The Extraction of Copper from Chalcopyrite Concentrate with Hydrogen Peroxide in Sulfuric Acid Solution
Abstract
:1. Introduction
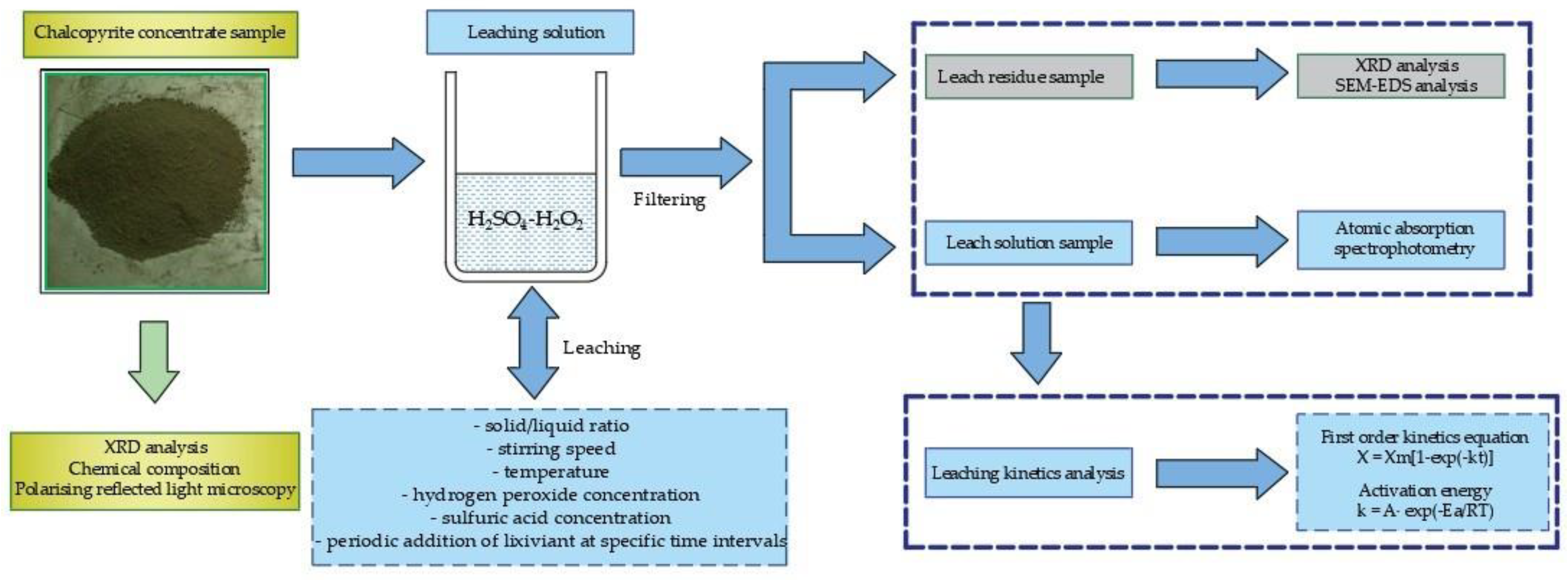
2. Materials and Methods
2.1. Material
2.2. Reagents and Experimental Procedure
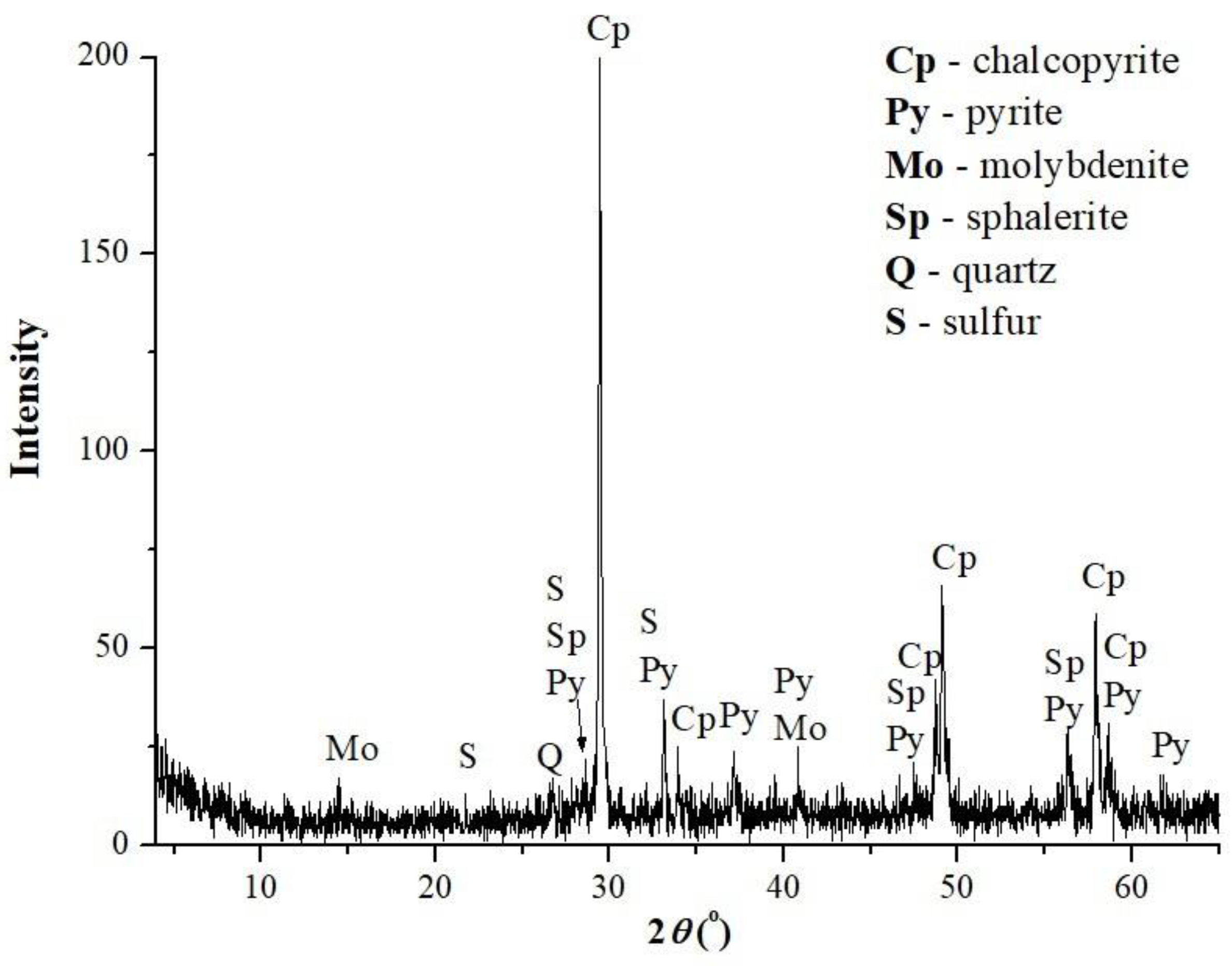
2.3. Leach Residue Characterization
3. Results and Discussion
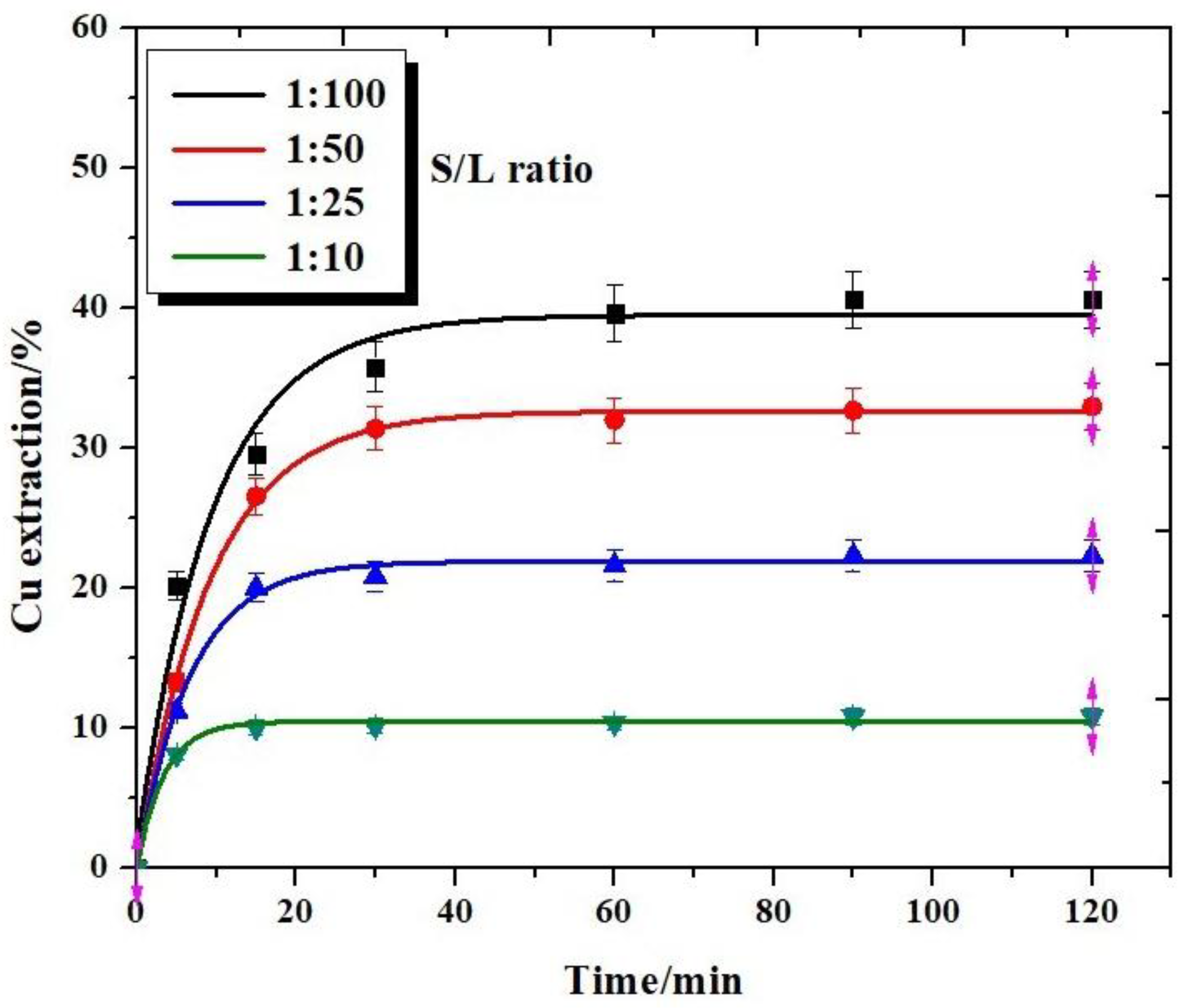
3.1. Effect of Solid/Liquid Ratio
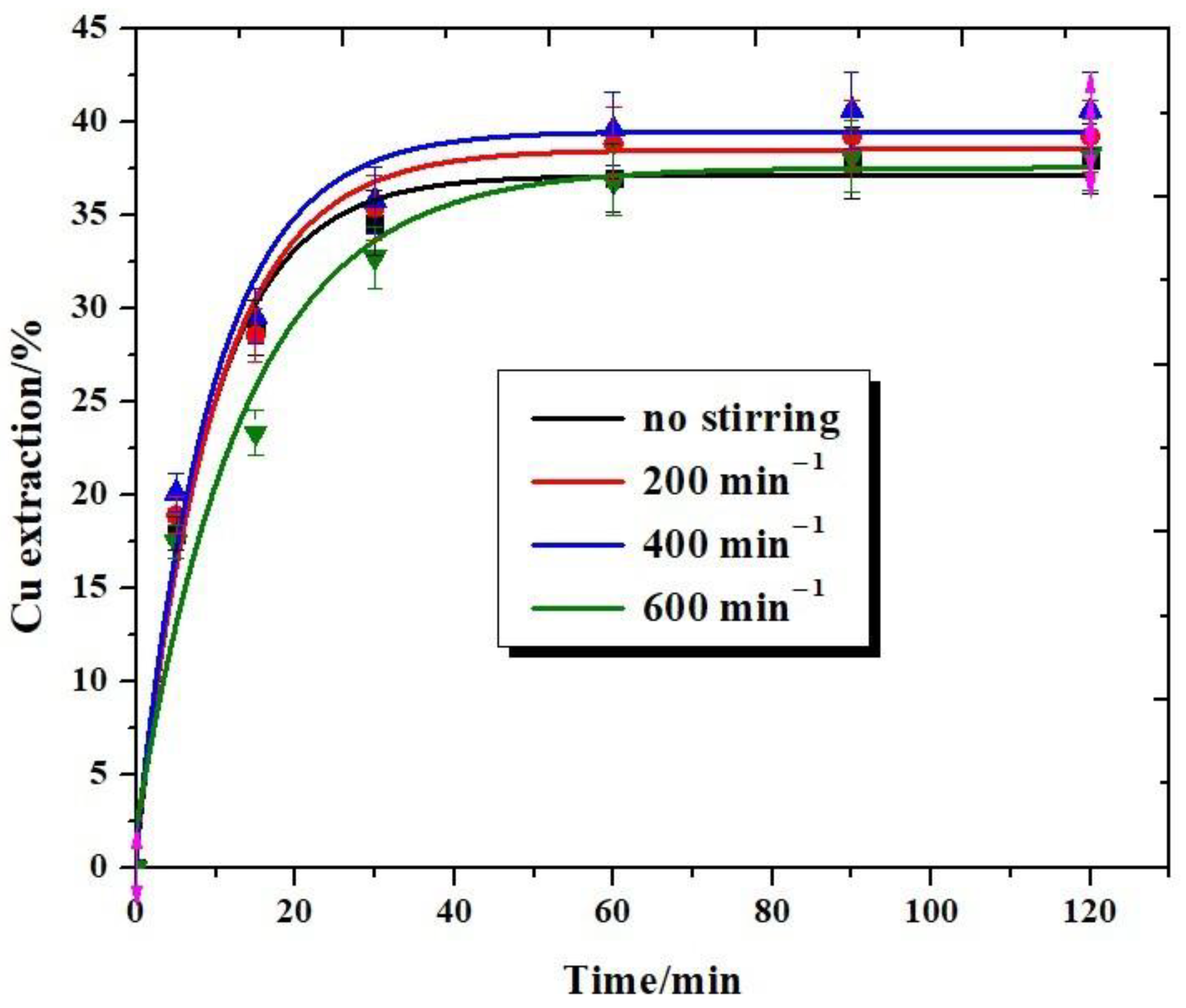
3.2. Effect of Stirring Speed
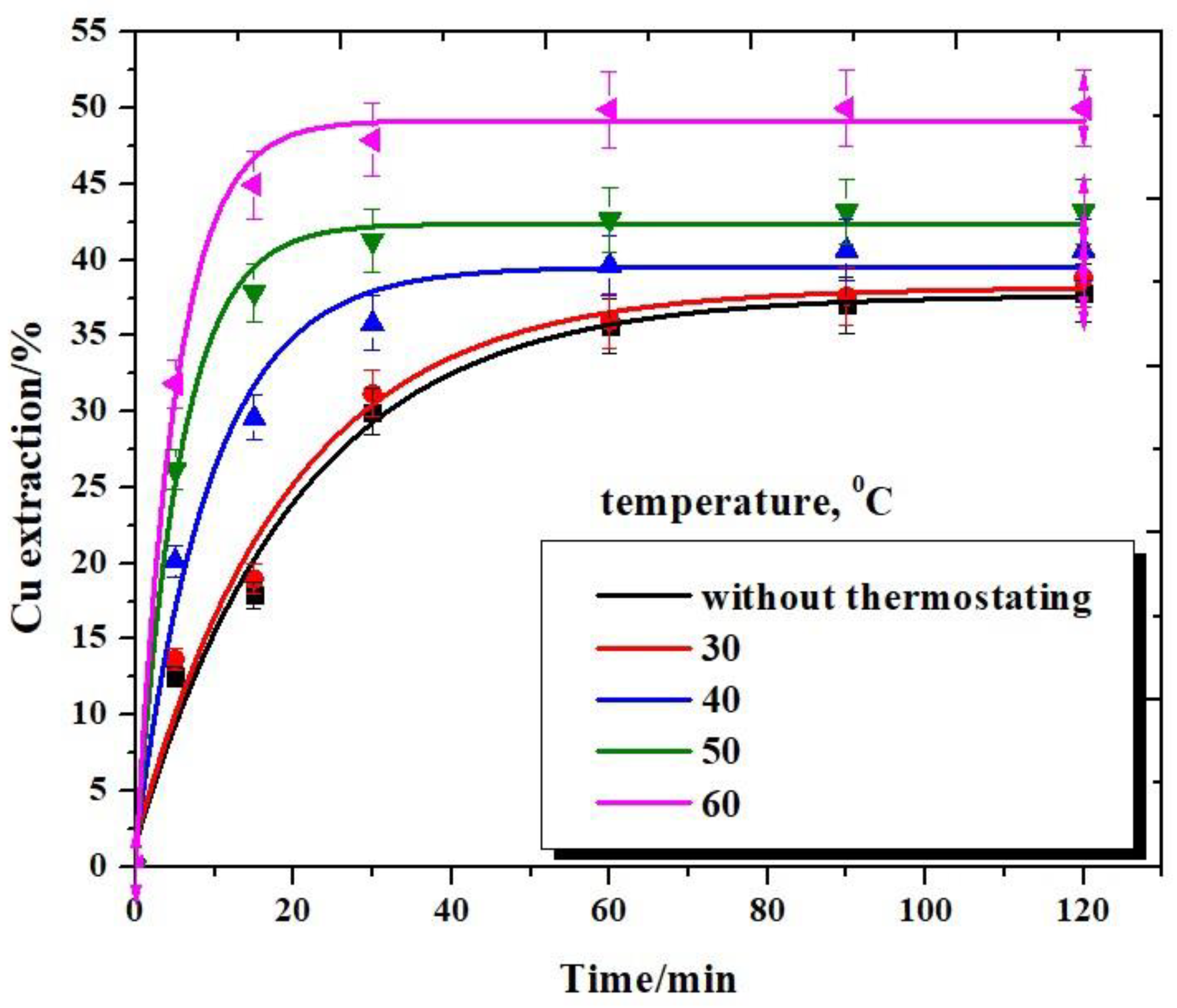
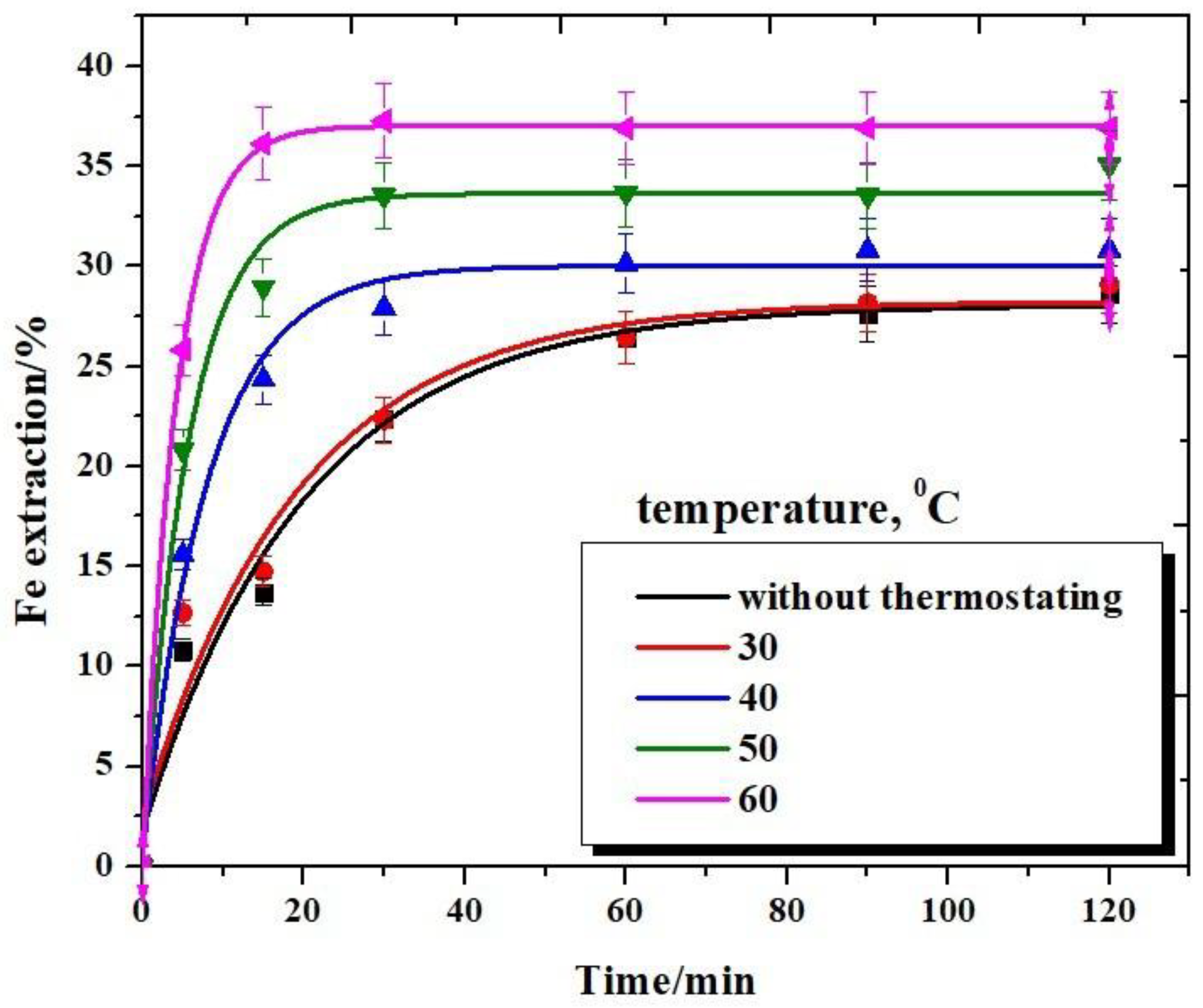
3.3. Effect of Reaction Temperature
Dissolution of Iron
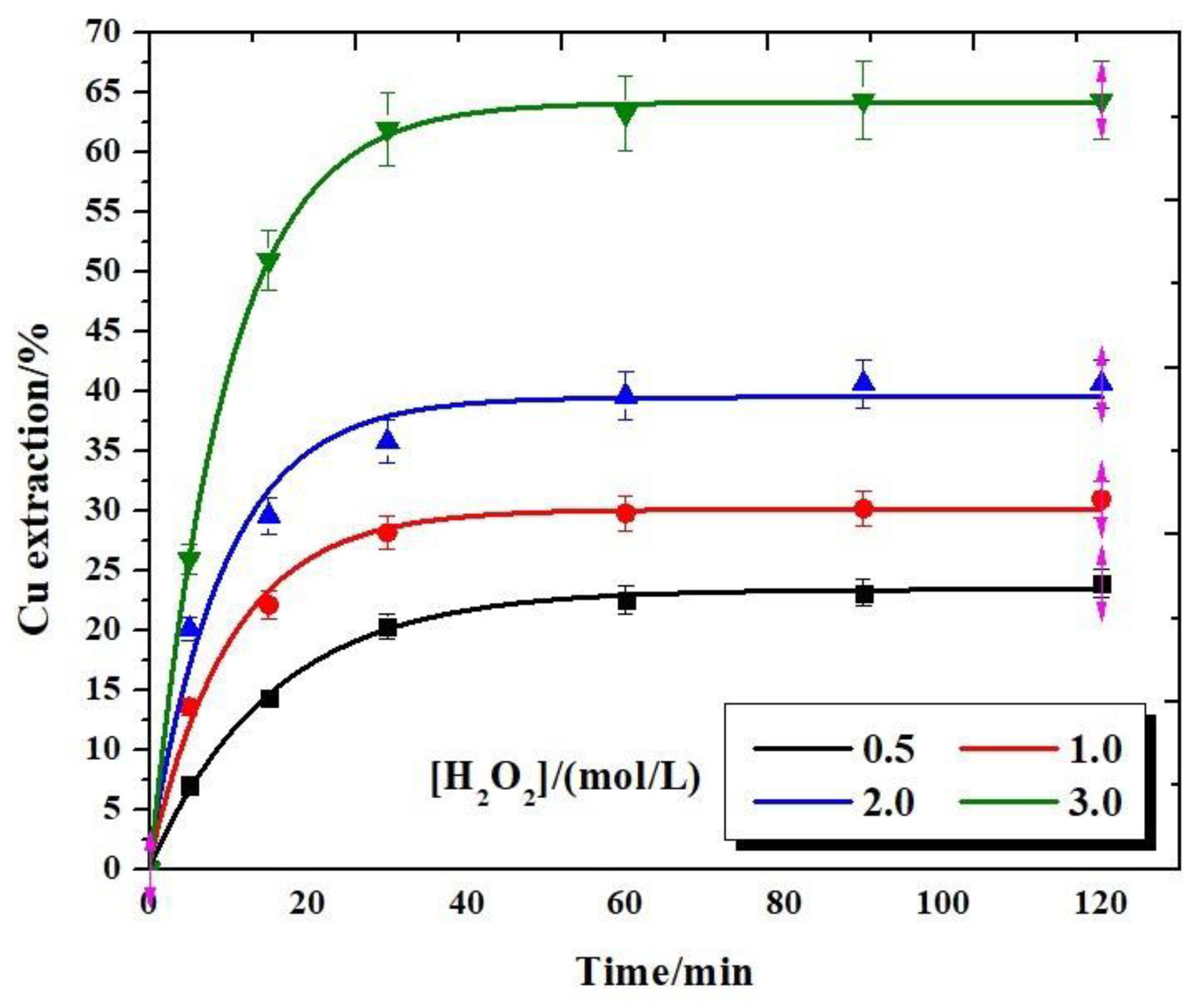
3.4. Effect of Hydrogen Peroxide Concentration
3.5. Effect of Sulfuric Acid Concentration
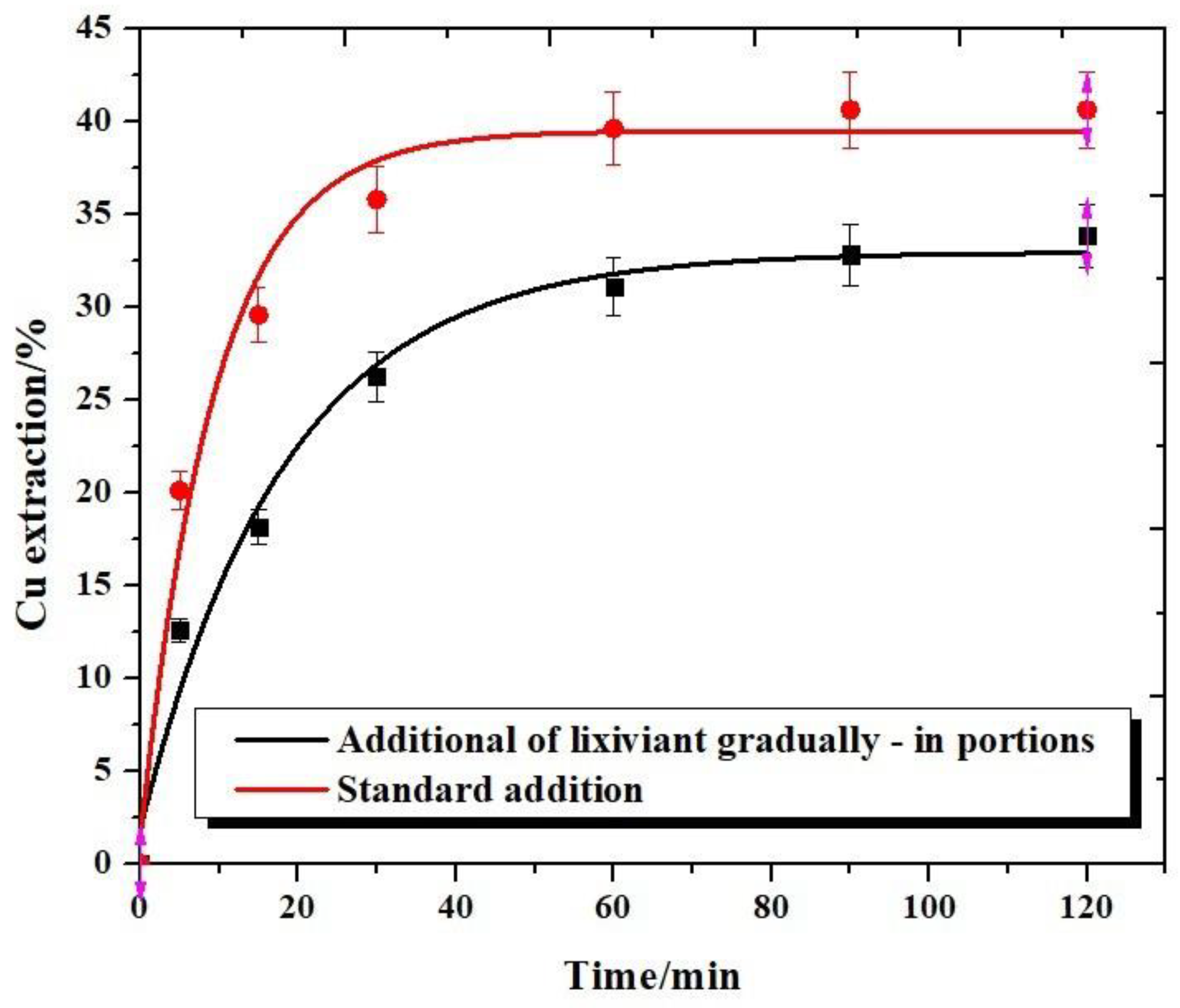
3.6. Effect of the Mode of Lixiviant Dosing
3.7. Kinetics of Chalcopyrite Leaching
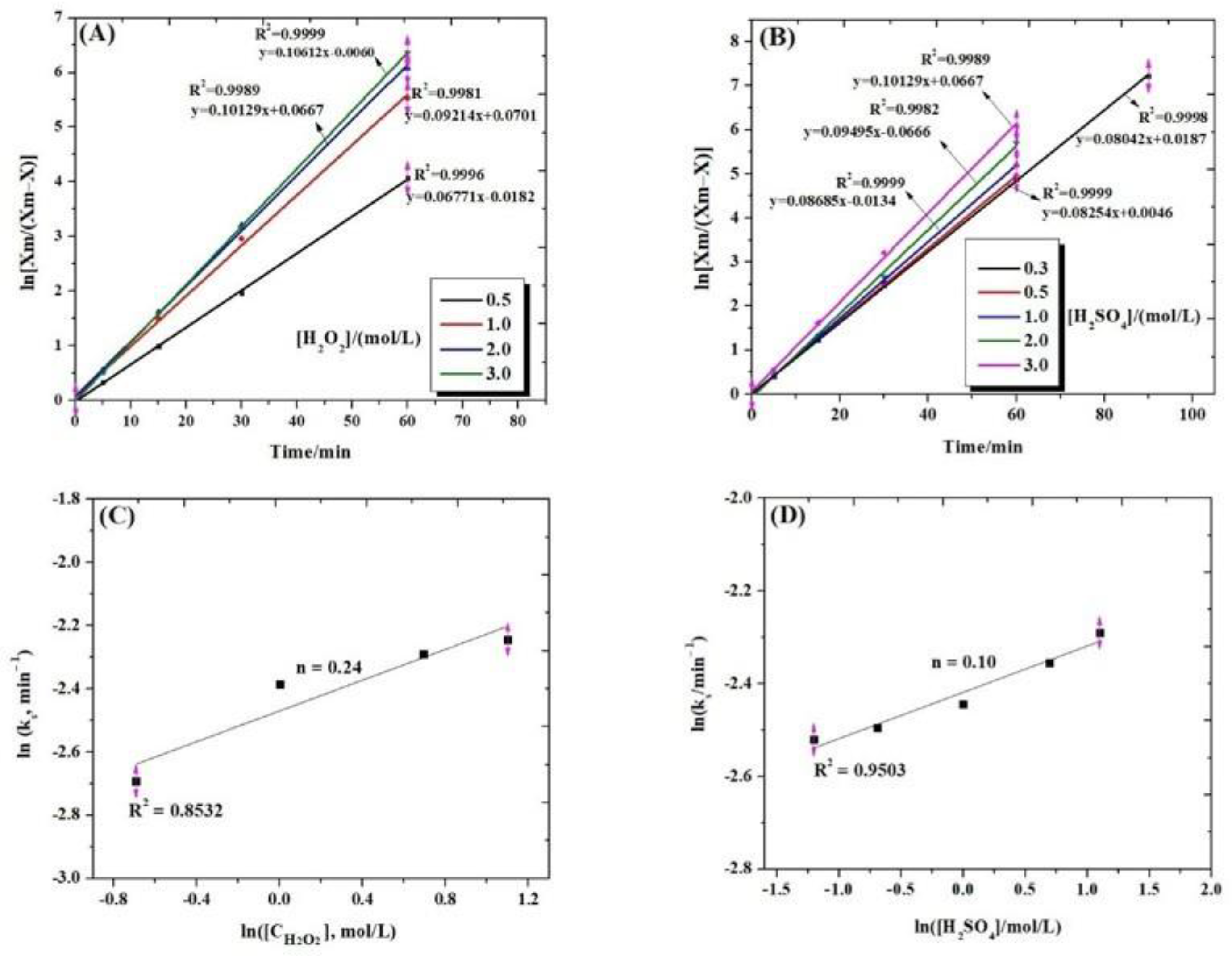
3.7.1. Effect of Temperature
3.7.2. Effect of Hydrogen Peroxide and Sulfuric Acid Concentrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez, M.; Ayala, L.; Robles, P.; Sepúlveda, R.; Torres, D.; Carrillo-Pedroza, F.R.; Jeldres, R.I.; Toro, N. Leaching Chalcopyrite with an Imidazolium-Based Ionic Liquid and Bromide. Metals 2020, 10, 183. [Google Scholar] [CrossRef]
- Takatori, K.; Kato, H.; Yoshimura, A.; Matsuno, Y. Chalcopyrite Leaching in a Dimethyl Sulfoxide Solution Containing Copper Chloride. Min. Metall. Explor. 2021, 38, 1477–1485. [Google Scholar] [CrossRef]
- Bogdanović, G.; Petrović, S.; Sokić, M.; Antonijević, M. Chalcopyrite Leaching in Acid Media: A Review. Metall. Mater. Eng. 2020, 26, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.D.; ·Sari, Z.A.; Erdemoğlu, M. Copper Enrichment in Solid with Selective Reverse Leaching with Oxalic Acid. J. Sustain. Metall. 2020, 6, 428–436. [Google Scholar] [CrossRef]
