The Microstructure and the Properties of 304 and 430 Steel Foams Prepared by Powder Metallurgy Using CaCl2 as a Space Holder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production Process
2.3. Characterization of SSF Samples
3. Results and Discussion
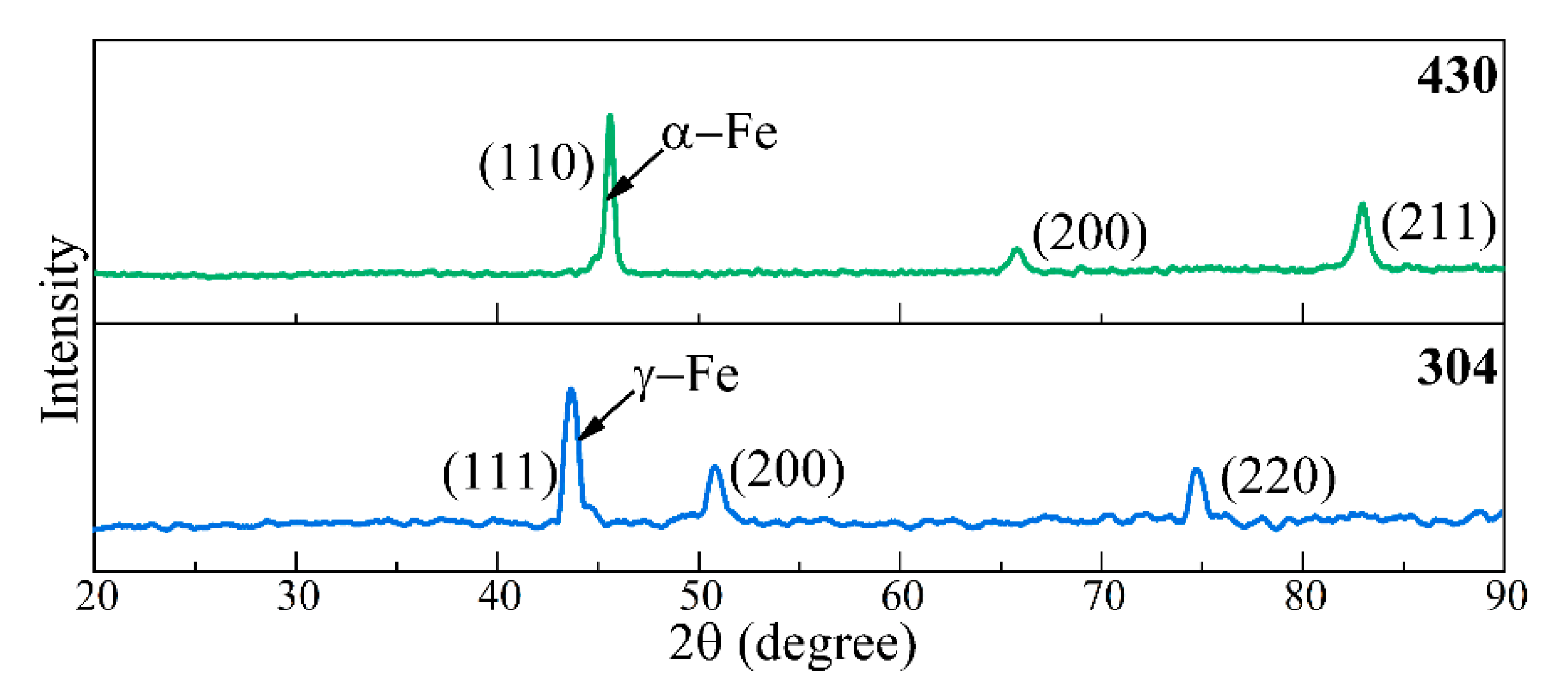
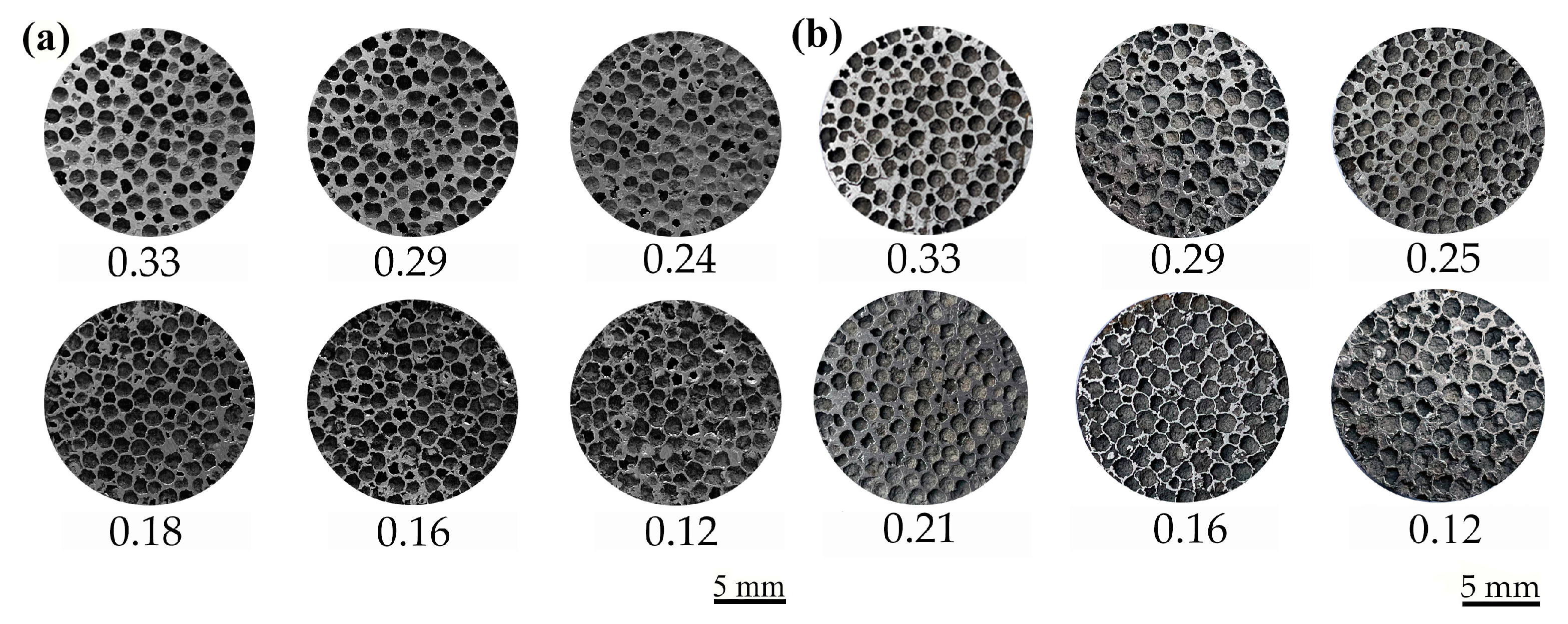
3.1. Microstructure
3.2. Compressive Behavior of SSF Samples
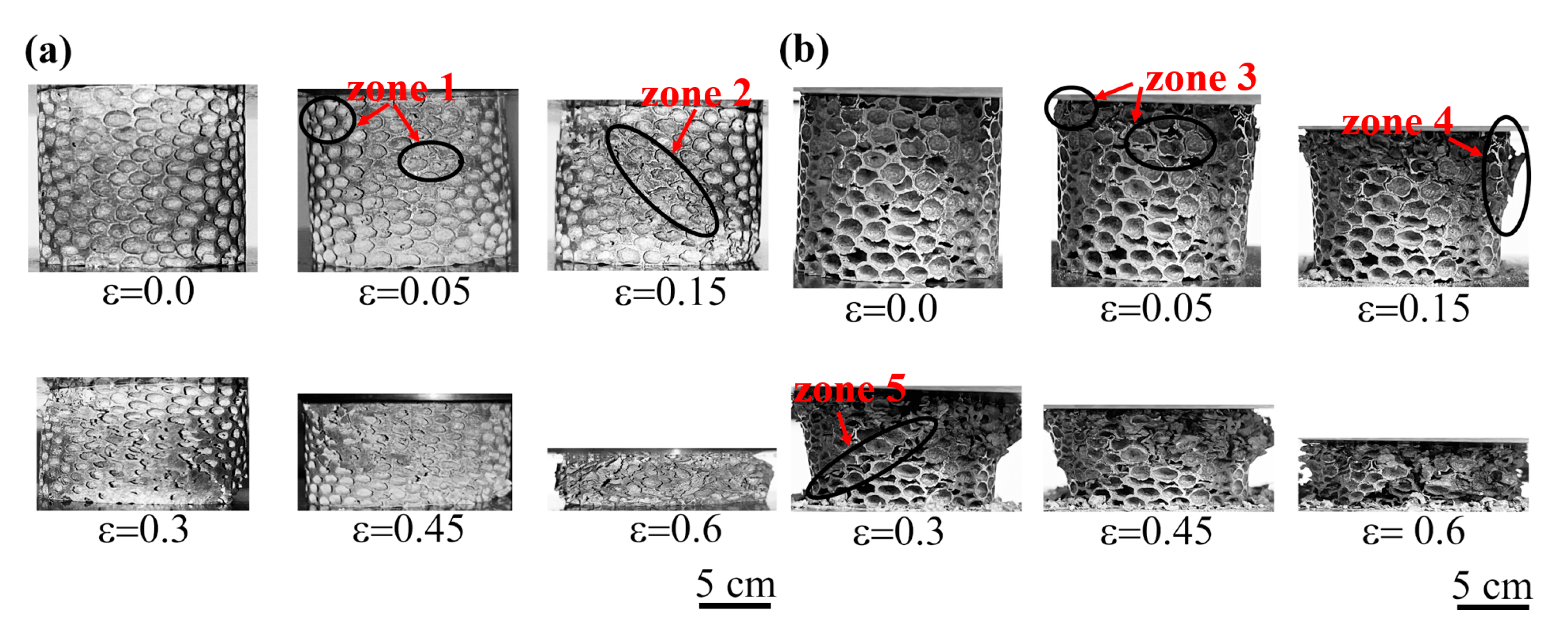
3.2.1. Cell Morphology and Evolution of Deformation
3.2.2. Stress–Strain Behavior
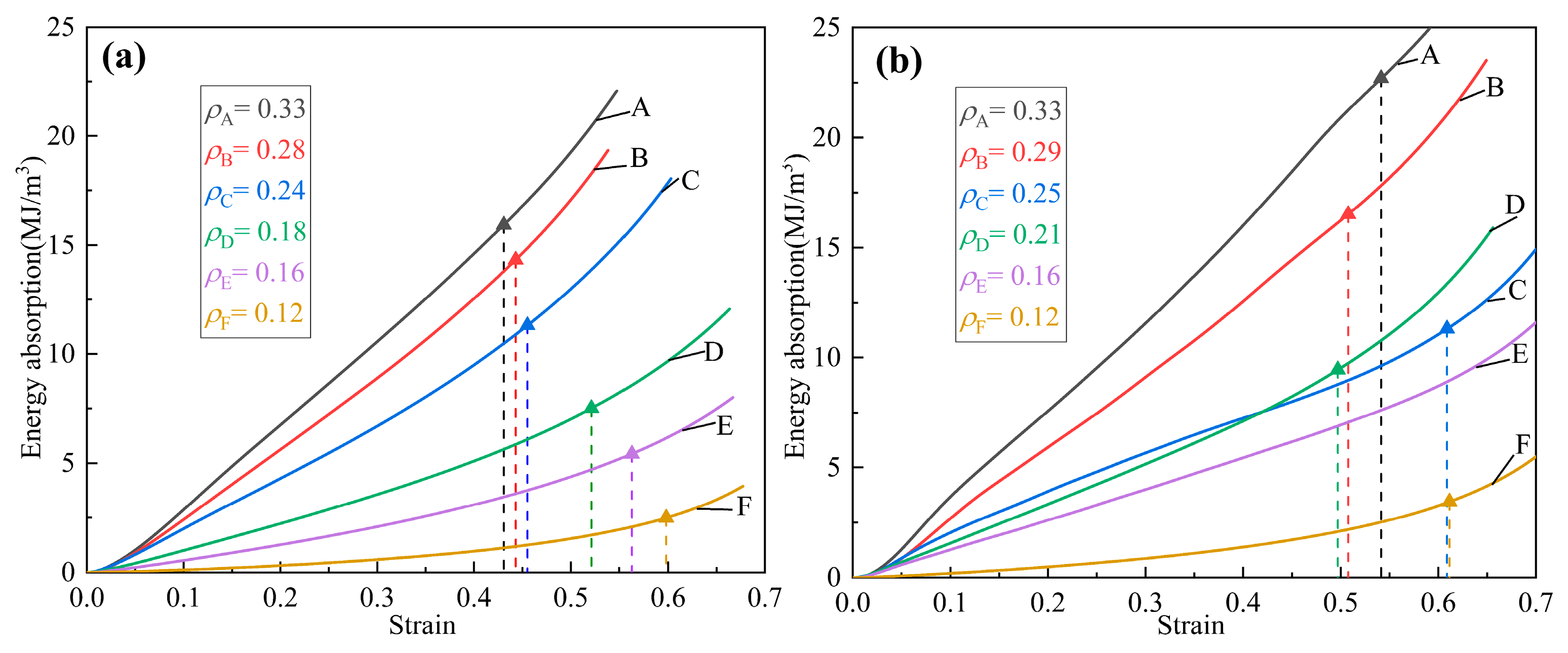
3.2.3. Energy Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, B.H.; Szyniszewski, S.; Hajjar, J.F.; Schafer, B.W.; Arwade, S.R. Steel foam for structures: A review of applications, manufacturing and material properties. J. Constr. Steel Res. 2012, 72, 276. [Google Scholar] [CrossRef]
