Preferential Locations of Hydrogen Accumulation and Damage in 1.2 GPa and 1.8 GPa Grade Hot-Stamped Steels: A Comparative Study
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Heat Treatments
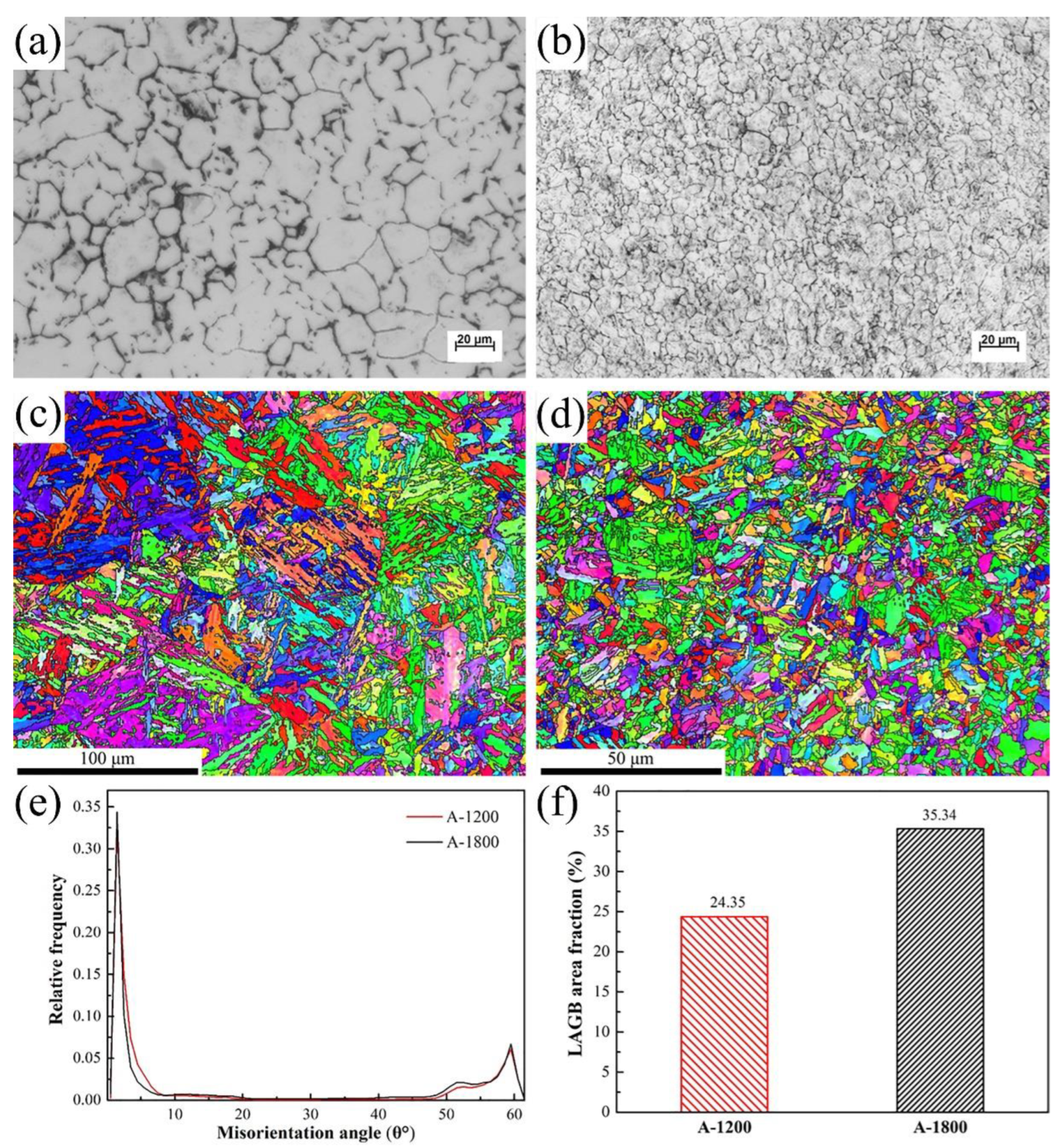
2.2. Microstructural Characterization
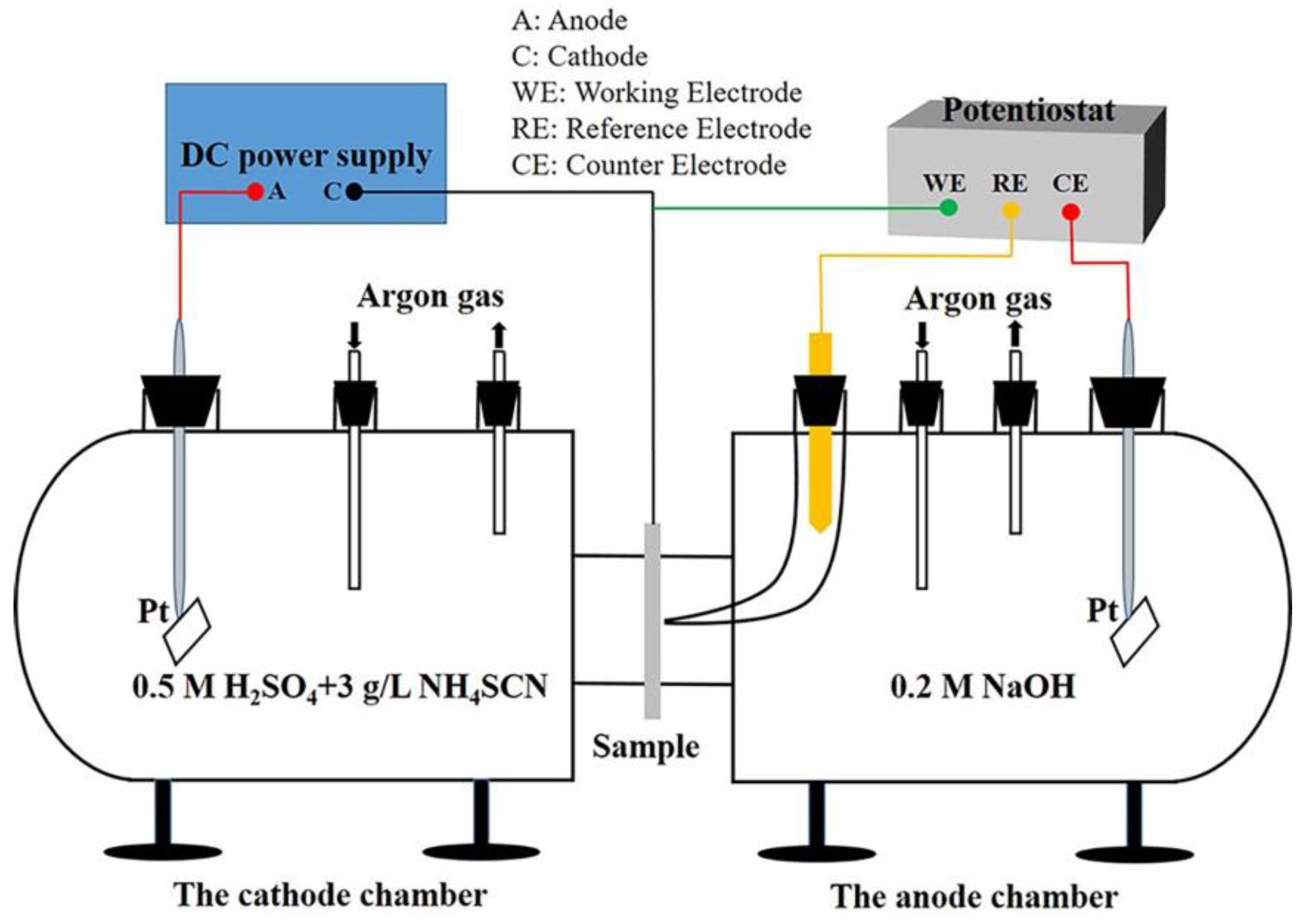
2.3. Hydrogen Permeation Test
2.4. Hydrogen Microprint Technique (HMT)
3. Results and Discussion
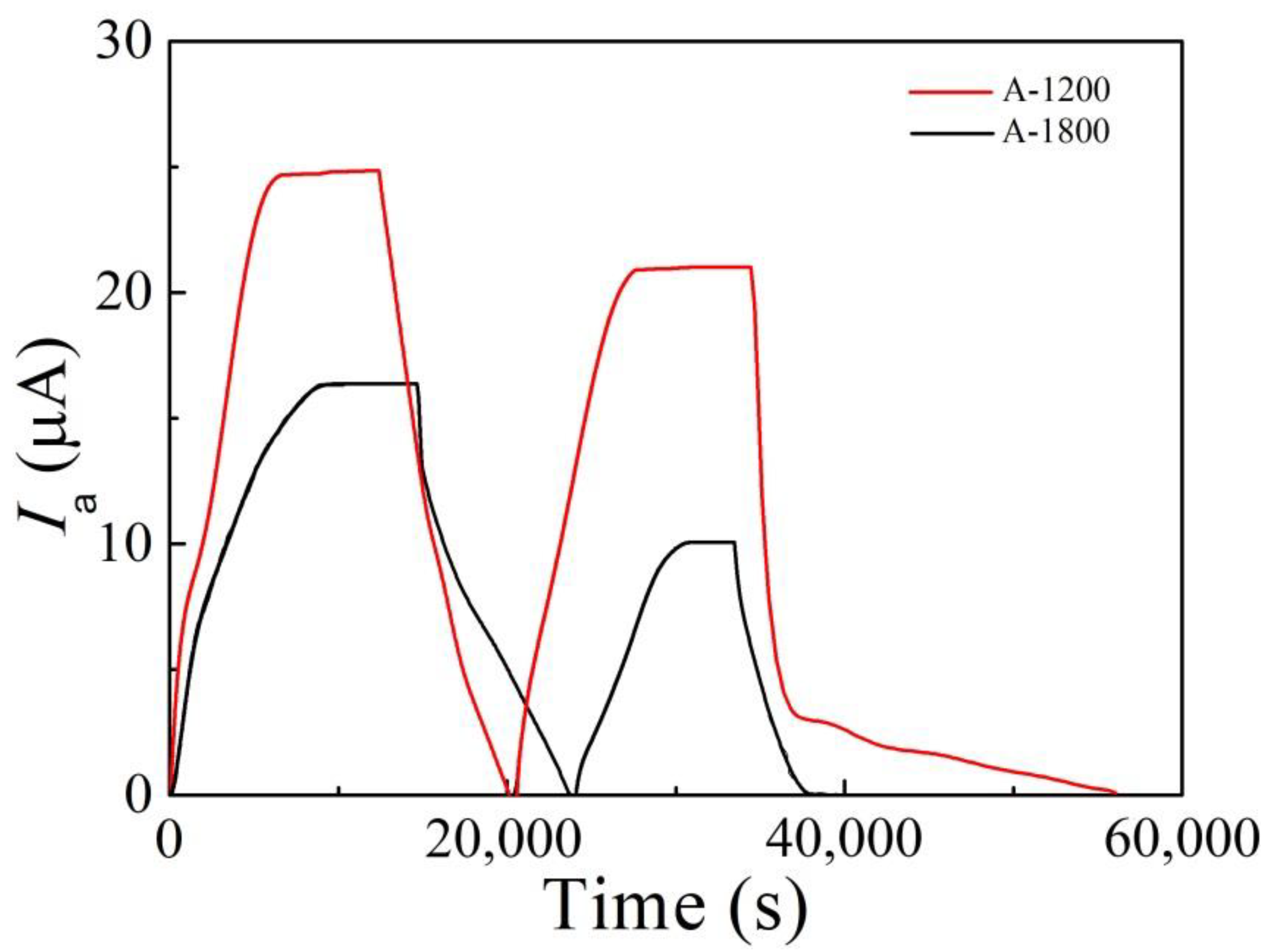
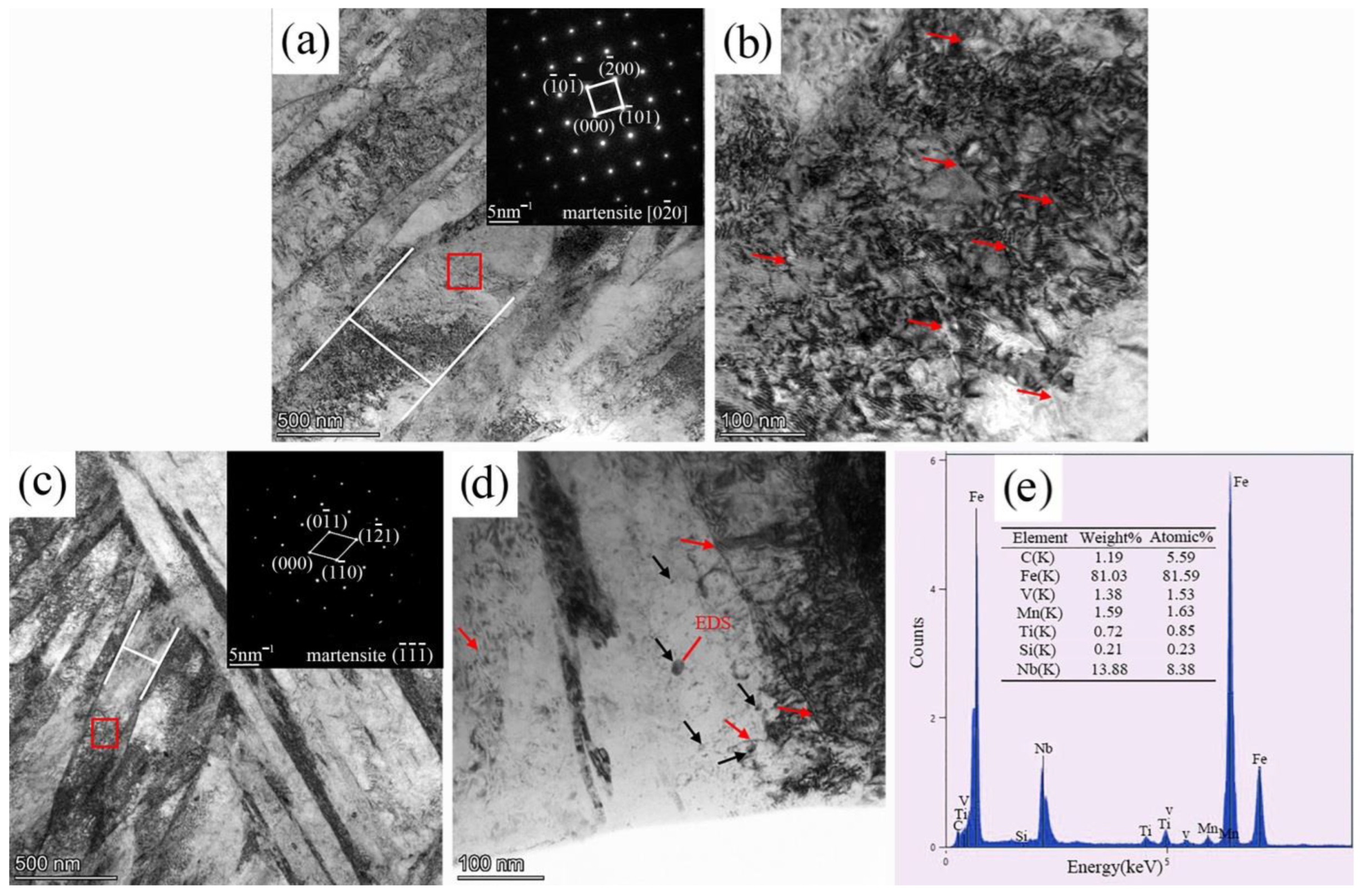
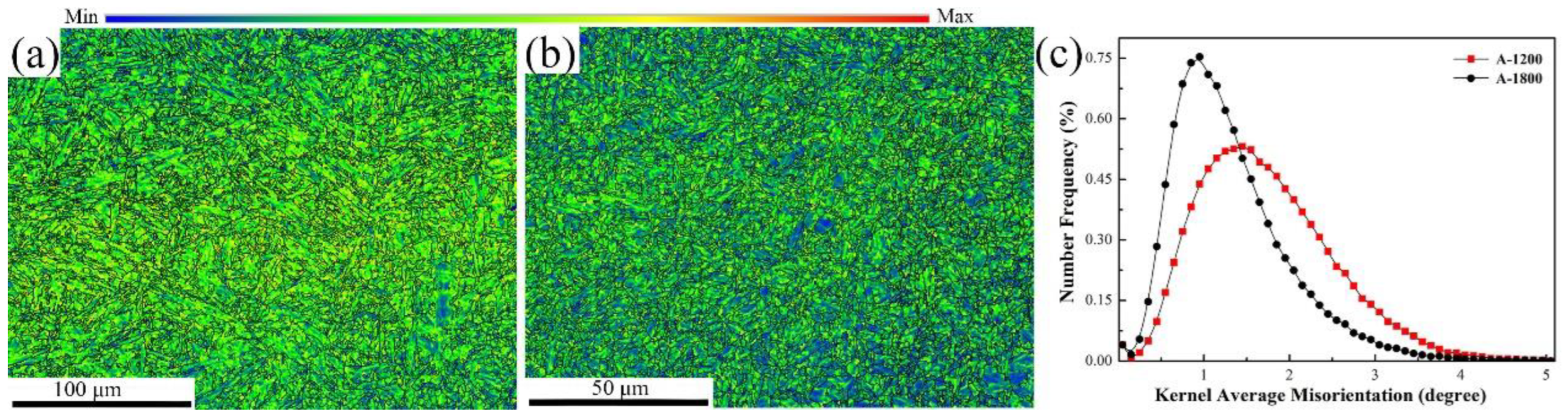
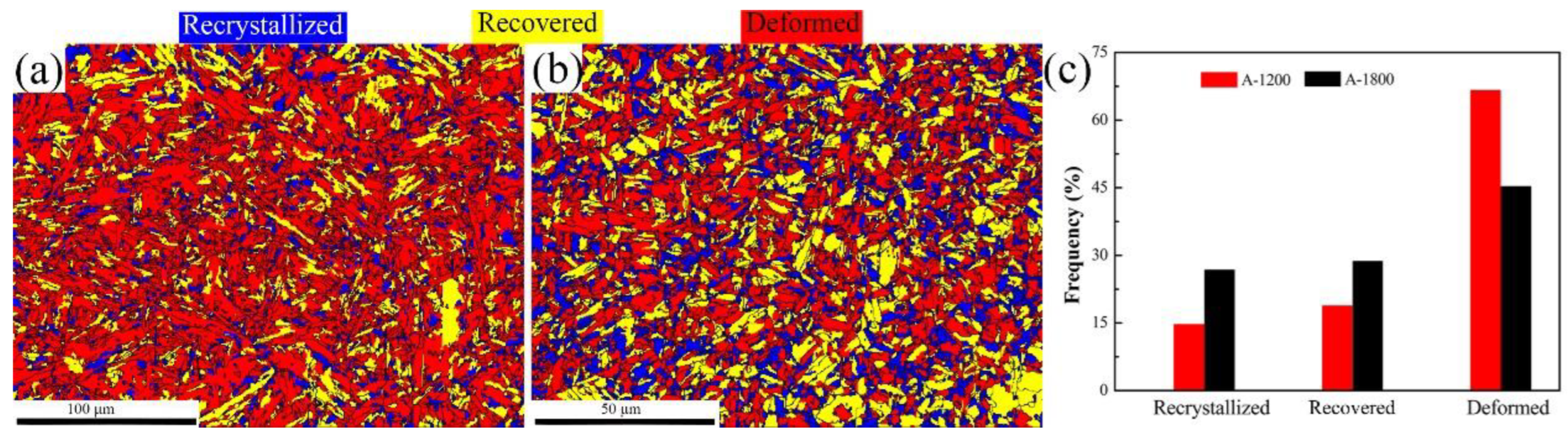
3.1. Hydrogen Diffusion Behaviors and Traps
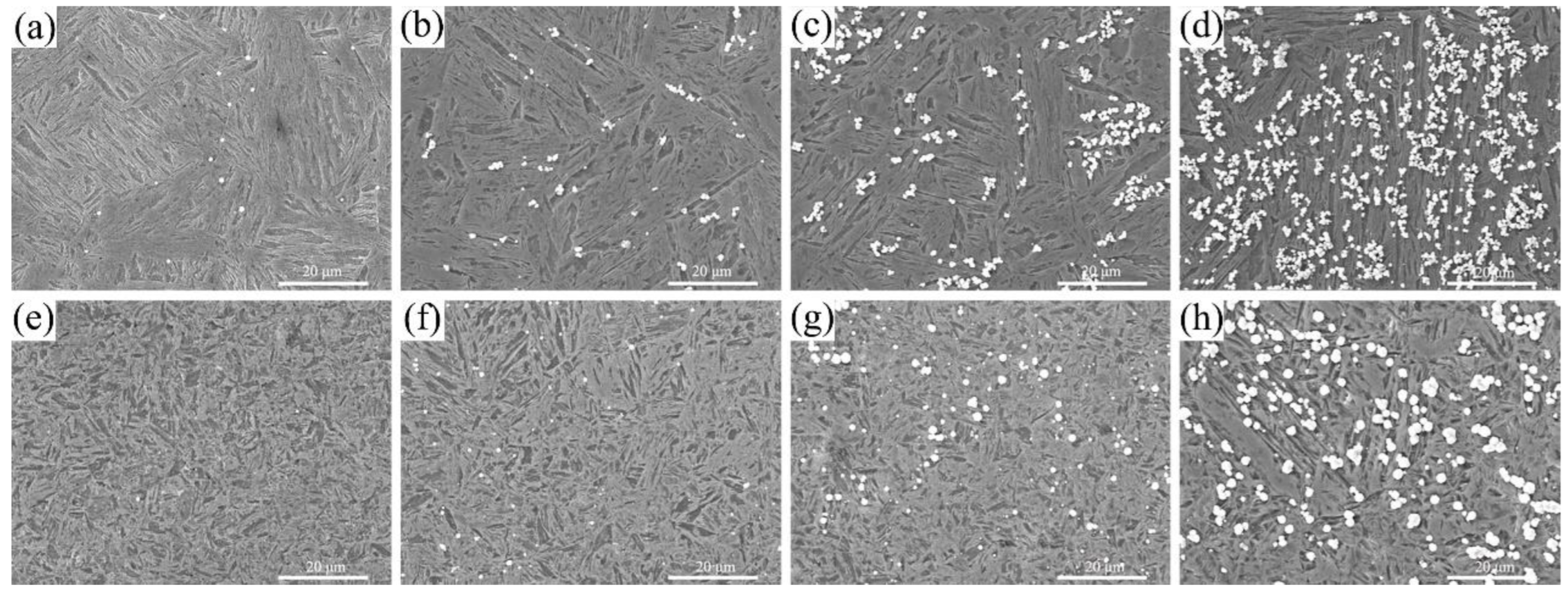
3.2. Locations of Hydrogen Accumulation
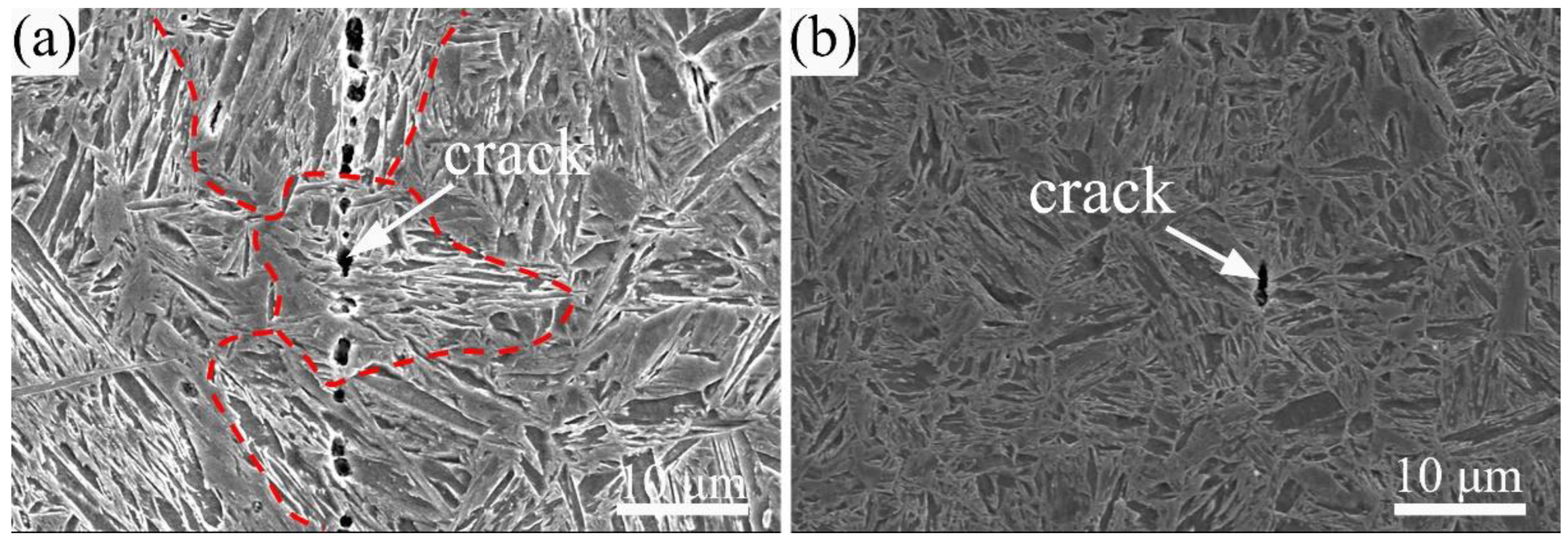
3.3. Hydrogen-Induced Crack Initiation and Propagation Behaviors
4. Conclusions
- (1)
- Compared with A-1200 steel, A-1800 steel had a lower hydrogen diffusion coefficient Deff (4.15 × 10−7 cm2/s) and a higher hydrogen trap density Nt (1.59 × 1021 cm−3). This was ascribed to its fine microstructure and the introduction of a large number of nano-sized carbides.
- (2)
- Both steels exhibited the initial hydrogen segregation in the grain boundary. However, the preferential locations of hydrogen accumulation were determined to be lath and grain boundaries for A-1200 and A-1800 steels, respectively. Moreover, markedly improved hydrogen aggregation also was found in A-1800 steel, owing to its fine microstructure and high LAGB area fraction.
