Binary Aluminum Alloys from 1-ethyl-3-methylimidazolium-based Ionic Liquids for Cathodic Corrosion Protection
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
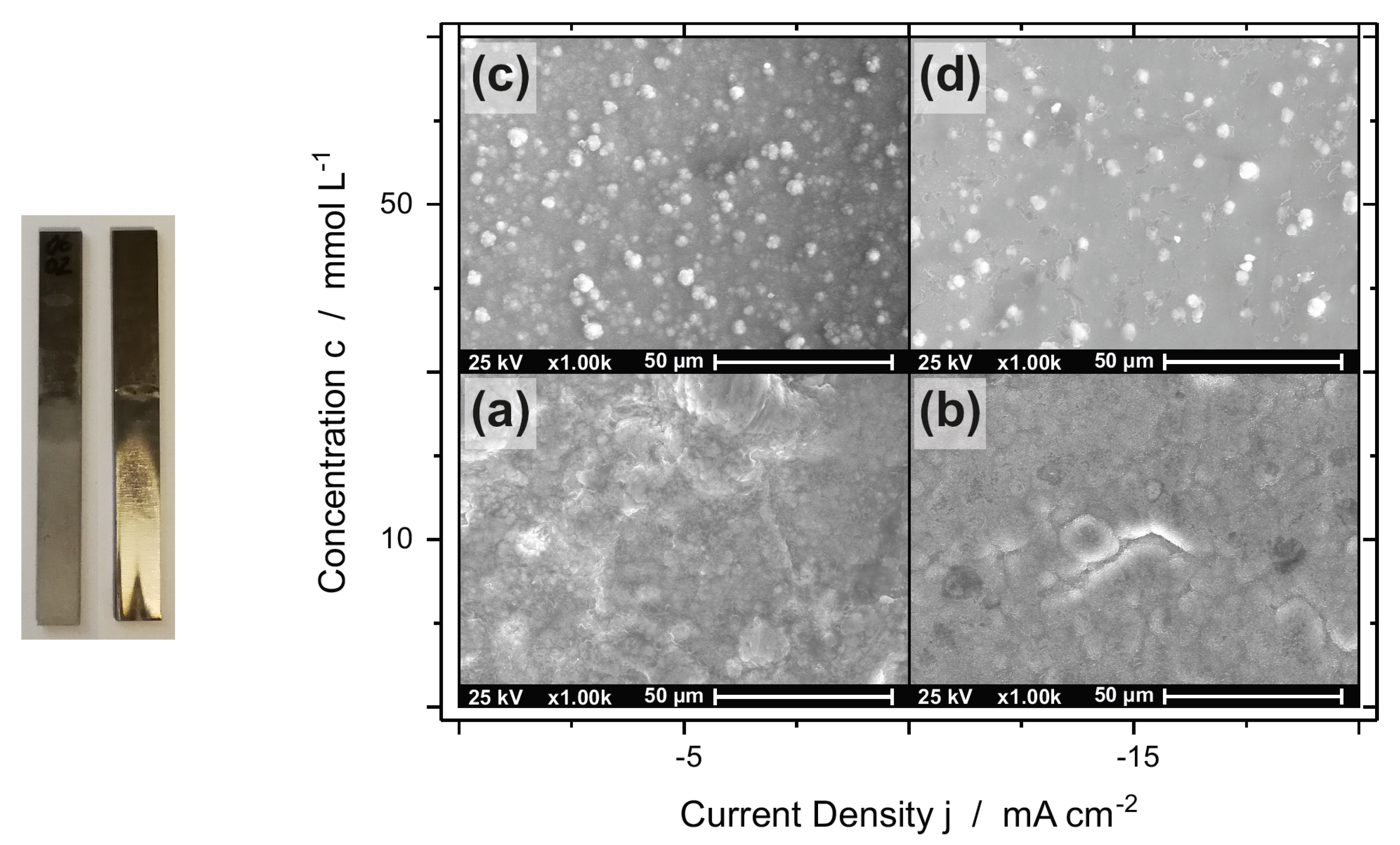
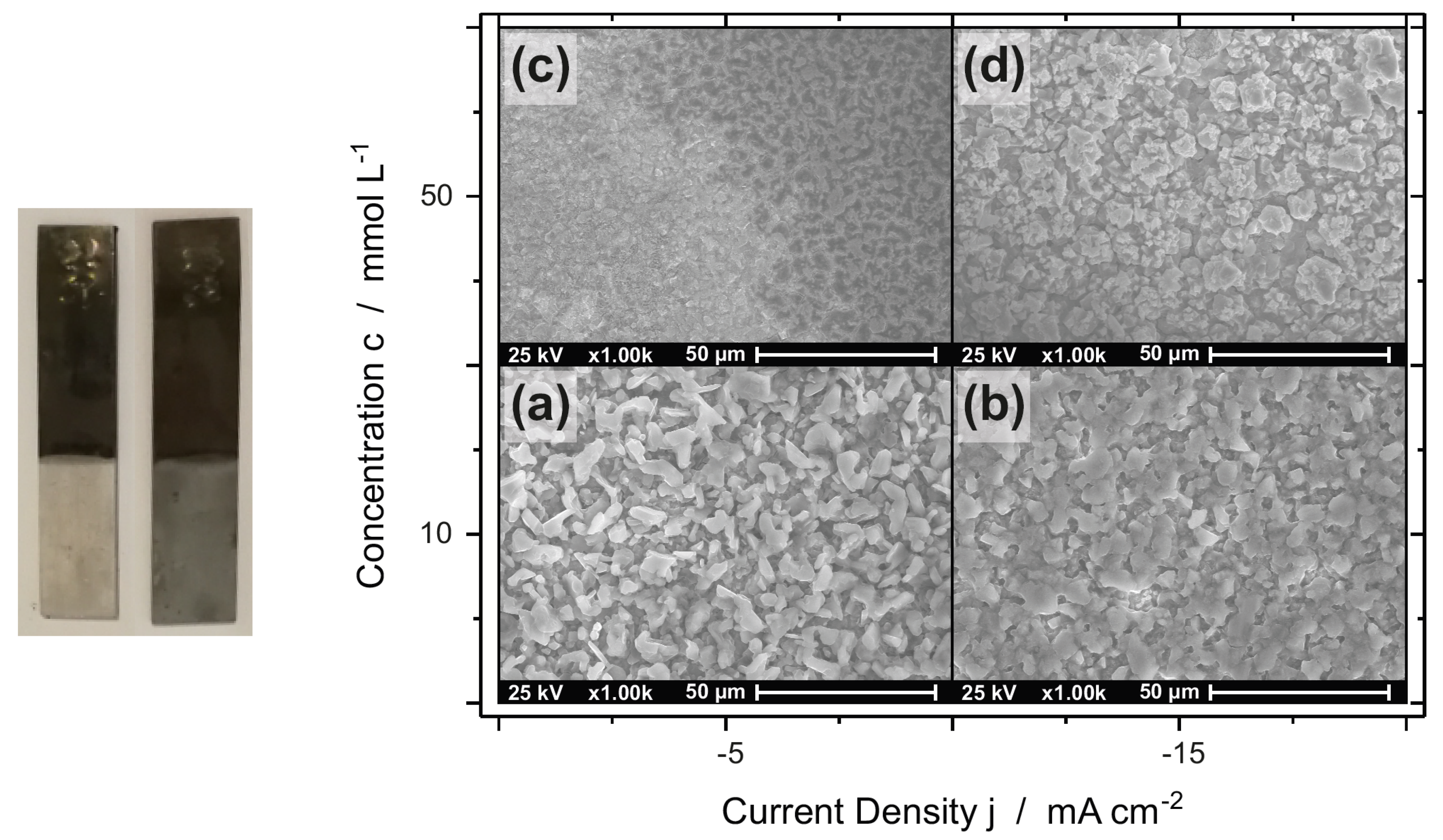
3.1. Morphology and Composition
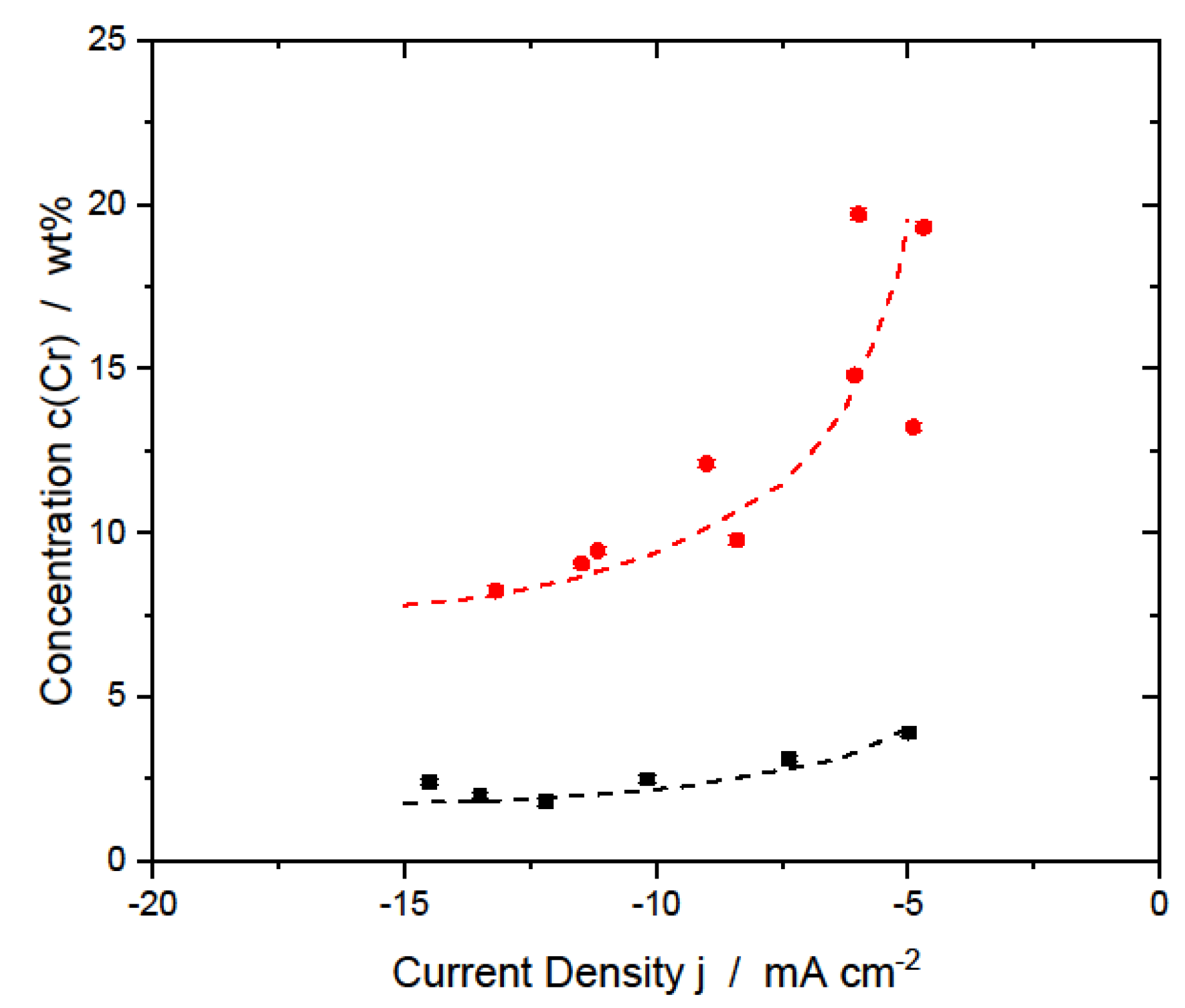
3.1.1. AlCr Alloys
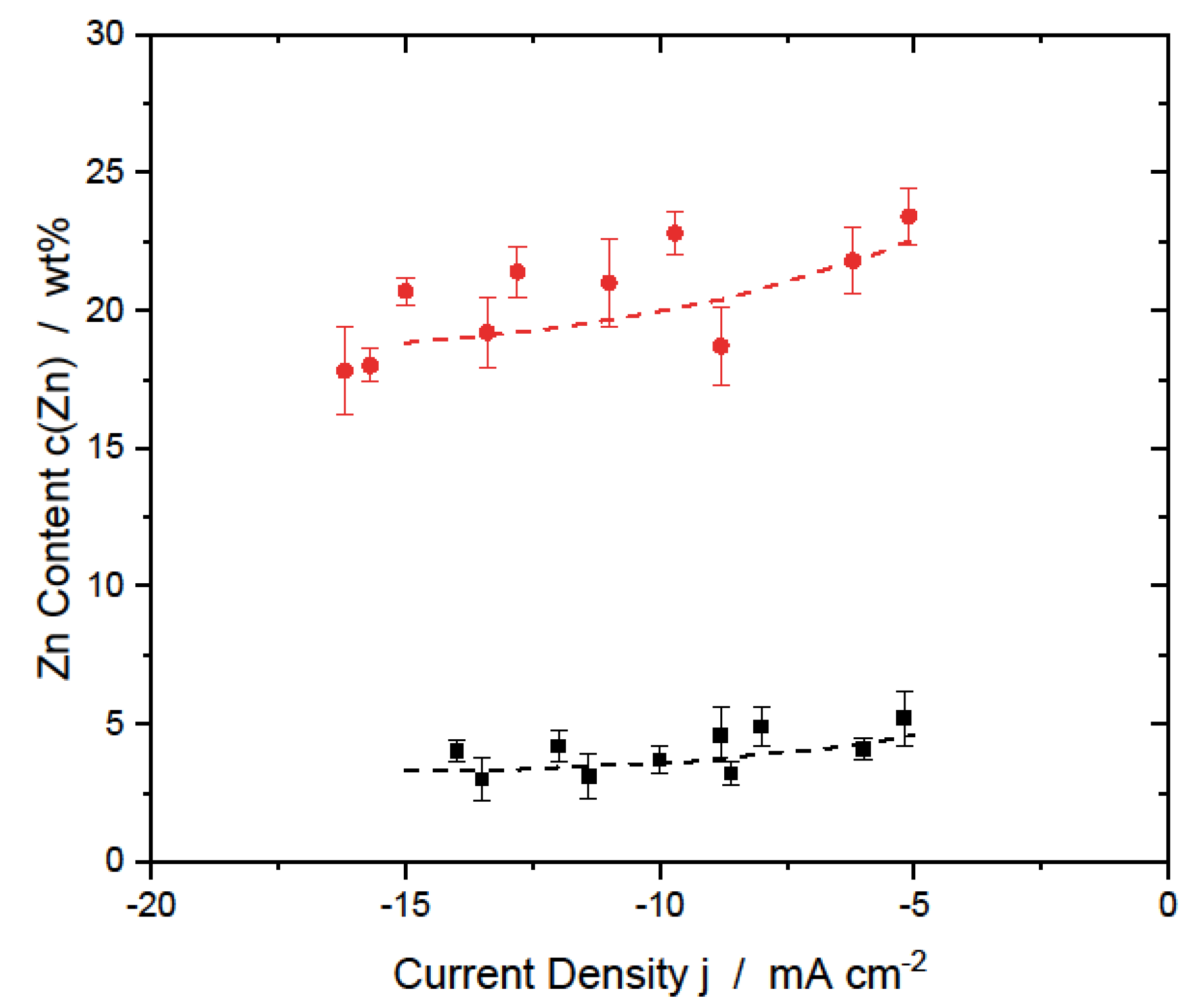
3.1.2. AlZn Alloys
3.1.3. AlSn Alloys
3.2. Corrosion Behavior
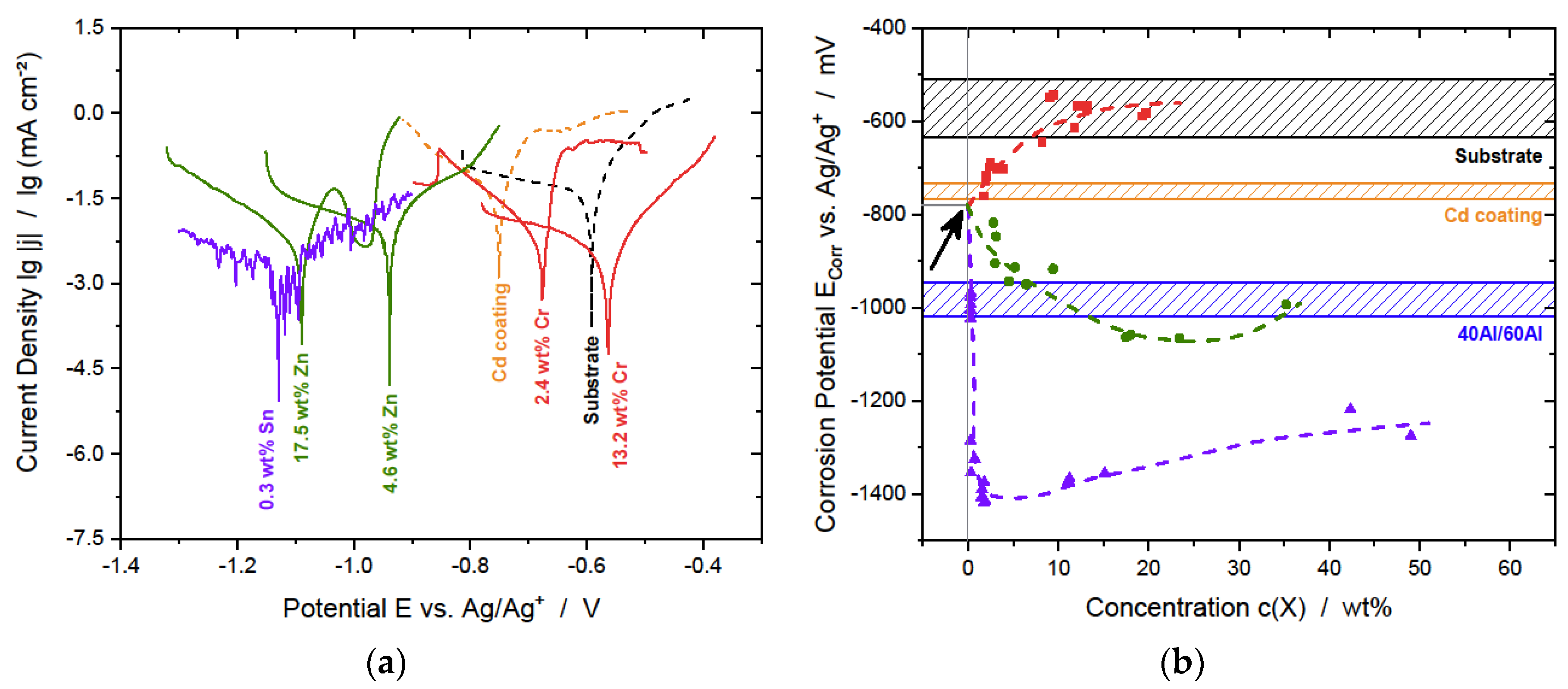
3.2.1. Potentiodynamic Polarization
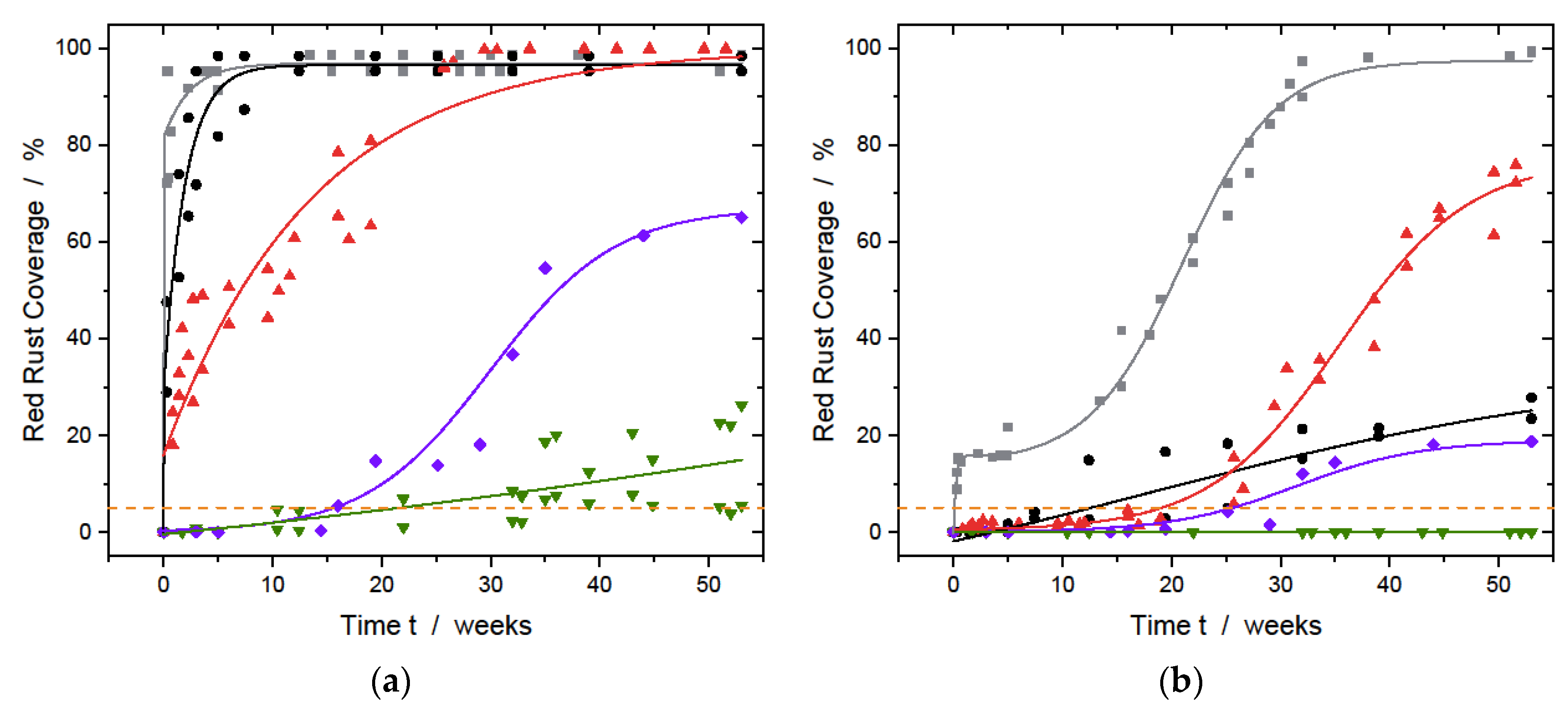
3.2.2. Neutral Salt Spray Test
3.2.3. Environmental Exposure Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bielewski, M. Corrosion Fatigue and Environmentally Assisted Cracking in Aging Military Vehicles (le fatigue-corrosion et la fissuration en milieu ambiant des vehicules militaires vieillissants); NATO Research and Technology: Neuilly-sur-Seine, France, 2011; Volume 140, pp. 1–22. [Google Scholar]
- Mason, R.; Neidbalson, M.; Klingenberg, M.; Khabra, P.; Handsy, C. Update on alternatives for cadmium coatings on military electrical connectors. Met. Finish. 2010, 108, 12–20. [Google Scholar] [CrossRef]
- Legg, K. Cadmium Replacement Alternatives for the Joint Strike Fighter; Report(3105JSF3); Rowan Technology Group: Libertyville, IL, USA, 2000. [Google Scholar]
