Analysis of the Use of Recycled Aluminum to Generate Green Hydrogen in an Electric Bicycle
Abstract
:1. Introduction
2. Experimental Section
2.1. Aluminum Test Tube, Caustic Soda and Drying Agent for Hydrogen Moisture
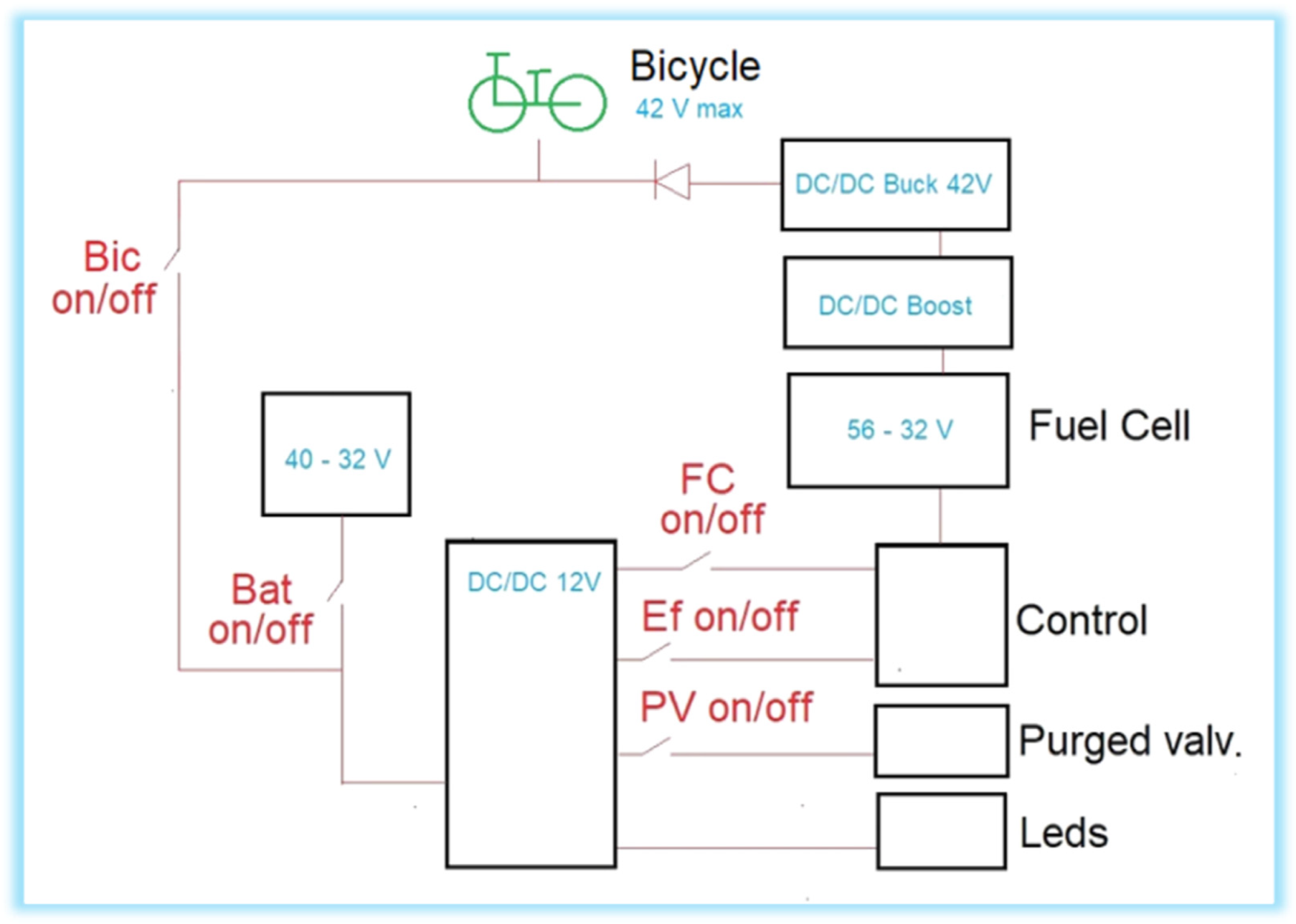
2.2. Fuel cell, Auxiliary Battery and Electronic Circuits
2.3. Bicycle and Rolling Resistance
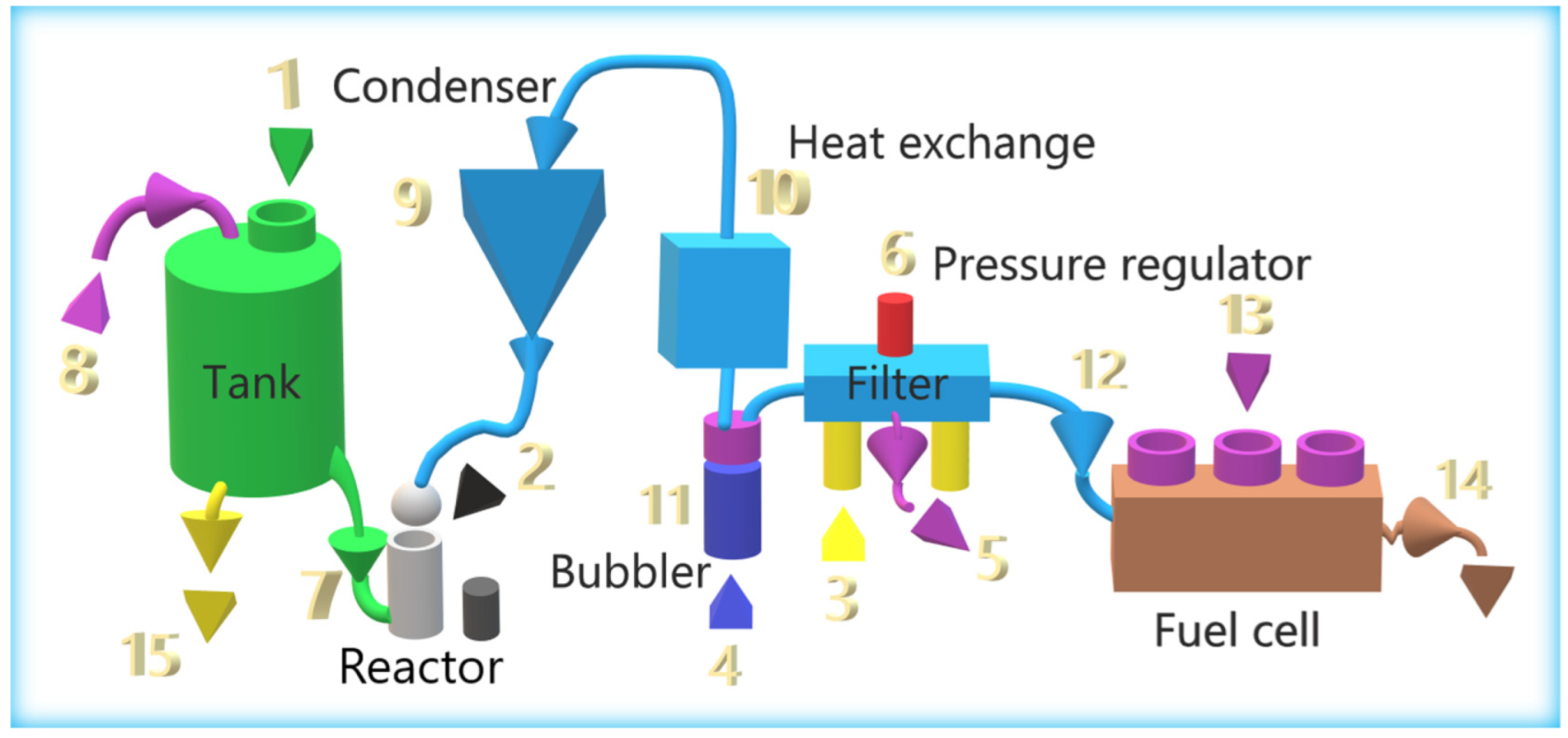
2.4. Assembly of the Hydrogen-Generation System from Aluminum Waste
2.5. Operation of the Hydrogen-Generation System
2.5.1. Loading Consumables
2.5.2. System Initialization
2.5.3. Fuel-cell Power
2.5.4. Hybrid-System Operation
3. Assays and Methodology Used in Tests
3.1. General Methodology
3.2. Battery Tests
3.3. Test with a Single Reactor and Hybrid FC System and 4.4 A hAuxiliary Battery
3.4. Test with Two Reactors and Hybrid FC System and 4.4 Ah Auxiliary Battery
4. Results and Discussion
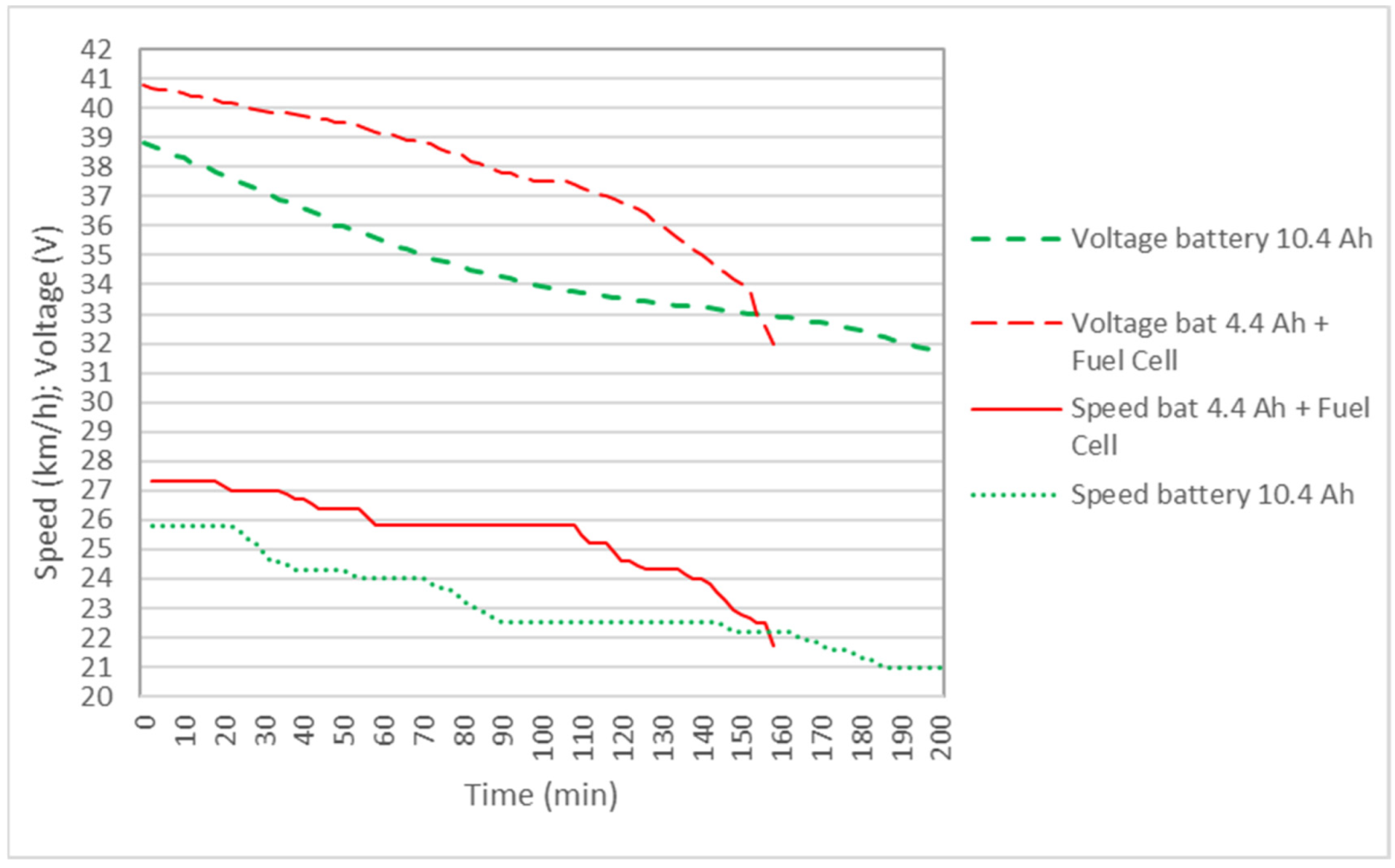
4.1. Results with a Single Reactor and Hybrid FC System and 4.4 Ah Auxiliary Battery
4.2. Results with Two Reactors and FC Hybrid System (4.4 Ah Auxiliary Battery)
4.3. Advantages, Problems and Improvements related to the Electric-Bicycle System with Aluminum Waste
