Abstract
Biocompatible titanium alloys are increasingly being used to make custom medical implants using additive manufacturing processes. This paper considered the welding reconditioning of a titanium-alloy customized additive manufactured hip implant with several manufacturing defects. The personalized implants are made starting from a Computer-Aided Design (CAD) model as a direct result from the medical imaging investigations of the areas of interest. Then the customized implant is fabricated using an additive manufacturing process (in this case Powder Bed Fusion—Direct Metal Laser Sintering—DMLS). The analysis of the chemical composition values as well as the values of the mechanical properties of the samples obtained via DMLS additive manufacturing process, revealed that such a manufacturing process can be successfully used to make customized surgical implants. The mechanical properties values of the DMLS samples are approximately equal to those specified by the manufacturer of the titanium powder used for sintering. On average, the tensile strength was found to be 24.75% higher, while yield strength 22.7% higher than the values provided in the standard for surgical implants applications. In case the additive manufacturing process produces products with defects one might want to try and recover the implant due to costs and time constraints. The Tungsten Inert Gas (TIG) welding reconditioning process with ERTi-5 Ti64 rod for welding titanium alloys with a content of 6% aluminum and 4% vanadium filler material was used to restore the geometric characteristics as well as the functional properties of a custom hip medical prosthesis. After welding depositing successive layers of materials, the surfaces of the prosthesis were machined to restore the functional properties according to the characteristics of the original 3D model. A 3D scan was used to compare the geometrical characteristics between the original part and reconditioned one. Deviations were less than 1 mm and were acceptable from the medical point of view.
1. Introduction
Medical implants and prostheses that replace different parts of the human body are made of biocompatible materials, which have mechanical and physical-chemical properties specific to these applications [1,2,3,4]. The materials used in medical implants manufacturing are, at best, inert to the bone tissue having the possibility to become bioactive by controlling the morphology and chemical composition at surface. The elements that must be considered when choosing the material used for medical implants such as compatibility, the material nature and degree of alloying, process and manufacturing method, mechanical properties, processing conditions significantly influence the interaction between the material and bone tissue. The long-term stability of the implant depends largely on all of the aspects presented above but also on its ability to integrate into the adjacent bone tissue [5].
Considering the possible problems caused by the incompatibility between the materials used to make the prosthesis on the bone tissue, scientists collaborate to create new biocompatible materials with a much higher acceptance rate [6,7].
Currently, a wide variety of biocompatible materials are used to make medical implants, such as metals and alloys (stainless steel, Ti-Al-V, Co-Cr-Mo) ceramics and glasses (alumina, zirconia), polymers (polyethylene, polyamides), composites (PMMA-glass fillers) [8,9].
In present, most medical prosthesis are made of titanium alloys, alloys that offer high mechanical properties (tensile strength, yield strength), low Young’s module, low specific weight (half the specific weight of stainless steel). In addition, such materials are not toxic (in comparison, stainless steel can cause several allergies), they are not ferromagnetic (the possibility of performing magnetic resonance imaging investigations) and have a good corrosion resistance in specific working environments [10,11].
The Ti6Al4V titanium alloy, is the most common titanium alloy used for making medical implants, being an α + β type of alloy initially used in the aerospace industry [12,13,14,15]. As in the case of stainless steels, the corrosion resistance of titanium alloys is given by the formation of an oxide layer on the surface of the material, in this case being the TiO2 layer [16]. The metallic materials used in the manufacture of medical implants are inert to human bone tissue [17,18]. To obtain customized implants having shapes and sizes specific to their use in case of surgery in areas with continuous growing tumors has led to the need of using new manufacturing methods. The classical methods of obtaining implants and medical instruments (forging, stamping) do not offer a high dimensional accuracy of the product [19]. To meet the dimensional and quality requirements for customized implants, the new trend is the use of additive manufacturing processes (3D printing), using the information obtained from the imaging examinations to which the patient was subjected [20,21]. Popov et al. [22] present the possibility of healthcare digitalization in the Industry 4.0 revolution using 3D printing which allows on-site printing of freeform shapes, which are potentially useful to develop custom-sized implants or prostheses.
Direct metal laser sintering (DMLS) is an additive manufacturing process used for printing metal products, which is based on using the energy of a laser beam to melt layers of metal powder (sintering) which, following solidification, finally forms the 3D model of the desired product. The size of the metal powder particles is between 20 and 40 μm. The size of the particles, the surfaces of the products, as well as their geometric configuration influence the final printing resolution [23,24,25,26,27].
In certain situations, when implants are made by classical technological processes, there are disturbing factors that appear and influence the result of the process. In such cases, the resulting product has a series of dimensional or shape (geometric) imperfections that may lead to the decision to classify the resulting product as scrap. In these situations, the prosthesis material must be remelted and subjected to a new processing process, which leads to increased costs related to obtaining implants in the final form [28,29]. In the case of additive manufacturing processes, the reuse of scrap is no longer possible, so the financial, time or resources loss is significant.
