Experimental Investigation of the Porous Free Zone of Silicon Cemented Layer Obtained through Pack Cementation
Abstract
1. Introduction
- -
- Alloying of the metallic matrix (which is to be silicon cemented) with elements having lower melting temperatures and characterized by the high mobility of atoms.
- -
- Simultaneous saturation of the surface with silicon and other elements with low diffusion mobility, e.g., chromium.
- -
- The generation of pressure that is uniformly distributed on the surface of the thermochemically processed product, leading to significant diffusion of vacancies and pores to the surface of the product.
2. Materials and Methods
- -
- Ferrosilicon (FeSi75C with 72–75% Si and below 0.1% C, with approximately 2% Al) produced by the Norwegian company FINNFJORD; particles with an average equivalent diameter of 40–50 mm, subsequently grounded into ball mills to an average equivalent diameter of 3–4 mm.
- -
- Alumina powders (Al2O3 > 98.5%) are produced at Alum. S.A. Romania, fraction > 150 μm max. 10%; fraction < 45 μm max. 12%.
- -
- Ammonium chloride (NH4Cl) of analytical purity, produced by SilverChemicals Romania.
- -
- Vickers HV0.05 (50gf) hardness tests were performed on the NEOPHOT 21 microscope N1096 series.
- -
- Optical Microscopy (OM) using a Zeiss Z1m Observer microscope-Axio Vision 4.8/038-12837.
- -
- Scanning Electron Microscopy (SEM) using a TESCAN VEGA XMU 8 microscope and Philips XL30 ESEM TMP microscope.
- -
- EDAX spectrometry (EDAX Sapphire type dispersive energy spectrometer with a resolution of 128 kV).
- -
- XRF analyses performed on the SPECTRO xSORT device no. 143714 used for the additional verification of silicon concentration in the superficial layers of the silicon cemented Fe-ARMCO.
3. Results
- bi; bij—the real coefficients of Equation (1).
- xi; xj—the coded values of the factors (independent parameters taken into analysis).
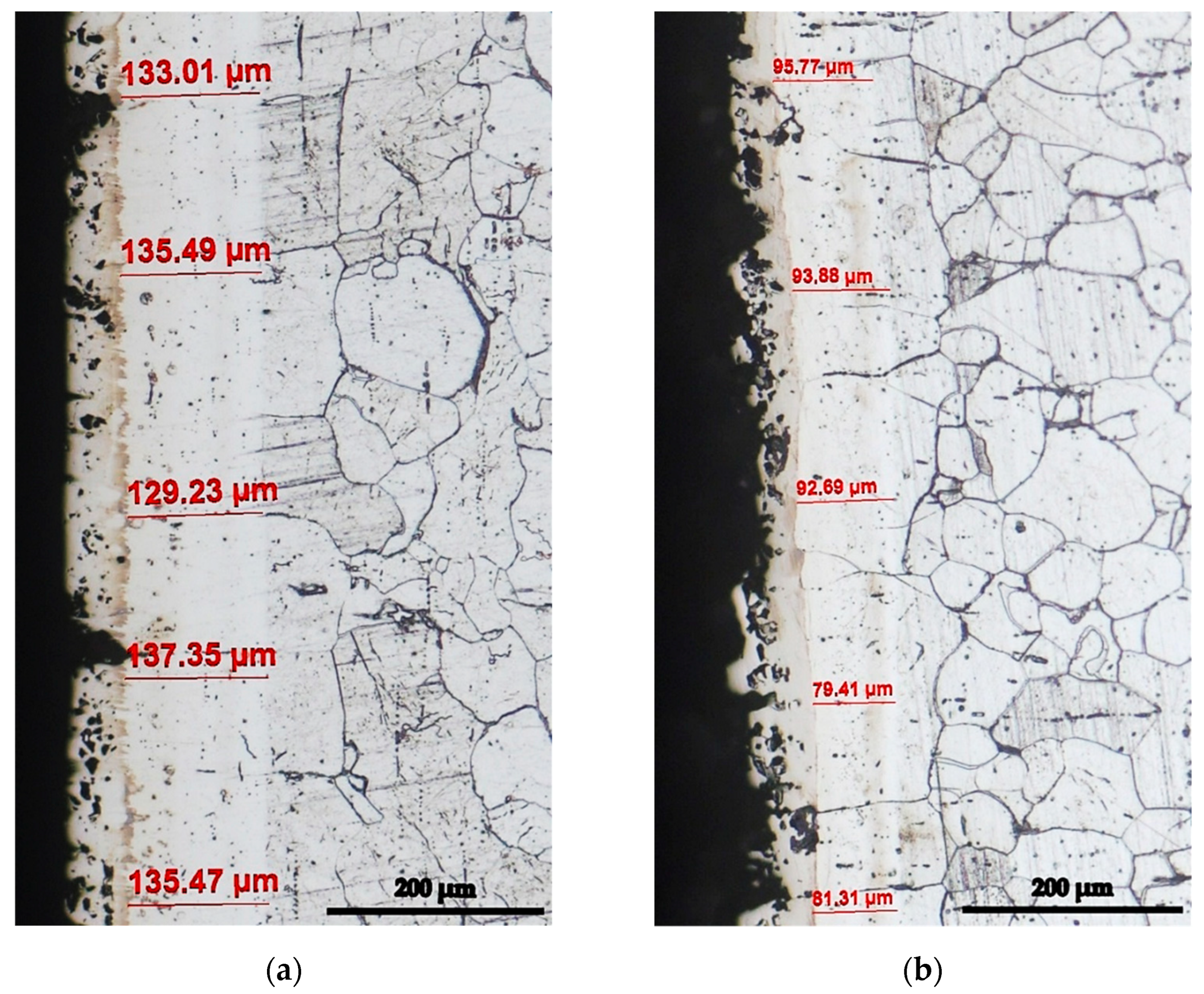
- Y—the real value of the dependent parameters (in the current situation, Y represents the dimension of the silicon cemented layer’s dense, porous free, and adherent zone, in direct contact with the surface of the thermochemically processed product)—see example in Figure 1.