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbara, W. Renewable Hydrogen implementations for combined energy storage, transportation, and stationary application. Therm. Sci. Eng. Prog. 2020, 16, 100460. [Google Scholar]
- Li, Y.; Taghizadeh-Hesary, F. The economic feasibility of green hydrogen and fuel cell electric vehicles for road transport in China. Energy Policy 2022, 160, 112703. [Google Scholar] [CrossRef]
- Ahmed IOsman Neha Mehta Ahmed, M.E. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar]
- Song, P.; Sui, Y.; Shan, T.; Hou, J.; Wang, X. Assessment of hydrogen supply solutions for hydrogen fueling station: A Shanghai case study. Int. J. Hydrogen Energy 2020, 45, 32884–32898. [Google Scholar] [CrossRef]
- Jorschick, H.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Hydrogenation of aromatic and heteroaromatic compounds—A key process for future logistics of green hydrogen using Liquid Organic Hydrogen Carrier systems. Sustain. Energy Fuels 2021, 5, 1311–1346. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, S.J. Recent Progress in Hydrogen Flammability Prediction for the Safe Energy Systems. Energies 2020, 13, 6263. [Google Scholar] [CrossRef]
- Rothuizen, E.; Rokni, M. Optimization of the overall energy consumption in cascade fueling stations for hydrogen vehicles. Int. J. Hydrogen Energy 2014, 39, 582–592. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Hoeflinger, J.; Hofmann, P. Air mass flow and pressure optimisation of a PEM fuel cell range extender system. Int. J. Hydrogen Energy 2020, 45, 29246–29258. [Google Scholar] [CrossRef]
- Wang, H.Z.; Leung, D.Y.C. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Xu, S.; Yang, X.-H.; Tang, S.-S.; Liu, J. Liquid metal activated hydrogen production from waste aluminum for power supply and its life cycle assessment. Int. J. Hydrogen Energy 2019, 44, 17505–17514. [Google Scholar] [CrossRef]
- Teng, H.-T.; Lee, T.-Y.; Chen, Y.-K.; Wang, H.-W.; Cao, G. Effect of Al(OH)3 on the hydrogen generation of aluminum-water system. J. Power Sources 2012, 219, 16–21. [Google Scholar] [CrossRef]
- Shaytura, N.S.; Laritchev, M.N.; Laritcheva, O.O.; Shkolnikov, E.I. Study of texture of hydroxides formed by aluminum oxidation with liquid water at various activation techniques. Curr. Appl. Phys. J. 2010, 10, S66–S68. [Google Scholar] [CrossRef]
