Characteristics of Pore Morphology in Aluminum Alloy Foams Fabricated by Semi-Solid Route among Multiple Experimental Runs
Abstract
:1. Introduction
2. Materials and Methods
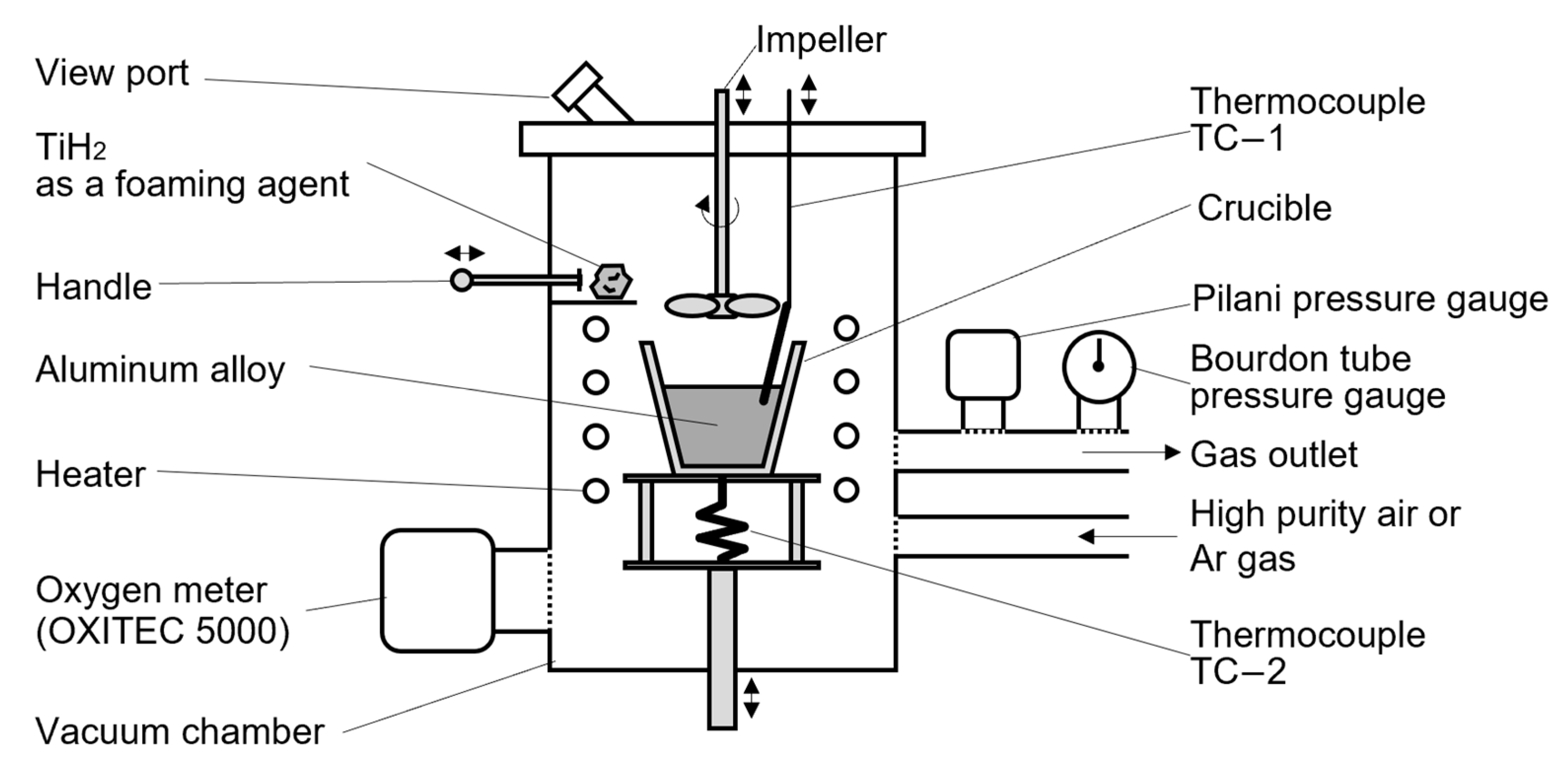
2.1. Fabrication Apparatus
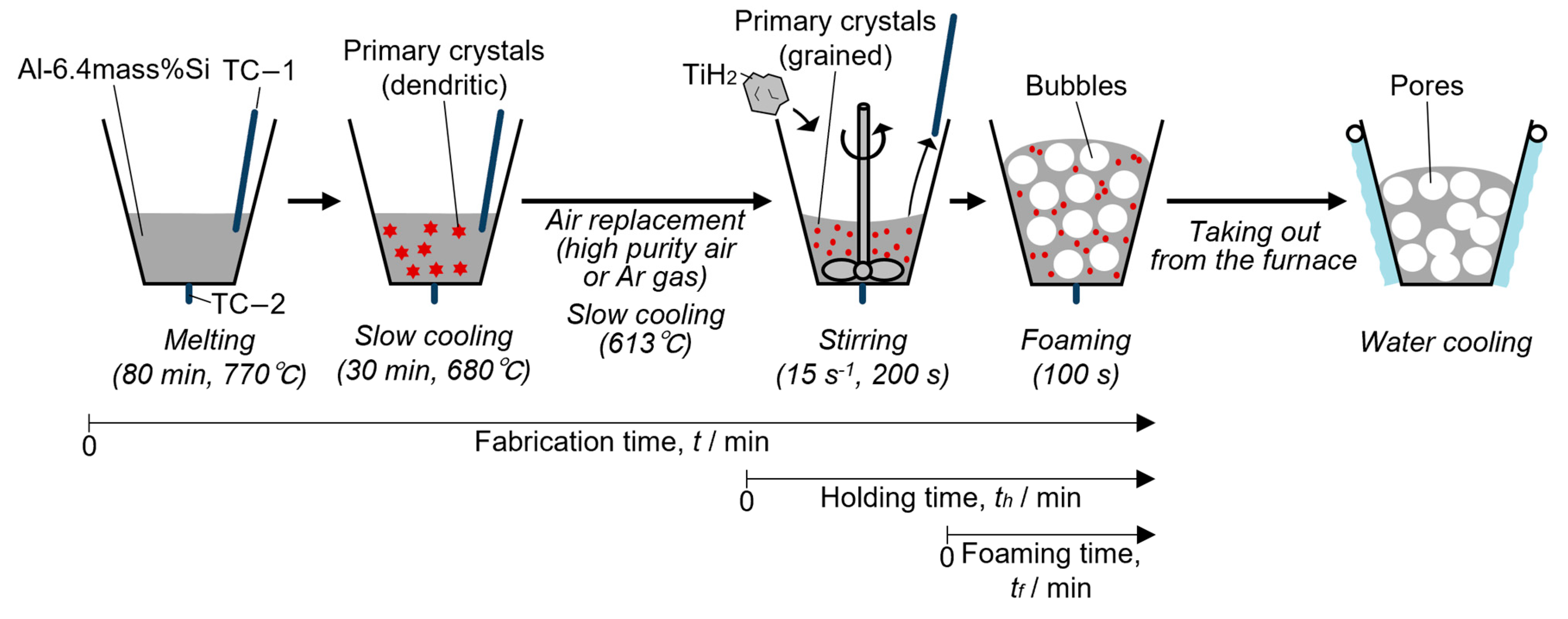
2.2. Fabrication of Aluminum Alloy Foam through the Semi-Solid Route
2.3. Analysis of Fabricated Foams
3. Results
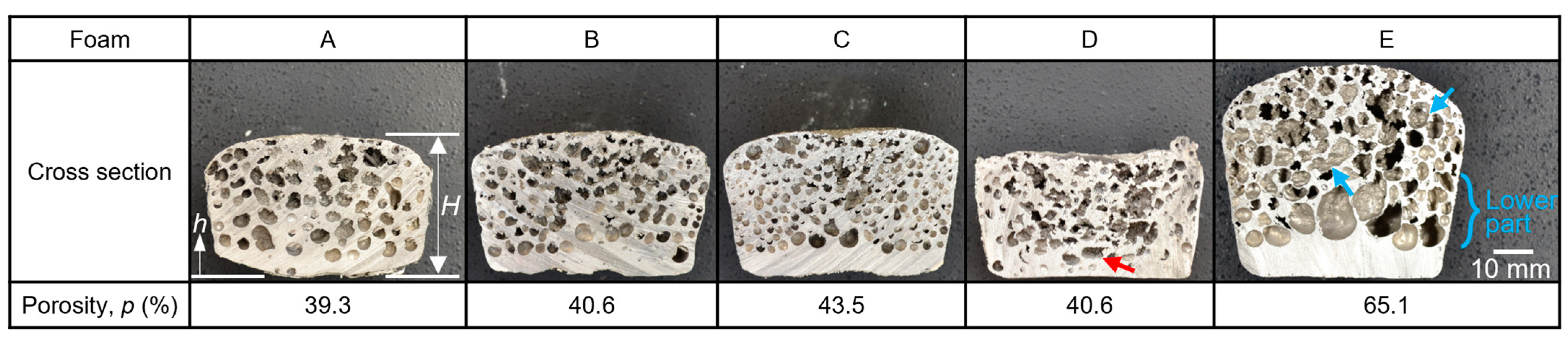
3.1. Fabricated Aluminum Alloy Foams
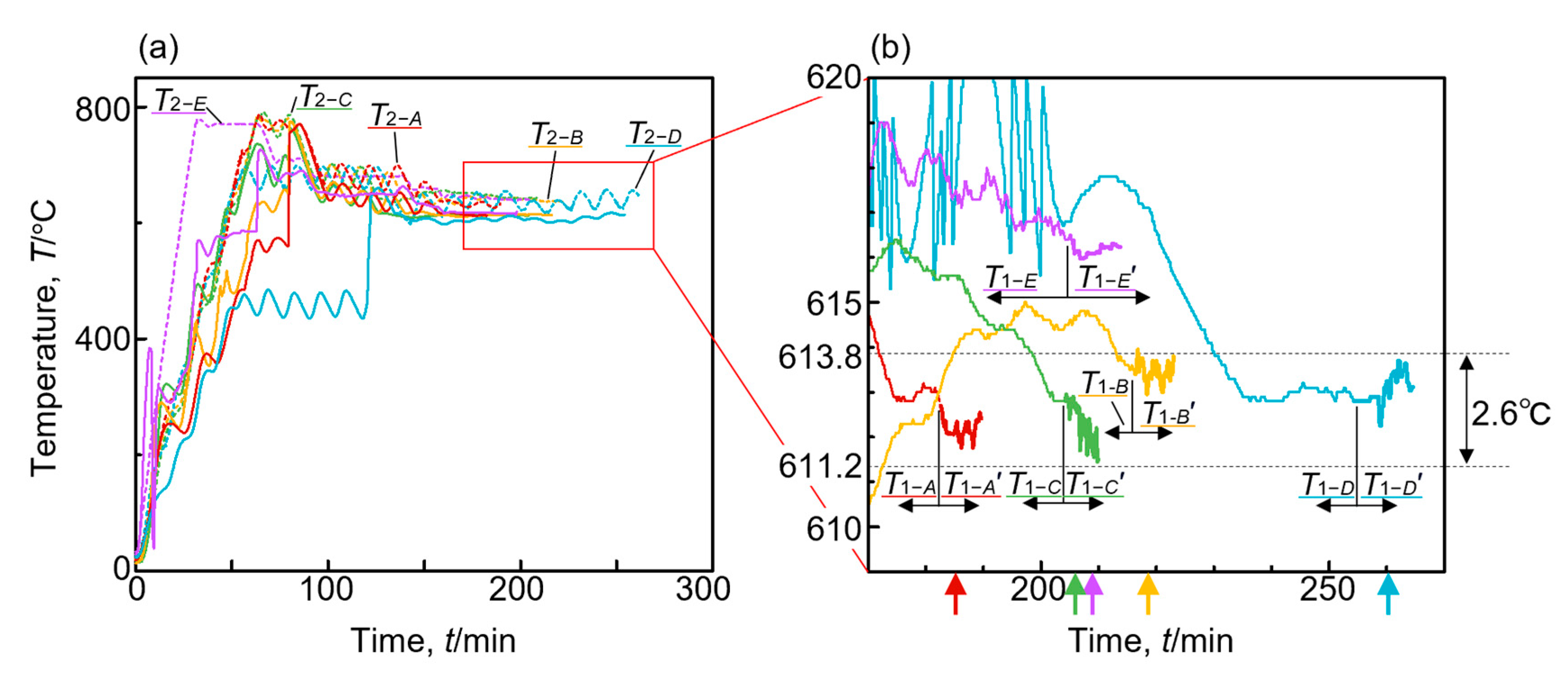
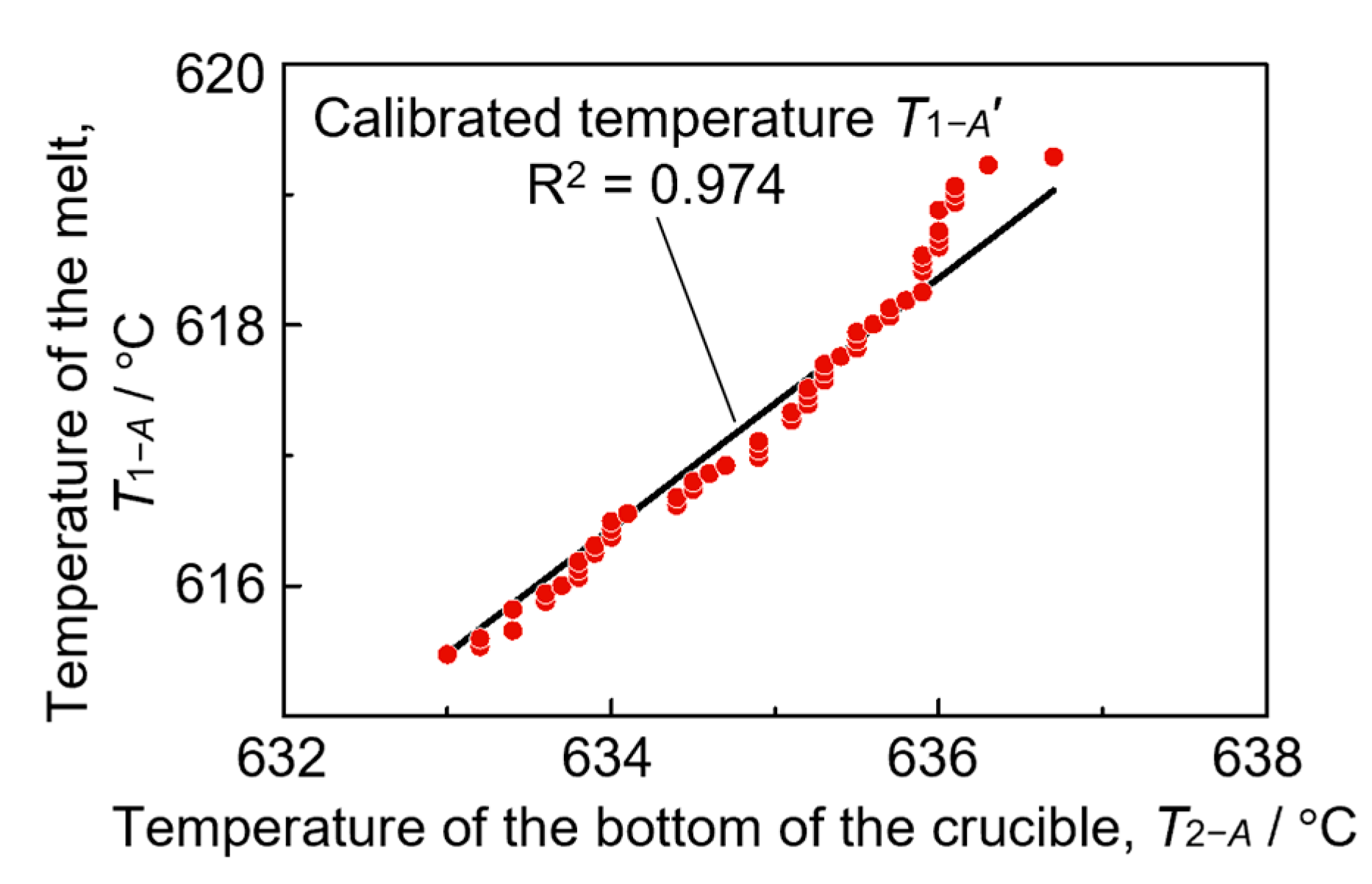
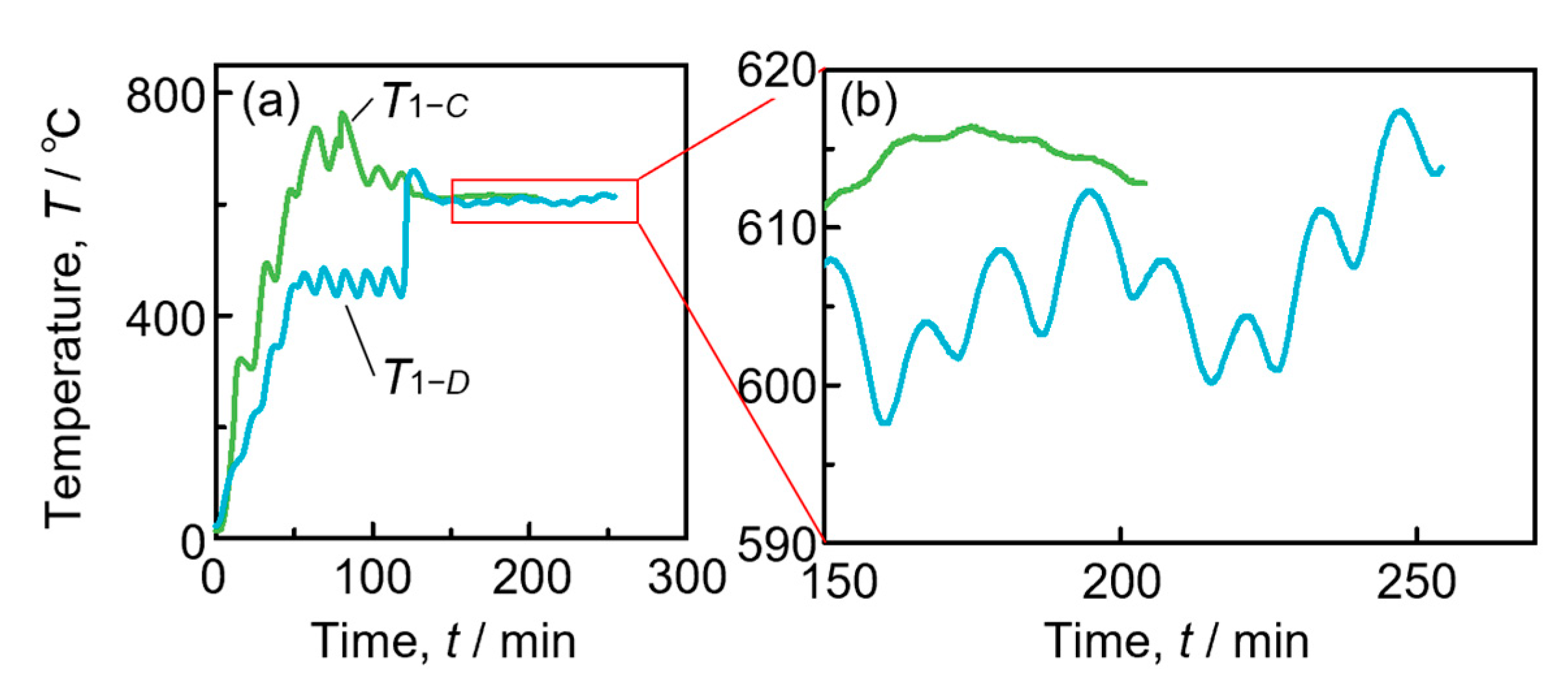
3.2. Temperature and Pressure history during fabrication
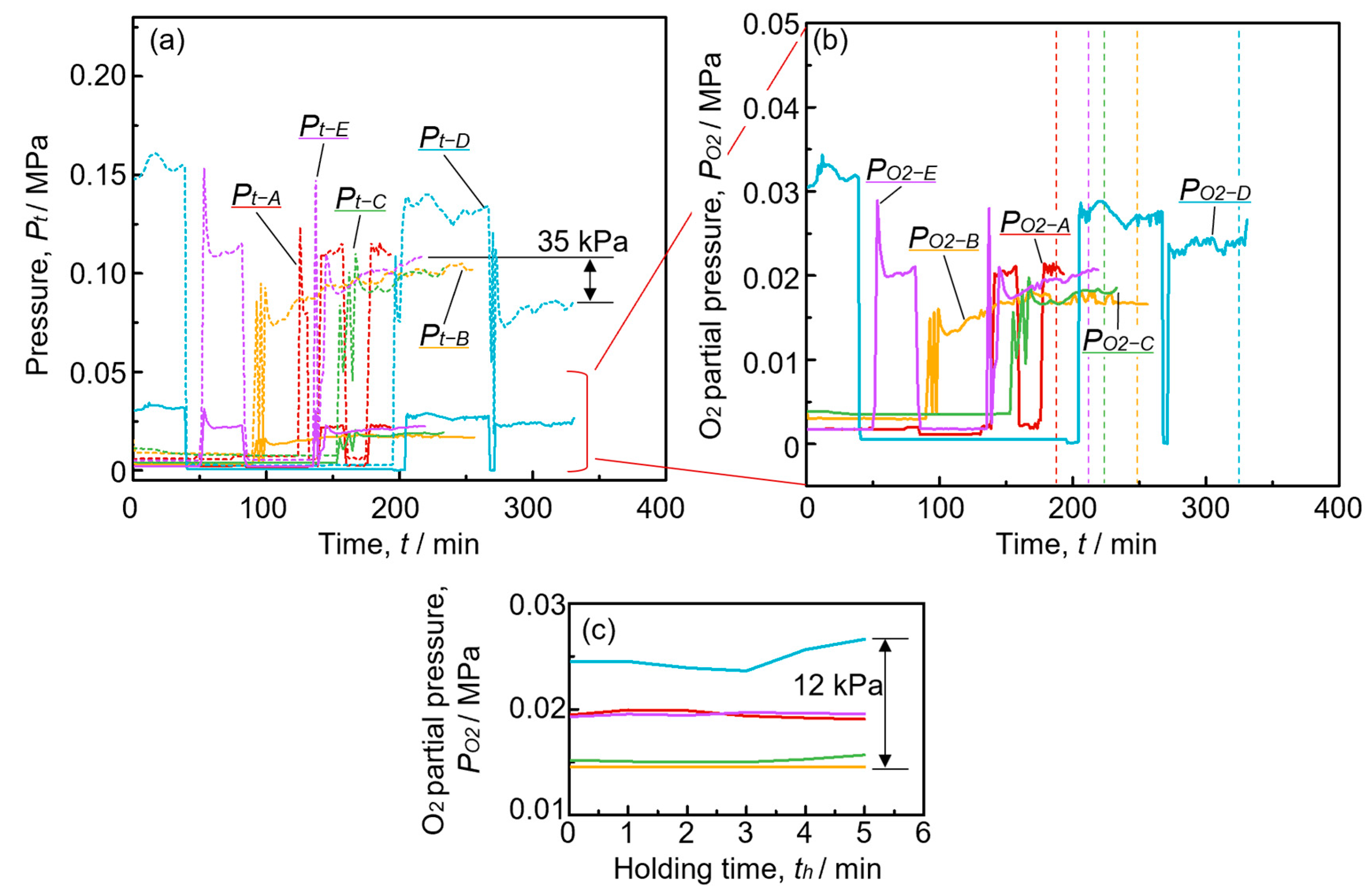
3.3. Pressure History during Fabrication
4. Discussion
4.1. Influence of H2 Generated through Decomposition of TiH2 during the Foaming Process
4.2. Reproductivity among Multiple Experimental Runs through the Semi-Solid Route
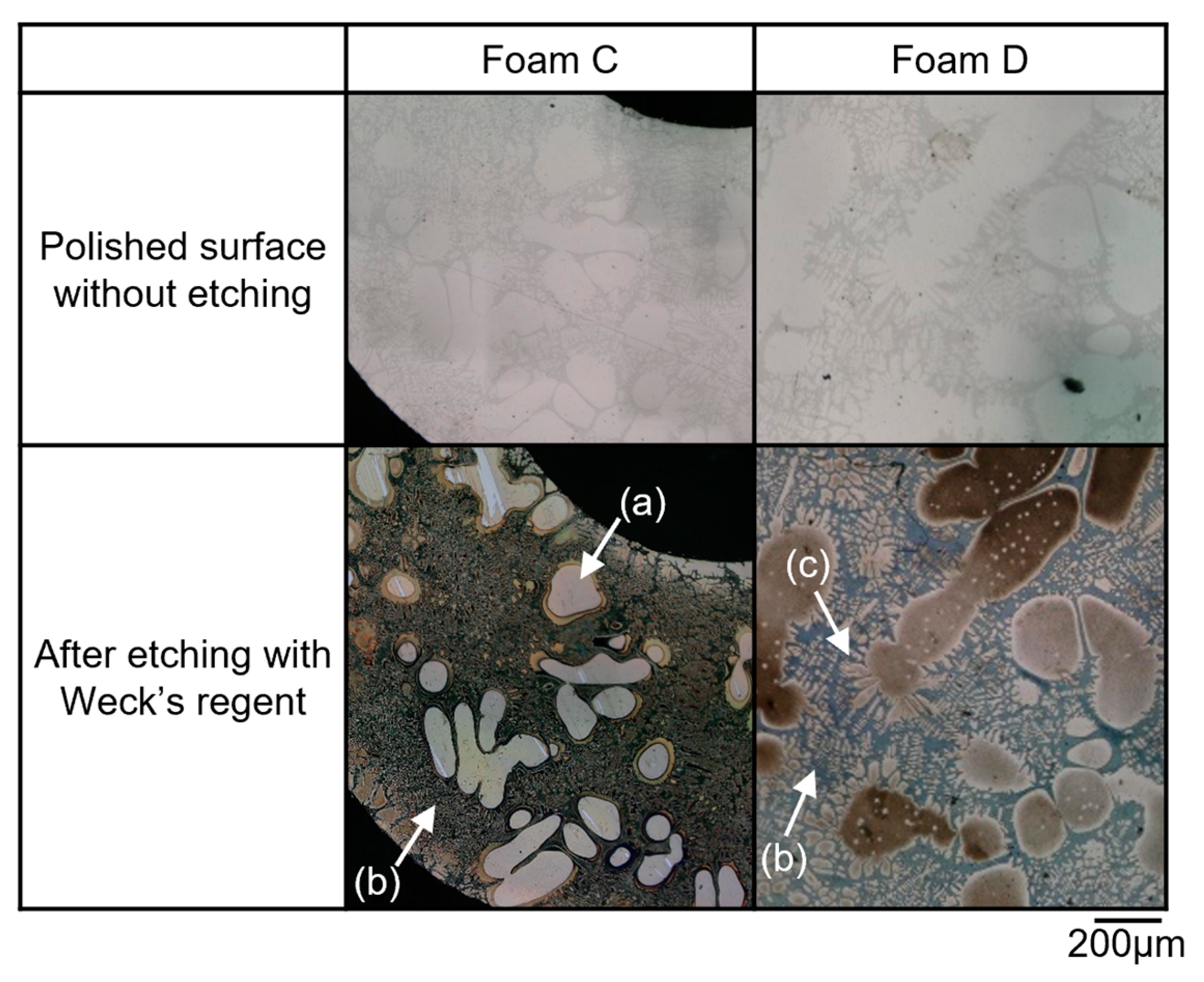
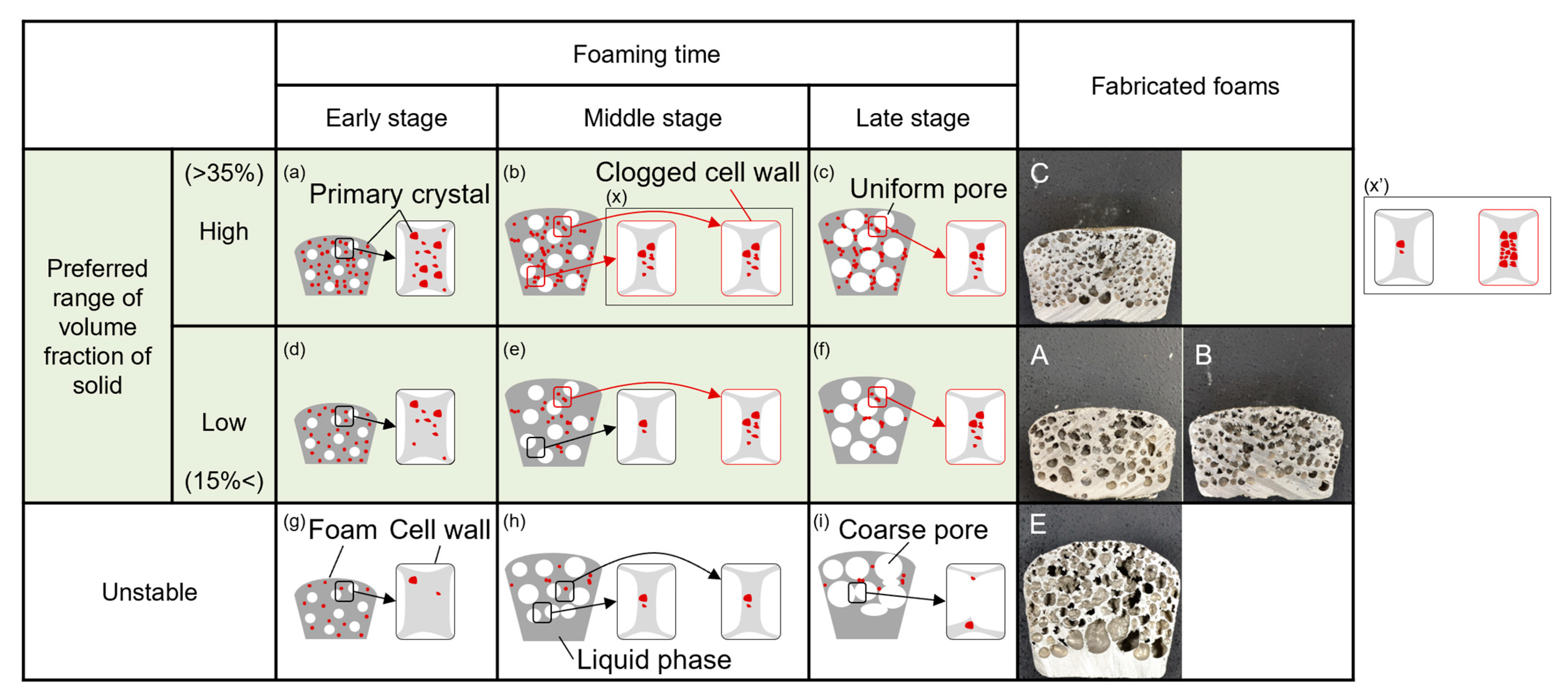
4.3. Characteristics of the Temperature and the Microstructure of the Foam with Poor Pore Morphology
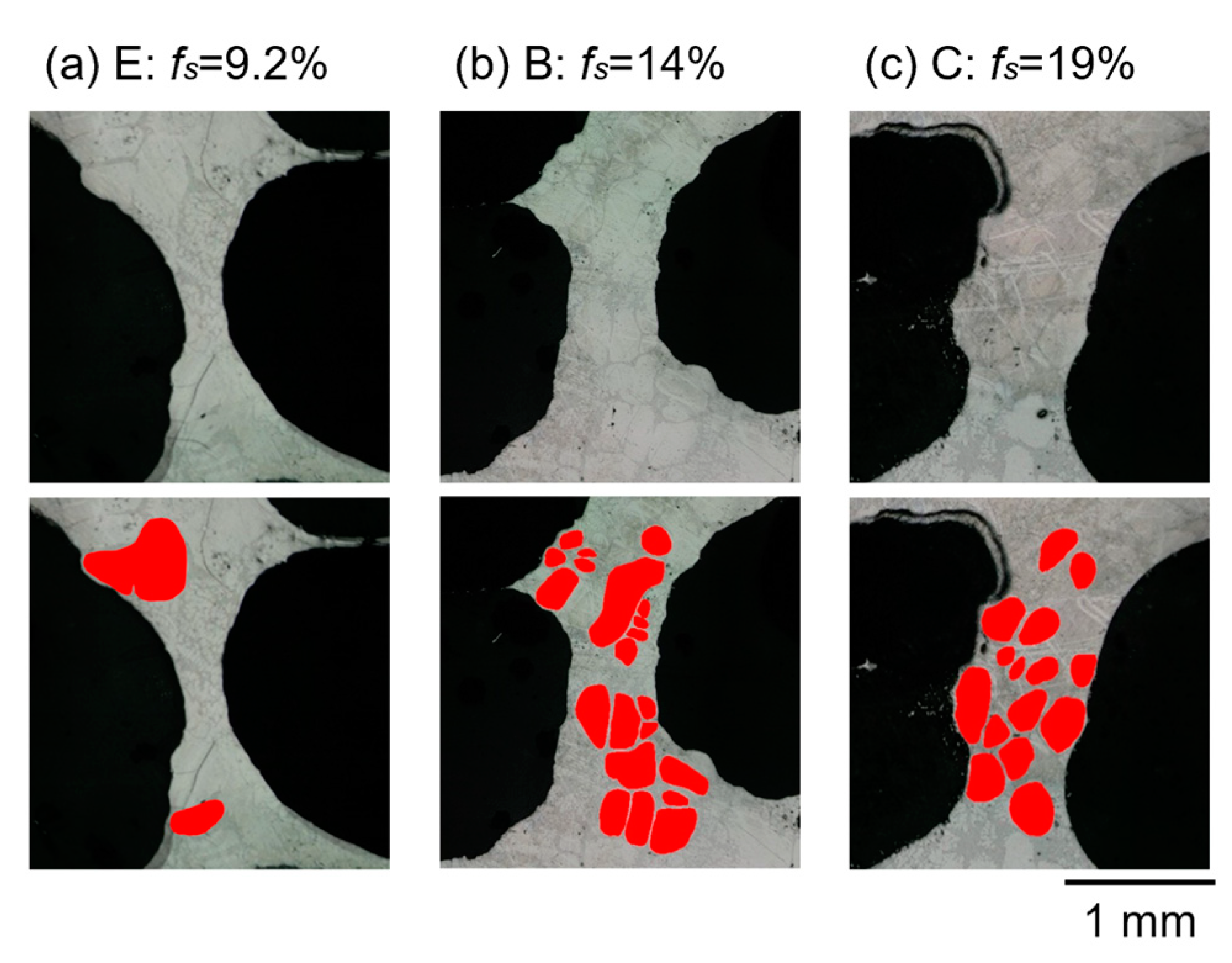
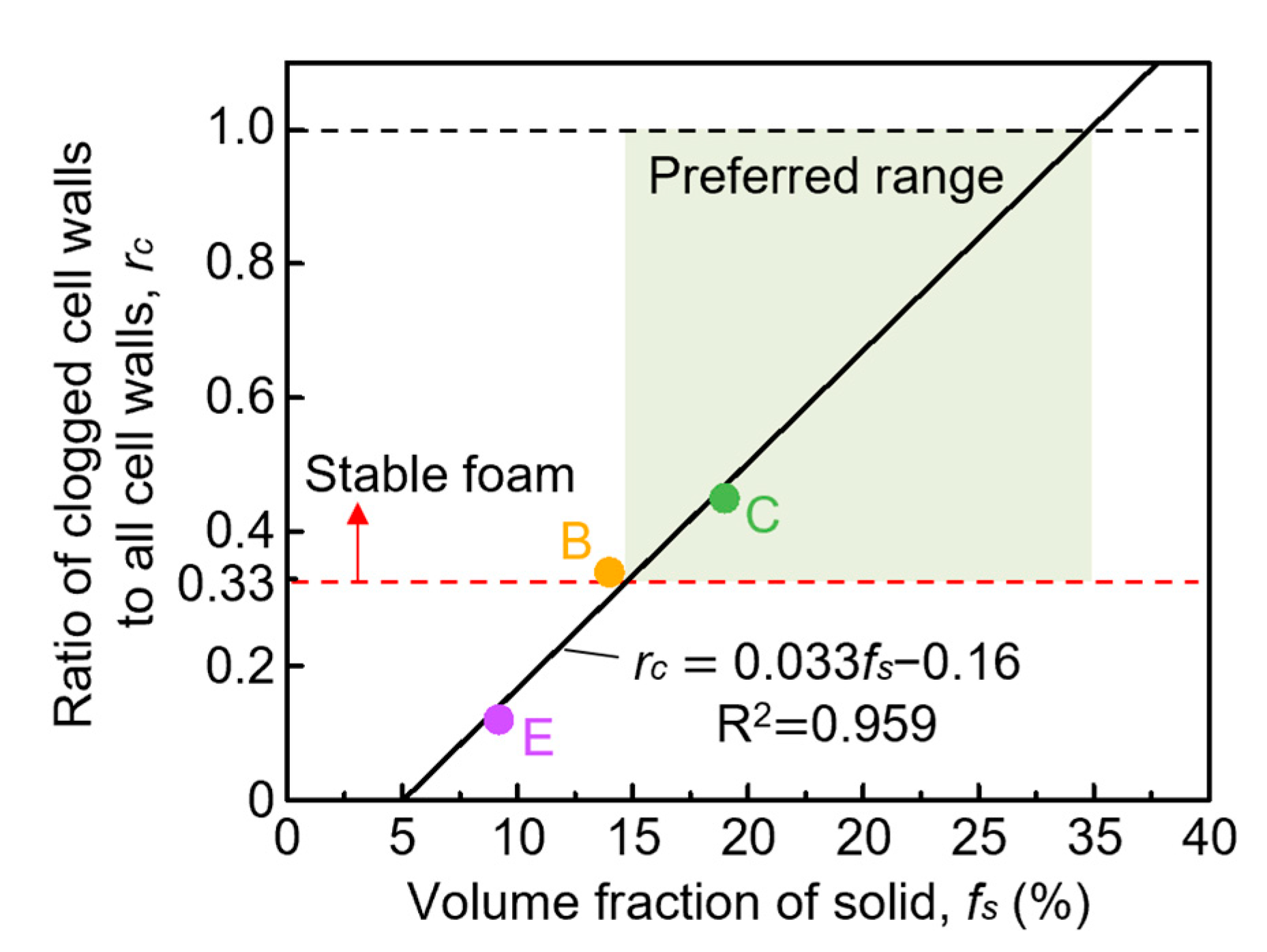
4.4. Relationship between Clogged Cell Wall and the Volume Fraction of the Solid