- (3)
- Hydrogen-induced cracking was mainly initiated at lath and grain boundaries for the A-1200 and A-1800 samples, respectively, charged at a current density of 50 mA/cm2 for 24 h. The greatly alleviated hydrogen damage of A-1800 steel was attributed to the low internal stress, high recrystallization fraction, and abundance of nano-sized carbides to weaken the process of HELP.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krbasian, H.; Tekkaya, A.E. A review on hot stamping. J. Mater. Process. Technol. 2010, 210, 2103–2118. [Google Scholar] [CrossRef]
- Taylor, T.; Clough, A. Critical review of automotive hot-stamped sheet steel from an industrial perspective. J. Mater. Sci. Technol. 2018, 34, 809–861. [Google Scholar] [CrossRef]
- Okayasu, M.; Fujiwara, T. Hydrogen embrittlement characteristics of hot-stamped 22MnB5 steel. Int. J. Hydrog. Energy 2021, 46, 19657–19669. [Google Scholar] [CrossRef]
- Zheng, Q.; Noorderhaven, N.; Du, J. Making the unlikely marriage work: The integration process of Chinese strategic asset-seeking acquisitions. J. World Bus. 2022, 57, 101305. [Google Scholar] [CrossRef]
- Jo, M.C.; Yoo, J.; Kim, S.; Kim, S.; Oh, J.; Bian, J.; Sohn, S.S.; Lee, S. Effects of Nb and Mo alloying on resistance to hydrogen embrittlement in 1.9 GPa-grade hot-stamping steels. Mater. Sci. Eng. A 2020, 789, 139656. [Google Scholar] [CrossRef]
- Garet, M.; Brass, A.M.; Haut, C.; Guttierez-Solana, F. Hydrogen trapping on non-metallic inclusions in Cr-Mo low alloy steels. Corros. Sci. 1998, 40, 1073–1086. [Google Scholar] [CrossRef]
- Szost, B.A.; Vegter, R.H.; Rivera-Díaz-Del-Castillo, P. Developing bearing steels combining hydrogen resistance and improved hardness. Mater. Des. 2013, 43, 499–506. [Google Scholar] [CrossRef]
- Michler, T.; Balogh, M.P. Hydrogen environment embrittlement of an ODS RAF steel—Role of irreversible hydrogen trap sites. Int. J. Hydrog. Energy 2010, 35, 9746–9754. [Google Scholar] [CrossRef]
- Huang, W.; Gu, H.; Liu, Q.; Si, T. Suppression of hydrogen-induced damage in 22MnB5 hot stamping steel by micro-alloying. Mater. Chem. Phys. 2020, 256, 123729. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. Evaluation of the effect of V4C3 precipitates on the hydrogen induced mechanical degradation in Fe-CV alloys. Mater. Sci. Eng. A 2016, 675, 299–313. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, J.; Zhao, Q.; Liu, J.; Huang, F.; Huang, Y.; Li, X. Dual role of nanosized NbC precipitates in hydrogen embrittlement susceptibility of lath martensitic steel. Corros. Sci. 2020, 164, 108345. [Google Scholar] [CrossRef]