- Hu, H.; Qiao, M.; Pei, Y.; Fan, K.; Li, H.; Zong, B.; Zhang, X. Kinetics of hydrogen evolution in alkali leaching of rapidly quenched Ni–Al alloy. Appl. Catal. A Gen. 2003, 252, 173–183. [Google Scholar] [CrossRef]
- Woodall, J.M.; Ziebarth, J.T.; Allen, C.R.; Jeon, J.; Choi, G.; Kramer, R. Generating hydrogen on demand by splitting water with Al rich alloys. Clean Technol. 2008, 5, 313–315. [Google Scholar]
- Fan, M.-Q.; Xu, F.; Sun, L.-X. Studies on hydrogen generation characteristics of hydrolysis of the ball milling Al-based materials in pure water. Int. J. Hydrogen Energy 2007, 32, 2809–2815. [Google Scholar] [CrossRef]
- Lin, Y.; Genzer, J.; Dickey, M.D. Attributes, Fabrication and Applications of Gallium-Based Liquid Metal Particles. Adv. Sci. 2020, 7, 2000192. [Google Scholar] [CrossRef]
- Tan, S.-C.; Gui, H.; Yang, X.-H.; Yuan, B.; Zhan, S.-H.; Liu, J. Comparative study on activation of aluminum with four liquid metals to generate hydrogen in alkaline solution. Int. J. Hydrogen Energy 2016, 41, 22663–22667. [Google Scholar] [CrossRef]
- Yuan, B.; Tan, S.; Liu, J. Dynamic hydrogen generation phenomenon of aluminum fed liquid phase Ga–In alloy inside NaOH electrolyte. Int. J. Hydrogen Energy 2016, 41, 1453–1459. [Google Scholar] [CrossRef]