5. Conclusions
- The fabrication process through the semi-solid route conducted in this study had reproductivity for the fabrication of the aluminum alloy foam. The fluctuation of ±1.3 °C (±2.5% in the volume fraction of the solid) around 612.5 °C, which is close to the setting temperature of 613 °C, does not have a major influence on the pore morphology.
- When the volume fraction of the solid increases, exceeding 15% in volume fraction of the solid, the number of clogged cell walls increases. An increase in the number of clogged cell walls is more effective for the clogging effect than the fraction of primary crystals in already clogged cell walls increases.
- The preferred range of the volume fraction of the solid for fabrication of stable foam, which was defined to have uniform pores with small diameter and high circularity in this study, is from around 15% to 35%. If the aluminum–silicon alloy foam is fabricated with a certain volume fraction of the solid, the clogging effect works most effectively, and the cell walls become most stable when the volume fraction of the solid is 35%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banhart, J. Manufacture, characterization and application of cellular metals and metals foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, T.; Fleck, N.A.; Hutchinson, J.W.; Wadley, H.N.G.; Gibson, L.J. Metal Foams: A Design Guide, 1st ed.; Butterworth-Heinemann: Boston, MA, USA, 2000; pp. 8–11. [Google Scholar]
- García-Moreno, F. Commercial Applications of Metals Foams: Their Properties and Production. Metals 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Stręk, A.M.; Lasowicz, N.; Kwiecien, A.; Zając, B.; Jankowski, R. Highly Dissipative Materials for Damage Protection against Earthquake-Induced Structural Pounding. Materials 2021, 14, 3231. [Google Scholar] [CrossRef]
- Latour, M.; D’Aniello, M.; Babcsan, N.; Capone, P.; Landolfo, R.; Rizzano, G. Bending response of sandwich panels with steel skins and aluminum foam core. Steel Constr. 2022, 15, 73–80. [Google Scholar] [CrossRef]
- Schäffler, P.; Hanko, G.; Mitterer, H.; Zach, P. Alulight Metal Foam Products. In Proceedings of the 5th International Conference on Porous Metals and Metallic Foams: MetFoam2007, Montreal, QC, Canada, 5–7 September 2007; Lefebvre, L.P., Banhart, J., Dunand, D.C., Eds.; DEStech Publications: Lancaster, PA, USA, 2008; pp. 7–10. [Google Scholar]
