Microstructure and Continuous Cooling Transformation of an Fe-7.1Al-0.7Mn-0.4C-0.3Nb Alloy
Abstract
:1. Introduction
2. Material and Methods
3. Results
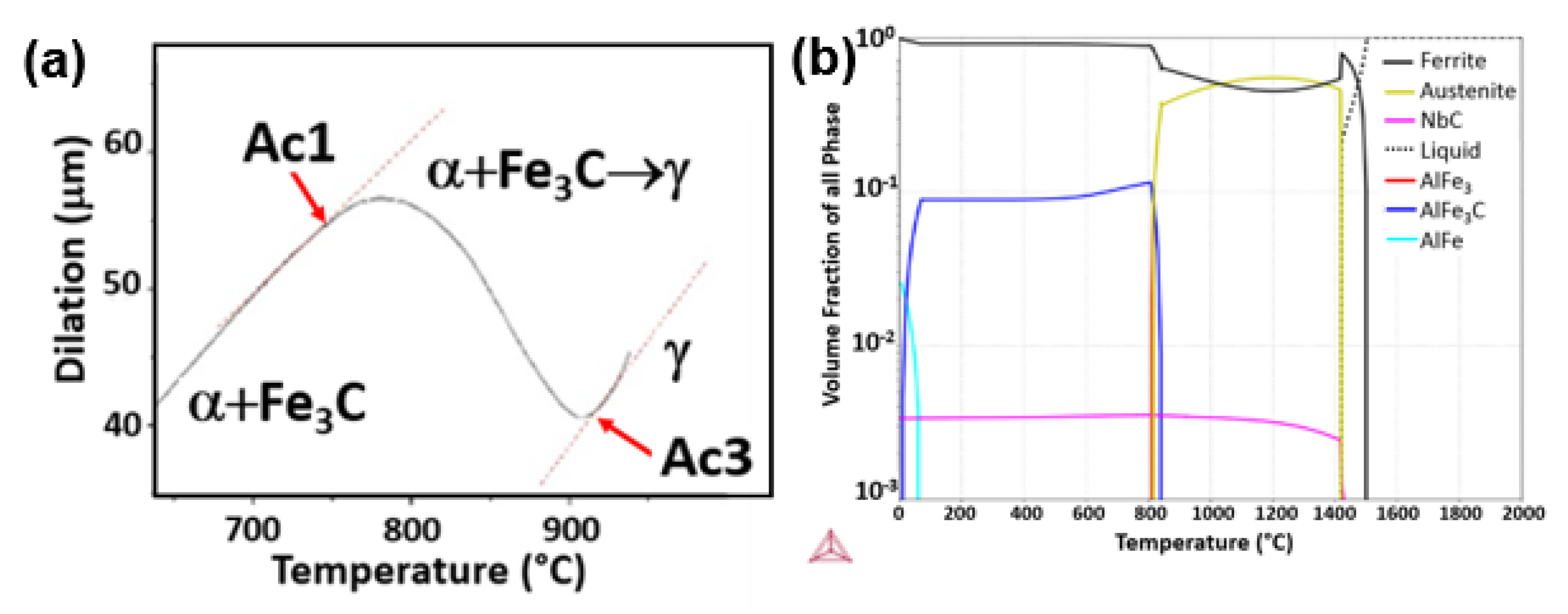
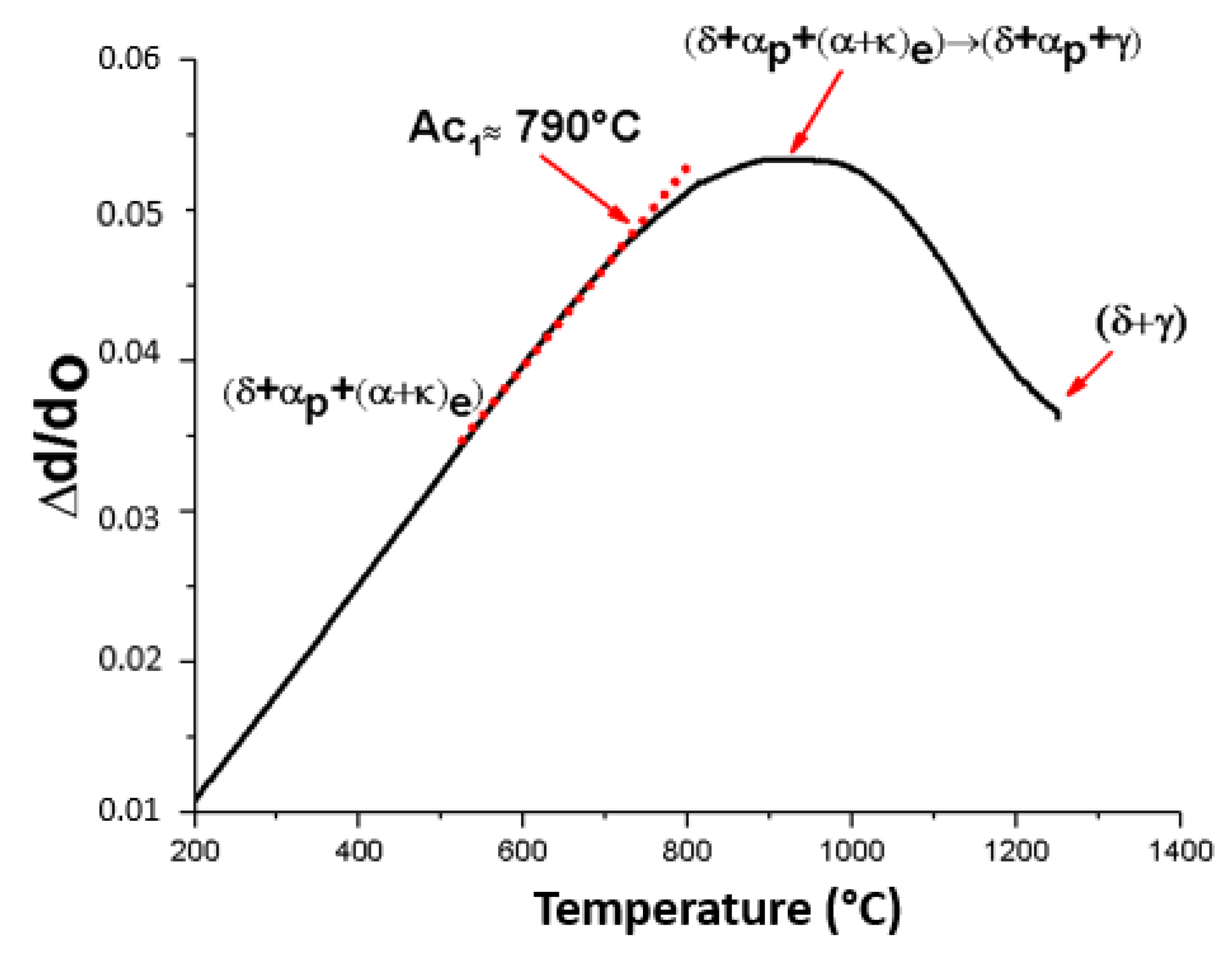
3.1. Dilatometric Curve during Heating
3.2. Microstructural Characterization
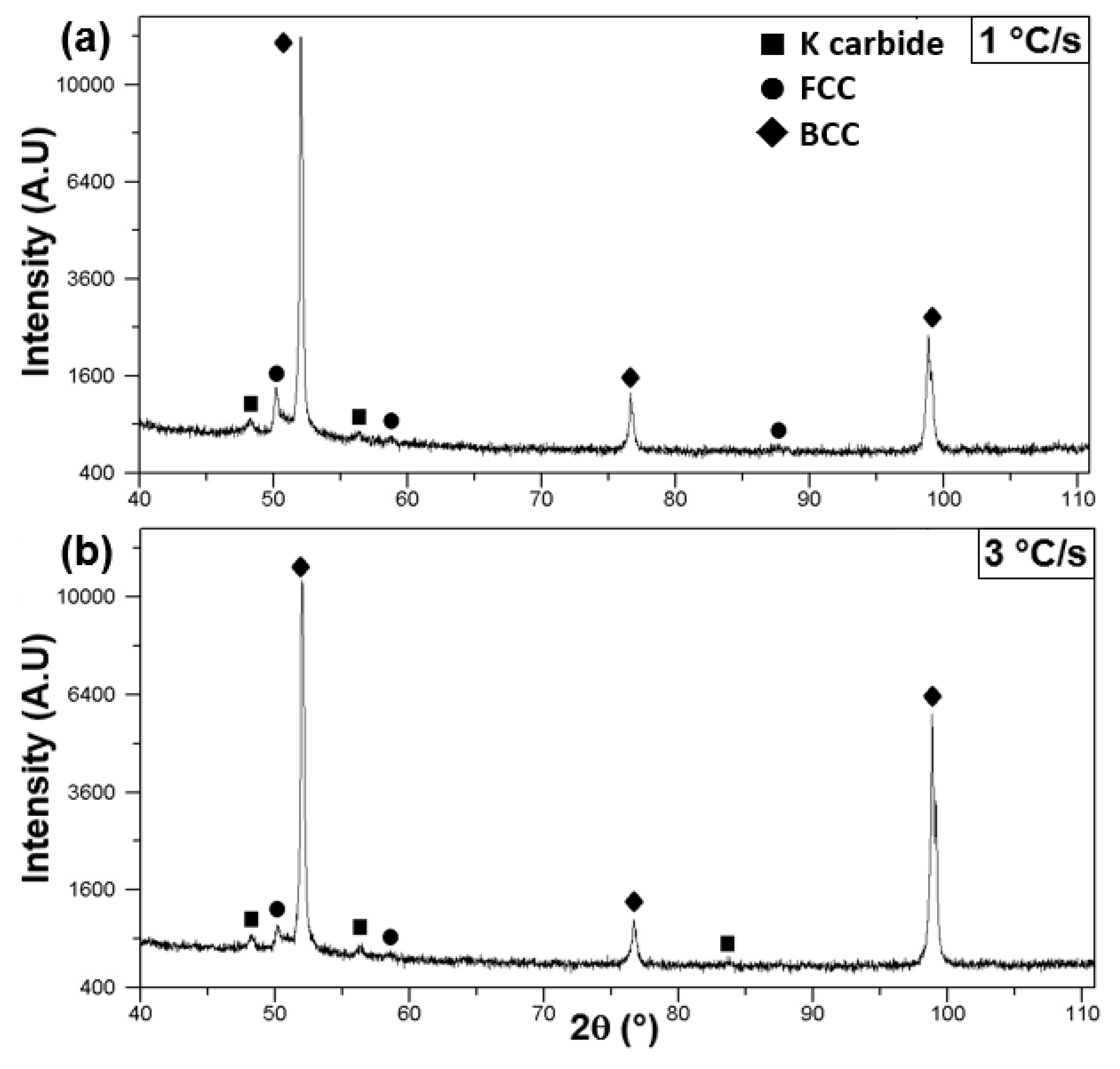
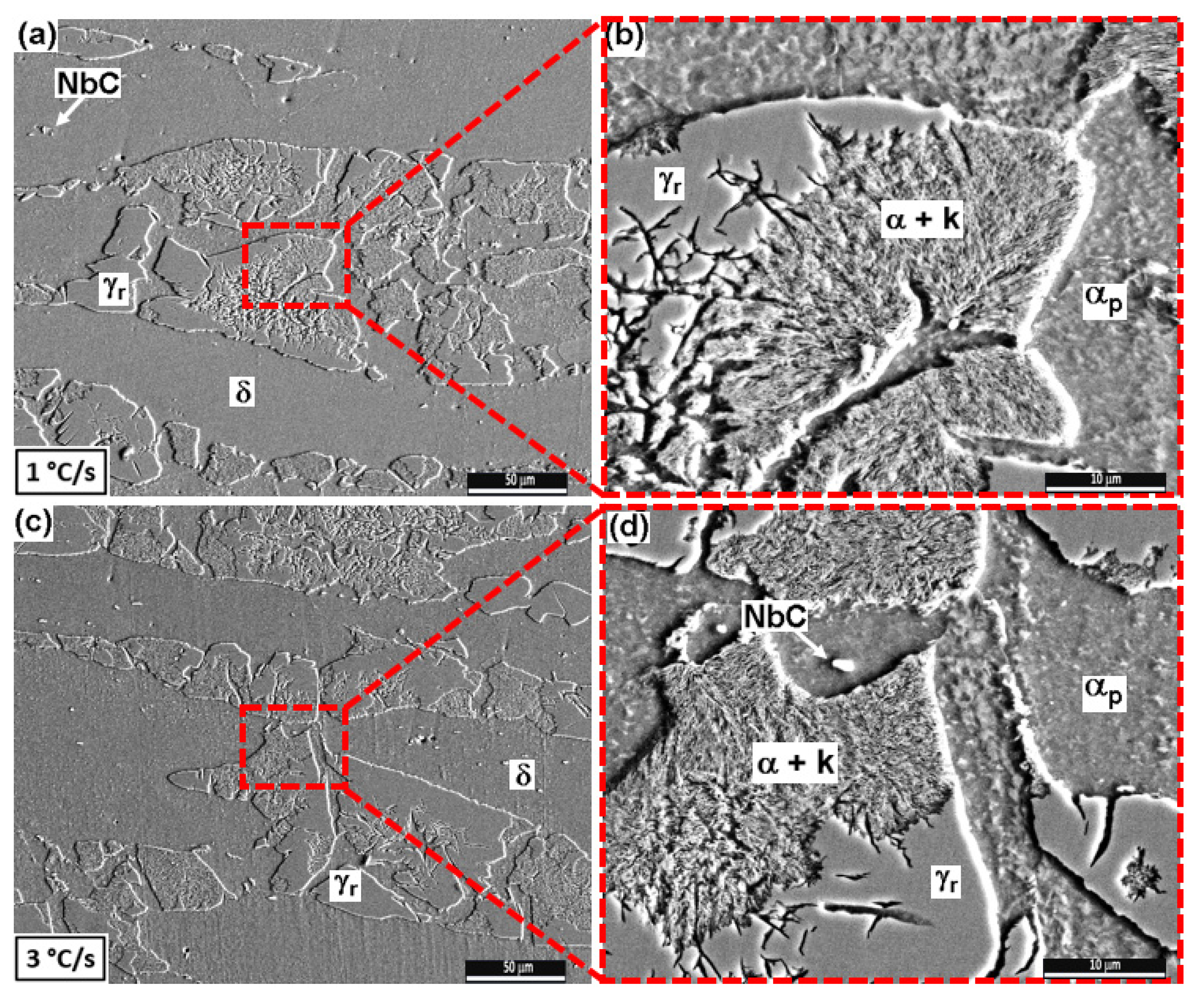
3.2.1. Low Cooling Rates: 1 and 3 °C/s
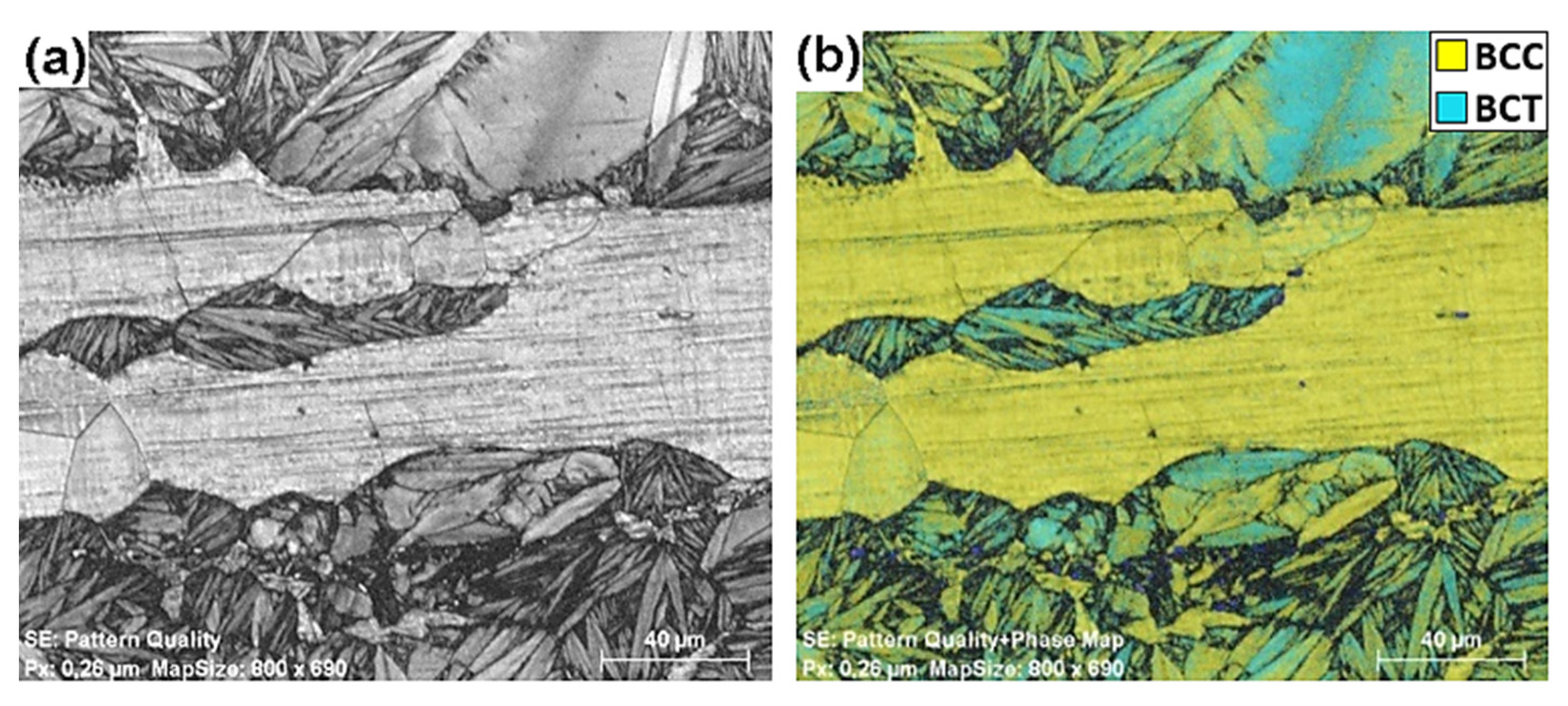
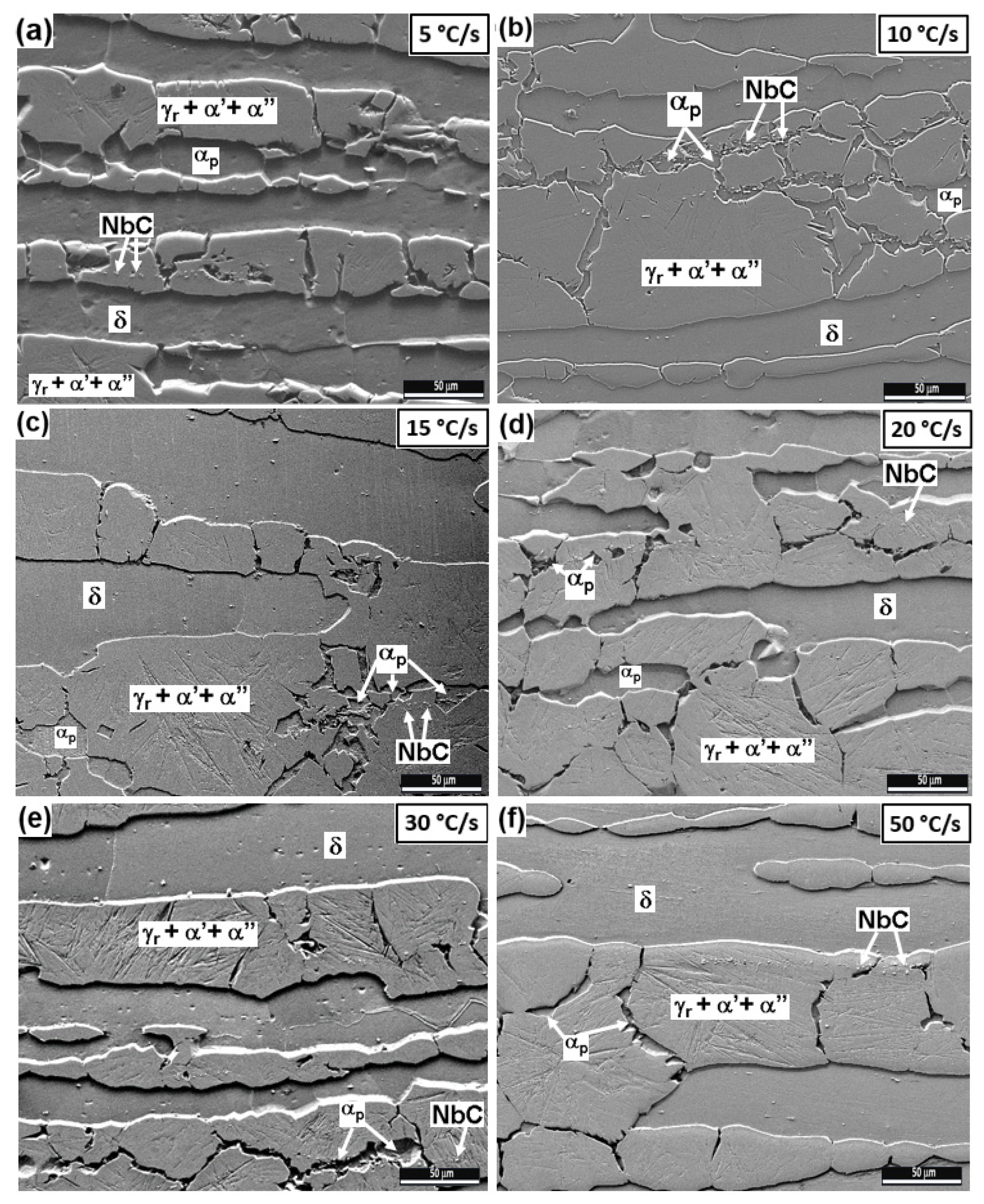
3.2.2. Intermediate and High Cooling Rates: 5 to 50 °C/s
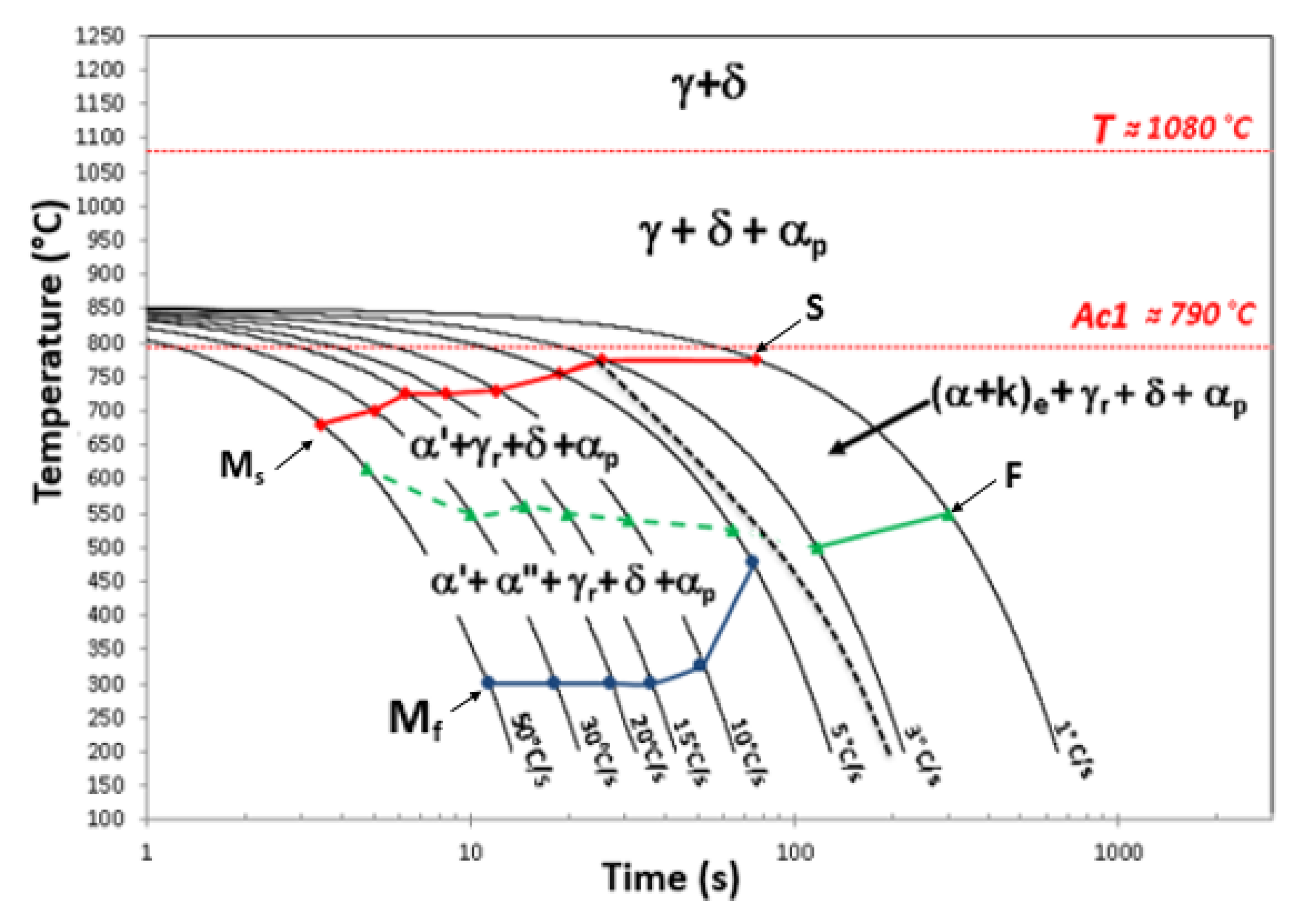
3.3. Continuous Cooling Transformation: CCT Diagram
4. Discussion
4.1. Dilatometric Curve Profile during Heating
4.2. Microstructural Characterization
4.3. Continuous Cooling Transformation: CCT Diagram
5. Conclusions
- -
- All investigated cooling rates applied in the dilatometry tests allowed the formation of a microstructure with proeutectoid α-ferrite, δ-ferrite, NbC, and retained austenite;