- Guo, K.S.; Li, M.C.; Gong, Q.; Li, C.S.; Li, C.H.; Zhong, H.; Zhou, Y. Experimental investigation on steel foams fabricated by sintering-dissolution process. Mater. Manuf. Process 2016, 31, 1597–1602. [Google Scholar] [CrossRef]
- Hsu, C.M.; Tzeng, Y.C.; Chen, S.F.; Chen, Y.L.; Lee, H.L. Fabrication of 17-4PH stainless steel foam by a pressureless powder space holder technique. Adv. Eng. Mater. 2021, 23, 2001202. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, T.; Ma, D.; Zhou, P.; Guo, C.; Zhou, Y.; Zuo, X. Comparision of structure and performance for foamed stainless steels 410L and 430L. Chin. J. Mater. Res. 2021, 35, 597–605. [Google Scholar]
- Jain, H.; Mondal, D.P.; Gupta, G.; Kumar, R.; Singh, S. Synthesis and characterization of 316L stainless steel foam made through two different removal process of space holder method. Manuf. Lett. 2020, 26, 33–36. [Google Scholar] [CrossRef]
- Rabiei, A.; Karimpour, K.; Basu, D.; Janssens, M. Steel-steel composite metal foam in simulated pool fire testing. Int. J. Therm. Sci. 2020, 153, 106336. [Google Scholar] [CrossRef]
