Electrochemical Analysis of the Influence of Purines on Copper, Steel and Some Other Metals Corrosion
Abstract
1. Introduction
2. Discussion
2.1. Copper and Alloys
2.1.1. Chloride Media
2.1.2. Sulfate and Other Media
2.1.3. Physiological Media
2.2. Steel and Iron
2.2.1. Chloride Media
2.2.2. Sulfate and Other Media
| Inhibitor | Concentration | Medium | IE % | Reference |
|---|---|---|---|---|
| Caffeine Theophylline | 200 ppm | 3 wt % NaCl | 57.4 EIS/77.4 PP 39.9 EIS/65.3 PP | [14] |
| Caffeine + Paraloid B-72 Theophylline + Paraloid B-72 | 200 mg m−2 | 86.4 EIS 99.7 EIS | ||
| Hybrid sol-gel + caffeine | 100 ppm | 3.5 wt % NaCl | 84.22 EIS | [66] |
| 88.79 PP | ||||
| Caffeine | 50 pm | 3 wt % NaCl | 82.7 EIS | [67] |
| 3 wt % NaCl + CO2 low carbon steel | 94.7 EIS | |||
| Theobromine | 1.0 g dm−3 | 1 M HCl mild steel | 90.0 WL,PP,EIS (308 K) | [68] |
| Adenine | 1 × 10−3 M | 200 ppm NaCl low carbon steel | 82 PP/83 EIS (305 K); 95 EIS (315 K); 88 EIS (325 K) | [64] |
| Guanine | 91 PP/93 EIS (305 K) | |||
| Guanine Adenine 2,6-dithiopurine 2,6-diaminopurine 6-thioguanine | 1 × 10−3 M | 1 M HCl mild steel | 68.3 PP/67.8 EIS 69.6 PP/69.9 EIS 85.9 PP/85.4 EIS 73.5 PP/70.9 EIS 77.9 PP/78.4 EIS | [57] |
| Comp 3 | 50 ppm | 1 M HCl steel API 5L X52 | 91.7 EIS/86.9 PP | [70] |
| Comp 4 | 86.9 EIS/87.0 PP | |||
| Comp 5 | 94.0 EIS/71.0 PP | |||
| Comp 6 | 91.8 EIS/93.5 PP | |||
| Comp 7 | 90.9 EIS/88.2 PP | |||
| Caffeine | 20 ppm | 1 M HCl steel API 5L X70 | 96.4 EIS | [69] |
| Theophylline | 88.6 EIS | |||
| Propargyl-theophylline | 91.0 EIS | |||
| Allyl-theophylline | 74.6 EIS | |||
| Caffeine | 50 ppm | 95.4 EIS | ||
| Theophylline | 90.6 EIS | |||
| Propargyl-theophylline | 90.0 EIS | |||
| Allyl-theophylline | 79.1 EIS | |||
| PVPO-TiO2-Adenine | 800 ppm | 1 M H2SO4 carbon steel 303 K | 83.50 WL/87.53 PP/92.18 EIS | [73] |
| 7-(ethylthiobenzimidazolyl) theophylline (7-ETBT) | 2 × 10−3 M | 1 M HCl mild steel | 90.73 WL/87.06 PP | [74] |
| FAP N-BAP | 0.8 × 10−3 M | 1 M HCl mild steel | 93.5 PP 97.7 PP | [13] |
| Adenine | 10 × 10−3 M | 1.1 M Cl- (NaCl, HCl) pH 1.5 25 °C 304 austenic stainless steel | 85 PP | [60] |
| Tenvir | 400 ppm | 1 M HCl mild steel | 96.05 WL/94.0 EIS/94.27 PP | [62] |
| Adenine Guanine Adenosine Guanosine | 25 ppm | 1 M HCl steel API 5L X52 | 91 EIS 88 EIS 94 EIS 93 EIS | [58] |
| Adenine KI | 5 × 10−2 M | 4 M H2SO4 low carbon steel | 83 EIS 75 EIS | [71] |
| Adenine + KI | 5 × 10−2 M + 1 × 10−3 M | 88 EIS |
2.3. Other Metals
2.3.1. Aluminum
2.3.2. Magnesium
2.3.3. Titanium, Tin, Indium, Nickel, and Alloys: CoCrMo and Sn-Ag Alloy
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tasić, Ž.Z.; Petrović Mihajlović, M.B.; Radovanović, M.B.; Antonijević, M.M. New trends in corrosion protection of copper. Chem. Pap. 2019, 73, 2103–2132. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Radovanović, M.B. Methods for Characterization of Protective Films on the Copper Surface—A Review. Zaštita Mater. Mater. Prot. 2010, 2, 111–122. [Google Scholar]