- Garai, F.; Béres, G.; Weltsch, Z. Development of tubes filled with aluminium foams for lightweight vehicle manufacturing. Mater. Sci. Eng. A 2020, 790, 139743–139751. [Google Scholar] [CrossRef]
- Chilla, V.; Mondal, D.P.; Janaki Ram, G.D.; Mukherjee, M.J. Processing of in-situ aluminum foam-filled stainless steel tube with foam-tube bonding for enhanced crashworthiness. Manuf. Process. 2022, 82, 488–500. [Google Scholar] [CrossRef]
- Sardari, P.T.; Babaei-Mahani, R.; Giddings, D.; Yasseri, S.; Moghimi, M.A.; Bahai, H. Energy recovery from domestic radiators using a compact composite metal Foam/PCM latent heat storage. J. Clean. Prod. 2020, 257, 120504–120515. [Google Scholar] [CrossRef]
- Kováčik, J.; Jerz, J.; Gopinathan, A.; Simančík, F.; Marsavina, L.; Linul, E. Effect of sample shape on compression behavior of aluminum foams. Mater. Today Proc. 2023, 78, 308–313. [Google Scholar] [CrossRef]
- Jafari, M.J.; Sharak, M.N.; Khavanin, A.; Ebadzadeh, T.; Fazlali, M.; Madvari, R.F. Improving the Morphological Parameters of Aluminum Foam for Maximum Sound Absorption Coefficient using Genetic Algorithm. Sound. Vib. 2021, 55, 117–130. [Google Scholar] [CrossRef]
- Gergely, V.; Clyne, T.W. Drainage in standing liquid metal foams: Modelling and experimental observations. Acta Mater. 2004, 52, 3047–3058. [Google Scholar] [CrossRef]