- Petrovic, J.; Thomas, G. Reaction of Aluminum with Water to Produce Hydrogen. In A Study of Issues Related to the Use of Aluminum for On-Board Vehicular Hydrogen Storage; U.S. Department of Energy: Washington, DC, USA, 2008. [Google Scholar]
- Macanás, J.; Soler, L.; Candela, A.M.; Muñoz, M.; Casado, J. Hydrogen generation by aluminium corrosion in aqueous alkaline solutions of inorganic promoters. The Alhydrox process. Energy 2011, 36, 2493–2501. [Google Scholar] [CrossRef]
- Fan, M.-Q.; Xu, F.; Sun, L.-X.; Zhao, J.-N.; Jiang, T.; Li, W.-X. Hydrolysis of ball milling Al–Bi–hydride and Al–Bi–salt mixture for hydrogen generation. J. Alloy. Compd. 2008, 460, 125–129. [Google Scholar] [CrossRef]
- Parmuzina, A.V.; Kravchenko, O.V.; Bulychev, B.M.; Shkolnikov, E.I.; Burlakova, A.G. Oxidation of activated aluminium with water as a method for hydrogen generation. Russ. Chem. Bull. 2009, 58, 493–498. [Google Scholar] [CrossRef]
- Salueña-Berna, X.; Marín-Genescà, M.; Massagués Vidal, L.; Dagà-Monmany, J.M. Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications. Materials 2021, 14, 7323. [Google Scholar] [CrossRef] [PubMed]
- Available online: Eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2002L0024:20070101:EN:PDF (accessed on 15 December 2022).