- xi—the coded value of the independent parameter i (or j, k … ij, ik … ijk).
- Xi0—the natural value of the independent parameter i (or j, k … ij, ik … ijk) corresponding to the chosen base level (Table 1).
- ➢
- ➢
- Determination of dispersion of reproducibility of the experimental values (S02) is achieved by several experimental values obtained in identical conditions (a minimum of three values).
- The active component of the powdered solid medium FeSi75C
- -
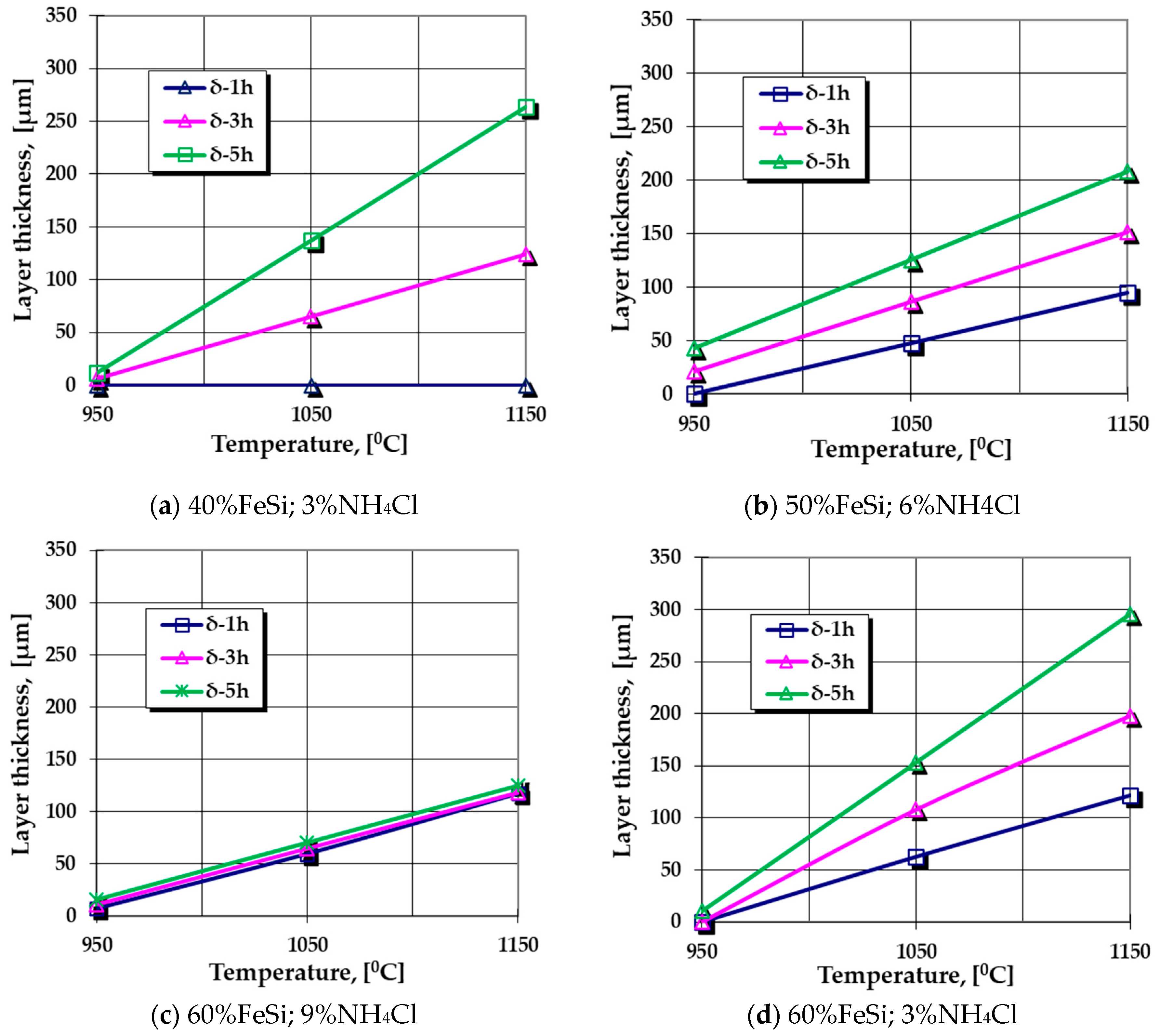
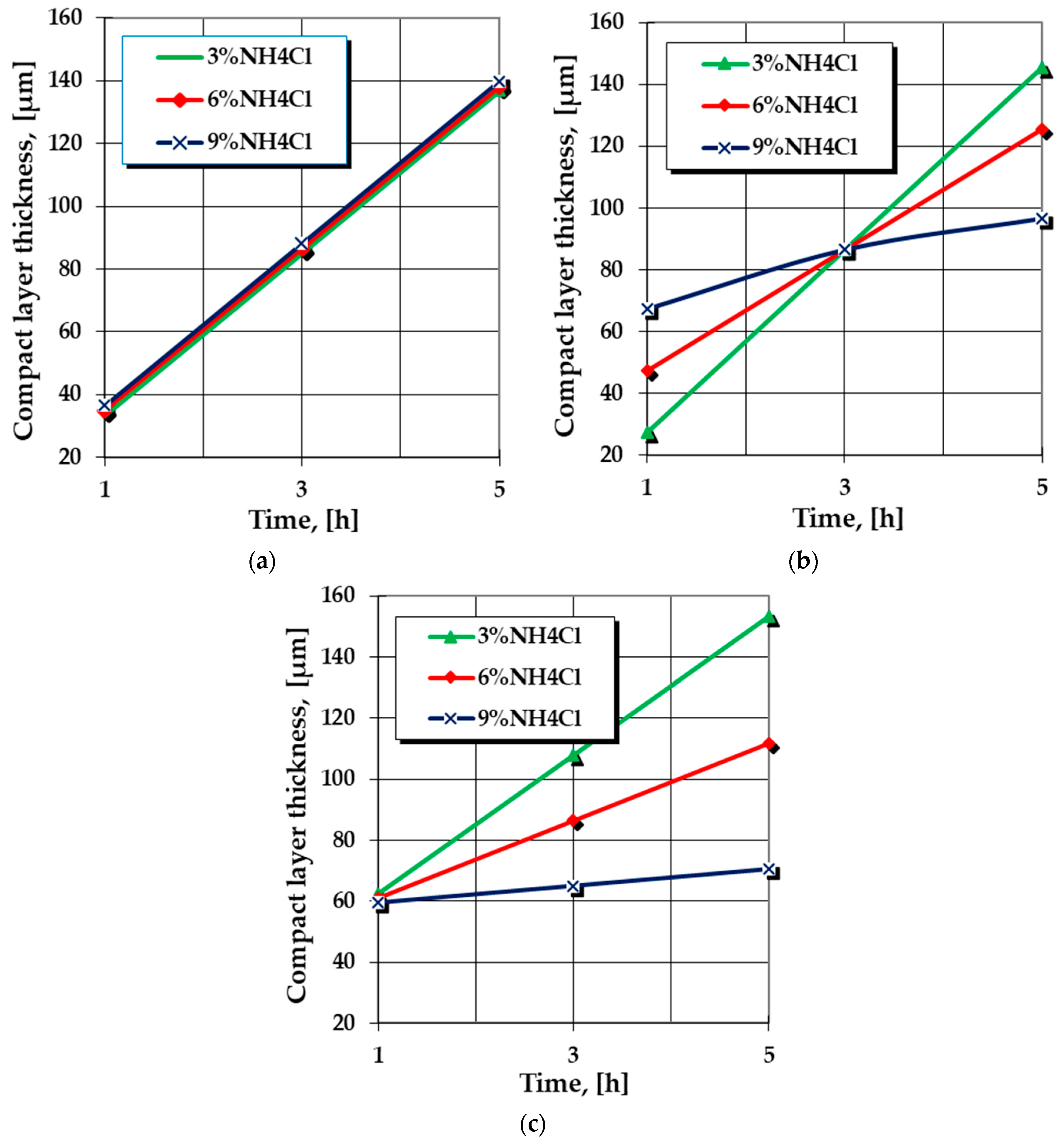
- The increase in the proportion of halide, utilized as an activator of the reactions between the components of the powdered solid medium, but also as an activator of the surface of the thermochemically processed metallic product, has a significant effect, especially for high values of the active component of the medium (ferrosilicon), as is shown in Figure 2a,c. Thus, for example, for 60% FeSi75C, the effects of the increase in the proportion of halide between 3 and 9% for a constant holding time on temperature is strictly dependent on the processing temperature—at 3% NH4Cl, the kinetics accelerates when the temperature is higher and the holding time is longer, as is shown in Figure 2d. At 9% NH4Cl, the variation of the holding time does not influence the kinetics, regardless of the processing temperature, as is shown in Figure 2c.