- Heim, K.; Vinod Kumar, G.S.; Garcia-Moreno, F.; Manke, I.; Banhart, J. Drainage of particle-stabilised aluminum composites through single films and Plateau borders. Colloids Surf. A Physicochem. Eng. Asp. 2013, 438, 85–92. [Google Scholar] [CrossRef]
- Miyoshi, T.; Itoh, M.; Akiyama, S.; Kitahara, A. ALPORAS Aluminum Foam: Production Process, Properties and Applications. Adv. Eng. Mater. 2000, 2, 179–183. [Google Scholar] [CrossRef]
- Hanafusa, T.; Ohishi, K. Making of Porous Metallic Material by the Semi-solid Aluminum Alloy. Bull. Hiroshima Prefect. Technol. Res. Inst. East. Reg. Ind. Res. Cen. 2010, 23, 28–29. [Google Scholar]
- Stanzick, H.; Wichmann, M.; Weise, J.; Helfen, L.; Baumbach, T.; Banhart, J. Process Control in Aluminum Foam Production Using Real-Time X-ray Radioscopy. Adv. Eng. Mater. 2002, 4, 814–823. [Google Scholar] [CrossRef]
- Jin, I.; Kenny, J.D.; Sang, H. Method of Producing Lightweight Foamed Metal. U.S. Patent 4,973,358, 27 November 1990. [Google Scholar]
- Heim, K.; García-Moreno, F.; Banhart, J. Particle size and fraction required to stabilize aluminum alloy foams created by gas injection. Scr. Mater. 2018, 153, 54–58. [Google Scholar] [CrossRef]