- von Baeckmann, W.; Schwenk, W.; Prinz, W. (Eds.) Handbuch des Kathodischen Korrosionsschutzes: Theorie und Praxis der Elektrochemischen Schutzfahren; VCH: Weinheim, Germany, 1989. [Google Scholar]
- Seré, P.; Zapponi, M.; Elsner, C.; Di Sarli, A. Comparative corrosion behaviour of 55Aluminium–zinc alloy and zinc hot-dip coatings deposited on low carbon steel substrates. Corrosion Sci. 1998, 40, 1711–1723. [Google Scholar] [CrossRef]
- Laverde, D.; Zubillaga, J.C.; Gil-Sevillano, J.; Villanueva, E. The influence of the primer layer on mechanical damage and loss of corrosion protection of deformed painted Zn-0.16% Al and Zn-5% Al galvanized sheet steel. Corrosion Sci. 1995, 37, 79–95. [Google Scholar] [CrossRef]
- Shih, H.; Hsu, J.; Sun, C.; Chung, S. The lifetime assessment of hot-dip 5% Al–Zn coatings in chloride environments. Surf. Coat. Technol. 2002, 150, 70–75. [Google Scholar] [CrossRef]
- Prosek, T.; Persson, D.; Stoulil, J.; Thierry, D. Composition of corrosion products formed on Zn–Mg, Zn–Al and Zn–Al–Mg coatings in model atmospheric conditions. Corrosion Sci. 2014, 86, 231–238. [Google Scholar] [CrossRef]
- Moffat, T.P. Electrodeposition of Al-Cr metallic glass. J. Electrochem. Soc. 1994, 141, L115–L117. [Google Scholar] [CrossRef]
- Ali, M.R.; Nishikata, A.; Tsuru, T. Electrodeposition of aluminum-chromium alloys from AlCl3-BPC melt and its corrosion and high temperature oxidation behaviors. Electrochim. Acta 1997, 42, 2347–2354. [Google Scholar] [CrossRef]