- -
- For moderate proportions of the active component (FeSi75C) in the powdered solid medium used for silicon cementation, the effects of variation of the proportion of halide in the medium may be different over time: From the kinetic accelerator to small reduced processing times, to become a brake at high values of holding time, as shown in Figure 3b, the amplitude of the effects is dependent on the proportion of halide in the media used for silicon cementation with an obvious correlation with the other parameters. The explanation of the dual characteristics is related to the ratio between the intensities with which the effects of activating the reactions between the environmental components and those of cleaning/activating the metal surface subjected to thermochemical processing are manifested.
4. Discussion
- -
- The silicon appears in the superficial zones of the thermochemically processed metallic products following a sequence of chemical reactions, either occurring often between its chlorides adsorbed in the superficial layers and the iron atoms (Equations (15) and (17)), or in the surface proximity following the reactions between its chlorides and the hydrogen resulting from the decomposition of the ammonia chloride (Equations (11) and (19)), etc.
- -
- Most of the reactions that occurred between the components of the powdered solid media utilized for silicon cementation develop with heat release (significant thermal effect).
- -
- The development of silicon chlorides is dependent on the ammonia chloride among the components of the powdered solid media and its decomposition at the processing temperature—Equation (11); however, the increase in the proportion of this component can have adverse effects such as the cleaning/corrosion of the surface, an aspect that must be considered when dosing its proportion.
- -
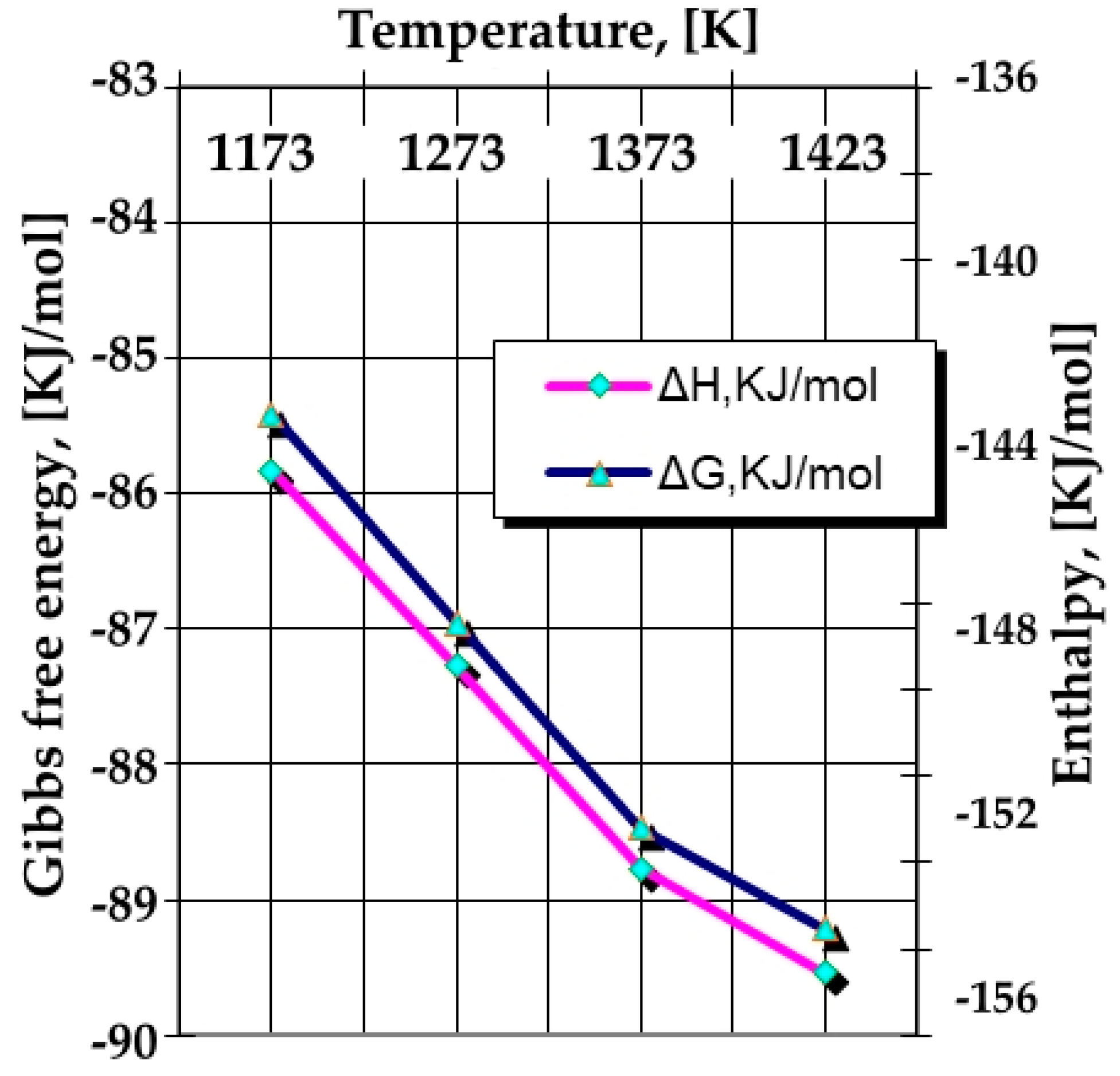
- The appearance of Si in a free state in the silicon cementation media, as defined in Equation (19), is likely to be determined from the thermodynamic point of view (see Figure 4), as is the formation of a nitride of Si2N type after a reaction of the following type:
5. Conclusions
- The statistical processing of the obtained experimental data led to the conclusion that ferrosilicon with higher than 60% silicon concentrations (FeSi75C) represent a significant active component, especially in the range of temperatures higher than 1100 °C.