- Shahid, M. Corrosion protection with eco-friendly inhibitors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 043001. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Petrović, M.B. Copper Corrosion Inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Petrović Mihajlović, M.B.; Antonijević, M.M. Copper Corrosion Inhibitors. Period 2008-2014. A Review. Int. J. Electrochem. Sci. 2015, 10, 1027–1053. [Google Scholar]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic biomolecules as green corrosion inhibitors. J. Mol. Liq. 2021, 341, 117265. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Alkaloids as green and environmental benign corrosion inhibitors: An overview. Int. J. Corros. Scale Inhib. 2019, 8, 512–528. [Google Scholar] [CrossRef]
- De Clercq, E. The acyclic nucleoside phosphonates from inception to clinical use: Historical perspective. Antivir. Res. 2007, 75, 1–13. [Google Scholar] [CrossRef]
- Domínguez-Martín, A.; del Pilar Brandi-Blanco, M.; Matilla-Hernández, A.; El Bakkali, H.; Nurchi, V.M.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. Unravelling the versatile metal binding modes of adenine: Looking at the molecular recognition patterns of deaza- and aza-adenines in mixed ligand metal complexes. Coordin. Chem. Rev. 2013, 257, 2814–2838. [Google Scholar] [CrossRef]
- Choquesillo-Lazarte, D.; del Pilar Brandi-Blanco, M.; García-Santos, I.; María González-Pérez, J.; Castiñeiras, A.; Niclós-Gutiérrez, J. Interligand interactions involved in the molecular recognition between copper(II) complexes and adenine or related purines. Coordin. Chem. Rev. 2008, 252, 1241–1256. [Google Scholar] [CrossRef]
- Vasseghi, S.; Ken, N. Effect of substituted purines on the corrosion behavior of iron. Corrosion 1979, 35, 300–303. [Google Scholar] [CrossRef]
- Abdel Aal, M.S.; Wahdan, M.H. Effect of c-content and microstructure of steel on the inhibiting action of purine and benzo(f)quinoline in HClO4 solution. In Proceedings of the 6th European Symposium on Corrosion Inhibitors, 133rd Manifestation of the European Federation of Corrosion, Ferrara, Italy, 16–20 September 1985; pp. 345–357. [Google Scholar]
- Jiang, Z.; Li, Y.; Zhang, Q.; Hou, B.; Xiong, W.; Liu, H.; Zhang, G. Purine derivatives as high efficient eco-friendly inhibitors for the corrosion of mild steel in acidic medium: Experimental and theoretical calculations. J. Mol. Liq. 2021, 323, 114809. [Google Scholar] [CrossRef]
- Espinoza Vázquez, A.; Figueroa, A.; Sánchez Molina, D.; Rodríguez-Gómeza, F.J.; Angeles Beltrán, D. Effects of corrosion inhibition with xanthines in gray cast iron protection with Paraloid B-72 in a saline medium. Prog. Org. Coat. 2021, 154, 106200. [Google Scholar] [CrossRef]
- Ismail, M.N.; Megahed, H.E.; Asmaa Ali, I.; El-Etre, M.A. Application of Theophylline Anhydrous as Inhibitor for Acid Corrosion of Aluminum. Egypt. J. Chem. 2017, 60, 95–107. [Google Scholar] [CrossRef][Green Version]
- Eddy, N.O.; Momoh-Yahaya, H.; Oguzie, E.E. Theoretical and experimental studies on the corrosion inhibition potentials of some purines for aluminum in 0.1M HCl. J. Adv. Res. 2015, 6, 203–217. [Google Scholar] [CrossRef]
- El-Sayed, A.-R.; Shaker, A.M.; Abd El-Lateef, H.M. Corrosion inhibition of tin, indium and tin–indium alloys by adenine or adenosine in hydrochloric acid solution. Corros. Sci. 2010, 52, 72–81. [Google Scholar] [CrossRef]
- El-Sayed, A.R.; Mohran, H.S.; Abd El-Lateef, H.M. Effect of some nitrogen-heterocyclic compounds on corrosion of tin, indium, and their alloys in HClO4. Monatsh. Chem. 2012, 143, 51–64. [Google Scholar] [CrossRef]