- Simanavičius, L.; Staknas, A.; Šarkis, A. Codeposition of aluminum with some metals from AlBr3–dimethylethylphenylammonium bromide solutions containing acetylacetonate of selected metal. Electrochim. Acta 2000, 46, 499–507. [Google Scholar] [CrossRef]
- Ueda, M.; Ebe, H.; Ohtsuka, T. Electrodeposition of Al-Cr-Ni layer by pulse electrolysis in AlCl3-EMIC molten salt. Electrochemistry 2005, 73, 739–741. [Google Scholar] [CrossRef]
- Pan, S.-J.; Tsai, W.-T.; Chang, J.-K.; Sun, I.-W. Co-deposition of Al–Zn on AZ91D magnesium alloy in AlCl3–1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2010, 55, 2158–2162. [Google Scholar] [CrossRef]
- Sato, Y.; Azumi, K. Al–Zn co-electrodeposition by a double counter electrode electrodeposition system from an AlCl3–1-ethyl-3-methylimidazolium chloride ionic liquid bath. Surf. Coat. Technol. 2016, 286, 256–261. [Google Scholar] [CrossRef]
- Cross, S.R.; Schuh, C.A. Ternary alloying additions and multilayering as strategies to enhance the galvanic protection ability of Al-Zn coatings electrodeposited from ionic liquid solution. Electrochim. Acta 2016, 211, 860–870. [Google Scholar] [CrossRef]