- -
- Cooling rates of 1 and 3 °C/s facilitated the decomposition of eutectoid austenite to form lamellar colonies, while cooling rates equal to or higher than 5 °C/s favored martensite formation with BCC and BCT structures;
- -
- The addition of Nb may influence the final microstructure of the alloy due to its contribution to stabilization of the ferritic matrix and the formation of niobium carbide;
- -
- The δ-ferrite and austenite coexistence at high temperatures and the added alloying elements promoted distinct variables that resulted in complex phase transformations observed in the continuous cooling transformation diagram obtained for the alloy under study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current State of Fe-Mn-Al-C Low Density Steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Zambrano, O.A. A General Perspective of Fe-Mn-Al-C Steels. J. Mater. Sci. 2018, 53, 14003–14062. [Google Scholar] [CrossRef] [Green Version]
- Mapelli, C.; Barella, S.; Gruttadauria, A.; Mombelli, D.; Bizzozero, M.; Veys, X. γ Decomposition in Fe-Mn-Al-C Lightweight Steels. J. Mater. Res. Technol. 2020, 9, 4604–4616. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, W.; Dong, H.; Zhu, J. Element Partitioning Effect on Microstructure and Mechanical Property of the Micro-Laminated Fe-Mn-Al-C Dual Phase Steel. Mater. Sci. Eng. A 2016, 654, 193–202. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Raabe, D. High Strength and Ductile Low Density Austenitic FeMnAlC Steels: Simplex and Alloys Strengthened by Nanoscale Ordered Carbides. Mater. Sci. Technol. 2014, 30, 1099–1104. [Google Scholar] [CrossRef] [Green Version]
- Frommeyer, G.; Brüx, U. Microstructures and Mechanical Properties of High-Strength Fe-Mn-AI-C Light-Weight TRIPLEX Steels. Steels Automot. Appl. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Kim, H.; Suh, D.W.; Kim, N.J. Fe-Al-Mn-C Lightweight Structural Alloys: A Review on the Microstructures and Mechanical Properties. Sci. Technol. Adv. Mater. 2013, 14, 014205. [Google Scholar] [CrossRef]