- Kuwahara, T.; Kaya, A.; Osaka, T.; Takamatsu, S.; Suzuki, S. Stabilization Mechanism of Semi-Solid Film Simulating the Cell Wall during Fabrication of Aluminum Foam. Metals 2020, 10, 333. [Google Scholar] [CrossRef]
- Takamatsu, S.; Kuwahara, T.; Kochi, R.; Suzuki, S. Percolation of Primary Crystals in Cell Walls of Aluminum Alloy Foam via Semi-Solid Route. Metals 2020, 10, 847. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Cluster Numbers. In Introduction to Percolation Theory, 2nd ed.; Taylor & Francis: London, UK, 1994; pp. 26–34. [Google Scholar]
- Kitazono, K.; Nishizawa, S.; Sato, E.; Motegi, T. Effect of ARB Cycle Number on Cell Morphology of Closed-Cell Al-Si Alloy Foam. Mater. Trans. 2004, 45, 2389–2394. [Google Scholar] [CrossRef]
- Kobashi, M.; Noguchi, M.; Kanetake, N. Observation of Foaming Behavior for Rolled Sheet Precursors Made of Various Aluminum Powders. Mater. Trans. 2011, 52, 934–938. [Google Scholar] [CrossRef]
- Campana, F.; Mancini, E.; Pilone, D.; Sasso, M. Strain rate and density-dependent strength of Al-Si7 alloy foams. Mater. Sci. Eng. A 2016, 651, 657–667. [Google Scholar] [CrossRef]
- Kanetake, N.; Kobashi, M.; Tsuda, S. Foaming Behavior of Aluminum Precursor Produced from Machined Chip Waste. Adv. Eng. Mater. 2008, 10, 840–844. [Google Scholar] [CrossRef]