- Nyembwe, K.J.; Fosso-Kankeu, E.; Waanders, F.; Mkandawire, M. pH-dependent Leaching Mechanism of Carbonatitic Chalcopyrite in Ferric Sulfate Solution. Trans. Nonferrous Met. Soc. China 2021, 31, 2139–2152. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Liu, H.; Fan, G.; Peng, W.; Cao, Y. Selective Complexation Leaching of Copper from Copper Smelting Slag with the Alkaline Glycine Solution: An Effective Recovery Method of Copper from Secondary Resource. Sep. Purif. Technol. 2023, 326, 124619. [Google Scholar] [CrossRef]
- Golik, V.I.; Klyuev, R.V.; Martyushev, N.V.; Zyukin, D.A.; Karlina, A.I. Prospects for Return of Valuable Components Lost in Tailings of Light Metals Ore Processing. Metallurgist 2023, 67, 96–103. [Google Scholar] [CrossRef]
- Santibáñez-Velásquez, L.E.; Guzmán, A.; Morel, M.J. Extraction of Iron and Other Metals from Copper Tailings through Leaching. Metals 2022, 12, 1924. [Google Scholar] [CrossRef]
- Nurtazina, N.; Uvarov, N.; Azhigulova, R.; Tyapkin, P. Chalcopyrite Leaching by Amino Acid Solutions in the Presence of Hydrogen Peroxide. Physicochem. Probl. Miner. Process. 2022, 58, 157067. [Google Scholar] [CrossRef]
- Sokić, M.; Stojanović, J.; Marković, B.; Kamberović, Ž.; Gajić, N.; Radosavljević-Mihajlović, A.; Milojkov, D. Modification of Structural-Textural Properties of Sulfide Minerals at Polymetallic Concentrate Leaching with Sulfuric Acid and Hydrogen Peroxide Solutions. Russ. J. Non-Ferr. Met. 2022, 63, 457–472. [Google Scholar] [CrossRef]
- Castellón, C.I.; Hernández, P.C.; Velásquez-Yévenes, L.; Taboada, M.E. An Alternative Process for Leaching Chalcopyrite Concentrate in Nitrate-Acid-Seawater Media with Oxidant Recovery. Metals 2020, 10, 518. [Google Scholar] [CrossRef]