- Hung, N.B.; Lim, O. A review of history, development, design and research of electric bicycles. Appl. Energy 2020, 260, 114323. [Google Scholar] [CrossRef]
- Moreira, M.V.; da Silva, G.E. A practical model for evaluating the performance of proton exchange membrane fuel cells. Renew. Energy 2009, 34, 1734–1741. [Google Scholar] [CrossRef]
- Najdoski, M. Gas Chemistry: A Microscale Kipp Apparatus. Chem. Educ. 2011, 16, 295–298. [Google Scholar]
- Kabalo, M.; Blunier, B.; Bouquain, D.; Miraoui, A. State-of-the-art of DC-DC converters for fuel cell vehicles. In Proceedings of the 2010 IEEE Vehicle Power and Propulsion Conference, Lille, France, 1–3 September 2010; pp. 1–6. [Google Scholar] [CrossRef]
- Alavi, O.; Rajabloo, T.; De Ceuninck, W.; Daenen, M. Non-Isolated DC-DC Converters in Fuel Cell Applications: Thermal Analysis and Reliability Comparison. Appl. Sci. 2022, 12, 5026. [Google Scholar] [CrossRef]
- Kheirandish, A.; Kazemi, M.S.; Dahari, M. Dynamic performance assessment of the efficiency of fuel cell-powered bicycle: An experimental approach. Int. J. Hydrogen Energy 2014, 39, 13276–13284. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.; Jiang, J.; Wu, B. An improved dynamic considering effect of temperature and equivalent internal resistance for PEM fuel cell power modules. J. Power Sources 2006, 161, 1062–1068. [Google Scholar] [CrossRef]
- Abdel-Rahim, O.; Chub, A.; Blinov, A.; Vinnikov, D.; Peftitsis, D. An efficient non-inverting buck-boost converter with improved step up/down ability. Energies 2022, 15, 4550. [Google Scholar] [CrossRef]





| Aluminum | Si (%) | Cu (%) | Zn (%) | Fe (%) | Mg (%) | Mn (%) | C (%) | O2 (%) | Others (%) | Al (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.3 | <0.5 | 97 |
| Aluminum | Weight (g) | Density (g/cm3) | Top Diameter (mm) | Lower Diameter (mm) | Height (mm) |
|---|---|---|---|---|---|
| Initial probe | 111.4 | 2.43 | 40 | 40 | 36.5 |
| Final residue | 10 | 2.43 | 15.5 | 20.5 | 16.1 |
| Aluminum | Weight (g) | Density (g/cm3) | Top Diameter (mm) | Lower Diameter (mm) | Height (mm) |
|---|---|---|---|---|---|
| Initial probe 1 | 108 | 2.43 | 40 | 40 | 35.4 |
| Initial probe 2 | 109 | 2.43 | 40 | 40 | 35.7 |
| Final residue 1 | 14.8 | 2.43 | 19 | 26 | 15.2 |
| Final residue 2 | 12.5 | 2.43 | 18 | 22 | 16.4 |
| Equivalent Capacity (Wh) | Average Speed (km/h) | Time (h) | Autonomy (km) | |
|---|---|---|---|---|
| Battery 4.4 Ah (only) | 132.1 | 24.6 | 1.28 | 32 |
| Battery 4.4 Ah + FC (1 reactor) | 226.1 (243.1) | 25.4 | 2.07 | 51.1 |
| Battery 4.4 Ah + FC (2 reactor) | 317.8 (339.7) | 25.6 | 2.67 | 67.3 |
| Battery 10.4 Ah (only) | 344.10 | 23.1 | 3.33 | 77.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salueña Berna, X.; Marín-Genescà, M.; Dagà-Monmany, J.M. Analysis of the Use of Recycled Aluminum to Generate Green Hydrogen in an Electric Bicycle. Metals 2023, 13, 357. https://doi.org/10.3390/met13020357
Salueña Berna X, Marín-Genescà M, Dagà-Monmany JM. Analysis of the Use of Recycled Aluminum to Generate Green Hydrogen in an Electric Bicycle. Metals. 2023; 13(2):357. https://doi.org/10.3390/met13020357
Chicago/Turabian StyleSalueña Berna, Xavier, Marc Marín-Genescà, and José María Dagà-Monmany. 2023. "Analysis of the Use of Recycled Aluminum to Generate Green Hydrogen in an Electric Bicycle" Metals 13, no. 2: 357. https://doi.org/10.3390/met13020357
APA StyleSalueña Berna, X., Marín-Genescà, M., & Dagà-Monmany, J. M. (2023). Analysis of the Use of Recycled Aluminum to Generate Green Hydrogen in an Electric Bicycle. Metals, 13(2), 357. https://doi.org/10.3390/met13020357