- Jain, H.; Kumar, R.; Gupta, G.; Mondal, D.P. Microstructure, mechanical and EMI shielding performance in open cell austenitic stainless steel foam made through PU foam template. Mater. Chem. Phys. 2020, 242, 122273. [Google Scholar] [CrossRef]
- Wang, C.H.; Jiang, F.C.; Shao, S.Q.; Yu, T.M.; Guo, C.H. Acoustic properties of 316L stainless steel hollow sphere composites fabricated by pressure casting. Metals 2020, 10, 1047. [Google Scholar] [CrossRef]
- Mutlu, I.; Oktay, E. Characterization of 17-4 PH stainless steel foam for biomedical applications in simulated body fluid and artificial saliva environments. Mater. Sci. Eng. C-Mater. 2013, 33, 1125–1131. [Google Scholar] [CrossRef]
- Xu, X.B.; Liu, P.S.; Chen, G.F.; Li, C.P. Sound absorption performance of highly porous stainless steel foam with reticular structure. Met. Mater. Int. 2021, 27, 3316–3324. [Google Scholar] [CrossRef]
- Nakajima, H. Fabrication, mechanical and physical properties, and its application of lotus-type porous metals. Mater. Trans. 2019, 60, 2481–2489. [Google Scholar]
- Ide, T.; Tane, M.; Ikeda, T.; Hyun, S.K.; Nakajima, H. Compressive properties of lotus-type porous stainless steel. J. Mater. Res. 2006, 21, 185–193. [Google Scholar]
- Ikeda, T.; Aoki, T.; Nakajima, H. Fabrication of lotus-type porous stainless steel by continuous zone melting technique and mechanical property. Metall. Mater. Trans. A 2005, 36, 77–86. [Google Scholar] [CrossRef]
- Szyniszewski, S.T.; Smith, B.H.; Hajjar, J.F.; Schafer, B.W.; Arwade, S.R. The mechanical properties and modeling of a sintered hollow sphere steel foam. Mater. Des. 2014, 54, 1083–1094. [Google Scholar]
- Fiedler, T.; Veyhl, C.; Belova, I.V.; Bernthaler, T.; Heine, B.; Murch, G.E. Mechanical properties and micro-deformation of sintered metallic hollow sphere structure. Comp. Mater. Sci. 2013, 74, 143–147. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Löber, L.; Klauss, H.