- Tsuda, T.; Hussey, C.L.; Stafford, G.R. Progress in surface finishing with lewis acidic room-temperature chloroaluminate ionic liquids. ECS Trans. 2006, 3, 217–231. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, J.; Qu, S.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of alloys and compounds from high-temperature molten salts. J. Alloys Compd. 2017, 690, 228–238. [Google Scholar] [CrossRef]
- Tsuda, T.; Stafford, G.R.; Hussey, C.L. Electrochemical surface finishing and energy storage technology with room-temperature haloaluminate ionic liquids and mixtures. J. Electrochem. Soc. 2017, 164, H5007–H5017. [Google Scholar] [CrossRef]
- Xu, X.-H.; Hussey, C.L. The Electrochemistry of Tin in the Aluminum Chloride-1-methyl-3-ethylimidazolium Chloride Molten Salt. J. Electrochem. Soc. 1993, 140, 618. [Google Scholar] [CrossRef]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Platis, F.S.; Capuano, G.A. Electrodeposition of Aluminum-Tin Alloys from Organic Electrolytes. Electrochem. Soc. 1987, 134, 2425–2429. [Google Scholar] [CrossRef]
- Ueda, M.; Inaba, R.; Ohtsuka, T. Composition and structure of Al–Sn alloys formed by constant potential electrolysis in an AlCl3–NaCl–KCl–SnCl2 molten salt. Electrochim. Acta 2013, 100, 281–284. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Ziegler, K.; Lehmkuhl, H.Z. Die elektrolytische abscheidung von aluminium aus organischen komplexverbindungen. Anorg. Allg. Chem. 1956, 283, 414–424. [Google Scholar] [CrossRef]