- Radovanović, M.B.; Tasić, Ž.Z.; Simonović, A.T.; Petrović Mihajlović, M.B.; Antonijević, M.M. Corrosion Behavior of Titanium in Simulated Body Solutions with the Addition of Biomolecules. ACS Omega 2020, 5, 12768–12776. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Putri, R.A.K.; Sunghun, B.; Ko, Y.G. Molecular structures in the inorganic-metal interactions for optimizing electrochemical performance. J. Mol. Liq. 2021, 326, 115344. [Google Scholar] [CrossRef]
- Park, J.; Park, M.; Seo, H.; Han, H.-S.; Lee, J.-Y.; Koo, D.; Kim, K.; Cha, P.-R.; Edwards, J.; Kim, Y.-W.; et al. A new corrosion-inhibiting strategy for biodegradable magnesium: Reduced nicotinamide adenine dinucleotide (NADH). Sci. Rep. 2018, 8, 17743. [Google Scholar] [CrossRef]
- Scendo, M. The effect of purine on the corrosion of copper in chloride solutions. Corros. Sci. 2007, 49, 373–390. [Google Scholar] [CrossRef]
- Scendo, M. The influence of adenine on corrosion of copper in chloride solutions. Corros. Sci. 2008, 50, 2070–2077. [Google Scholar] [CrossRef]
- Zeng, N.; Zhao, H.; Luo, C.; Liu, Y.; Wang, C.; Ma, T.; Wang, W. Roles and mechanistic analysis of adenine as a green inhibitor in chemical mechanical polishing. J. Appl. Electrochem. 2021, 51, 1479–1489. [Google Scholar] [CrossRef]
- Petrović Mihajlović, M.B.; Radovanović, M.B.; Tasić, Ž.Z.; Antonijević, M.M. Imidazole based compounds as copper corrosion inhibitors in seawater. J. Mol. Liq. 2017, 225, 127–136. [Google Scholar] [CrossRef]
- Radovanović, M.B.; Simonović, A.T.; Petrović, M.B.; Milić, S.M.; Antonijević, M.M. Influence of Purine on Brass Behavior in Neutral and Alkaline Sulphate Solutions. Int. J. Electrochem. Sci. 2012, 7, 11796–11810. [Google Scholar]
- Ebadi, M.; Basirun, W.J.; Leng, S.Y.; Mahmoudian, M.R. Investigation of Corrosion Inhibition Properties of Caffeine on Nickel by Electrochemical Techniques. Int. J. Electrochem. Sci. 2012, 7, 8052–8063. [Google Scholar]
- El-Taib Heakal, F.; Fekry, A.M. Experimental and Theoretical Study of Uracil and Adenine Inhibitors in Sn-Ag Alloy/Nitric Acid Corroding System. J. Electrochem. Soc. 2008, 155, C534–C542. [Google Scholar] [CrossRef]
- Romas, M.; Munoz, A.; Mareci, D.; Vidal, C.; Curteanu, S.; Sutiman, D. Influence of caffeine and temperature on corrosion-resistance of CoCrMo alloy. Chem. Pap. 2014, 68, 1066–1078. [Google Scholar] [CrossRef]
- Levin, M.; Wiklund, P.; Leygraf, C. Bioorganic compounds as copper corrosion inhibitors in hydrocarbon media. Corros. Sci. 2012, 58, 104–114. [Google Scholar] [CrossRef]
- Alvarez, F.; Schilardi, P.L.; De Mele, M.F.L. Reduction of the “burst release” of copper ions from copper-based intrauterine devices by organic inhibitors. Contraception 2012, 85, 91–98. [Google Scholar] [CrossRef]
- Alvarez, F.; Grillo, C.; Schilardi, P.; Rubert, A.; Benítez, G.; Lorente, C.; De Mele, M.F.L. Decrease in cytotoxicity of copper-based intrauterine devices (IUD) pretreated with 6-mercaptopurine and pterin as biocompatible corrosion inhibitors. ACS Appl. Mater. Interfaces. 2013, 5, 249–255. [Google Scholar] [CrossRef]
- Alonso, C.; Casero, E.; Román, E.; Campos, S.F.P.; De Mele, M.F.L. Effective inhibition of the early copper ion burst release by purine adsorption in simulated uterine fluids. Electrochim. Acta 2016, 189, 54–63. [Google Scholar] [CrossRef]