- Both the atomic adsorption mechanism responsible for silicon saturation and the ionic mechanism responsible for nitrogen saturation contribute to the formation of the silicon-cemented layer in a powdery solid environment consisting of FeSi75C-Al2O3-NH4Cl.
- During the holding time at the silicon cementation temperature, the Si2N silicon nitride synthesis reaction is very likely from a thermodynamic point of view, but its presence seems not to influence the final silicon content of the layer.
- The maximum thickness of the silicon cemented layer zone free of porosity, in direct contact with the substrate, namely, Fe-ARMCO, subjected to processing, is obtained by associating a proportion of the active component as high as possible (60%) and as low as possible (at the lower limit imposed of the programming method chosen, namely, 3%) for the component with the role of activator of the reactions in the powdered solid media. For moderate proportions of the active component (FeSi75C) in the powdered solid media used for silicon cementation, the variation of the proportion of halide in the media may register different effects over time, becoming a kinetic accelerator for low processing times or a barrier for high processing times. Thus, at reduced holding times, the maximum kinetic is recorded by the mixtures that have the highest proportion of halide (approximately 9%), and once the holding time increases, the maximum effect will be recorded by the mixtures with low concentrations of halide in the powdery mixture (approximately 3%).
- The calculated and statistically verified mathematical models allow anticipation via the calculation of the modality of combining the parameters with a significant influence on the growth kinetics of the free porous zone of the silicon cemented layer in powdered solid media.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lakhtin, I.M.; Arzamasov, B.N. Thermochemical Treatments of Steels; Metallurghia (Metallurgy Publishing House): Moscow, Russia, 1985; pp. 141–154. (In Russian) [Google Scholar]
- Minkevici, A.N. Thermochemical Treatments of Metals and Alloys; Tehnica, Ed.; Technical Publishing House: Bucharest, Romania, 1968; pp. 295–308. (In Russian) [Google Scholar]
- Barey, D.M.O.A. Concentrations and Reactions of Iron in Crystalline Silicon after Aluminum Gettering. Ph.D. Thesis, Georg-August University Goettingen, Göttingen, Germany, 29 November 2011; p. 52. (In German). [Google Scholar]
- Kubachewski von Goldbeck, O. Fe-Si Iron-Silicon. In IRON-Binary Phase Diagrams; Springer: Berlin/Heidelberg, Germany, 1982; pp. 136–139. [Google Scholar] [CrossRef]
- Hauser, H.H. Coatings of High-Temperature Materials; Samsonov, G.V., Epik, A.P., Eds.; Springer Science & Business Media: New York, NY, USA, 1966; Part 1; pp. 59–62. [Google Scholar] [CrossRef]
- Dueva, T.Y. Herald of the Machine Industry; USSR: Moscow, Russia, 1937; Volume 16–17, p. 35. (In Russian) [Google Scholar]
- Haslam, R.T.; Carlsmith, L.E. The Cementation of Iron by Silicon. Ind. Eng. Chem. 1924, 16, 1110–1113. [Google Scholar] [CrossRef]
- Balandin, Y.A.; Kolpakov, A.S. Diffusion Siliconizing in a Fluidized Bed. Metal Sci. Heat Treat. 2006, 48, 127–130. [Google Scholar] [CrossRef]
- Fitzer, E. The siliconizing of steel as a solid state reaction. Arch. Das Eisenhuettenwesen 1954, 25, 455–463. [Google Scholar] [CrossRef]
- Leahovici, L.S.; Voroşnin, L.G.; Şcerbakov, E.R.D.; Panin, G.G. Siliconization of Metals and Alloys; Tehnica, N.I., Ed.; Scientific and Technical Publishing House: Minsk, Russia, 1972; pp. 193, 255–261. (In Russian) [Google Scholar]
- Taloi, D.; Florian, E.; Bratu, C.; Berceanu, E. Optimization of Metallurgical Processes; Pedagogica, D.S., Ed.; Didactic and Pedagogical Publishing House: Bucharest, Romania, 1983; pp. 79–85. (In Romanian) [Google Scholar]
- Dimitriu, S.; Taloi, D. Mathematical Modeling Methods of Technological Processes; Printech, Ed.; Printech Publishing House: Bucuharest, Romania, 2014; pp. 194–206. (In Romanian) [Google Scholar]
- Hics, C. Basic Principles of Experiment Planning; Izd.Mir (World Publishing House): Moscow, Russia, 1967. (In Russian) [Google Scholar]
- Himmelbau, D. Process Analysis by Statistical Methods; Izd.Mir (World Publishing House): Moscow, Russia, 1975. (In Russian) [Google Scholar]
- Johnson, N.; Leone, F. Statistics and Experimental Programming in Technology and Science. Data Processing Methods; Izd.Mir (World Publishing House): Moscow, Russia, 1980. (In Russian) [Google Scholar]
- Mehrer, H.; Eggersmann, M.; Gude, A.; Salamon, M.; Sepiol, B. Diffusion in intermetallic phases of the Fe-Al and Fe-Si systems. Mater. Sci. Eng. A 1997, 239–240, 889–898. [Google Scholar] [CrossRef]
- Batz, W.; Mead, H.V.; Birchenall, C.E. Diffusion of Silicon in Iron. J. Met. 1952, 10, 1070. [Google Scholar] [CrossRef]
- Mirani, H.V.M.; Maaskant, P. Diffusion of Si in Fe-Si Containing 8 to 11 at% Si. Phys. Status Solidi A 1972, 14, 521–525. [Google Scholar] [CrossRef]
- HSC Chemistry Software, Version 6.12; Outotec Research Oy: Pori, Finland, 2007.