- Kimura, Y.; Handac, K.; Hayashid, K.; Mishima, Y. Microstructure Control and Ductility Improvement of the Two-Phase γ-Fe/κ-(Fe, Mn)3AlC Alloys in the Fe-Mn-Al-C Quaternary System. Intermetallics 2004, 12, 607–617. [Google Scholar] [CrossRef]
- Woo, S.D.; Kim, N.J. Low-Density Steels. Scr. Mater. 2013, 68, 337–338. [Google Scholar] [CrossRef]
- Zuazo, I.; Hallstedt, B.; Lindahl, B.; Selleby, M.; Soler, M.; Etienne, A.; Perlade, A.; Hasenpouth, D.; Massardier-Jourdan, V.; Cazottes, S.; et al. Low-Density Steels: Complex Metallurgy for Automotive Applications. JOM 2014, 66, 1747–1758. [Google Scholar] [CrossRef]
- Frommeyer, G.; Drewes, E.J.; Engl, B. Physical and Mechanical Properties of Iron-Aluminium-(Mn, Si) Lightweight Steels. Rev. Métall. 2000, 97, 1245–1253. [Google Scholar] [CrossRef]
- Suh, D.W.; Park, S.J.; Lee, T.H.; Oh, C.S.; Kim, S.J. Influence of Al on the Microstructural Evolution and Mechanical Behavior of Low-Carbon, Manganese Transformation-Induced-Plasticity Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Bausch, M.; Frommeyer, G.; Hofmann, H.; Balichev, E.; Soler, M.; Didier, M.; Samek, L. Ultra High-Strength and Ductile FeMnAlC Light-Weight Steels; The Publications Office of the European Union: Luxembourg, 2013; ISBN 9789279222450. [Google Scholar]
- Brüx, U.; Frommeyer, G.; Jimenez, J. Light-Weight Steels Based on Iron-Aluminium—Influence of Micro Alloying Elements (B, Ti, Nb) on Microstructures, Textures and Mechanical Properties. Mater. Technol. Steel Res. 2002, 73, 543–548. [Google Scholar] [CrossRef]
- Zheng, W.; He, S.; Selleby, M.; He, Y.; Li, L.; Lu, X.G.; Ågren, J. Thermodynamic Assessment of the Al-C-Fe System. Calphad Comput. Coupling Phase Diagr. Thermochem. 2017, 58, 34–49. [Google Scholar] [CrossRef]