-J.; Kühn, U.; Eckert, J. Characterization of 316L steel cellular dodecahedron structures produced by selective laser melting. Technologies 2016, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Caiazzo, F.; Campanelli, S.L.; Cardaropoli, F.; Contuzzi, N.; Sergi, V.; Ludovico, A.D. Manufacturing and characterization of similar to foam steel components processed through selective laser melting. Int. J. Adv. Manuf. Technol. 2017, 92, 2121–2130. [Google Scholar] [CrossRef] [Green Version]
- Capek, J.; Machova, M.; Fousova, M.; Kubasek, J.; Vojtech, D.; Fojt, J.; Jablonska, E.; Lipov, J.; Ruml, T. Highly porous, low elastic modulus 316L stainless steel scaffold prepared by selective laser melting. Mater. Sci. Eng. C-Mater. 2016, 69, 631–639. [Google Scholar] [CrossRef]
- Sazegaran, H.; Naserian Nik, A.M.; Akbari, M.R.; Akbari Nejad Fogh, A. Investigation on the compressive behavior of steel foams manufactured by powder metallurgy route. J. Metall. Mater. Eng. 2021, 32, 45–56. [Google Scholar]
- Sazegaran, H. Investigation on production parameters of steel foam manufactured through powder metallurgical space holder technique. Met. Mater. Int. 2021, 27, 3371–3384. [Google Scholar] [CrossRef]
- Jain, H.; Mondal, D.P.; Gupta, G.; Kumar, R. Effect of compressive strain rate on the deformation behaviour of austenitic stainless steel foam produced by space holder technique. Mater. Chem. Phys. 2021, 259, 124010. [Google Scholar] [CrossRef]
- Mondal, D.P.; Jain, H.; Das, S.; Jha, A.K. Stainless steel foams made through powder metallurgy route using NH4HCO3 as space holder. Mater. Des. 2015, 88, 430–437. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, T.; Fleck, N.A.; Hutchinson, J.W.; Wadley, H.N.G.; Gibson, L.J. Properties of metal foams. In Metal Foams: A Design Guide; Butterworth Heinemann: Oxford, UK, 2000; Volume 4, pp. 40–54. [Google Scholar]
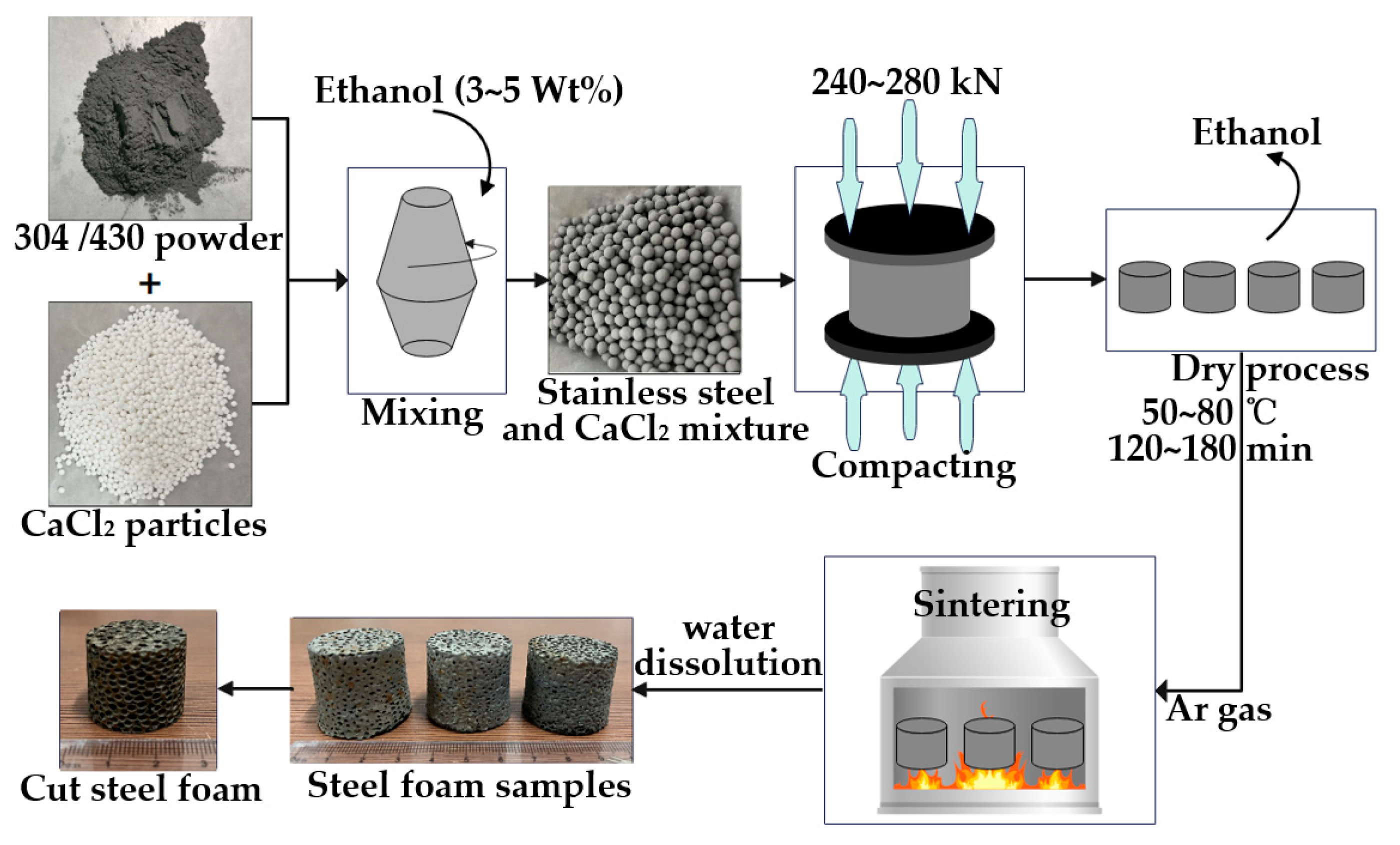
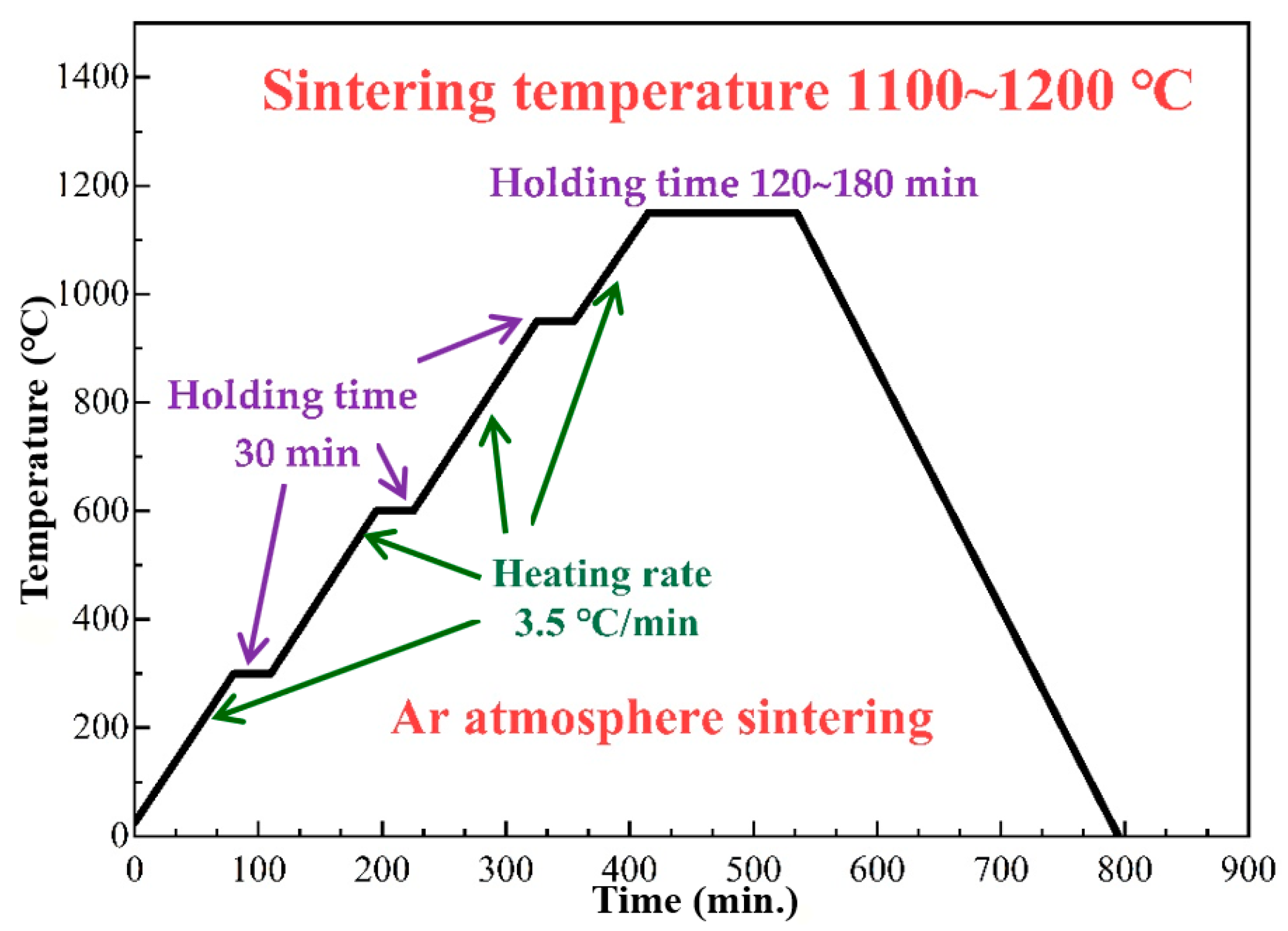