- Cojocaru, M.O. Nitriding in an Electrostatic Field. Ph. D. Thesis, Moscovskii Avto-Dorojnii Institut MADI (Moscow Road Vehicle Institute), Moscow, Russia, 1976. (In Russian). [Google Scholar]
- Cojocaru, M.O.; Ciuca, I.; Druga, L.N.; Cosmeleata, G. Empirical Exposition of the Adsorption’s Ionic Mechanism on Gaseous Nitriding. Surf. Eng. Appl. Electrochem. 2008, 44, 63–68. [Google Scholar] [CrossRef]
- Vedeneev, V.I. Breaking Energy of Chemical Bonds. Ionization Potentials and Electron and Proton Affinity; Izd. AN-SSSR (SSSR Publishing House): Moscow, Russia, 1962. (In Russian) [Google Scholar]
- Paukstisand, S.J.; Gole, J.L. The Ionization Potential of Si2N and Si2O+. J. Phys. Chem. A 2002, 106, 8435–8441. [Google Scholar] [CrossRef]
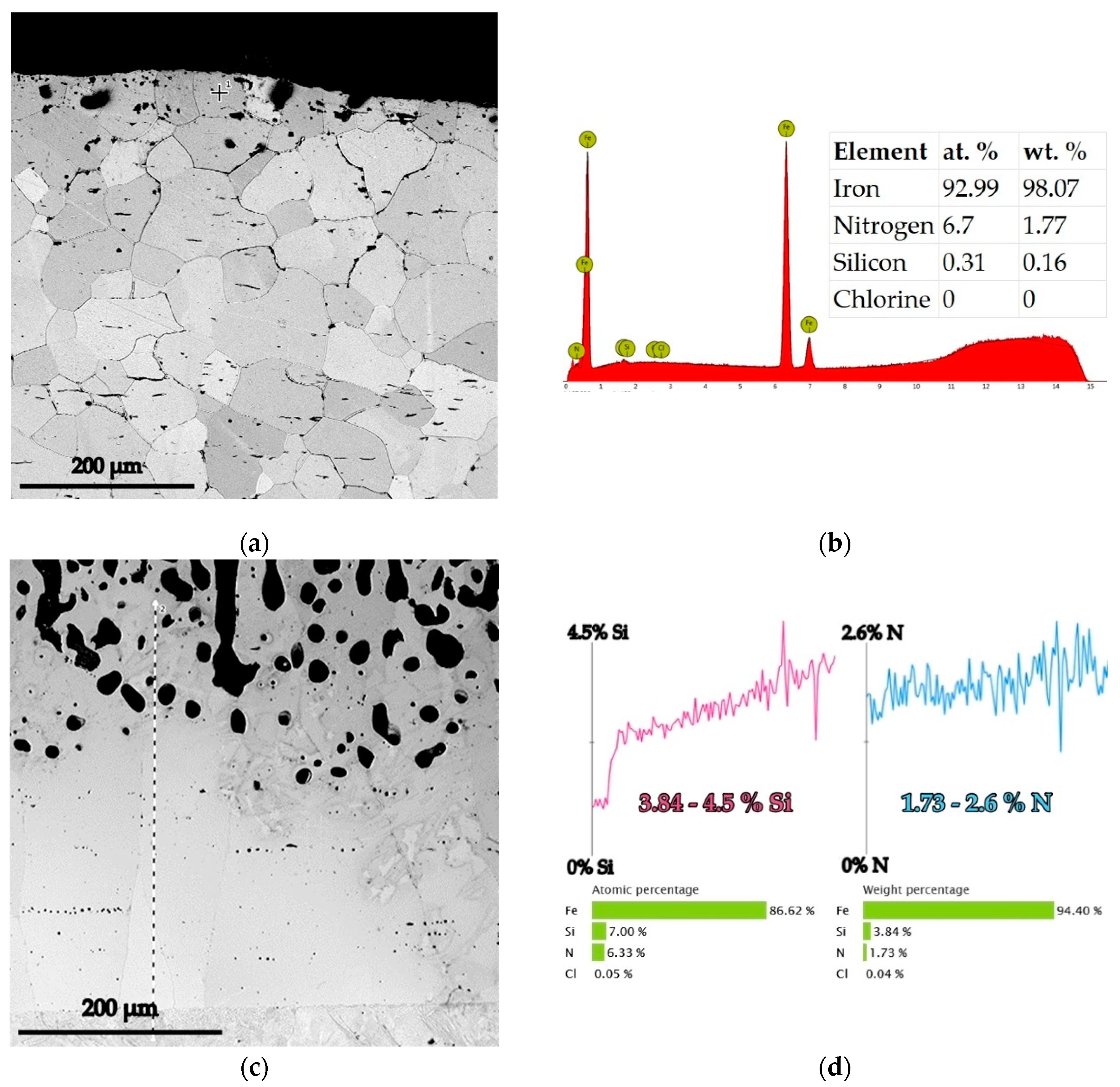
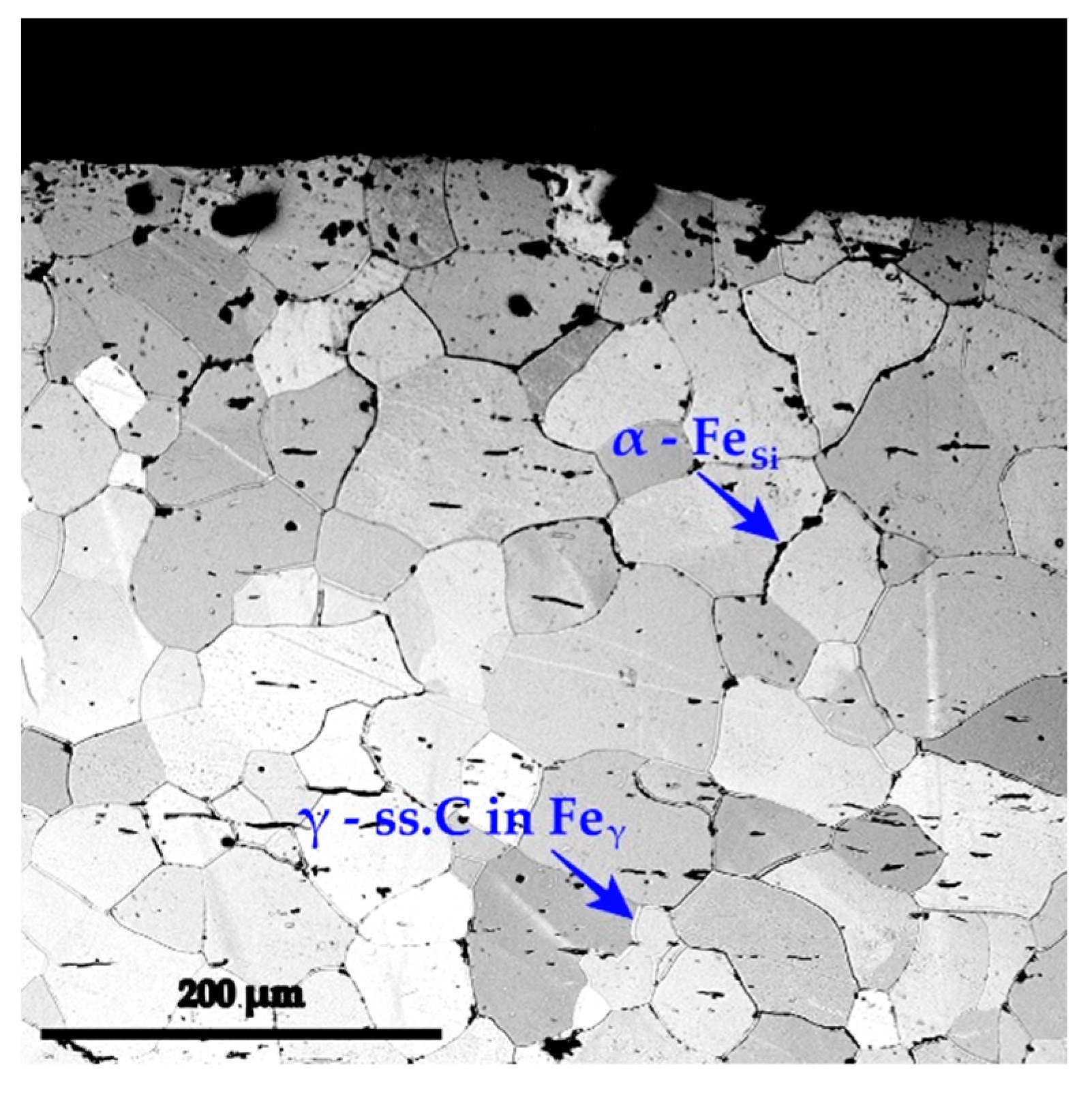
| El. % | C | Si | Mn | P | S | Cu | Ni | Cr | Mo | V | Al | N | Fe |
| 0.02 | 0.07 | 0.12 | 0.017 | 0.011 | 0.01 | 0.01 | 0.003 | 0.004 | 0.0005 | 0.006 | 0.008 | 99.70 |
| Factors | T, [°C] X1 | T, [h] X2 | [%], FeSi X3 | [%], NH4Cl X4 | δ [μm] Active Component: FeSi75C |
|---|---|---|---|---|---|
| Y | |||||
| Range of Variation (ΔXi) | 100 °C | 2 h | 10% | 3% | - |
| Higher level (Xi0 + ΔXi) | (+1)/1150 °C | (+1)/5 h | (+1) 60% | (+1) 9% | - |
| Base level (Xi0) | (0)/1050 °C | (0)/3 h | (0) 50% | (0) 6% | - |