- Kartal, M.; Xia, F.; Ralph, D.; Rickard, W.D.A.; Renard, F.; Li, W. Enhancing Chalcopyrite Leaching by Tetrachloroethylene-Assisted Removal of Sulphur Passivation and the Mechanism of Jarosite Formation. Hydrometallurgy 2020, 191, 105192. [Google Scholar] [CrossRef]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Melo, E.; Hernández, M. Pretreatment to Leaching for a Primary Copper Sulphide Ore in Chloride Media. Metals 2021, 11, 1260. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Zhu, L.; Liu, B.; Zhou, K.; Xu, J.; Guo, C. Eco-friendly Oxidation Leaching from Chalcopyrite Powder and Kinetics Assisted by Sodium Chloride in Organic Acid Media. Adv. Powder Technol. 2022, 33, 103547. [Google Scholar] [CrossRef]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Melo, E. The Effects of Sulphuric Acid and Sodium Chloride Agglomeration and Curing on Chalcopyrite Leaching. Metals 2021, 11, 873. [Google Scholar] [CrossRef]
- Adebayo, A.O.; Ipinmoroti, K.O.; Ajayi, O.O. Dissolution Kinetics of Chalcopyrite with Hydrogen Peroxide in Sulphuric Acid Medium. Chem. Biochem. Eng. Q. 2003, 17, 213–218. [Google Scholar]
- Aydogan, S.; Ucar, G.; Canbazoglu, M. Dissolution Kinetics of Chalcopyrite in Acidic Potassium Dichromate Solution. Hydrometallurgy 2006, 81, 45–51. [Google Scholar] [CrossRef]
- Dakubo, F.; Baygents, J.C.; Farrell, J. Peroxodisulfate Assisted Leaching of Chalcopyrite. Hydrometallurgy 2012, 121–124, 68–73. [Google Scholar] [CrossRef]
- Cordoba, E.M.; Munoz, J.A.; Blazquez, M.L.; Gonzalez, F.; Ballester, A. Leaching of Chalcopyrite with Ferric ion. Part II: Effect of redox potential. Hydrometallurgy 2008, 93, 88–96. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Stanković, S.; Kamberović, Ž.; Štrbac, N.; Manojlović, V.; Petronijević, N. Kinetics of Chalcopyrite Leaching by Hydrogen Peroxide in Sulfuric Acid. Metals 2019, 9, 1173. [Google Scholar] [CrossRef]