In the case of recoverable scrap, there is the possibility of using welding reconditioning to restore the geometric configuration and the functional properties of the implants.
Through the advantages offered by the welding processes, it is possible to reduce the costs related to the production of implants, but also those related to the long-term use of surgical instruments or cosmetic implants. The costs related to welding reconditioning of instruments and medical implants, depending on their complexity and the type of defect, are between 15 and 50% of the costs of new products.
In the literature, there is a limited number of studies that refer to welding reconditioning possibilities of different types of products, made of steel, cast iron [30,31,32,33,34,35,36,37,38] or non-ferrous materials [39]. In terms of welding reconditioning products made of titanium alloys, Petrik I.A. [40] presents the possibility of reconditioning by welding the components of gas turbine rotors and Paton B.E. [41] uses various welding or reconditioning technologies for welding components made of titanium alloys. Graf B. and Liu Q. show [42,43] the possibility of welding repair with the help of laser metal deposition technology. Yu J.H. [44], Onuike B. [45] and Rahito [46] use the notion of additive manufacturing for laser metal-layer deposit reconditioning.
Following the desktop research, it was found that there is no research conducted on the possibility of modifying the characteristics of the products obtained with the help of AM processes. In some cases, and especially in the case of medical implants, the rapid evolution of malignant bone tumors can lead to changes in the bone area that must be ex-removed and replaced. In such cases, considering the rapid evolution of the tumor, the time from the analysis of the imaging results to the design and realization of the implant with the help of an AM procedure, makes it necessary to modify the constructive form of the implant, by adding additional elements to compensate the excised bone area or of some additional elements for the attachment of the implant on the healthy bone structure.
The purpose of this paper is to analyze the possibilities of restoring the geometric characteristics by welding of implants made by additive manufacturing processes that would otherwise be unusable and unrecoverable. In this case, the use of the DMLS additive manufacturing process of a custom hip implant, resulted in a geometric configuration that did not meet the requirements of the fabrication specifications. To restore the geometric characteristics of the implant, the tungsten inert gas (TIG) welding deposition process was used.
The novelty of the proposed research consists in the restoration and/or modification of the geometric and functional characteristics of an AM made implant with manufacturing errors with the help of welding reconditioning processes by. Welding reconditioning is presented, in some cases, as a fast and inexpensive alternative for remanufacturing the implant according to the new functional requirements.
2. Materials and Methods
2.1. Materials
The implant demonstrator has been additively manufactured using Ti-6Al-4V powder supplied by EOS Gmbh (Krailling, Germany). For the TIG welding deposition the ERTi-5 Ti64 rod filler material was used.
The ERTi-5 Ti64 rod is used for welding titanium alloys with a content of 6% aluminum and 4% vanadium, providing high fatigue strength, toughness, and ductility of the weld bead material. It has good weldability and can be heat treated to reach a higher strength or toughness.
The ERTi-5 Ti64 rod is currently used in the aircraft and motor-sports competition applications for the manufacture of airframes and chassis structures, turbine engine parts, exhaust systems and ducting, discs, wheels, spacer rings, but also in medical industry [47].
The chemical composition and mechanical properties of the materials used, according to the manufacturer’s quality certificates, are presented in Table 1 and Table 2.

Table 1.
Chemical composition of the used materials in wt%.

Table 2.
Mechanical properties of the used materials.
2.2. Mechanical Measurements and Microstructural Characterization Methods
Considering that the parts obtained with the help of the DMLS additive manufacturing process are made by a successive deposition of the sintered layers of metallic powder, the resulting parts may not correspond in terms of mechanical properties. To analyze the values of the mechanical properties of the resulting components, four samples (T1, T2, T3, T4) obtained by DMLS with the help of EOSINT M270 equipment (EOS GmbH, Krailling, Germany) were subjected to tensile testing. The tensile test was performed, using H10KT test machine (TINIUS OLSEN, Salfords, England) with a 2 mm/s (23 N/mm2 s) working speed value. The Rockwell, HRC test method, 120° cone angle, 1471N compressive force (206× hardness tester model from Affri System, Varese, Italy) was used to measure the hardness of the sample material obtained by additive manufacturing. The shape of the samples, in accordance with ASTM E8/E8M:2021 [48], is presented in Figure 1. Microscopic analysis of the samples was performed using an optical microscope (OLYMPUS GX51, Tokyo, Japan) as well as an electron microscope (SEM INSPECT S, FEI, Eindhoven, The Netherlands) in high-vacuum working mode for imaging and microanalysis of conductive samples and/or conventional prepared samples (coated) and low-vacuum working mode for imaging and microanalysis of unprepared samples.
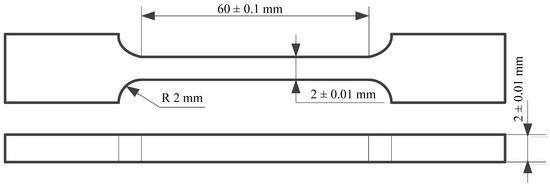
Figure 1.