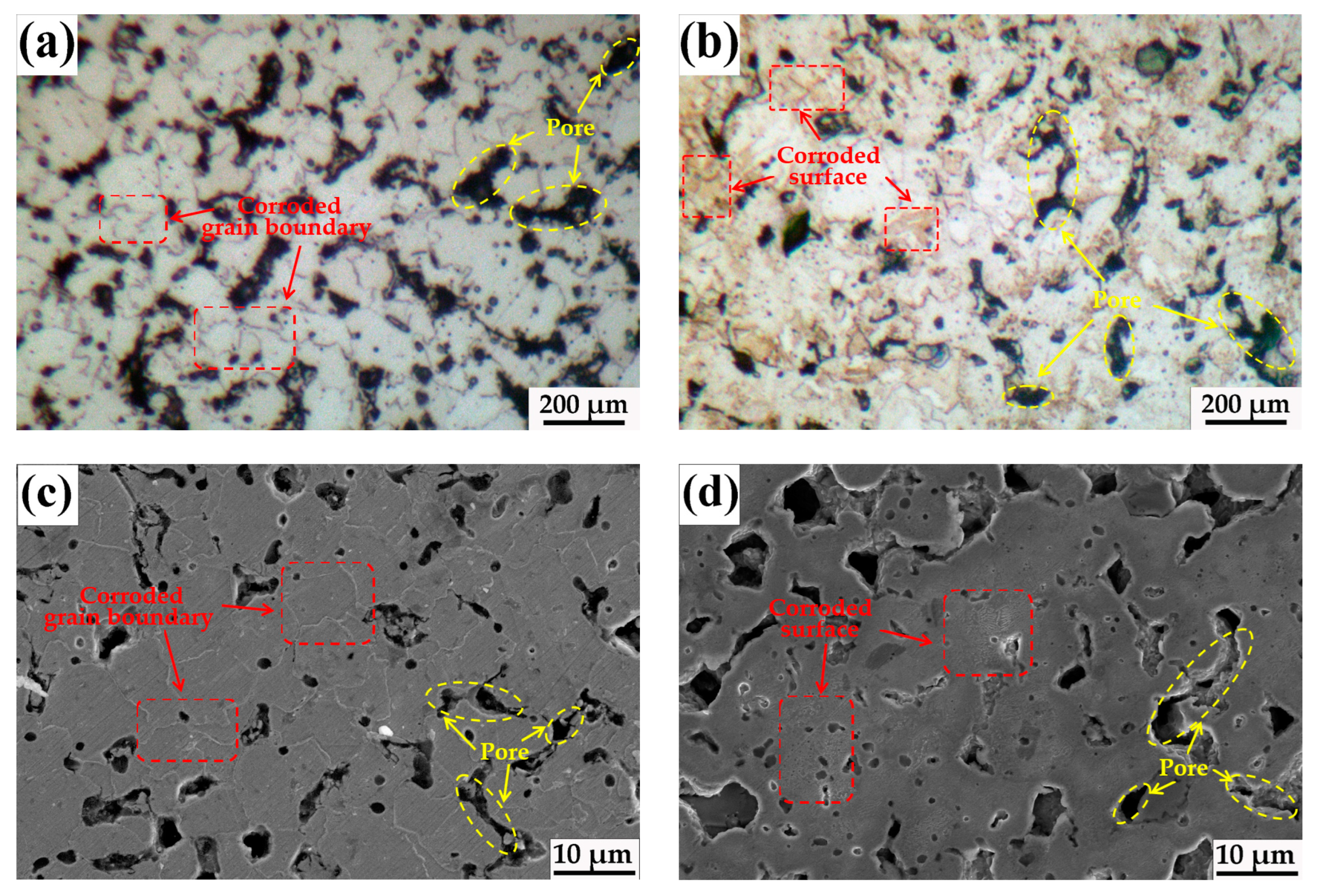
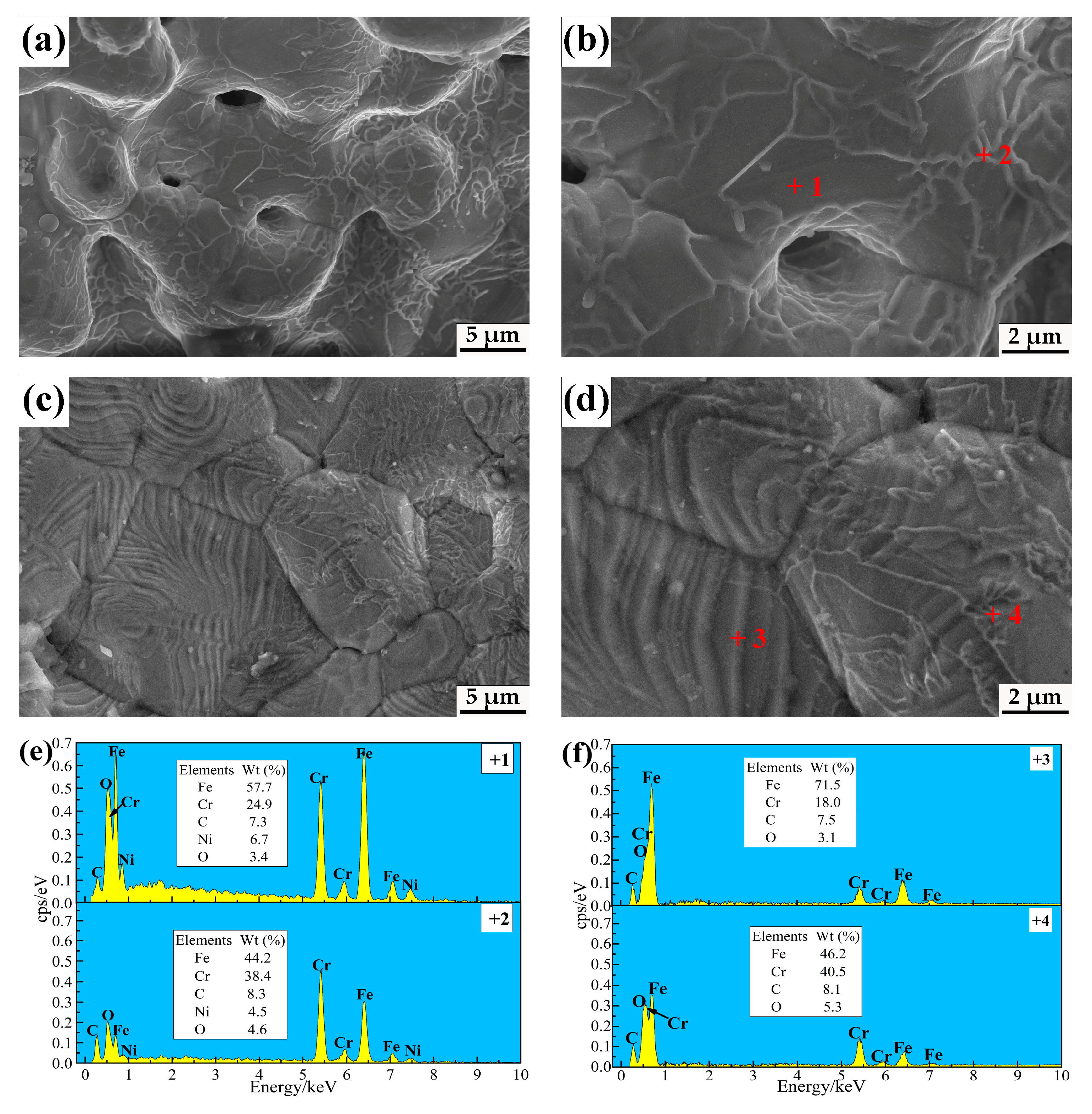

| Materials | C | Si | Mn | P | S | Cr | Ni | Fe |
|---|---|---|---|---|---|---|---|---|
| 304 | ≤0.08 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 18–20 | 8–10.5 | Bal. |
| 430 | ≤0.12 | ≤1.00 | ≤2.00 | ≤0.04 | ≤0.030 | 16–18 | 0 | Bal. |
| Compressive Strength | Compressive Strength | ||
|---|---|---|---|
| 0.33 | 40.29 | 0.33 | 49.79 |
| 0.28 | 32.30 | 0.29 | 38.00 |
| 0.24 | 23.70 | 0.25 | 26.65 |
| 0.18 | 13.53 | 0.21 | 17.91 |
| 0.16 | 6.20 | 0.16 | 15.32 |
| 0.12 | 0.81 | 0.12 | 1.50 |
| Energy Absorption Value | Energy Absorption Value | ||
|---|---|---|---|
| 0.331 | 15.94 | 0.33 | 22.70 |
| 0.28 | 14.31 | 0.29 | 16.51 |
| 0.24 | 11.32 | 0.25 | 11.34 |
| 0.18 | 7.51 | 0.21 | 9.47 |
| 0.16 | 5.44 | 0.16 | 7.82 |
| 0.12 | 2.47 | 0.12 | 3.