- Chen, P.; Li, X.; Yi, H. The κ -Carbides in Low-Density Fe-Mn-Al-C Steels: A Review on Their Structure, Precipitation and Deformation Mechanism. Metals 2020, 10, 1021. [Google Scholar] [CrossRef]
- Wang, C.J.; Chang, Y.C. TEM Study of the Internal Oxidation of an Fe-Mn-Al-C Alloy after Hot Corrosion. Oxid. Met. 2002, 57, 363–378. [Google Scholar] [CrossRef]
- Sozańska-Jędrasik, L.; Mazurkiewicz, J.; Matus, K.; Borek, W. Structure of Fe-Mn-Al-C Steels after Gleeble Simulations and Hot-Rolling. Materials 2020, 13, 739. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.J.; Zhang, X.F.; Qin, R.S. Structure and Properties of κ -Carbides in Duplex Lightweight Steels. Ironmak. Steelmak. Process. Prod. Appl. 2016, 42, 626–631. [Google Scholar] [CrossRef] [Green Version]
- De Souza Baêta Júnior, E. Análise Temodinâmica e Cristalográfica Aplicada Na Produção de Aço Leve Ligado Com Al e Nb Contendo Ferrita Delta, Instituto Militar de Engenharia—IME, 2020. Available online: http://aquarius.ime.eb.br/~debiasi/tese%20Eustaquio.pdf (accessed on 14 June 2022).