- Mahajan, V.; Misra, M.; Zhong, K.; Fuerstenau, M.C. Enhanced Leaching of Copper from Chalcopyrite in Hydrogen peroxide–Glycol system. Miner. Eng. 2007, 20, 670–674. [Google Scholar] [CrossRef]
- Jones, S.W. Applications of Hydrogen Peroxide and Derivatives; Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Olubambi, P.A.; Potgieter, J.H. Investigations on the Mechanisms of Sulfuric Acid Leaching of Chalcopyrite in the Presence of Hydrogen Peroxide. Miner. Process. Extr. Metall. Rev. 2009, 30, 327–345. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Janković, Z.D.; Dimitrijević, M.D. Kinetics of Chalcopyrite Dissolution by Hydrogen Peroxide in Sulphuric Acid. Hydrometallurgy 2004, 71, 329–334. [Google Scholar] [CrossRef]
- Agacayak, T.; Aras, A.; Aydogan, S.; Erdemoglu, M. Leaching of Chalcopyrite Concentrate in Hydrogen Peroxide Solution. Physicochem. Probl. Miner. Process. 2014, 50, 657–666. [Google Scholar] [CrossRef]
- Turan, M.D.; Altundogan, H.S. Leaching of Chalcopyrite Concentrate with Hydrogen Peroxide and Sulfuric Acid in an Autoclave System. Metall. Mater. Trans. B 2013, 44, 809–819. [Google Scholar] [CrossRef]
- Turan, M.D.; Sari, Z.A.; Nizamoglu, H. Pressure Leaching of Chalcopyrite with Oxalic Acid and Hydrogen Peroxide. J. Taiwan Inst. Chem. Eng. 2021, 118, 112–120. [Google Scholar] [CrossRef]
- Solis-Marcíal, O.J.; Lapidus, G.T. Improvement of Chalcopyrite Dissolution in Acid Media Using Polar Organic Solvents. Hydrometallurgy 2013, 131–132, 120–126. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, A.; Lapidus, G.T. Study of Chalcopyrite Leaching from a Copper Concentrate with Hydrogen Peroxide in Aqueous Ethylene Glycol Media. Hydrometallurgy 2017, 169, 192–200. [Google Scholar] [CrossRef]