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Zhang, G.; Zhou, Y.; Song, S.; Zuo, X.; Wang, H. The Microstructure and the Properties of 304 and 430 Steel Foams Prepared by Powder Metallurgy Using CaCl2 as a Space Holder. Metals 2022, 12, 1182. https://doi.org/10.3390/met12071182
Liang J, Zhang G, Zhou Y, Song S, Zuo X, Wang H. The Microstructure and the Properties of 304 and 430 Steel Foams Prepared by Powder Metallurgy Using CaCl2 as a Space Holder. Metals. 2022; 12(7):1182. https://doi.org/10.3390/met12071182
Chicago/Turabian StyleLiang, Jichao, Guangcheng Zhang, Yun Zhou, Shaowei Song, Xiaoqing Zuo, and Hui Wang. 2022. "The Microstructure and the Properties of 304 and 430 Steel Foams Prepared by Powder Metallurgy Using CaCl2 as a Space Holder" Metals 12, no. 7: 1182. https://doi.org/10.3390/met12071182
APA StyleLiang, J., Zhang, G., Zhou, Y., Song, S., Zuo, X., & Wang, H. (2022). The Microstructure and the Properties of 304 and 430 Steel Foams Prepared by Powder Metallurgy Using CaCl2 as a Space Holder. Metals, 12(7), 1182. https://doi.org/10.3390/met12071182