- Gurgel, M.A.M. Comportamento Mecânico a Quente de Um Aço Do Sistema Fe-Mn-Al-C Com Adição de Nióbio, Instituto Militar de Engenharia—IME, 2021. Available online: http://aquarius.ime.eb.br/~debiasi/Tese%20Monica%20Magalhaes.pdf (accessed on 14 June 2022).
- Dynamic Systems Inc. Gleeble Operations Manual; Dynamic Systems Inc.: Poughkeepsie, NY, USA, 2014. [Google Scholar]
- ASTM-E3-11; Standard Guide for Preparation of Metallographic Specimens. ASTM: West Conshohocken, PA, USA, 2017; Volume 03.01, pp. 1–12.
- Rana, R.; Lahaye, C.; Ray, R.K. Overview of Lightweight Ferrous Materials: Strategies and Promises. JOM 2014, 66, 1734–1746. [Google Scholar] [CrossRef] [Green Version]
- Krizan, D.; Steineder, K.; Kaar, S.; Hebesberger, T. Development of Third Generation Advanced High Strength Steels for Automotive Applications. In Proceedings of the 18th International Scientific Conference, Rajecke Teplice, Slovakia, 10–11 October 2018; pp. 1–15. [Google Scholar]
- Gurgel, M.A.M.; Baêta Júnior, E.d.S.; da silva Teixeira, R.; Moreira, L.P.; Brandao, L.P.; Paula, A.d.S. Caracterização Microestrutural de Uma Liga Fe-7,1Al-0,7Mn-0,4C-0,3Nb Do Sistema Fe-Mn-Al-C. Rev. Matér. 2022, 27, 1–10. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, H.; Han, S.Y.; Seo, C.H.; Choi, K.; Lee, S.; Kim, N.J.; Kwak, J.H.; Chin, K.G. Correlation of Microstructure and Cracking Phenomenon Occurring during Hot Rolling of Lightweight Steel Plates. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Ding, H.; Cai, M.; Han, D. Hot Deformation Behavior and Processing Map of a Fe-11Mn-10Al-0.9C Duplex Low-Density Steel Susceptible to κ-Carbides. J. Mater. Eng. Perform. 2019, 28, 5116–5126. [Google Scholar] [CrossRef]
- Han, S.Y.; Shin, S.Y.; Lee, H.J.; Lee, B.J.; Lee, S.; Kim, N.J.; Kwak, J.H. Effects of Annealing Temperature on Microstructure and Tensile Properties in Ferritic Lightweight Steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 43, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Kaar, S.; Krizan, D.; Schwabe, J.; Hofmann, H.; Hebesberger, T.; Commenda, C.; Samek, L. Influence of the Al and Mn Content on the Structure-Property Relationship in Density Reduced TRIP-Assisted Sheet Steels. Mater. Sci. Eng. A 2018, 735, 475–486. [Google Scholar] [CrossRef]
- Khaple, S.; Prakash, U.; Golla, B.R.; Satya Prasad, V.V. Effect of Niobium Addition on Microstructure and Mechanical Properties of Fe–7Al–0.35C Low-Density Steel. Metallogr. Microstruct. Anal. 2020, 9, 127–139. [Google Scholar] [CrossRef]
- Sinha, M.; Ahad, S.; Chaudhry, A.K.; Ghosh, S. Kinetic Constraints of δ-Ferrite to the Formation of Kappa (κ) Carbide in a Fe-4Mn-9Al-0.3C Wt Pct Low-Density Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 809–817. [Google Scholar] [CrossRef]
- Zhou, N.; Song, R.; Yang, F.; Li, X.; Li, J. Influence of Annealing Temperature on Microstructure and Three-Stage Strain Hardening Behavior in Cold-Rolled Fe-Mn-Al-C Steel. JOM 2019, 71, 4105–4113. [Google Scholar] [CrossRef]
- Choi, Y.; Suh, D.W.; Bhadeshia, H.K.D.H. Retention of δ-Ferrite in Aluminium-Alloyed TRIP-Assisted Steels. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 2904–2914. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, C. Research and Development of 780MPa Grade Low-Density Steels. IOP Conf. Ser. Mater. Sci. Eng. 2019, 493, 012120. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, C.Y.; Park, I.J.; Lee, Y.K. Isothermal Precipitation Behavior of J-Carbide in the Fe-9Mn-6Al-0.15C Lightweight Steel with a Multiphase Microstructure. J. Alloys Compd. 2013, 574, 299–304. [Google Scholar] [CrossRef]
- Sozańska-Jędrasik, L.; Mazurkiewicz, J.; Borek, W.; Matus, K. Carbides Analysis of the High Strength and Low Density Fe-Mn-Al-Si Steels. Arch. Metall. Mater. 2018, 63, 265–276. [Google Scholar] [CrossRef]
- Jimenez-Melero, E.; van Dijk, N.H.; Zhao, L.; Sietsma, J.; Offerman, S.E.; Wright, J.P.; van der Zwaag, S. Martensitic Transformation of Individual Grains in Low-Alloyed TRIP Steels. Scr. Mater. 2007, 56, 421–424. [Google Scholar] [CrossRef]