- Ghomi, M.A.; Mozammel, M.; Moghanni, H.; Shahkar, L. Atmospheric Leaching of Chalcopyrite in the Presence of Some Polar Organic Reagents: A Comparative Study and Optimization. Hydrometallurgy 2019, 189, 105120. [Google Scholar] [CrossRef]
- Wu, J.; Ahn, J.; Lee, J. Kinetic and Mechanism Studies using Shrinking Core Model for Copper Leaching from Chalcopyrite in Methanesulfonic Acid with Hydrogen Peroxide. Miner. Process. Extr. Metall. Rev. 2021, 42, 38–45. [Google Scholar] [CrossRef]
- Barton, I.F.; Hiskey, B.J. Chemical, Crystallographic, and Electromagnetic Variability in Natural Chalcopyrite and Implications for Leaching. Min. Eng. 2022, 189, 107867. [Google Scholar] [CrossRef]
- Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M. Leaching of Chalcopyrite with Hydrogen Peroxide in Hydrochloric Acid Solution. Trans. Nonferrous Met. Soc. China 2018, 28, 1444–1455. [Google Scholar] [CrossRef]
- Arslanoglu, H.; Yaras, Y. Chalcopyrite Leaching with Hydrogen Peroxide in Formic Acid Medium. Trans. Indian Inst. Met. 2020, 73, 785–792. [Google Scholar] [CrossRef]
- Hu, J.; Tian, G.; Zi, F.; Hu, X. Leaching of Chalcopyrite with Hydrogen Peroxide in 1-hexyl-3-methyl-imidazolium Hydrogen Sulfate Ionic Liquid Aqueous Solution. Hydrometallurgy 2017, 169, 1–8. [Google Scholar] [CrossRef]
- Antonijević, M.; Dimitrijević, D.; Janković, Z. Leaching of Pyrite with Hydrogen Peroxide in Sulphuric Acid. Hydrometallurgy 1997, 46, 71–83. [Google Scholar] [CrossRef]
- Carrillo-Pedroza, F.R.; Davalos-Sanchez, A.; Soria-Aguilar, M.; Pecina-Trevino, E.T. Coal Desulfurization in Oxidative Acid Media using Hydrogen Peroxide and Ozone: A Kinetic and Statistical Approach. Energy Fuels 2009, 23, 3703–3710. [Google Scholar] [CrossRef]
- Kremer, M.L. Promotion of the Fenton reaction by Cu2+ ions: Evidence for Intermediates. Int. J. Chem. Kinet. 2006, 38, 725–736. [Google Scholar] [CrossRef]