The shape of the samples used to perform the tensile test.
2.3. Methods
2.3.1. Additive Manufacturing
Additive manufacturing processes are a rapid prototyping technology that provides the fastest way from product idea to market launch. Innovative companies around the world use this technology for very fast, flexible and cost-effective parts production, directly from electronic format. The DMLS additive manufacturing process was used to make the custom hip implant. For this, the 3D CAD model is divided into layers using EOS RP Tools software (version 5.2, Cambridge, UK). This conversion is necessary as the additive manufacturing equipment can achieve the desired prototype only by layer-by-layer deposition. Furthermore, these specialized software packages ensure that the layers do not form in the air without having the support of the previous layers or special structures (called supports). Therefore, supports are generated where considered necessary. In the DMLS case, the model and the supports are automatically divided by the software into layers (bottom to top) with a thickness of 0.02 or 0.03 mm depending on the type of material used. The 0.03 mm layer thickness is used only for titanium and titanium alloy Ti6Al4V, for all other materials the 0.02 mm thickness is valid. The 3D model of the implant presented in Figure 2a, can be made based on the information obtained from the imaging investigations of the person for whom it is desired to customize the implant. The equipment used to make the implant was a rapid prototyping equipment by laser sintering, EOSINT M270 type (EOS GmbH, Krailling, Germany), which has the possibility of processing titanium powders, by using argon as a protecting atmosphere. The working parameters used for the manufacture of the hip implant using the DMLS process are presented in Table 3. Following the additive manufacturing process, the implant presented in Figure 2b was obtained.
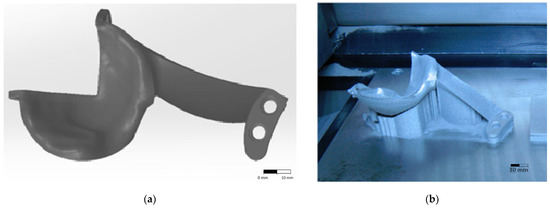
Figure 2.
Custom hip implant model (a) custom 3D model (b) model resulted from the DMLS additive manufacturing process.

Table 3.
Working parameters of the EOS M270.
2.3.2. Welding Process
During the additive manufacturing process of the custom implant, an error occurred (Figure 3) which led to improper geometric characteristics of the final product. From the analysis of the model presented in Figure 3 one can observe that the clamping ear as well as the fixing holes of the implant do not meet the initial specifications of the 3D model. This error led to the impossibility of using the implant for surgery and classify it as scrap.
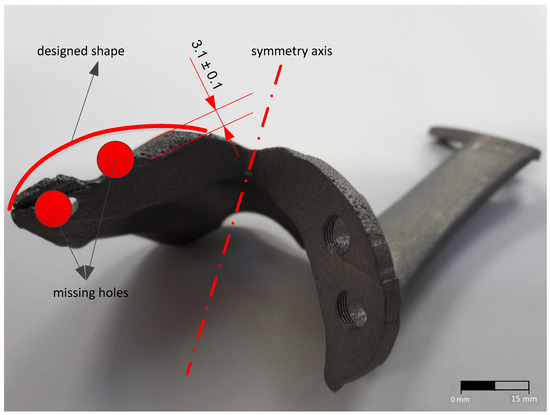
Figure 3.
Custom implant obtained by the DMLS additive manufacturing process.
To restore the geometric characteristics of the implant, a TIG welding deposition process was proposed [49]. The removal of the inappropriate layer was carried out by cutting machining. On the resulted surface, successive layers were deposited to reach the shape required for further cutting machining and restoring the implant to the nominal size of the initial 3D drawing. The welding deposition parameters are presented in Table 4.

Table 4.
The welding parameters used in the TIG welding process.
To restore the geometric shape of the implant, six layers of welding were deposited. The first layer was applied directly to the surface resulted from cutting machining to melt and homogenize all of the micro asperities of the inappropriate surface. The next four layers were deposited on the side surfaces of the clamping ear, and layer six was deposited on the upper surface.
The decision to restore the functional properties by welding reconditioning processes must consider the technological possibilities of production and includes a series of steps specific to the manufacturing processes. The welding reconditioning flowchart for customized implants is presented in Figure 4.
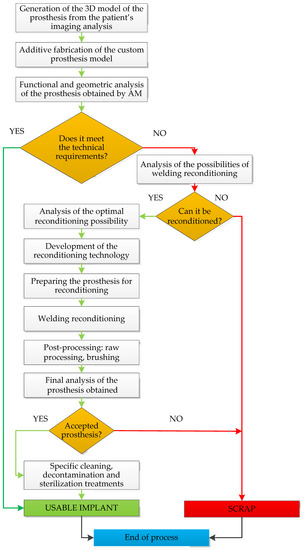
Figure 4.
Welding reconditioning flowchart for customized implants.