- Murray, J.L.; McAlister, A.J. The Al-Si (Aluminum-Silicon) system. Bull. Alloy Phase Diagr. 1984, 5, 74–84. [Google Scholar] [CrossRef]
- Nikanorov, S.P.; Volkov, M.P.; Gurin, V.N.; Burenkov, Y.A.; Derkachenko, L.I.; Kardashev, B.K.; Regel, L.L.; Wilcox, W.R. Structural and mechanical properties of Al-Si alloys obtained by fast cooling of a levitated melt. Mater. Sci. Eng. A 2005, 390, 63–69. [Google Scholar] [CrossRef]
- Weck, E.; Leistner, E. Metallographic Instructions for Colour Etching by immersion Pt. 3., 1st ed.; DVS-Verlag GmbH: Düsseldorf, Germany, 1986; pp. 6–19. [Google Scholar]
- Lei, S.; Gao, L.; Harada, Y.; Kumai, S. Microstructure Analysis of Quenched Semi-Solid A356 Aluminum Alloy Slurry by Using Weck’s Reagent. Mater. Trans. 2020, 61, 1077–1083. [Google Scholar] [CrossRef]
- Hangai, Y.; Takagi, T.; Koyama, S.; Suzuki, R.; Kamakoshi, Y. Refoaming of deformed aluminum foam fabricated by precursor foaming process using remaining foaming agent and densification using friction stir welding. Mater. Lett. 2023, 351, 135008–135011. [Google Scholar] [CrossRef]
- Smith, L.N.; Lobb, C.J. Percolation in two-dimensional conductor-insulator networks with controllable anisotropy. Phys. Rev. B 1979, 20, 3653–3658. [Google Scholar] [CrossRef]