- Mlasi, B.; Glasser, D.; Hildebrandt, D. Kinetics of the Decomposition of Hydrogen Peroxide in Acidic Copper Sulfate Solutions. Ind. Eng. Chem. Res. 2015, 54, 5589–5597. [Google Scholar] [CrossRef]
- Ahn, J.; Wu, J.; Lee, J. Investigation on Chalcopyrite Leaching with Methanesulfonic Acid (MSA) and Hydrogen Peroxide. Hydrometallurgy 2019, 187, 54–62. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Urošević, D.; Milić, S.; Sokić, M.; Marković, R. Dissolution of Copper from Smelting Slag by Leaching in Chloride Media. J. Min. Metall. Sect. B 2017, 53, 407–412. [Google Scholar] [CrossRef]
- Mojtahedi, B.; Rasouli, S.; Yoozbashizadeh, H. Pressure Leaching of Chalcopyrite Concentrate with Oxygen and Kinetic Study on the Process in Sulfuric Acid Solution. Trans. Indian. Inst. Met. 2020, 73, 975–987. [Google Scholar] [CrossRef]
- Popovič, L.; Raschman, P.; Spišák, J.; Fedoročková, A.; Sučik, G. Generalized Shrinking Particle Model of Leaching: Effect of the Order of Surface Chemical Reaction, Liquid-to-Solid Ratio and Non-Ideal Behaviour of the Liquid Phase. Hydrometallurgy 2020, 196, 105441. [Google Scholar] [CrossRef]
- Turan, M.D.; Sari, Z.A.; Demiraslan, A. Ultrasound-Assisted Leaching and Kinetic Study of Blended Copper Slag. Metall. Mater. Trans. B 2019, 50, 1949–1956. [Google Scholar] [CrossRef]
- Cháidez, J.; Parga, J.; Valenzuela, J.; Carrillo, R.; Almaguer, I. Leaching Chalcopyrite Concentrate with Oxygen and Sulfuric Acid Using a Low-Pressure Reactor. Metals 2019, 9, 189. [Google Scholar] [CrossRef]
- Saldaña, M.; Gálvez, E.; Robles, P.; Castillo, J.; Toro, N. Copper Mineral Leaching Mathematical Models—A Review. Materials 2022, 15, 1757. [Google Scholar] [CrossRef]
- Dreisinger, D.; Abed, N. A Fundamental Study of the Reductive Leaching of Chalcopyrite Using Metallic Iron. Part I: Kinetic Analysis. Hydrometallurgy 2002, 66, 37–57. [Google Scholar] [CrossRef]