- Lehmkuhl, H.; Mehler, K.; Landau, U. Advances in Electrochemical Science and Engineering; Gerischer, H., Tobias, C.W., Eds.; Wiley-VCH: Weinheim, Germany, 1993; p. 163. [Google Scholar]
- Zhao, Y.; VanderNoot, T.J. Electrodeposition of aluminium from nonaqueous organic electrolytic systems and room temperature molten salts. Electrochim. Acta 1997, 42, 3–13. [Google Scholar] [CrossRef]
- Ishibashi, N.; Yoshio, M. Electrodeposition of aluminium from the NBS type bath using tetrahydrofuran—benzene mixed solvent. Electrochim. Acta 1972, 17, 1343–1352. [Google Scholar] [CrossRef]
- Yoshio, M.; Ishibashi, N. High-rate plating of aluminium from the bath containing aluminium chloride and lithium aluminium hydride in tetrahydrofuran. J. Appl. Electrochem. 1973, 3, 321–325. [Google Scholar] [CrossRef]
- Abbott, A.P.; MacFarlane, D.R.; Endres, F. (Eds.) Electrodeposition from Ionic Liquids; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Fox, D.M.; Gilman, J.W.; Morgan, A.B.; Shields, J.R.; Maupin, P.H.; Lyon, R.E.; de Long, H.C.; Trulove, P.C. Flammability and thermal analysis characterization of imidazolium-based ionic liquids. Ind. Eng. Chem. Res. 2008, 47, 6327–6332. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Gao, X.-H.; Chen, J.-C.; Li, Z.-W.; Li, C.-L.; Zhang, S.-C. Imidazolium-organic solvent–alkali metal salt mixtures as nonflammable electrolytes incorporated into PVDF–PEG polymer electrolyte. J. Appl. Polym. Sci. 2009, 113, 2492–2498. [Google Scholar] [CrossRef]