| Lower level (Xi0 − ΔXi) | (−1)/950 °C | (−1)/1 h | (−1) 40% | (−1) 3% | - |
| Exp.1 | (+1)/1150 °C | (+1)/5 h | (+1) 60% | (+1) 9% | 125.59 |
| Exp.2 | (+1)/1150 °C | (+1)/5 h | (+1) 60% | (−1) 3% | 301.368 |
| Exp.3 | (+1)/1150 °C | (+1)/5 h | (−1) 40% | (+1) 9% | 134.11 |
| Exp.4 | (+1)/1150 °C | (−1)/1 h | (+1) 60% | (+1) 9% | 88.61 |
| Exp.5 | (−1)/950 °C | (+1)/5 h | (+1) 60% | (+1) 9% | 0.00 |
| Exp.6 | (+1)/1150 °C | (+1)/5 h | (−1) 40% | (−1) 3% | 270.94 |
| Exp.7 | (+1)/1150 °C | (−1)/1 h | (+1) 60% | (−1) 3% | 134.39 |
| Exp.8 | (+1)/1150 °C | (−1)/1 h | (−1) 40% | (+1) 9% | 156.27 |
| Exp.9 | (−1)/950 °C | (−1)/1 h | (+1) 60% | (+1) 9% | 137.78 |
| Exp.10 | (−1)/950 °C | (+1)/5 h | (−1) 40% | (+1) 9% | 0.00 |
| Exp.11 | (−1)/950 °C | (+1)/5 h | (+1) 60% | (−1) 3% | 0.00 |
| Exp.12 | (−1)/950 °C | (−1)/1 h | (−1) 40% | (+1) 9% | 0.00 |
| Exp.13 | (−1)/950 °C | (−1)/1 h | (+1) 60% | (−1) 3% | 0.00 |
| Exp.14 | (−1)/950 °C | (+1)/5 h | (−1) 40% | (−1) 3% | 35.02 |
| Exp.15 | (+1)/1150 °C | (−1)/1 h | (−1) 40% | (−1) 3% | 0.00 |
| Exp.16 | (−1)/950 °C | (−1)/1 h | (−1) 40% | (−1) 3% | 0.00 |
| Coefficient | Active Part: FeSi |
|---|---|
| b0 | 86.48 |
| b1 | 64.88 |
| b2 | 39.07 |
| b3 | −5.28 |
| b4 | −6.23 |
| b12 | 17.4 |
| b13 | 16.3 |
| b14 | −19.0 |
| b23 | −13.6 |
| b24 | −20.0 |
| b34 | −21.5 |
| b123 | 7.98 |
| b124 | −32.9 |
| b234 | 3.75 |
| b134 | −8.65 |
| b1234 | 16.6 |