3. Results and Discussion
3.1. Machanical Properties
The values of the hardnesses as well as of the mechanical properties of the Ti6Al4V samples obtained by DMLS additive manufacturing process are presented in Table 5. Analyzing the values presented in Table 5 one can observe that the samples obtained by DMLS show values within the limits prescribed by the manufacturer of EOS Ti64 powder. The modulus of elasticity varies between 99.41 GPa (T1) and 107.86 GPa (T4), lower values compared to the values of the Ti6Al4V sheets modulus of elasticity obtained by cold-rolling or hot-rolling. The values of the mechanical properties are slightly higher than those obtained by the classical manufacturing processes (tensile strength Rm = 892 MPa and Rp0.2 = Yield strength 828 MPa [47]). On average, the tensile strength was found to be 24.75% higher, while yield strength 22.7% higher than the values provided in the standard for surgical implants applications [49,50,51,52,53,54]. The results validated the EOS datasheet specifications, also presented in Table 5.

Table 5.
Mechanical properties of the DMLS Ti6Al4V sample.
3.2. Composition and Microstructure of Ti64 DMLS Samples
Table 6 presents the average chemical composition of the Ti64 powder samples, obtained by DMLS additive manufacturing process, as measured by energy dispersive x-ray (EDX) spectroscopy analysis. Following the analysis, one can notice that the samples are homogeneous, without imperfections and the values of the chemical composition, measured at different points, are approximately equal throughout their mass.

Table 6.
Chemical composition of the laser-sintered powder and weld deposit material wt%.
Figure 5 shows the microstructure of the Ti6Al4V samples obtained by the DMLS additive manufacturing process. The microscopical analysis of the images presented in Figure 5 shows a lamellar structure of type (α + β) combined with a martensitic acicular structure of type α’, which could explain the slight increase of the hardness values compared to the specifications of the metal powder manufacturer. The formation of the martensitic structure is caused by the principle of the DMLS process which consists in heating to the melting temperature followed by a rapid cooling process. The microstructure obtained, as well as the values of the mechanical and chemical composition characteristics of the analyzed samples obtained by DMLS, are similar to those of the products fabricated by classical manufacturing processes. Figure 6 presents the results of the EDS analysis for the implant obtained by additive manufacturing process (Figure 6a) and by welding deposition (Figure 6b) made to restore the geometric characteristics of the implant.
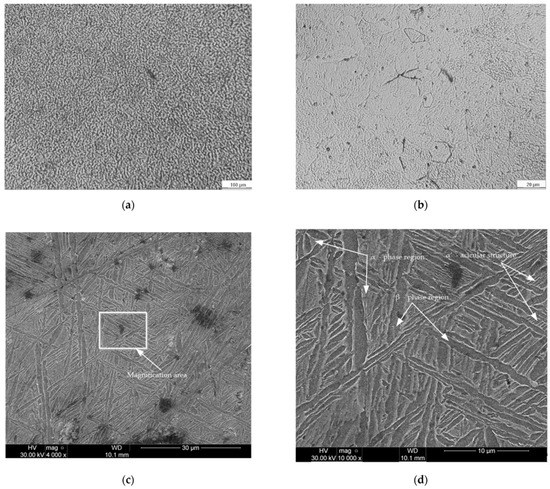
Figure 5.
Microscopy image of the Ti64 DMLS samples: (a) 100×; (b) 800×; (c) 4000×; (d) 10,000×.
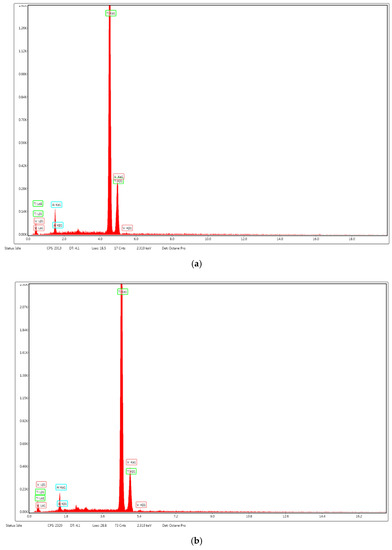
Figure 6.
EDS analysis of sintered samples of Ti6Al4V alloy fabricated using DMLS process: (a) sintered samples; (b) weld deposit zone.
Following the analysis of the values of mechanical and chemical composition properties obtained, one can observe that they are similar to those of products obtained by classical manufacturing processes, which may lead to the decision of using additive manufacturing methods to obtain customized medical implants. The implant resulted from the welding deposition process, using the technological parameters presented in Table 4, is presented in Figure 7. The analysis of the sample presented in Figure 3 shows that the width of the deposition area is relatively small (3.1 ± 0.1 mm wide). After processing the affected area, by burnishing, the implant clamping edge was reconstructed by welding deposition. Figure 7 shows the layer-by-layer welding deposition process until reaching the dimensions that allow further processing by cutting machining (6.2 ± 0.1 mm average width) and restoring the area to the geometric configuration and tolerances prescribed in the fabrication drawing of the 3D model.