- Ali, M.R.; Abbott, A.P.; Ryder, K.S. Electrodeposition of Al-Mg Alloys from Acidic AlCl3-EMIC-MgCl2 Room Temperature Ionic Liquids. J. Electrochem. 2015, 21, 172. [Google Scholar]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Hussey, C.L.; Scheffler, T.B.; Wilkes, J.S.; Fannin, A.A. Chloroaluminate equilibria in the aluminum chloride-1-methyl-3-ethylimidazolium chloride ionic liquid. J. Electrochem. Soc. 1986, 133, 1389–1391. [Google Scholar] [CrossRef]
- Lai, P.K.; Skyllas-Kazacos, M. Electrodeposition of aluminium in aluminium chloride/1-methyl-3-ethylimidazolium chloride. J. Electroanal. Chem. 1988, 248, 431–440. [Google Scholar] [CrossRef]
- Böttcher, R.; Valitova, A.; Ispas, A.; Bund, A. Electrodeposition of aluminium from ionic liquids on high strength steel. Trans. Inst. Met. Finish. 2019, 97, 82–88. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Liu, J.S.Y.; Chen, P.Y.; Sun, I.W.; Hussey, C.L. Electrochemical Studies of Chromium (III) and Chromium (II) Chloride Complexes in Basic Aluminum Chloride-1-Methyl-3-ethylimidazolium Chloride Room Temperature Molten Salts. J. Electrochem. Soc. 1997, 144, 2388–2392. [Google Scholar] [CrossRef]