- Seol, J.B.; Raabe, D.; Choi, P.; Park, H.S.; Kwak, J.H.; Park, C.G. Direct Evidence for the Formation of Ordered Carbides in a Ferrite-Based Low-Density Fe-Mn-Al-C Alloy Studied by Transmission Electron Microscopy and Atom Probe Tomography. Scr. Mater. 2013, 68, 348–353. [Google Scholar] [CrossRef]
- Cheng, W.C.; Song, Y.S.; Lin, Y.S.; Chen, K.F.; Pistorius, P.C. On the Eutectoid Reaction in a Quaternary Fe-C-Mn-Al Alloy: Austenite → Ferrite + Kappa-Carbide + M23C6 Carbide. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 1199–1216. [Google Scholar] [CrossRef]
- Sohn, S.S.; Lee, S.; Lee, B.J.; Kwak, J.H. Microstructural Developments and Tensile Properties of Lean Fe-Mn-Al-C Lightweight Steels. JOM 2014, 66, 1857–1867. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformation in Metals and Alloys, 3rd ed.; Routledge: London, UK, 1992; ISBN 9788578110796. [Google Scholar]
- Da Silva De Souza, S.; Moreira, P.S.; De Faria, G.L. Austenitizing Temperature and Cooling Rate Effects on the Martensitic Transformation in a Microalloyed-Steel. Mater. Res. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, C.; Qu, F.; Wang, Y.; Qiao, Z. Martensite Transformation Kinetics in 9Cr-1.7W-0.4Mo-Co Ferritic Steel. J. Alloys Compd. 2014, 610, 322–330. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C.; Sietsma, J. Kinetics of Martensite Formation in Plain Carbon Steels: Critical Assessment of Possible Influence of Austenite Grain Boundaries and Autocatalysis. Mater. Sci. Technol. 2014, 30, 1024–1033. [Google Scholar] [CrossRef]
- Baligidad, R.G. Effect of Niobium on Microstructure and Mechanical Properties of High Carbon Fe–10.5 Wt.% Al Alloys. Mater. Sci. Eng. A 2004, 368, 131–138. [Google Scholar] [CrossRef]
- Kwon, M.H.; Kim, J.K.; Bian, J.; Mohrbacher, H.; Song, T.; Kim, S.K.; De Cooman, B.C. Solidification Microsegregation and Hot Ductility of Fe-Mn-C-Al-XNb TWIP Steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 5509–5523. [Google Scholar] [CrossRef]
- Zargaran, A.; Kim, H.S.; Kwak, J.H.; Kim, N.J. Effects of Nb and C Additions on the Microstructure and Tensile Properties of Lightweight Ferritic Fe-8Al-5Mn Alloy. Scr. Mater. 2014, 89, 37–40. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 48, ISBN 9780750680844. [Google Scholar]
- Baêta Júnior, E.d.S.; Botelho, R.A.; Araújo, L.S.; Brandão, L.P.M.; Monteiro, S.N. Microstructure Optimization in δ-Trip Steels with Al and Nb. Mater. Sci. Forum 2018, 930, 501–506. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, W.; Veys, X.; Huyberechts, G.; Springer, H.; Selleby, M. Prediction of Martensite Start Temperature for Lightweight Fe-Mn-Al-C Steels. J. Phase Equilibria Diffus. 2018, 39, 476–489. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurgel, M.A.M.; de Souza Baêta Júnior, E.; da Silva Teixeira, R.; do Nascimento, G.O.; Oliveira, S.S.; Ferronatto Leite, D.N.; Moreira, L.P.; Brandao, L.P.; dos Santos Paula, A. Microstructure and Continuous Cooling Transformation of an Fe-7.1Al-0.7Mn-0.4C-0.3Nb Alloy. Metals 2022, 12, 1305. https://doi.org/10.3390/met12081305
Gurgel MAM, de Souza Baêta Júnior E, da Silva Teixeira R, do Nascimento GO, Oliveira SS, Ferronatto Leite DN, Moreira LP, Brandao LP, dos Santos Paula A. Microstructure and Continuous Cooling Transformation of an Fe-7.1Al-0.7Mn-0.4C-0.3Nb Alloy. Metals. 2022; 12(8):1305. https://doi.org/10.3390/met12081305
Chicago/Turabian StyleGurgel, Mônica Aline Magalhães, Eustáquio de Souza Baêta Júnior, Rodolfo da Silva Teixeira, Gabriel Onofre do Nascimento, Suzane Sant’Ana Oliveira, Duílio Norberto Ferronatto Leite, Luciano Pessanha Moreira, Luiz Paulo Brandao, and Andersan dos Santos Paula. 2022. "Microstructure and Continuous Cooling Transformation of an Fe-7.1Al-0.7Mn-0.4C-0.3Nb Alloy" Metals 12, no. 8: 1305. https://doi.org/10.3390/met12081305
APA StyleGurgel, M. A. M., de Souza Baêta Júnior, E., da Silva Teixeira, R., do Nascimento, G. O., Oliveira, S. S., Ferronatto Leite, D. N., Moreira, L. P., Brandao, L. P., & dos Santos Paula, A. (2022). Microstructure and Continuous Cooling Transformation of an Fe-7.1Al-0.7Mn-0.4C-0.3Nb Alloy. Metals, 12(8), 1305. https://doi.org/10.3390/met12081305