- Petrović Mihajlović, M.B.; Radovanović, M.B.; Simonović, A.T.; Tasić, Ž.Z.; Antonijević, M.M. Evaluation of purine based compounds as the inhibitors of copper corrosion in simulated body fluid. Results Phys. 2019, 14, 102357. [Google Scholar] [CrossRef]
- Scendo, M. Inhibitive action of the purine and adenine for copper corrosion in sulphate solutions. Corros. Sci. 2007, 49, 2985–3000. [Google Scholar] [CrossRef]
- Scendo, M. Corrosion inhibition of copper by purine or adenine in sulphate solutions. Corros. Sci. 2007, 49, 3953–3968. [Google Scholar] [CrossRef]
- Scendo, M. Inhibition of copper corrosion in sodium nitrate solutions with nontoxic inhibitors. Corros. Sci. 2008, 50, 1584–1592. [Google Scholar] [CrossRef]
- Petrović, M.B.; Simonović, A.T.; Radovanović, M.B.; Milić, S.M.; Antonijević, M.M. Influence of purine on copper behavior in neutral and alkaline sulfate solutions. Chem. Pap. 2012, 66, 664–676. [Google Scholar] [CrossRef]
- Guo, X.; Huang, H.; Liu, D. The inhibition mechanism and adsorption behavior of three purine derivatives on the corrosion of copper in alkaline artificial seawater: Structure and performance. Colloids Surf. A 2021, 622, 126644. [Google Scholar] [CrossRef]
- Gudić, S.; Oguzie, E.E.; Radonić, A.; Vrsalović, L.; Smoljko, I.; Kliškić, M. Inhibition of copper corrosion in chloride solution by caffeine isolated from black tea. Maced. J. Chem. Chem. Eng. 2014, 33, 13–25. [Google Scholar] [CrossRef]
- de Souza, F.S.; Giacomelli, C.; Gonçalves, R.S.; Spinelli, A. Adsorption behavior of caffeine as a green corrosion inhibitor for copper. Mater. Sci. Eng. C 2012, 32, 2436–2444. [Google Scholar] [CrossRef]
- Silva, E.F.; Wysard, J.S.; Bandeira, M.C.E.; Mattos, O.R. Electrochemical and surface enhanced Raman spectroscopy study of Guanine as corrosion inhibitor for copper. Corros. Sci. 2021, 191, 109714. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS Investigation of the Corrosion Behavior of Steel Bars Embedded into Modified Concretes with Eggshell Contents. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Duarte, T.; Meyer, Y.A.; Osório, W.R. The Holes of Zn Phosphate and Hot Dip Galvanizing on Electrochemical Behaviors of Multi-Coatings on Steel Substrates. Metals 2022, 12, 863. [Google Scholar] [CrossRef]
- Verissimo, N.C.; Freitas, E.S.; Cheung, N.; Garcia, A.; Osório, W.R. The effects of Zn segregation and microstructure length scale on the corrosion behavior of a directionally solidified Mg-25 wt.%Zn alloy. J. Alloys Compd. 2017, 723, 649–660. [Google Scholar] [CrossRef]
- Vida, T.A.; Freitas, E.S.; Cheung, N.; Garcia, A.; Osório, W.R. Electrochemical Corrosion Behavior of as-cast Zn-rich Zn-Mg Alloys in a 0.06M NaCl Solution. Int. J. Electrochem. Sci. 2017, 12, 5264–5283. [Google Scholar] [CrossRef]
- Zhao, L.; Li, K.; Yao, J.; Yuan, Y.; Du, B. The Laser Deposited Nickel-Aluminum Bronze Coatings on SUS630 Stainless Steel and Its Corrosion Resistance in 3.5 wt.% NaCl Solution. Metals 2022, 12, 781. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Imidazole derivatives as corrosion inhibitors for copper: A DFT and reactive force field study. Corros. Sci. 2020, 171, 108724. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Zhang, Y.; Wang, P.; Zhang, J. Theoretical evaluation of inhibition performance of purine corrosion inhibitors. Mol. Simul. 2013, 39, 1034–1041. [Google Scholar] [CrossRef]