- Lin, L.; Li, B.; Zhu, G.; Kang, Y.-L.; Liu, R.-D. Effect of niobium precipitation behavior on microstructure and hydrogen induced cracking of press hardening steel 22MnB5. Mater. Sci. Eng. A 2018, 721, 38–46. [Google Scholar] [CrossRef]
- Turk, A.; San Martín, D.; Rivera-Díaz-Del-Castillo, P.E.; Galindo-Nava, E.I. Correlation between vanadium carbide size and hydrogen trapping in ferritic steel. Scr. Mater. 2018, 152, 112–116. [Google Scholar] [CrossRef]
- Thomas, A.; Szpunar, J.A. Hydrogen diffusion and trapping in X70 pipeline steel. Int. J. Hydrog. Energy 2020, 45, 2390–2404. [Google Scholar] [CrossRef]
- Chen, S.S.; Wu, T.I.; Wu., J.K. Effects of deformation on hydrogen degradation in a duplex stainless steel. J. Mater. Sci. 2004, 39, 67–71. [Google Scholar] [CrossRef]
- Ichitani, K.; Kanno, M. Visualization of hydrogen diffusion in steels by high sensitivity hydrogen microprint technique. Sci. Technol. Adv. Mater. 2003, 4, 545–551. [Google Scholar] [CrossRef]
- Okayasu, M.; Yang, L. Influence of microstructure on the mechanical properties and hydrogen embrittlement characteristics of 1800 MPa grade hot-stamped 22MnB5 steel. J. Mater. Sci. 2019, 54, 5061–5073. [Google Scholar] [CrossRef]
- Park, J.; Seong, H.G.; Kim, S.J. Effect of heat treatment conditions on corrosion and hydrogen diffusion behaviors of ultra-strong steel used for automotive applications. Corros. Sci. Technol. 2019, 18, 267–276. [Google Scholar]