- Böttcher, R.; Ispas, A.; Bund, A. On the electrodeposition of tantalum from three different ionic liquids with the bis (trifluoromethyl sulfonyl) amide anion. Electrochem. Commun. 2020, 115, 106720. [Google Scholar] [CrossRef]
- Noda, M.; Morimitsu, M.; Matsunaga, M. Electrodeposition of In-Sn alloys in EMI-BF4-Cl ambient temperature melts with current pulses. ECS Trans. 2007, 3, 249–252. [Google Scholar] [CrossRef]
- Ispas, A.; Vlaic, C.A.; Camargo, M.K.; Bund, A. Electrochemical deposition of aluminum and aluminum-manganese alloys in ionic liquids. ECS Trans. 2016, 75, 657–665. [Google Scholar] [CrossRef]
- Caporali, S.; Fossati, A.; Lavacchi, A.; Perissi, I.; Tolstogouzov, A.; Bardi, U. Aluminium electroplated from ionic liquids as protective coating against steel corrosion. Corrosion Sci. 2008, 50, 534–539. [Google Scholar] [CrossRef]
- Chang, J.-K.; Chen, S.-Y.; Tsai, W.-T.; Deng, M.-J.; Sun, I.-W. Improved corrosion resistance of magnesium alloy with a surface aluminum coating electrodeposited in ionic liquid. J. Electrochem. Soc. 2008, 155, C112–C116. [Google Scholar] [CrossRef]
- Chang, J.-K.; Chen, S.-Y.; Tseng, C.-H.; Tsai, W.-T.; Deng, M.-J.; Sun, I.-W. Electrodeposition of Al on magnesium alloy from aluminum chloride/1-ethyl-3-methylimidazolium chloride ionic liquids. Electrochemistry 2009, 77, 585–587. [Google Scholar] [CrossRef]



| C | Mn | Si | P | S | Al |
|---|---|---|---|---|---|