Figure 7.
Custom implant subjected to the welding deposition process to restore the geometric characteristics prescribed by the 3D model. (a) front view; (b) back view.
After cutting machining on a CNC machine (Figure 8), the surfaces of the ”recovered” implant must be brushed to reach the rugosity imposed by its functional role. In the end it is necessary to process the tapped holes to the dimensions of the special fastening screws on the bone structure. The custom implant resulted from post-processing (cutting machining) is presented in Figure 9.
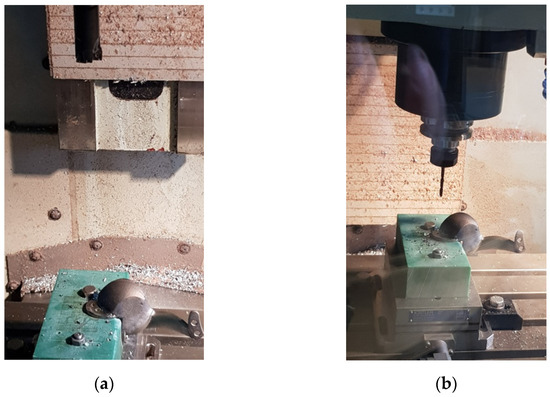
Figure 8.
Cutting machining for the dimensional restoration of the personalized implant: (a) fixing the implant in the device to mill the edges of the implant; (b) drilling holes.

Figure 9.
Custom implant resulting from the cutting machining.
One area of interest is the actual interface between the metallic layer deposited by welding and the main body of the implant (the connection area between the AM part and the welded part). This area was also analyzed under a microscope, as explained in previous section. As a result, Figure 10 shows the structural changes in the reconditioning area. Considering the value of the temperature reached during the welding process, but also the rapid cooling of the material, in the fusion zone (FZ), but also in the thermally influenced zone (HAZ), one can observe the occurrence of the α acicular phase and the recrystallized β phase. In the area of the cord material, a higher percentage of martensite (α’ phase) can be observed, as well as a lamellar α structure.
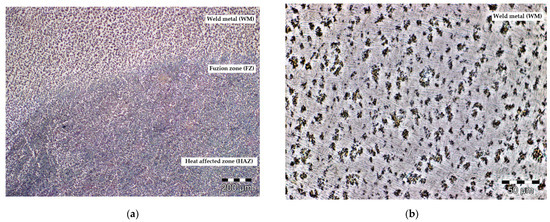
Figure 10.
Microscopy image of the hip prosthesis reconditioning zone: (a) 100×; (b) 50×.
3.3. Geometrical Analysis of the Corrected Holes
To verify the accuracy of the shape and the geometric dimensions of the reconditioned implant, the 3d model of the implant was reverse engineered by using a Hexagon (ROMER) Absolute arm with 3d scanner and touch equipment. A set of surfaces (2394422) were generated over the cloud points by using the Designer 2022.0 R2 software package with reverse capabilities (Figure 11). After a simple re-mesh (automatically surface) a fully 3d triangle mesh surfaces were obtained. This model has high accuracy of the real scanned implant. By generating parametric surfaces with high accuracy of the points cloud, precise measurements are possible to be made. The scanned holes were reversed to cylindrical surfaces and measured by using the software capabilities.
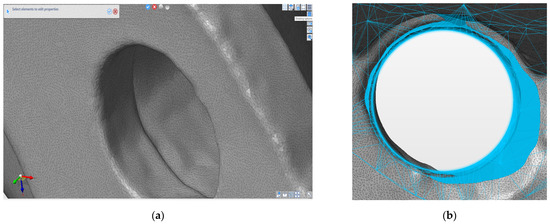
Figure 11.
(a) 3D scanned hole (b) comparative of the corrected hole and the CAD model (overlap between the scanned hole and the original CAD model).
By comparative analysis with the original CAD model some errors were identified as deviations between components and holes shape. A solution was discussed together with an orthopedic surgeon specialist. The measured deviations were accepted (under 1 mm deviations were measured as explained above), and for the holes the next thread bigger diameter was accepted. The technical solution was obtained in two steps: firstly, enlargement of the hole diameter by drilling up to 6.2 mm (shape correction to cylindrical face) and then the generation of the thread by taping to M7. Then a NC program for machining on the Fanuc equipment mentioned above was designed and implemented.
After completing the process of restoring the construction of the medical implant and processing it, using the TIG welding reconditioning process and the post-processing through cutting machining, the samples will be subjected to specific cleaning, decontamination and sterilization treatments specified in the medical quality standards regarding handling and use of medical instruments and equipment.
From the point of view of TIG welding reconditioning possibilities, it is found that there are certain limitations of using the process caused by large dimensional deviations of the obtained surfaces, reduced accessibility to interior areas and the possibility of reconditioning products with complex surfaces. Another possible issue is related to ensuring inert protection in the reconditioning area, protection that influences the weldability of the material but also the properties of the reconditioned product.
