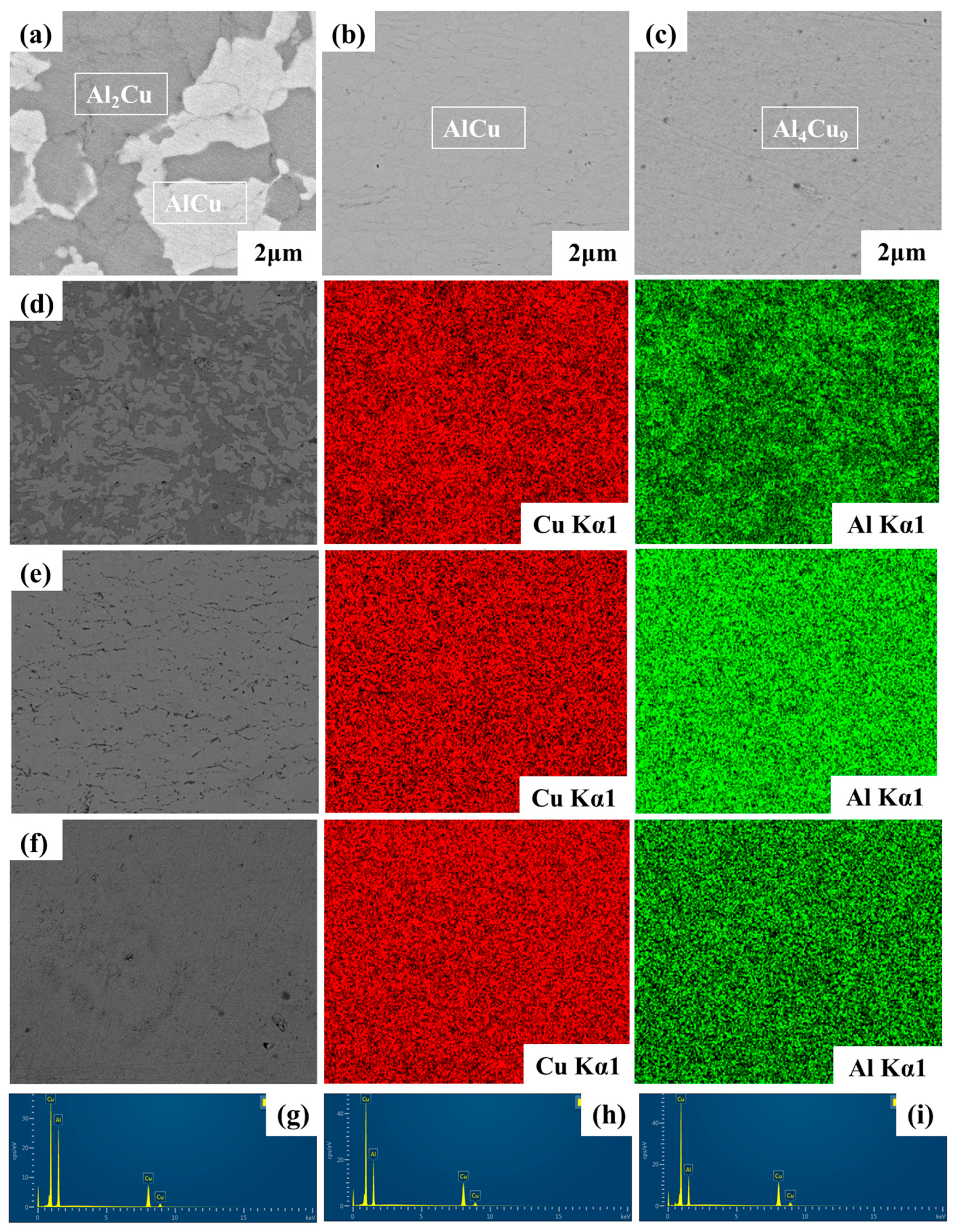


| Dealloyed Samples | Cu Contents (wt.%) | Al Contents (wt.%) |
|---|---|---|
| Cu40Al60 | 99.76 | 0.24 |
| Cu50Al50 | 99.79 | 0.21 |
| Cu60Al40 | 99.77 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Zhao, J.; Wang, H.; Li, H.; Yin, G.; Cai, M.; Wang, Y.; Shen, Q. Oil-Retention and Oil-Bearing Tribological Properties of Nanoporous Copper Prepared Using a Chemical Dealloying Method. Metals 2023, 13, 1232. https://doi.org/10.3390/met13071232
Chen F, Zhao J, Wang H, Li H, Yin G, Cai M, Wang Y, Shen Q. Oil-Retention and Oil-Bearing Tribological Properties of Nanoporous Copper Prepared Using a Chemical Dealloying Method. Metals. 2023; 13(7):1232. https://doi.org/10.3390/met13071232
Chicago/Turabian StyleChen, Fei, Jiahao Zhao, Hao Wang, Honglin Li, Guanchao Yin, Meirong Cai, Yangwei Wang, and Qiang Shen. 2023. "Oil-Retention and Oil-Bearing Tribological Properties of Nanoporous Copper Prepared Using a Chemical Dealloying Method" Metals 13, no. 7: 1232. https://doi.org/10.3390/met13071232
APA StyleChen, F., Zhao, J., Wang, H., Li, H., Yin, G., Cai, M., Wang, Y., & Shen, Q. (2023). Oil-Retention and Oil-Bearing Tribological Properties of Nanoporous Copper Prepared Using a Chemical Dealloying Method. Metals, 13(7), 1232. https://doi.org/10.3390/met13071232