| 0.07 | 1.60 | 0.15 | <0.015 | <0.010 | 0.020 |
| – | – | – | – | ||
| 0.15 | 2.10 | 0.30 | 0.050 | ||
| Ti | Cr | B | Mo | V | Fe |
| 0.010 | 0.50 | 0.0015 | 0.10 | 0.12 | balance |
| – | – | – | – | – | |
| 0.050 | 1.00 | 0.0040 | 0.30 | 0.20 |
| Type | Fe | Cu | Mn | Zn | Ti | In | Al |
|---|---|---|---|---|---|---|---|
| 40Al | 0.084 | <0.001 | 0.223 | 2.94 | 0.036 | 0.028 | balance |
| 60Al | 0.130 | 0.001 | 0.337 | 3.83 | 0.033 | 0.035 | Balance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böttcher, R.; Ispas, A.; Bund, A. Binary Aluminum Alloys from 1-ethyl-3-methylimidazolium-based Ionic Liquids for Cathodic Corrosion Protection. Metals 2023, 13, 377. https://doi.org/10.3390/met13020377
Böttcher R, Ispas A, Bund A. Binary Aluminum Alloys from 1-ethyl-3-methylimidazolium-based Ionic Liquids for Cathodic Corrosion Protection. Metals. 2023; 13(2):377. https://doi.org/10.3390/met13020377
Chicago/Turabian StyleBöttcher, Rene, Adriana Ispas, and Andreas Bund. 2023. "Binary Aluminum Alloys from 1-ethyl-3-methylimidazolium-based Ionic Liquids for Cathodic Corrosion Protection" Metals 13, no. 2: 377. https://doi.org/10.3390/met13020377
APA StyleBöttcher, R., Ispas, A., & Bund, A. (2023). Binary Aluminum Alloys from 1-ethyl-3-methylimidazolium-based Ionic Liquids for Cathodic Corrosion Protection. Metals, 13(2), 377. https://doi.org/10.3390/met13020377