4. Conclusions
The present study considered welding reconditioning of a titanium-alloy customized additive manufactured implant with several manufacturing defects. The personalized implants are made starting from a CAD model as a direct result from the imaging investigations of the areas of interest. Then the implant is fabricated using Additive Manufacturing process (in this case, DMLS). The analysis of the chemical composition values as well as the values of the mechanical properties of the samples obtained via DMLS additive manufacturing process, showed that such a manufacturing process can be successfully used to make customized surgical implants. The mechanical properties values of the DMLS samples are approximately equal to those specified by the manufacturer of the titanium powder used for sintering. On average the tensile strength was found to be 24.75% higher, while yield strength 22.7% higher than the values provided in the standard for surgical implants applications [49,50,51,52,53,54].
If at the end of the AM production process results an inappropriate implant (for example it does not correspond from a functional, geometric, or dimensional point of view), it is considered scrap. The implant cannot be melted and reused, so this study aimed to recover such implants by restoring their geometric and functional characteristics through specific welding reconditioning processes. Several stages are proposed for this reconditioning process. The process starts with depositing successive layers by welding, followed by post-processing using a CNC cutting machining, brushing, or polishing the surfaces to achieve the roughness required by the medical field, processing tapped holes for special screws on the bone structure, followed by specific cleaning decontamination and sterilization treatments. These modifications on the implants can be made to correct certain dimensional and shape deviations resulting from the additive manufacturing process, as well as to adapt according to the continuous evolution of the tumors in the implant area. The mechanical properties of obtained implant, with regards to the welded area, are similar to the original AM obtained implant and hence usable for its original purpose.
There are several limitations to the use of welding technologies for reconditioning of defective parts. If large dimensional deviations occurred, it is difficult, if not impossible, to recondition the part mainly since the large area required for adhesion between the filler material and the main body of the implant creates stress points and the occurrence of large areas that will suffer from heating and rapid cooling processes during welding. Further investigations are underway to solve this aspect by using additive manufactured micro and mini scaffolds to help the welded area maintain its original intended mechanical properties and to increase the adhesion of the filler material to the main body of the implant.
For this study we could not perform destructive testing on the implant, especially in the welded area. Therefore, further work is required to determine the changes in mechanical properties of the deposited layers of the welded materials (as this is affected by heat that can influence the interface between the AM part and the welded-on areas).
Author Contributions
Writing—original draft preparation, C.R., methodology, C.R., C.-G.A., A.-M.B. and D.-T.C., investigation F.D.A. and A.B., writing—review and editing, C.R., C.-G.A. and A.-M.B., visualization, D.-T.C., F.D.A. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded under the framework of the Erasmus+ KA2 Strategic partnership project 2021-1-CZ01-KA220-VET-000033007—“EuropeAn 3d printinG poLymer opErators” (EAGLE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Mierzejewska, Ż.A.; Hudák, R.; Sidun, J. Mechanical Properties and Microstructure of DMLS Ti6Al4V Alloy Dedicated to Biomedical Applications. Materials 2019, 12, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiel-Jamrozik, M.; Jamrozik, W.; Witkowska, I. The heat treatment influence on the structure and mechanical properties of Ti6Al4V alloy manufactured by SLM technology. Innov. Biomed. Eng. 2018, 623, 319–327. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, W.Y.; Zhou, Y.; Li, X.; Chen, D.L. Tensile and fatigue behavior of electron beam welded dissimilar joints of Ti-6Al-4V and IMI834 titanium alloys. Mater. Sci. Eng. A 2016, 649, 146–152. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, J.; Guo, S.; Tan, H.; Lin, X.; Huang, W. Influence of alpha/beta interface phase on the tensile properties of laser cladding deposited Ti-6Al-4V titanium alloy. J. Mater. Sci. Technol. 2017, 33, 675–681. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Spataru, M.C.; Cojocaru, F.D.; Sandu, A.V.; Solcan, C.; Duceac, I.A.; Baltatu, M.S.; Voiculescu, I.; Geanta, V. Assessment of the Effects of Si Addition to a New TiMoZrTa System. Materials 2021, 14, 7610. [Google Scholar] [CrossRef] [PubMed]
- Verestiuc, L.; Spataru, M.C.; Baltatu, M.S.; Butnaru, M.; Solcan, C.; Sandu, A.V.; Voiculescu, I.; Geanta, V.; Vizureanu, P. New Ti–Mo–Si materials for bone prosthesis applications. J. Mech. Behav. Biomed. Mater. 2021, 113, 104198. [Google Scholar] [CrossRef]
- Davis, J.R. Overview of Biomaterials and Their Use in Medical Devices. In Handbook of Materials for Medical Devices; Davis & Associates, ASM International: Materials Park, OH, USA, 2003; pp. 1–9. Available online: https://www.asminternational.org/documents/10192/1849770/06974G_Chapter_1.pdf (accessed on 8 February 2022).