- Fatemi, N.; Whitehead, R.; Price, D.; Dollimore, D. Some Comments on the use of Avrami−Erofeev Expressions and Solid State Decomposition Rate Constants. Thermochim. Acta 1986, 104, 93–100. [Google Scholar] [CrossRef]
- Kasongo, K.B.; Mwanat, M.H.M.; Malenga, N.E.; Makhatha, M.E. Kinetic Study of Copper and Cobalt Dissolution from Sulfidic Ores in Sulfate–Chloride Media. Min. Metall. Explor. 2022, 39, 2209–2219. [Google Scholar] [CrossRef]
- Klyuev, R.V.; Morgoev, I.D.; Morgoeva, A.D.; Gavrina, O.A.; Martyushev, N.V.; Efremenkov, E.A.; Mengxu, Q. Methods of Forecasting Electric Energy Consumption: A Literature Review. Energies 2022, 15, 8919. [Google Scholar] [CrossRef]
- Stoll, A.; Benner, P. Machine Learning for Material Characterization with an Application for Predicting Mechanical Properties. GAMM-Mitteilungen. GAMM-Mitteilungen 2021, 44, e202100003. [Google Scholar] [CrossRef]
- Athamena, A.; Gaagai, A.; Aouissi, H.A.; Burlakovs, J.; Bencedira, S.; Zekker, I.; Krauklis, A.E. Chemometrics of the Environment: Hydrochemical Characterization of Groundwater in Lioua Plain (North Africa) Using Time Series and Multivariate Statistical Analysis. Sustainability 2023, 15, 20. [Google Scholar] [CrossRef]
- Kurnyta, A.; Baran, M.; Kurnyta-Mazurek, P.; Kowalczyk, K.; Dziendzikowski, M.; Dragan, K. The Experimental Verification of Direct-Write Silver Conductive Grid and ARIMA Time Series Analysis for Crack Propagation. Sensors 2021, 21, 6916. [Google Scholar] [CrossRef] [PubMed]
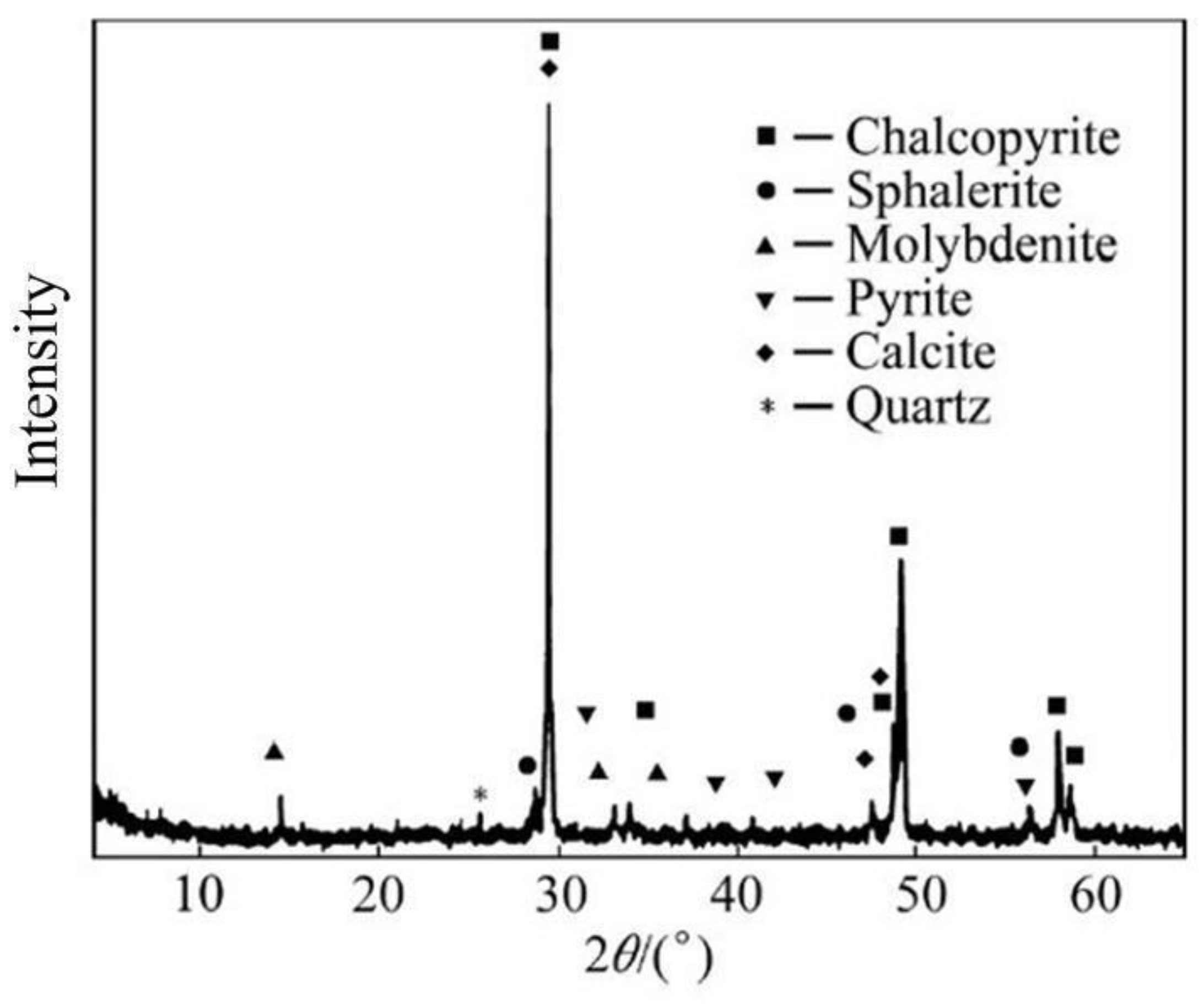
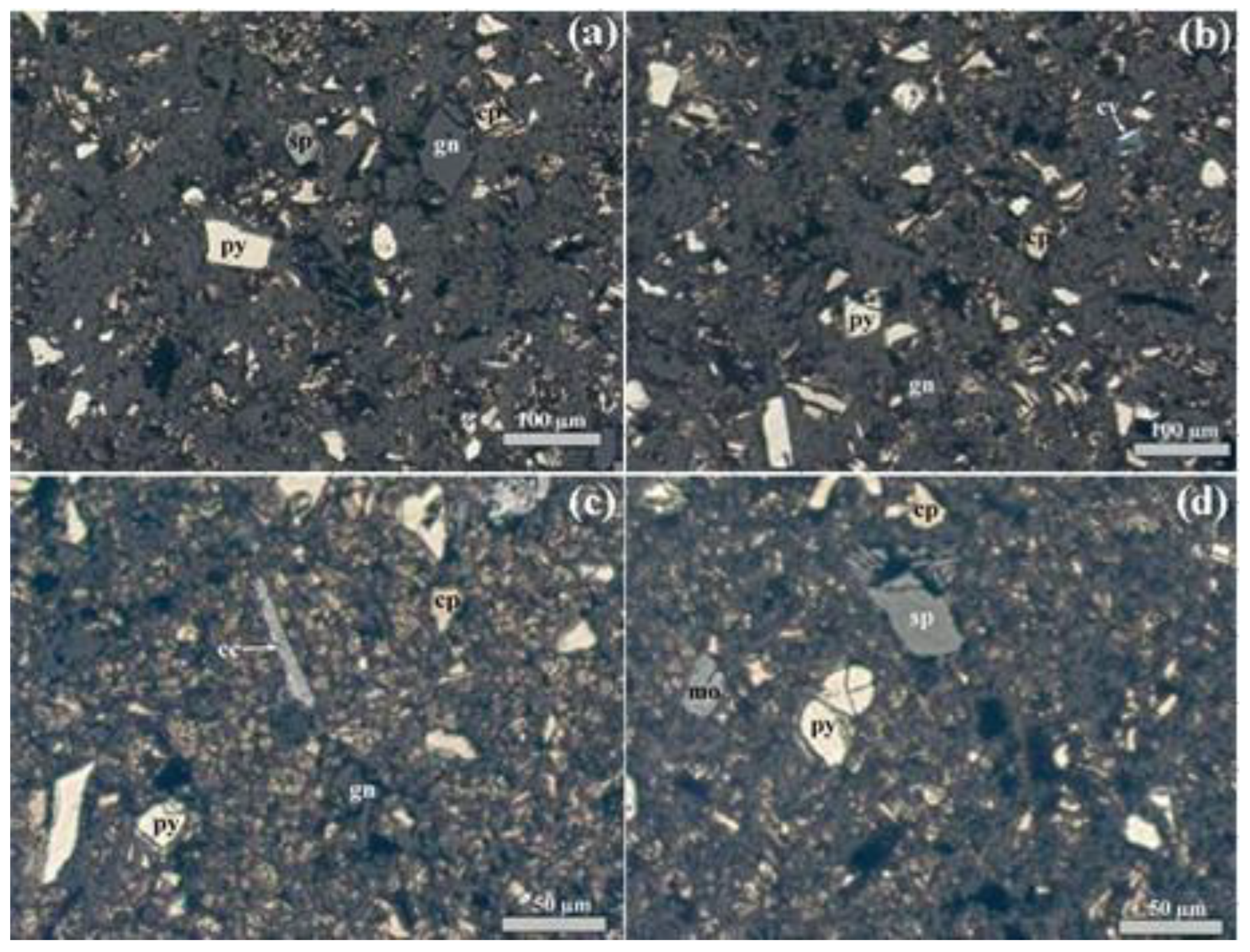



| Element | Content (wt %) |
|---|---|
| Cu | 24.84 |
| Fe | 29.52 |
| S | 32.92 |
| Mo | 0.57 |
| Zn | 0.60 |
| Pb | 0.076 |
| SiO2 | 2.67 |
| Al2O3 | 1.06 |