| Parameter | Active Component: FeSi | |
|---|---|---|
| S02 | 54.31 | t = 2.12 |
| Sbi2; Sbij2 | 3.394 | |
| Δbi; Δbij… | ±3.905 | |
| Statistical Parameter | Active Component: FeSi75C | |
|---|---|---|
| Ftabular | 19.4 | S02 = 54.31 |
| S2conc | 313.62 · 10−4 | |
| Fcalculated | 5.77 · 10−4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, M.O.; Branzei, M.; Morariu, M.D.; Cotrut, C.M.; Dragomir, D. Experimental Investigation of the Porous Free Zone of Silicon Cemented Layer Obtained through Pack Cementation. Metals 2023, 13, 162. https://doi.org/10.3390/met13010162
Cojocaru MO, Branzei M, Morariu MD, Cotrut CM, Dragomir D. Experimental Investigation of the Porous Free Zone of Silicon Cemented Layer Obtained through Pack Cementation. Metals. 2023; 13(1):162. https://doi.org/10.3390/met13010162
Chicago/Turabian StyleCojocaru, Mihai Ovidiu, Mihai Branzei, Mircea Dan Morariu, Cosmin Mihai Cotrut, and Daniela Dragomir. 2023. "Experimental Investigation of the Porous Free Zone of Silicon Cemented Layer Obtained through Pack Cementation" Metals 13, no. 1: 162. https://doi.org/10.3390/met13010162
APA StyleCojocaru, M. O., Branzei, M., Morariu, M. D., Cotrut, C. M., & Dragomir, D. (2023). Experimental Investigation of the Porous Free Zone of Silicon Cemented Layer Obtained through Pack Cementation. Metals, 13(1), 162. https://doi.org/10.3390/met13010162