- Williams, M.M.R. The Mathematics of Diffusion; Crank, J., Ed.; Clarendon Press: Oxford, UK, 1975; 414p. [Google Scholar]
- Yen, S.K.; Huang, I.B. Critical hydrogen concentration for hydrogen-induced blistering on AISI 430 stainless steel. Mater. Chem. Phys. 2003, 80, 662–666. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The mechanism of hydrogen evolution on iron in acid solutions by determination of permeation rates. J. Electrochem. Soc. 1964, 111, 619–623. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Characterization of inclusions of X80 pipeline steel and its correlation with hydrogen-induced cracking. Corros. Sci. 2011, 53, 1201–1208. [Google Scholar] [CrossRef]
- Ovejero-García, J. Hydrogen microprint technique in the study of hydrogen in steels. J. Mater. Sci. 1985, 20, 2623–2629. [Google Scholar] [CrossRef]
- Ichitani, K.; Kuramoto, S.; Kanno, M. Quantitative evaluation of detection efficiency of thehydrogen microprint technique applied to steel. Corros. Sci. 2003, 45, 1227–1241. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Collins, L.; Stankievech, R. Evaluation of hydrogen induced cracking behavior of API X70 pipeline steel at different heat treatments. Int. J. Hydrog. Energy 2014, 39, 6076–6088. [Google Scholar] [CrossRef]