- Pandey, E.; Srivastava, K.; Gupta, S.; Srivastava, S.; Mishra, N. Some Biocompatible Materials Used In Medical Practices-A Review. Int. J. Pharm. Sci. Res. 2016, 7, 2748–2755. [Google Scholar] [CrossRef]
- Klimas, J.; Lukaszewicz, A.; Szota, M.; Nabiałek, M. Modification of the structure and properties of the titanium alloy Ti6Al4V in biomedical applications. Arch. Metall. Mater. 2015, 60, 2013–2018. [Google Scholar] [CrossRef]
- Shah, F.A.; Snis, A.; Matic, A.; Thomsen, P.; Palmquist, A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016, 30, 357–367. [Google Scholar] [CrossRef]
- Shunyu, L.; Shin, C.Y. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2000; pp. 1–24. ISBN 978-0-87170-686-7. [Google Scholar]
- Cui, C.; Hu, B.; Zhao, L.; Liu, S. Titanium alloy production technology, market prospects and industry development. Mater. Des. 2011, 32, 1684–1691. [Google Scholar] [CrossRef]
- Inagaki, I.; Takechi, T.; Shira, Y.; Ariyasu, N. Application and features of titanium for the aerospace industry. Nippon. Steel Sumitomo Met. Tech. Rep. 2014, 106, 22–27. [Google Scholar]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M.P. Corrosion of titanium: Part 2: Effects of surface treatments. J. Appl. Biomater. Funct. Mater. 2018, 16, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.Y.; Li, T.H.; Yeh, T.K. Corrosion behavior of TiO2-treated type 304 stainless steels in high temperature water containing with hydrogen peroxide. J. Nucl. Sci. Technol. 2016, 53, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M. Buture challenges in the in vitro and in vivo evaluation of biomaterial biocompatibility. Regen. Biomater. 2016, 3, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Brooks, P.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [Green Version]
- So, S.; Harris, I.A.; Naylor, J.M.; Adie, S.; Mittal, R. Correlation between metal allergy and treatment outcomes after ankle fracture fixation. J. Orthop. Surg. 2011, 19, 309–313. [Google Scholar] [CrossRef]
- Willemsen, K.; Nizak, R.; Noordmans, H.J.; Castelein, R.M.; Weinans, H.; Kruyt, M.C. Challenges in the design and regulatory approval of 3D-printed surgical implants: A two-case series. Lancet Digit. Health 2019, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Popov, V.V.; Kudryavtseva, E.V.; Kumar Katiyar, N.; Shishkin, A.; Stepanov, S.I.; Goel, S. Industry 4.0 and Digitalisation in Healthcare. Materials 2022, 15, 2140. [Google Scholar] [CrossRef]
- Bucking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12, e0178540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- All3DP.com. Available online: https://all3dp.com/2/direct-metal-laser-sintering-dmls-simply-explained/ (accessed on 7 February 2022).
- EOS.info. Available online: https://www.eos.info/en/industrial-3d-printing (accessed on 7 February 2022).
- Băilă, D.; Vițelaru, C.; Trușcă, R.; Constantin, L.R.; Păcurar, A.; Parau, C.A.; Păcurar, R. Thin Films Deposition of Ta2O5 and ZnO by E-Gun Technology on Co-Cr Alloy Manufactured by Direct Metal Laser Sintering. Materials 2021, 14, 3666. [Google Scholar] [CrossRef] [PubMed]
- Băilă, D. Experimental Researches of Co-Cr alloys powders manufactured by sintering process DMLS and Ni-Cr alloys used in Dentistry. Adv. Mater. Res. 2015, 1119, 433–437. [Google Scholar] [CrossRef]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Söderholm, P.; Ekvall, T. Metal markets and recycling policies: Impacts and challenges. Miner. Econ. 2020, 33, 257–272. [Google Scholar] [CrossRef] [Green Version]
- Belenyi, A.; Achimas, G. Studies and investigations on the possibilities of reconditioning an eccentric shaft. Acta Tech. Napoc. Ser. Appl. Math. Mech. Eng. 2018, 61, 241–246. [Google Scholar]
- Caltaru, M.; Badicioiu, M.; Ripeanu, R.G.; Dinita, A.; Minescu, M.; Laudacescu, E. Tribological characterization of the drill pipe tool joints reconditioned by using welding technologies. IOP Conf. Ser. Mater. Sci. Eng. 2018, 295, 1–10. [Google Scholar] [CrossRef]
- Nafikov, M.Z.; Aipov, R.S.; Konnov, A.Y. Powder Metallurgy Reconditioning of Food and Processing Equipment Components. Russ. Metall. 2017, 2017, 1057–1062. [Google Scholar] [CrossRef]
- Sheikhi, S.; Mayer, E.; Maaß, J.; Wagner, F. Automated Reconditioning of Thin Wall Structures Using Robot-Based Laser Powder Coating. Sustainability 2020, 12, 1477. [Google Scholar] [CrossRef] [Green Version]