| Fe3O4 | <0.03 |
| CaO | 3.23 |
| MgO | 0.29 |
| K2O | 0.18 |
| Mineral | Chemical Formula | Content, % |
|---|---|---|
| Chalcopyrite | CuFeS2 | 64.79 |
| Pyrite | FeS2 | 18.78 |
| Covellite | CuS | 0.86 |
| Chalcocite | Cu2S | 0.37 |
| Bornite | Cu5FeS4 | 0.08 |
| Sphalerite | ZnS | 0.91 |
| Galena | PbS | 0.09 |
| Molybdenite | MoS2 | 0.88 |
| Magnetite | Fe3O4 | 0.07 |
| Rutile | TiO2 | 0.02 |
| Cassiterite | SnO2 | 0.03 |
| Gangue | CaCO3, SiO2 | 13.12 |
| Total | 100.00 |
| Element | Spectrum 1 | Spectrum 2 | Spectrum 3 |
|---|---|---|---|
| O | 4.00 | 8.84 | 2.70 |
| Al | / | 0.90 | / |
| Si | 0.30 | 5.30 | 1.70 |
| K | / | 1.40 | / |
| Cu | 1.25 | 13.50 | 6.40 |
| Fe | 49.20 | 24.80 | 32.85 |
| S | 45.25 | 44.46 | 53.65 |
| Zn | / | 0.80 | 2.70 |
| Σ | 100.00 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M.; Vukčević, M.; Kovačević, R. The Extraction of Copper from Chalcopyrite Concentrate with Hydrogen Peroxide in Sulfuric Acid Solution. Metals 2023, 13, 1818. https://doi.org/10.3390/met13111818
Petrović SJ, Bogdanović GD, Antonijević MM, Vukčević M, Kovačević R. The Extraction of Copper from Chalcopyrite Concentrate with Hydrogen Peroxide in Sulfuric Acid Solution. Metals. 2023; 13(11):1818. https://doi.org/10.3390/met13111818
Chicago/Turabian StylePetrović, Sanja J., Grozdanka D. Bogdanović, Milan M. Antonijević, Marija Vukčević, and Renata Kovačević. 2023. "The Extraction of Copper from Chalcopyrite Concentrate with Hydrogen Peroxide in Sulfuric Acid Solution" Metals 13, no. 11: 1818. https://doi.org/10.3390/met13111818
APA StylePetrović, S. J., Bogdanović, G. D., Antonijević, M. M., Vukčević, M., & Kovačević, R. (2023). The Extraction of Copper from Chalcopyrite Concentrate with Hydrogen Peroxide in Sulfuric Acid Solution. Metals, 13(11), 1818. https://doi.org/10.3390/met13111818