- Si, T.; Liu, Y.; Zhang, Q.; Liu, D.; Li, Y. Effect of Microstructure on Hydrogen Permeation in EA4T and 30CrNiMoV12 Railway Axle Steels. Metals 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xiong, L.; Liu, S. Rapid Hydrogen Transportation Along Grain Boundary in Nickel. Acta. Metall. Sin. 2014, 27, 615–620. [Google Scholar] [CrossRef]
- Tian, H.; Li, Y.; Wang, X.; Cui, Z. Combined effect of cathodic potential and sulfur species on calcareous deposition, hydrogen permeation and hydrogen embrittlement of a low carbon bainite steel in artificial seawater. Corros. Sci. 2019, 158, 108089. [Google Scholar] [CrossRef]
- Harris, T.M.; Latanision, M. Grain boundary diffusion of hydrogen in nickel. Metall. Trans. A 1991, 22, 351–355. [Google Scholar] [CrossRef]
- Brass, A.M.; Chanfreau, A. Accelerated diffusion of hydrogen along grain boundaries in nickel. Acta Mater. 1996, 44, 3823–3831. [Google Scholar] [CrossRef]
- Li, L.; Song, B.; Cai, Z.; Liu, Z.; Cui, X. Effect of vanadium content on hydrogen diffusion behaviors and hydrogen induced ductility loss of X80 pipeline steel. Mater. Sci. Eng. A 2019, 742, 712–721. [Google Scholar] [CrossRef]
- Du, Y.A.; Ismer, L.; Rogal, J.; Hickel, T.; Neugebauer, J.; Drautz, R. First-principles study on the interaction of H interstitials with grain boundaries in α-and γ-Fe. Phys. Rev. B 2011, 84, 144121. [Google Scholar] [CrossRef]