- Hadisaputra, S.; Purwoko, A.A.; Savalas, L.R.T.; Prasetyo, N.; Yuanita, E.; Hamdiani, S. Quantum Chemical and Monte Carlo Simulation Studies on Inhibition Performance of Caffeine and Its Derivatives against Corrosion of Copper. Coatings 2020, 10, 1086. [Google Scholar] [CrossRef]
- Ogunyemi, B.T.; Latona, D.F.; Adejoro, I.A. Molecular modeling and quantitative structure–property relationships (QSPRs) of purine derivatives as corrosion inhibitor in acid medium. Sci. Afr. 2020, 8, e00336. [Google Scholar] [CrossRef]
- Hara, M.; Watanabe, D.; Kimura, C.; Aoki, H.; Sugino, T. Suppression of Cu Oxidation Using Environmentally Friendly Inhibitors under Conditions of High Temperature and High Humidity for Cu/Low-k. Jpn. J. Appl. Phys. 2009, 48, 04C016. [Google Scholar] [CrossRef]
- Jakeria, M.R.; Fazal, M.A.; Haseeb, A.S.M.A. Effect of corrosion inhibitors on corrosiveness of palm biodiesel. Corros. Eng. Sci. Technol. 2015, 50, 56–62. [Google Scholar] [CrossRef]
- Ghoneim, A.A.; El-Kamel, R.S.; Fekry, A.M. Hydrogen evolution and quantum calculations for potassium sorbate as an efficient green inhibitor for biodegradable magnesium alloy staples used for sleeve gastrectomy surgery. Int. J. Hydrogen Energy 2020, 45, 24370–24382. [Google Scholar] [CrossRef]
- Tasić, Ž.Z.; Antonijević, M.M.; Petrović Mihajlović, M.B.; Radovanović, M.B. The influence of synergistic effects of 5-methyl-1H-benzotriazole and potassium sorbate as well as 5-methyl-1H-benzotriazole and gelatin on the copper corrosion in sulphuric acid solution. J. Mol. Liq. 2016, 219, 463–473. [Google Scholar] [CrossRef]
- Aladag, N.; Trnkova, L.; Kourilova, A.; Ozsoz, M.; Jelen, F. Voltammetric study of aminopurines on pencil graphite electrode in the presence of copper ions. Electroanalysis 2010, 22, 1675–1681. [Google Scholar] [CrossRef]
- Yan, Y.; Li, W.; Cai, L.; Hou, B. Electrochemical and quantum chemical study of purines as corrosion inhibitors for mild steel in 1 M HCl solution. Electrochim. Acta 2008, 53, 5953–5960. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, D.Y.; Negrón Silva, G.; Angeles Beltrán, D.; Palomar Pardavé, M.; Romero Romo, M.; Herrera Hernández, H. Adenine and guanine derivative bases of purines and their corresponding nucleosides as corrosion inhibitors in 1M hydrochloric acid. ECS Trans. 2011, 36, 179. [Google Scholar] [CrossRef]
- Scendo, M.; Trela, J.; Radek, N. Purine as an effective corrosion inhibitor for stainless steel in chloride acid solutions. Corros. Rev. 2012, 30, 33–45. [Google Scholar] [CrossRef]
- Scendo, M.; Trela, J. Adenine as an Effective Corrosion Inhibitor for Stainless Steel in Chloride Solution. Int. J. Electrochem. Sci. 2013, 8, 9201–9221. [Google Scholar]
- Hu, K.; Gu, H.; Zhuang, J.; Zeng, X.; Yan, L.; Jiang, Y.; Guo, Q.; Ding, J. Synthesis and anti-corrosion performance of Adenine-L-Alanine ramification. J. Adhes. Sci. Technol. 2016, 30, 851–865. [Google Scholar] [CrossRef]
- Shahi, G.; Verma, C.B.; Ebenso, E.E.; Quraishi, M.A. Thermodynamic and Electrochemical investigation of (9-[(R)2[[bis [[(isopropoxycarbonyl)oxy]methoxy] phosphinyl] methoxy] propyl] adenine fumarate) as Green Corrosion Inhibitor for Mild Steel in 1M HCl. Int. J. Electrochem. Sci. 2015, 10, 1102–1116. [Google Scholar]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E. Adsorption and Inhibition Behavior of Purine based Drugs on Mild Steel Corrosion in Hydrochloric Acid Solution: DFT Study. Anal. Bioanal. Electrochem. 2018, 10, 98–110. [Google Scholar]