- Dobrotă, D.; Petrescu, V. Use of Ultrasound in Reconditioning by Welding of Tools Used in the Process of Regenerating Rubber. Materials 2018, 11, 276. [Google Scholar] [CrossRef] [Green Version]
- Konovodov, V.V.; Valentov, A.V.; Grigoryeva, E.G.; Abdrasulov, K.A. Analysis of the Retailoring Methods and the Workability of Deposited Surfaces. IOP Conf. Ser. Mater. Sci. Eng. 2016, 125, 012035. [Google Scholar] [CrossRef] [Green Version]
- Lyalyakin, V.P.; Murzaev, V.P.; Slinko, D.B.; Kudryashova, E.Y. Advancement of the Process of Cold Welding of Cast Iron. Met. Sci. Heat Treat. 2014, 56, 420–423. [Google Scholar] [CrossRef]
- Machedon Pisu, T.; Vas, A.; Magyari, M.; Iordache, A. Research on cladding (CMT MIG, WIG arc mechanized pulse) for molds used for casting. Metal. Int. 2013, 18, 89–94. [Google Scholar]
- Candea, V.N.; Iovanas, R.; Ploscariu, C.; Binchiciu, H. Research regarding the elaboration of a martensitic stainless-steel electrode for reconditioning the rotors of power water units. Metal. Int. 2009, 14, 175–178. [Google Scholar]
- Stanila, S.D. Research on the Reconditioning of Conveyor Troughs by Using Non-Ferrous Alloys. In Proceedings of the 1st WSEAS International Conference on Materials Science, Bucharest, Romania, 7–9 November 2008; pp. 74–77. [Google Scholar]
- Petrik, I.A.; Kovalenko, T.A.; Ovchinnikov, A.V. Reconditioning rotor components of gas turbine engines produced from titanium alloys by welding using modified submicrocrystalline filler materials. Weld. Int. 2016, 30, 123–128. [Google Scholar]
- Paton, B.E.; Akhonin, S.V.; Prilutsky, V.P. Development of welding technologies in titanium component manufacturing. In Proceedings of the 12th World Conference on Titanium, Beijing, China, 19–24 June 2011; Volume 2, pp. 1585–1591. [Google Scholar]
- Graf, B.; Gumenyuk, A.; Rethmeier, M. Laser Metal Deposition as Repair Technology for Stainless Steel and Titanium Alloys. Phys. Procedia 2012, 39, 376–381. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Zheng, H.; Tang, K.; Li, H.; Gong, S. TC17 titanium alloy laser melting deposition repair process and properties. Opt. Laser Technol. 2016, 82, 1–9. [Google Scholar] [CrossRef]
- Yu, J.-H.; Choi, Y.-S.; Shim, D.-S.; Park, S.-H. Repairing casting part using laser assisted additive metal-layer deposition and its mechanical properties. Opt. Laser Technol. 2018, 106, 87–93. [Google Scholar] [CrossRef]
- Onuike, B.; Bandyopadhyay, A. Additive manufacturing in repair: Influence of processing parameters on properties of Inconel 718. Mater. Lett. 2019, 252, 256–259. [Google Scholar] [CrossRef]
- Wasono, R.S.; Wahab, D.A.; Azman, A.H. Additive Manufacturing for Repair and Restoration in Remanufacturing: An Overview from Object Design and Systems Perspectives. Processes 2019, 7, 802. [Google Scholar]
- ASTM F1472; Standard Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications. ASTM International: Washington, DC, USA, 2020.
- ASTM E8/E8M-21; Standard Test Methods For Tension Testing Of Metallic Materials. ASTM International: Washington, DC, USA, 2021.
- Cicic, D.T.; Rontescu, C.; Amza, C.G.; Chivu, O.R. The Combined Effect of Chemical Elements on the Properties of a Layer Deposited by Welding. Rev. Chim. 2015, 66, 1299–1301. [Google Scholar]
- ASTM B348; Standard Specification for Titanium and Titanium Alloy Bars and Billets. ASTM International: Washington, DC, USA, 2021.
- ISO 5832-3; Implants for surgery—Metallic materials—Wrought titanium 6-aluminum 4-vanadium alloy. International Organization for Standardization: Geneva, Switzerland, 2021.
- Gatto, M.L.; Groppo, R.; Bloise, N.; Fassina, L.; Visai, L.; Galati, M.; Iuliano, L.; Mengucci, P. Topological, Mechanical and Biological Properties of Ti6Al4V Scaffolds for Bone Tissue Regeneration Fabricated with Reused Powders via Electron Beam Melting. Materials 2021, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Dolev, O.; Osovski, S.; Shirizly, A. Ti-6Al-4V hybrid structure mechanical properties—Wrought and additive manufactured powder-bed material. Addit. Manuf. 2021, 37, 101657. [Google Scholar] [CrossRef]
- EOS Titanium Ti64 Material Data Sheet. EOS.net. Available online: http://www.eos.info (accessed on 8 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).