- Hojo, T.; Akiyama, E.; Saitoh, H.; Shiro, A.; Yasuda, R.; Shobu, T.; Kinugasa, J.; Yuse, F. Effects of residual stress and plastic strain on hydrogen embrittlement of a stretch-formed TRIP-aided martensitic steel sheet. Corros. Sci. 2020, 177, 108957. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, D.; Huang, F.; Gao, W.; Wan, J.; Liu, J. Mitigation of hydrogen embrittlement in ultra-high strength lath martensitic steel via Ta microalloying. Mater. Des. 2021, 210, 110090. [Google Scholar] [CrossRef]


| Sample | C | Si | Mn | P | S | Cr | B | Ti | Nb | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A-1200 | 0.23 | 0.29 | 1.27 | 0.01 | 0.006 | 0.18 | 0.0035 | - | - | - | Bal. |
| A-1800 | 0.32 | 0.25 | 1.00 | 0.01 | 0.003 | 0.20 | 0.0037 | 0.030 | 0.035 | 0.035 | Bal. |
| Parameters | A-1200 Steel | A-1800 Steel | ||
|---|---|---|---|---|
| 1st Charging | 2nd Charging | 1st Charging | 2nd Charging | |
| L (mm) | 1.03 | 1.03 | 1.05 | 1.05 |
| T0.63 (s) | 3305 | 3010 | 4430 | 4010 |
| I∞ (μA) | 23.86 | 20.01 | 17.37 | 12.08 |
| J∞L (mol m−1 s−1) | 1.44 × 10−11 | 1.21 × 10−11 | 1.07 × 10−11 | 0.74 × 10−11 |
| Deff (cm2 s−1) | 5.35 × 10−7 | 5.43 × 10−7 | 4.15 × 10−7 | 4.58 × 10−7 |
| Capp (wppm) | 3.54 ± 0.02 | 2.92 ± 0.01 | 3.39 ± 0.04 | 2.13 ± 0.02 |
| Nt (cm−3) | 1.29 × 1021 | 1.05 × 1021 | 1.59 × 1021 | 0.91 × 1021 |
| Nr (cm−3) | 1.05 × 1021 | 0.91 × 1021 | ||