| Component | Si | Fe | Cu | Al |
|---|---|---|---|---|
| mass% | 6.4 | 0.0 | 0.0 | Bal. |
| Component | O2 | CO | CO2 | H2O | N2 |
|---|---|---|---|---|---|
| volppm | 20 × 104 | <0.1 | <0.1 | <0.54 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takamatsu, S.; Arai, T.; Sayama, A.; Suzuki, S. Characteristics of Pore Morphology in Aluminum Alloy Foams Fabricated by Semi-Solid Route among Multiple Experimental Runs. Metals 2023, 13, 1654. https://doi.org/10.3390/met13101654
Takamatsu S, Arai T, Sayama A, Suzuki S. Characteristics of Pore Morphology in Aluminum Alloy Foams Fabricated by Semi-Solid Route among Multiple Experimental Runs. Metals. 2023; 13(10):1654. https://doi.org/10.3390/met13101654
Chicago/Turabian StyleTakamatsu, Satomi, Takahiro Arai, Akane Sayama, and Shinsuke Suzuki. 2023. "Characteristics of Pore Morphology in Aluminum Alloy Foams Fabricated by Semi-Solid Route among Multiple Experimental Runs" Metals 13, no. 10: 1654. https://doi.org/10.3390/met13101654
APA StyleTakamatsu, S., Arai, T., Sayama, A., & Suzuki, S. (2023). Characteristics of Pore Morphology in Aluminum Alloy Foams Fabricated by Semi-Solid Route among Multiple Experimental Runs. Metals, 13(10), 1654. https://doi.org/10.3390/met13101654