- Kassou, O.; Galai, M.; Ballakhmima, R.A.; Dkhireche, N.; Rochdi, A.; Ebn Touhami, M.; Touir, R.; Zarrouk, A. Comparative study of low carbon steel corrosion inhibition in 200 ppm NaCl by amino acid compounds. J. Mater. Environ. Sci. 2015, 6, 1147–1155. [Google Scholar]
- Tüzün, B.; Kaya, C. Investigation of DNA–RNA Molecules for the Efficiency and Activity of Corrosion Inhibition by DFT and Molecular Docking. J. Bio- Tribo-Corros. 2018, 4, 69. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Hazwan Hussin, M. Susceptibility of hybrid sol-gel (TEOS-APTES) doped with caffeine as potent corrosion protective coatings for mild steel in 3.5 wt.% NaCl. Prog. Org. Coat. 2020, 140, 105478. [Google Scholar] [CrossRef]
- Espinoza-Vazquez, A.; Rodríguez-Gomez, F.J. Caffeine and nicotine in 3% NaCl solution with CO2 as corrosion inhibitors for low carbon steel. RSC Adv. 2016, 6, 70226. [Google Scholar] [CrossRef]
- Elmsellem, H.; Elyoussfi, A.; Steli, H.; Sebbar, N.K.; Essassi, E.M.; Dahmani, M.; El Ouadi, Y.; Aouniti, A.; El Mahi, B.; Hammouti, B. The theobromine (chocolate) as green inhibitor of mild steel corrosion in hydrochloric acid: Electrochemical and theoretical quantum studies. Der. Pharma Chem. 2016, 8, 248–256. [Google Scholar]
- Espinoza-Vázquez, A.; Rodríguez Gómez, F.J.; Martinez Cruz, I.K.; Negrón Silva, G.E.; Palomar-Pardavé, M. Determination of Inhibition Properties of Caffeine, Theophylline and Their Allylic and Propargylic Derivatives on API 5L X70 Steel Immerse in 1M HCl. ECS Trans. 2018, 84, 165. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Rodríguez-Gomez, F.J.; Martínez-Cruz, I.K.; Ángeles-Beltrán, D.; Negrón-Silva, G.E.; Palomar-Pardavé, M.; Romero, L.L.; Pérez-Martínez, D.; Navarrete-López, A.M. Adsorption and corrosion inhibition behaviour of new theophylline–triazole -based derivatives for steel in acidic medium. R. Soc. Open Sci. 2019, 6, 181738. [Google Scholar] [CrossRef]
- Abdel Rehim, S.S.; Hazzazi, O.A.; Amin, M.A.; Khaled, K.F. On the corrosion inhibition of low carbon steel in concentrated sulphuric acid solutions. Part I: Chemical and electrochemical (AC and DC) studies. Corros. Sci. 2008, 50, 2258–2271. [Google Scholar] [CrossRef]
- Bagga, M.K.; Gadi, R.; Yadav, O.S.; Kumar, R.; Chopra, R.; Singh, G. Investigation of phytochemical components and corrosion inhibition property of Ficus racemosa stem extract on mild steel in H2SO4 medium. J. Environ. Chem. Eng. 2016, 4, 4699–4707. [Google Scholar] [CrossRef]
- Saini, N.; Pahuja, P.; Lgaz, H.; Chung, I.-M.; Selwal, K.; Singhal, S.; Lata, S. PVP oxime-TiO2-adenine as a hybrid material: Decent synthesis and depiction with advanced theoretical measurements for anticorrosive behavior and antibacterial potentiality. J. Mol. Liq. 2019, 278, 438–451. [Google Scholar] [CrossRef]
- Diki, N.Y.S.; Coulibaly, N.H.; Kassi, K.F.; Trokourey, A. Mild steel corrosion inhibition by 7-(ethylthiobenzimidazolyl) theophylline. J. Electrochem. Sci. Eng. 2021, 11, 97–106. [Google Scholar] [CrossRef]
- Su, P.; Li, L.; Li, W.; Huang, C.; Wang, X.; Liu, H.; Singh, A. Expired Drug Theophylline as Potential Corrosion Inhibitor for 7075 Aluminium Alloy in 1M NaOH Solution. Int. J. Electrochem. Sci. 2020, 15, 1412–1425. [Google Scholar] [CrossRef]