| Nir (cm−3) | 0.24 × 1021 | 0.68 × 1021 | ||
| Sample | Number of Cracks | Average Length (μm) | Average Width (μm) | Initiation Site (number) | Propagation Method |
|---|---|---|---|---|---|
| A-1200 | 11 | 12.25 | 1.37 | Lath (8) | Transgranular |
| A-1800 | 3 | 3.71 | 0.74 | Grain boundary (2) | Intergranular |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Gu, H.; Dai, Q.; Shen, H.; Si, T. Preferential Locations of Hydrogen Accumulation and Damage in 1.2 GPa and 1.8 GPa Grade Hot-Stamped Steels: A Comparative Study. Metals 2022, 12, 1075. https://doi.org/10.3390/met12071075
Wei P, Gu H, Dai Q, Shen H, Si T. Preferential Locations of Hydrogen Accumulation and Damage in 1.2 GPa and 1.8 GPa Grade Hot-Stamped Steels: A Comparative Study. Metals. 2022; 12(7):1075. https://doi.org/10.3390/met12071075
Chicago/Turabian StyleWei, Pengfei, Hairong Gu, Qingpeng Dai, Hui Shen, and Tingzhi Si. 2022. "Preferential Locations of Hydrogen Accumulation and Damage in 1.2 GPa and 1.8 GPa Grade Hot-Stamped Steels: A Comparative Study" Metals 12, no. 7: 1075. https://doi.org/10.3390/met12071075
APA StyleWei, P., Gu, H., Dai, Q., Shen, H., & Si, T. (2022). Preferential Locations of Hydrogen Accumulation and Damage in 1.2 GPa and 1.8 GPa Grade Hot-Stamped Steels: A Comparative Study. Metals, 12(7), 1075. https://doi.org/10.3390/met12071075