- Amin, M.A.; Mohsen, Q.; Hazzazi, O.A. Synergistic effect of I− ions on the corrosion inhibition of Al in 1.0M phosphoric acid solutions by purine. Mater. Chem. Phys. 2009, 114, 908–914. [Google Scholar] [CrossRef]
- Jakovljević, S.; Alar, V.; Ivanković, A. Electrochemical Behaviour of PACVD TiN-Coated CoCrMo Medical Alloy. Metals 2017, 7, 231. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, M.A.L.; Laverde-Cataño, D.A.; Lozano, D.; Martinez-Cazares, G.; Bedolla-Gil, Y. Influence of Boron Addition on the Microstructure and the Corrosion Resistance of CoCrMo Alloy. Metals 2019, 9, 307. [Google Scholar] [CrossRef]
- Heimann, R.B. Osseoconductive and Corrosion-Inhibiting Plasma-Sprayed Calcium Phosphate Coatings for Metallic Medical Implants. Metals 2017, 7, 468. [Google Scholar] [CrossRef]
- Mirea, R.; Biris, I.M.; Ceatra, L.C.; Ene, R.; Paraschiv, A.; Cucuruz, A.T.; Sbarcea, G.; Popescu, E.; Badea, T. In Vitro Physical-Chemical Behaviour Assessment of 3D-Printed CoCrMo Alloy for Orthopaedic Implants. Metals 2021, 11, 857. [Google Scholar] [CrossRef]
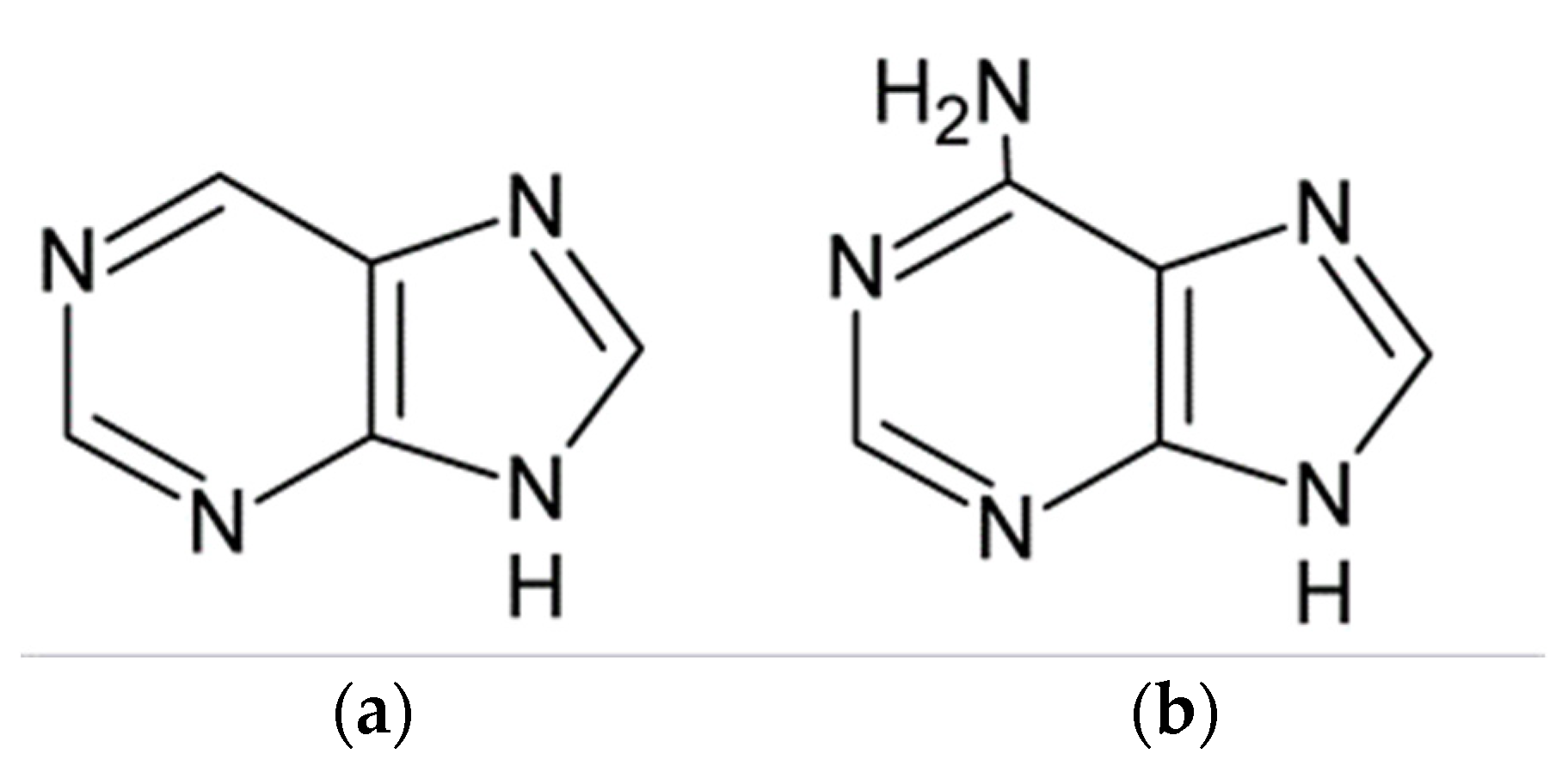
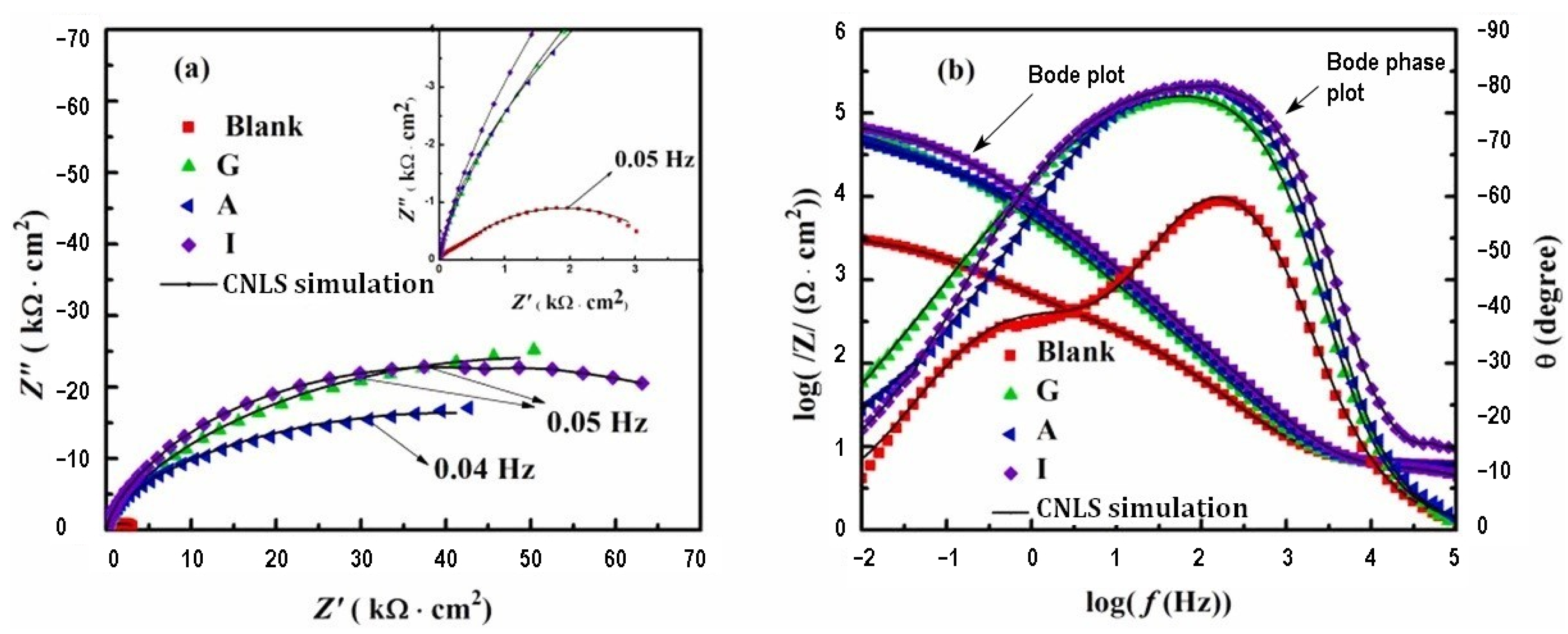
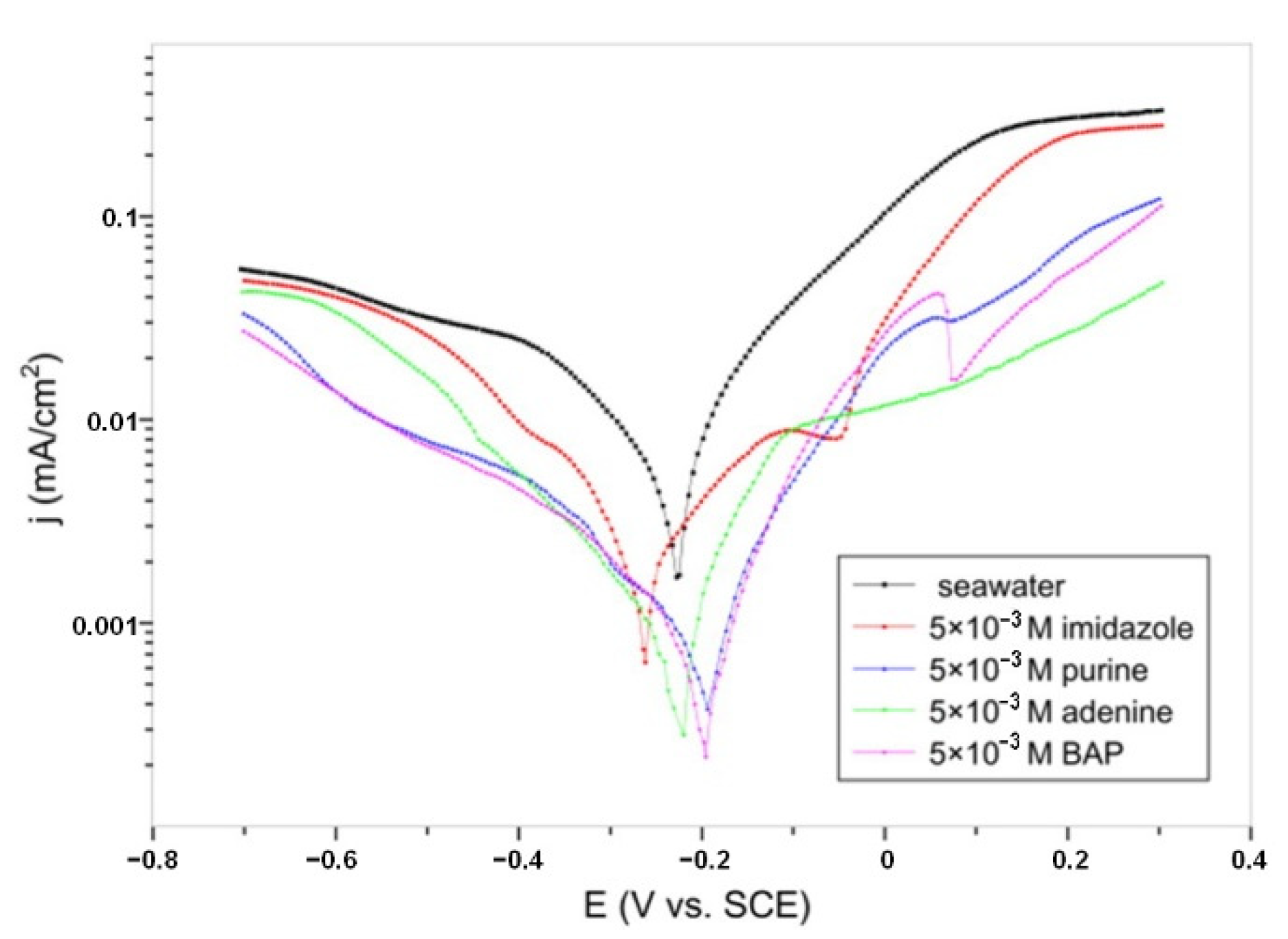
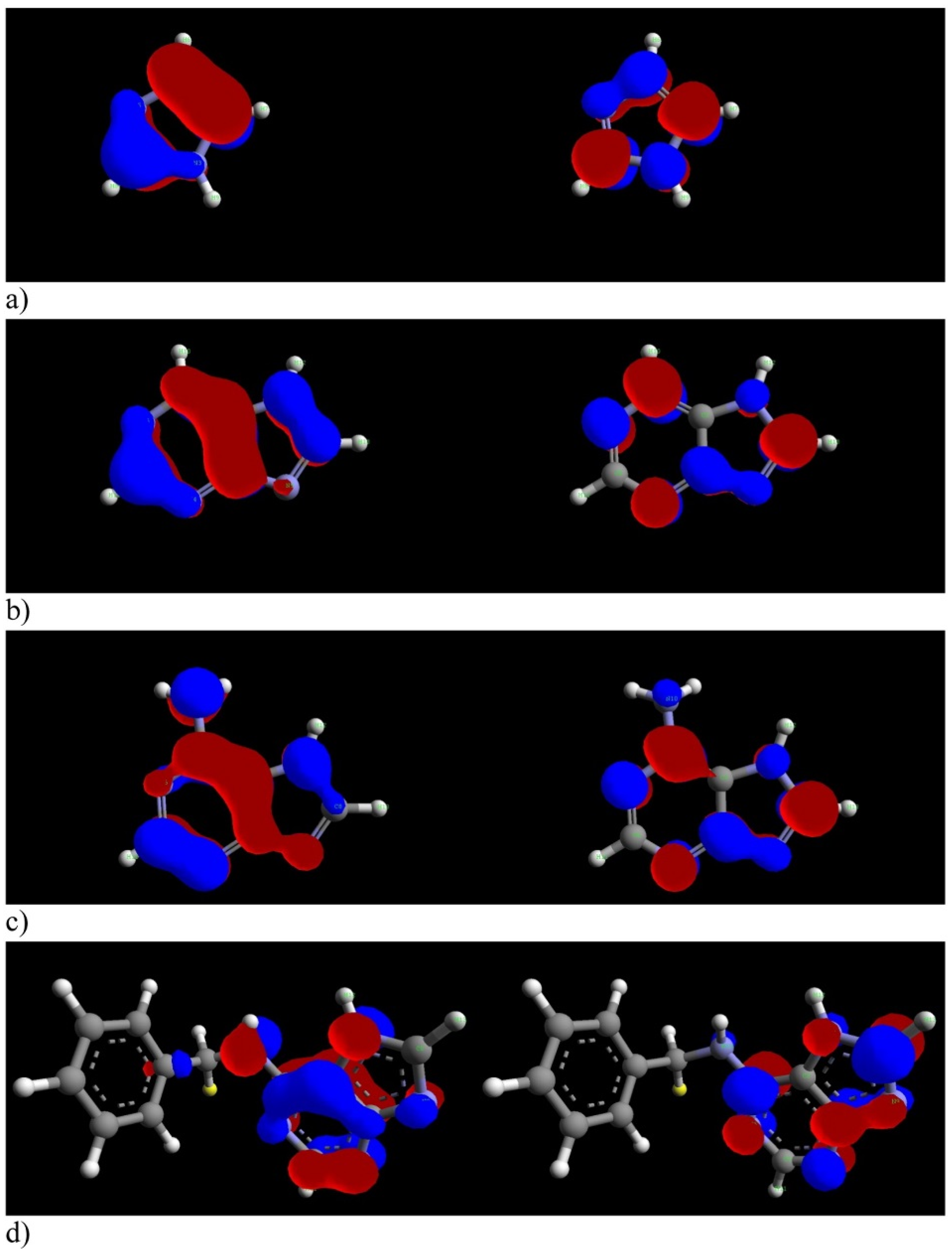

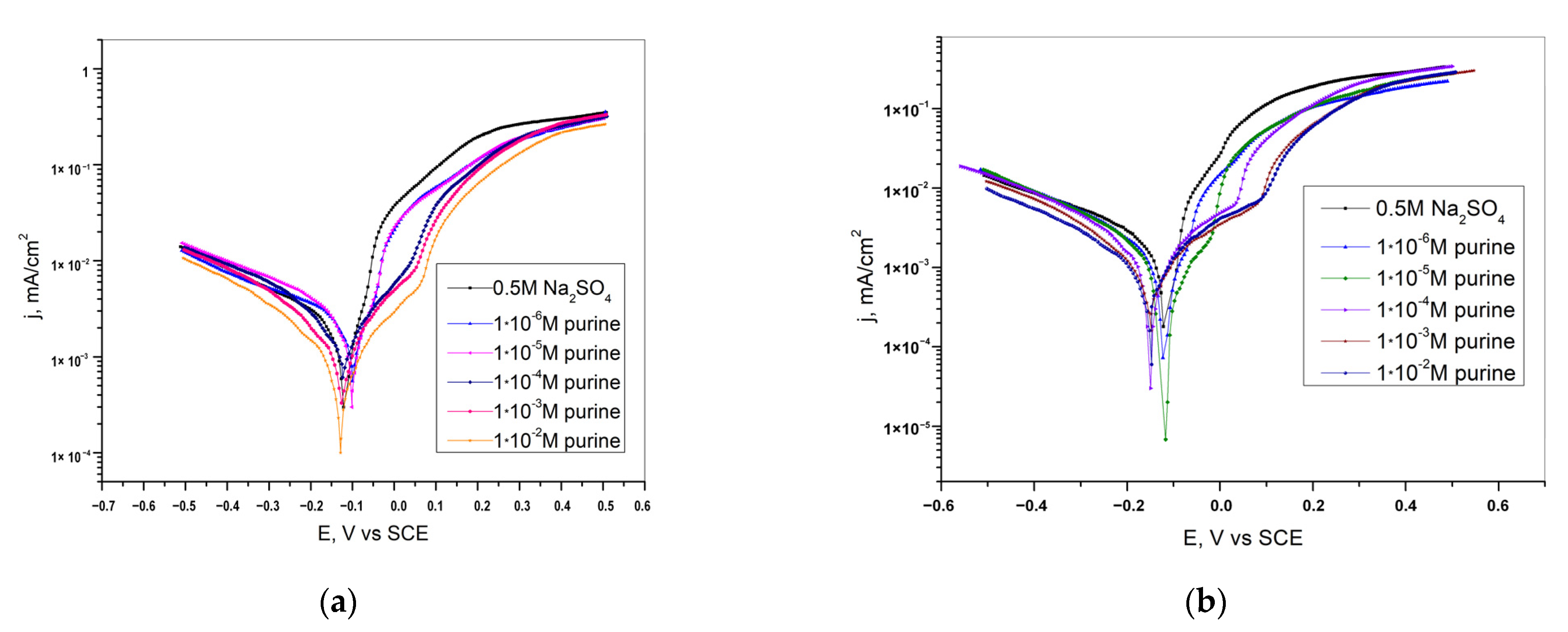


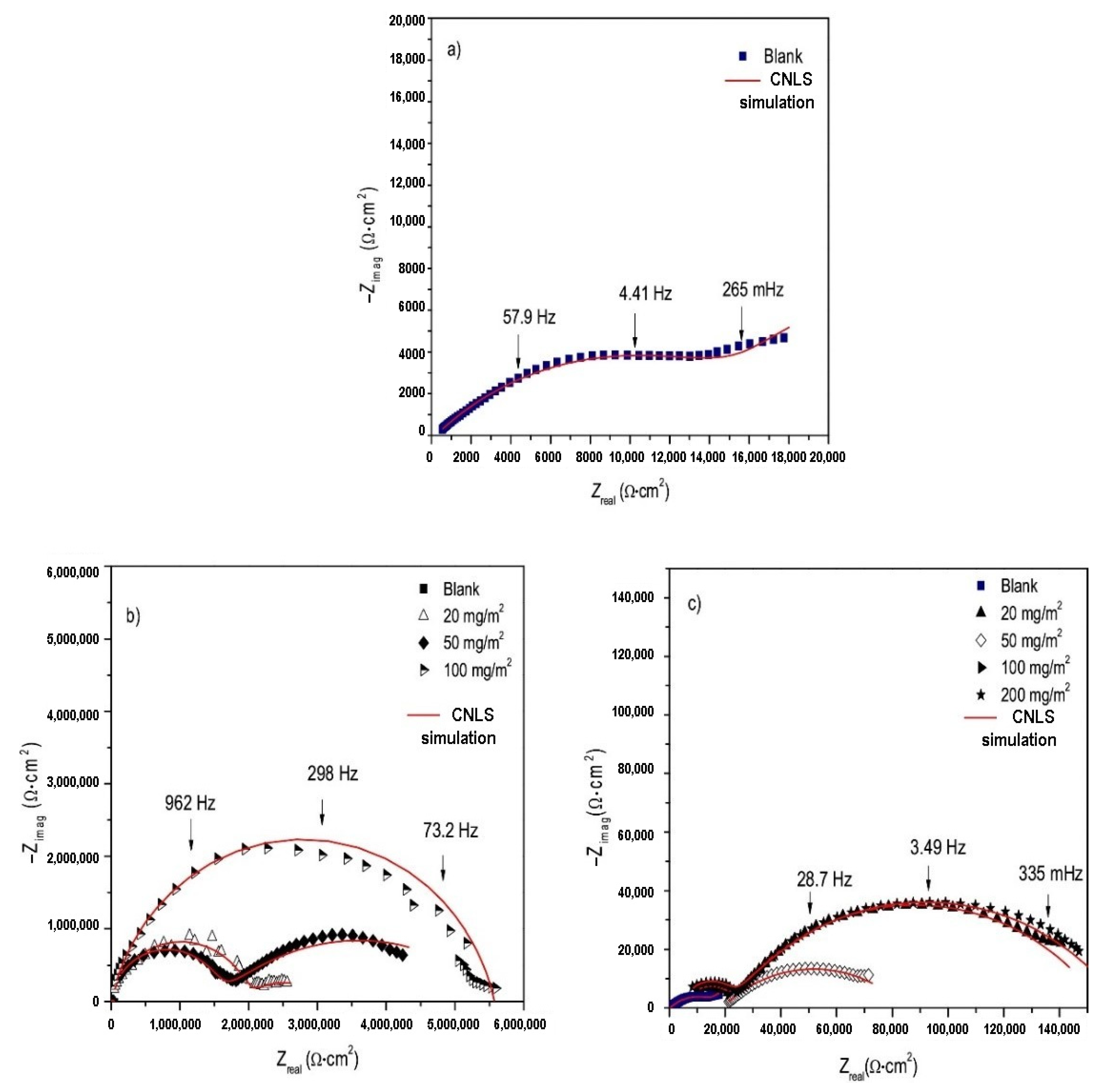
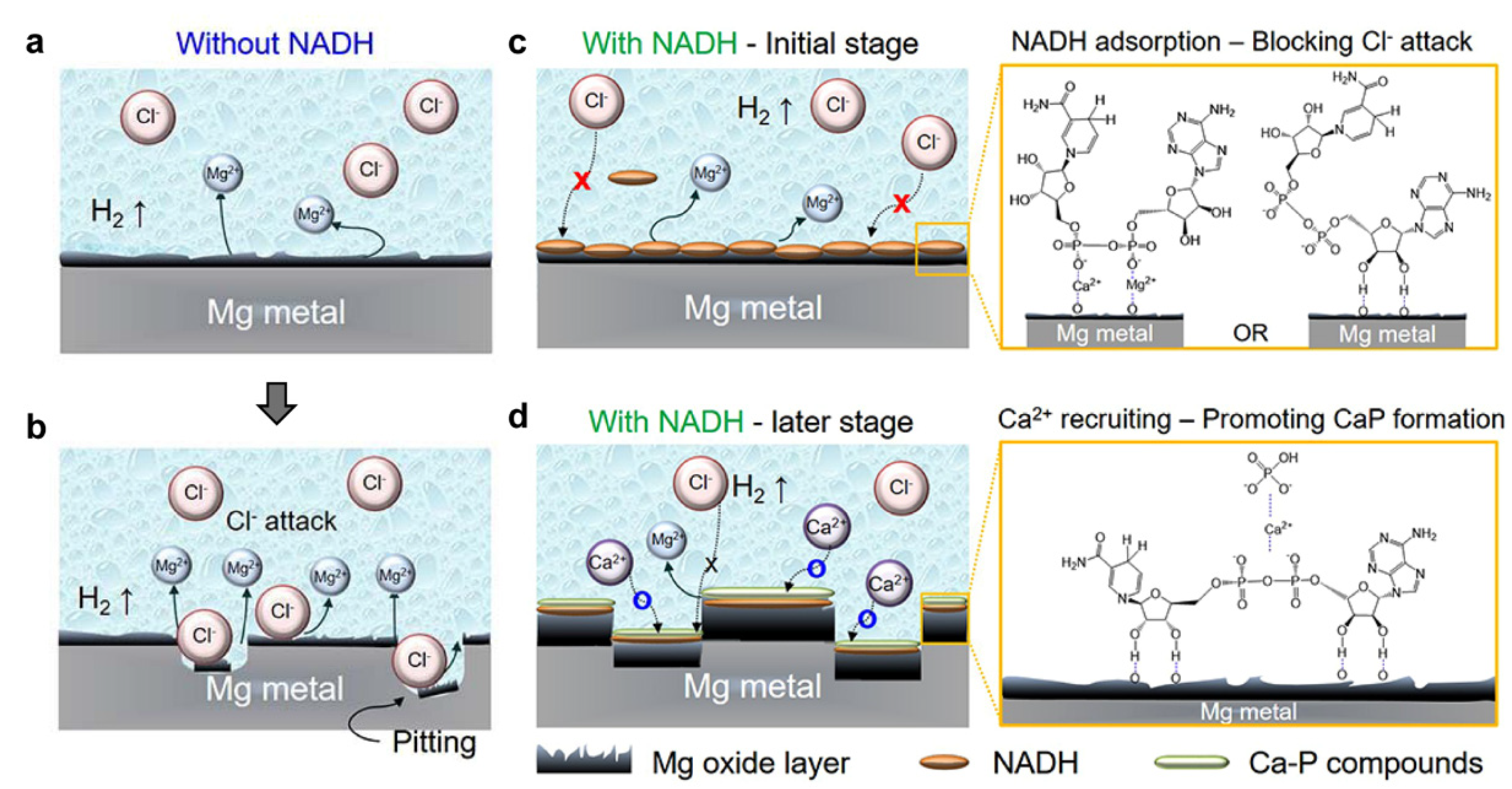
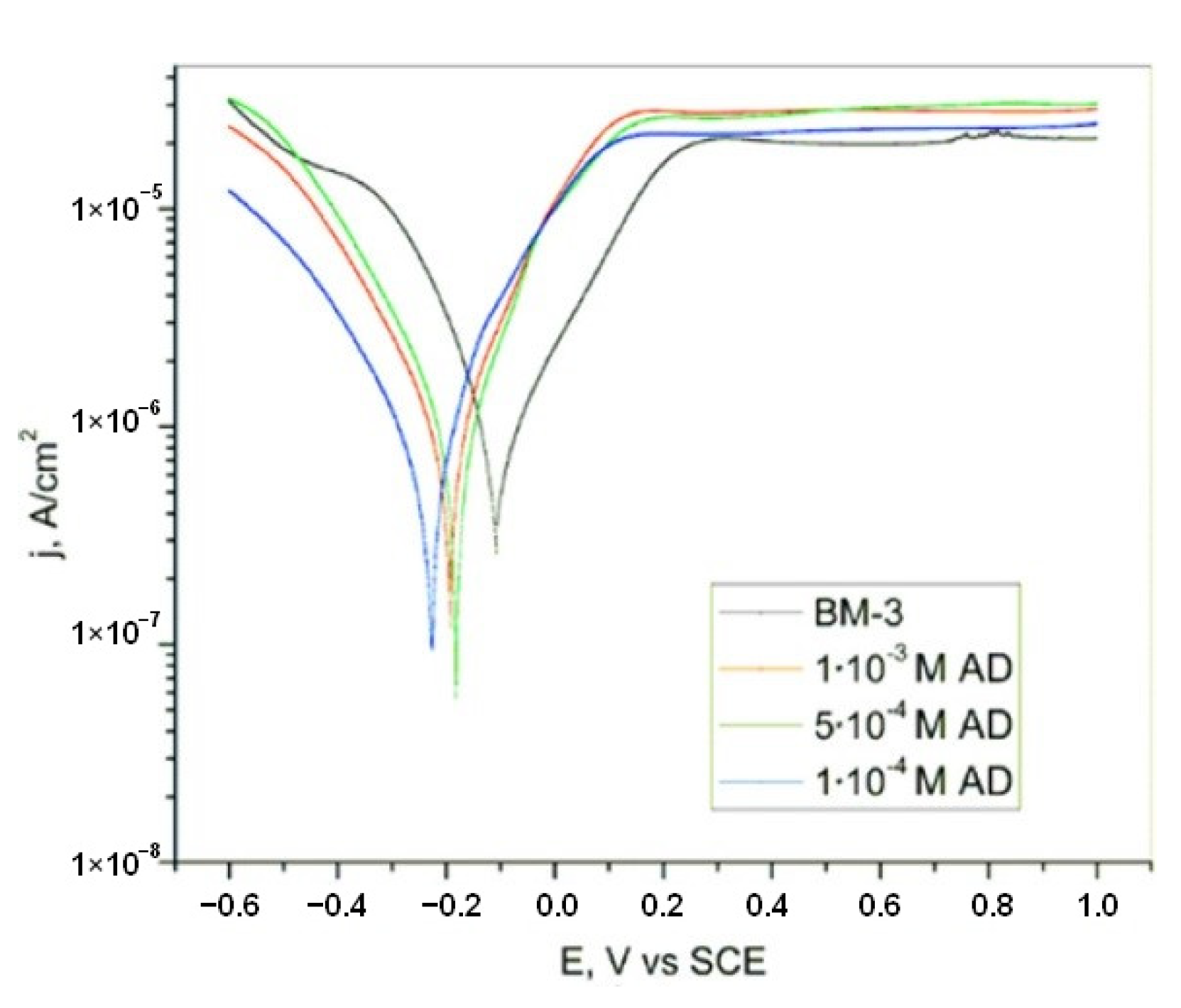
| Inhibitor | Concentration | Medium | IE % | Reference |
|---|---|---|---|---|
| Purine | 1 × 10−2 M | 1 M NaCl | 76 PP | [22] |
| 5 × 10−3 M | 97 WL | |||
| Adenine | 1 × 10−2 M | 1 M NaCl | 92 PP | [23] |
| 5 × 10−3 M | 98 WL | |||
| Purine | 1 × 10−2 M | 0.5 M NaNO3 | 90 PP/91 WL | [37] |
| Adenine | 91 PP/96 WL | |||
| Purine | 1 × 10−2 M | 0.5 M Na2SO4 pH 6.8 | 91 WL | [35] |
| Adenine | 94 WL | |||
| Purine | 0.5 M Na2SO4 pH 1.0 | 79 WL | ||
| Adenine | 88 WL | |||
| Purine | 1 × 10−2 M | 0.5 M Na2SO4 pH 6.8 | 76 PP | [36] |
| Adenine | 91 PP | |||
| Purine | 1 × 10−2 M | 0.5 M Na2SO4 pH 7.0 | 91 PP | [38] |
| 0.5 M Na2SO4 pH 9.0 | 89 PP | |||
| Purine | 5 × 10−3 M | Artificial seawater | 91.91 PP | [25] |
| Adenine | 5 × 10−3 M | 92.17 PP | ||
| 6-benzylaminopurine | 5 × 10−3 M | 94.43 PP | ||
| Adenine | 0.1 wt % | 1 wt % glycine + 0.3 wt % H2O2 pH 10 | 37.5 PP | [24] |
| Adenine | 1 × 10−2 M | BM3 | 88.10 PP/89.69 EIS | [34] |
| 2,6-diaminopurine | 1 × 10−2 M | 87.18 PP/90.10 EIS | ||
| AD + PS | 1 × 10−2 M + 0.01% | 91.17 PP | ||
| DAP + PS | 1 × 10−2 M + 0.01% | 93.02 PP | ||
| AD + PS | 1 × 10−3 M + 1% | 88.56 PP | ||
| DAP + PS | 1 × 10−3 M + 1% | 87.87 PP | ||
| Purine | 1 × 10−2 M in NaCl 3 h | SUF | 85 PR | [33] |
| Purine | 1 × 10−3 M in 5 g/L NaCl 1 h | SUF pH 6−0.1 V(SCE) | 61.20 PP | [32] |
| 1 × 10−3 M in 5 g/L NaCl 3 h | 98.61 PP | |||
| 1 × 10−2 M in 5 g/L NaCl 1 h | 98.10 PP | |||
| 1 × 10−2 M in 5 g/L NaCl 3 h | 99.18 PP | |||
| Purine | 1 × 10−2 M | 0.5 M Na2SO4 pH 7.0 | 85.2 PP | [26] |
| 1 × 10−2 M | 0.5 M Na2SO4 pH 9.2 | 91.0 PP | ||
| Adenine | 0.1 g/L | Alkaline artificial seawater | 95.99 PP/95.89 EIS | [39] |
| 0.2 g/L | 97.22 PP/96.23 EIS | |||
| Guanine | 0.1 g/L | 98.21 PP/97.27 EIS | ||
| Hypoxanthine | 0.1 g/L | 98.69 PP/97.59 EIS | ||
| Caffeine | 1 × 10−3 M | 0.5 M NaCl | 91.19 PP/92.16 EIS | [40] |
| Caffeine | 1 × 10−2 M | 0.1 M H2SO4 | 57.0 PP/71.0 EIS | [41] |
| Guanine | 1 × 10−3 M | 0.1 M HCl (pH 2) | 87.0 WL (298 K) 92.0 WL (313 K) | [42] |
| Purine Derivative | IE % Experiments | IE % Predicted | Purine Derivative | IE % Experiments | IE % Predicted |
|---|---|---|---|---|---|
| Adenine | 91 | 91.3 | Diaminopurine | 70.9 | 70.4 |
| Guanine | 86 | 85.8 | Hypo-xanthine | 90.61 | 91.61 |
| Theophylline | 88.6 | 88.7 | Xanthine | 84.38 | 81.30 |
| Propargyl-theophylline | 91 | 91 | Ally-theophylline | 79.10 | 78.36 |
| Adenosine | 93 | 93 | Guanosine | 93.00 | 89.02 |
| Caffeine | 96.4 | 96.2 | Dithiopurine | 78 | 83.91 |
| Metal | Inhibitor | Concentration | Media | IE % | Reference |
|---|---|---|---|---|---|
| Aluminum | Theophylline | 1 × 10−4 M | 2.0 M HCl | 99.99 PP | [15] |
| Theophylline | 2.5% | 1.0 M NaOH | 90 PP/91 EIS | [75] | |
| Adenine | 0.01 M | 0.1 M HCl | 72.58 WL/88.87 EIS/88.85 PP | [16] | |
| Guanine | 73.50 WL/94.25 EIS/94.37 PP | ||||
| Hypoxanthine | 71.01 WL/91.41 EIS/91.22 PP | ||||
| Purine | 1 × 10−2 M | 1 M H3PO4 | 55.01 PP/53.36 EIS | [76] | |
| Purine + KI | 1 × 10−2 M + 1 × 10−4 M | 94.07 PP/92.05 EIS | |||
| Magnesium | IO | 3.5 wt % NaCl | 99.36 | [20] | |
| IO-MEL | 0.023 M | 99.94 | |||
| IO-AD | 0.023 M | 99.85 | |||
| IO-TEMD | 0.023 M | 99.99 | |||
| Titanium | Adenine | 1 × 10−3 M | BM-3 | 82.2 PP/77.4 EIS | [19] |
| Tin | Adenine | 1 × 10−3 M | 0.5 M HClO4 | 65 PP | [18] |
| Indium | 73 PP | ||||
| Alloy | CE% | ||||
| I 0.5 wt % In | 152 PP | ||||
| II 2 wt % In | 210 PP | ||||
| III 5 wt % In | 170 PP | ||||
| IV 10 wt % In | 160 PP | ||||
| V 20 wt % In | 155 PP | ||||
| Tin | Adenosine | 1 × 10−3 M | 0.5 M HClO4 | 72 PP | [18] |
| Indium | 79 PP | ||||
| Alloy | CE % | ||||
| I 0.5 wt % In | 205 PP | ||||
| II 2 wt % In | 350 PP | ||||
| III 5 wt % In | 250 PP | ||||
| IV 10 wt % In | 230 PP | ||||
| V 20 wt % In | 210 PP | ||||
| Tin | Adenine | 1 × 10−3 M | 0.5 M HCl | 64 PP | [17] |
| Indium | 69 PP | ||||
| III 5 wt % In | 66 PP | ||||
| Tin | Adenosine | 1 × 10−3 M | 73 PP | ||
| Indium | 72 PP | ||||
| III 5 wt % In | 73 PP | ||||
| Sn–Ag alloy | Adenine | 3 × 10−3 M | 1.5 M HNO3 | 85 PP | [28] |
| Nickel | Caffeine | 5 × 10−2 | 3.5% NaCl | 96.80 PP | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović Mihajlović, M.B.; Tasić, Ž.Z.; Radovanović, M.B.; Simonović, A.T.; Antonijević, M.M. Electrochemical Analysis of the Influence of Purines on Copper, Steel and Some Other Metals Corrosion. Metals 2022, 12, 1150. https://doi.org/10.3390/met12071150
Petrović Mihajlović MB, Tasić ŽZ, Radovanović MB, Simonović AT, Antonijević MM. Electrochemical Analysis of the Influence of Purines on Copper, Steel and Some Other Metals Corrosion. Metals. 2022; 12(7):1150. https://doi.org/10.3390/met12071150
Chicago/Turabian StylePetrović Mihajlović, Marija B., Žaklina Z. Tasić, Milan B. Radovanović, Ana T. Simonović, and Milan M. Antonijević. 2022. "Electrochemical Analysis of the Influence of Purines on Copper, Steel and Some Other Metals Corrosion" Metals 12, no. 7: 1150. https://doi.org/10.3390/met12071150
APA StylePetrović Mihajlović, M. B., Tasić, Ž. Z., Radovanović, M. B., Simonović, A. T., & Antonijević, M. M. (2022). Electrochemical Analysis of the Influence of Purines on Copper, Steel and Some Other Metals Corrosion. Metals, 12(7), 1150. https://doi.org/10.3390/met12071150








