The Effect of Cathodic Cage Plasma TiN Deposition on Surface Properties of Conventional Plasma Nitrided AISI-M2 Steel
Abstract
:1. Introduction
2. Experimental Details
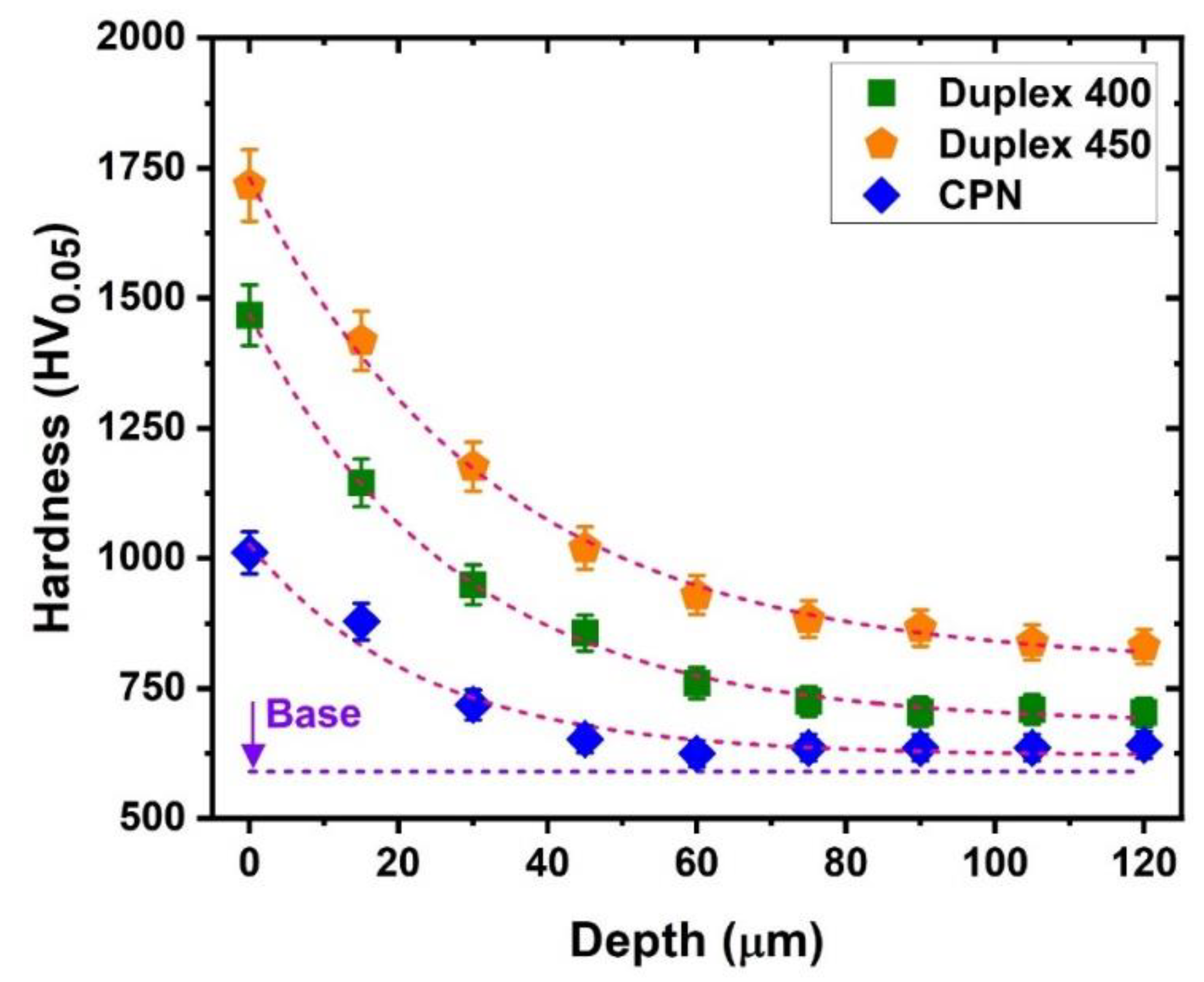
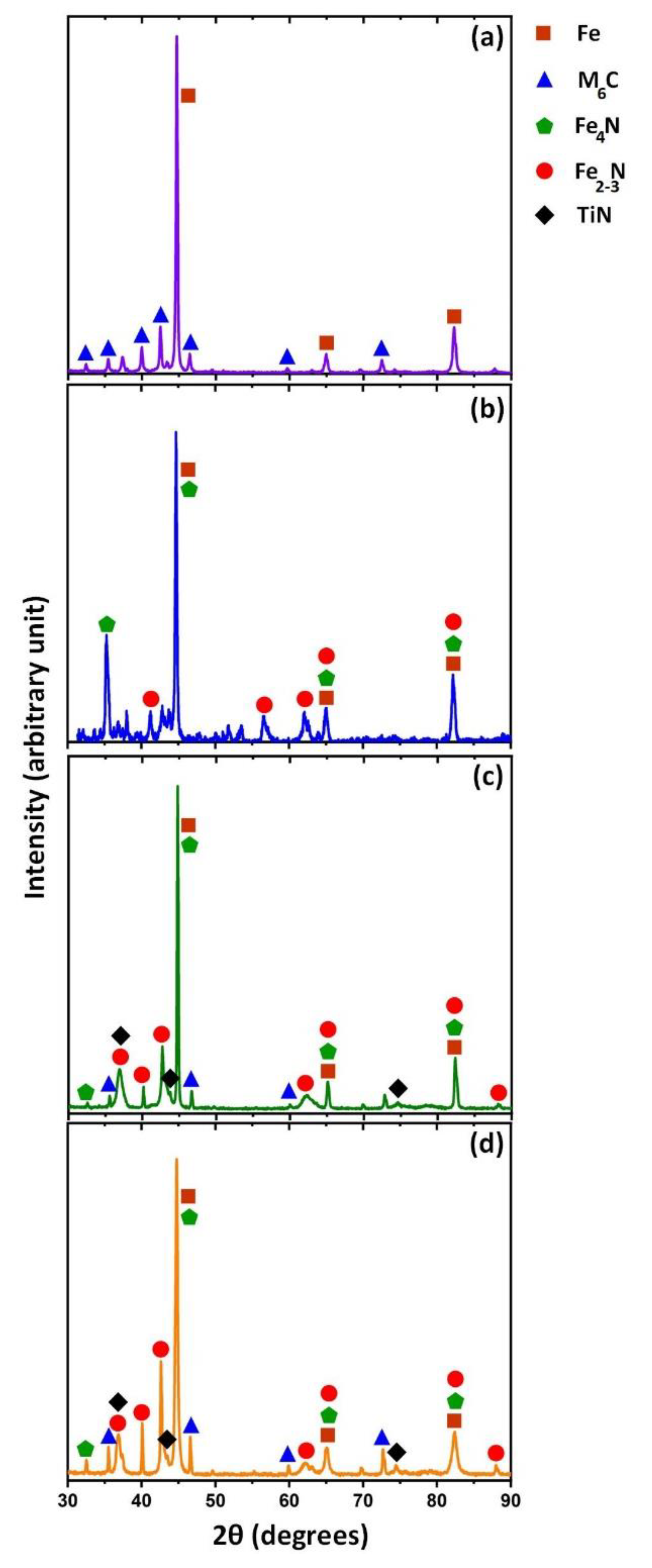
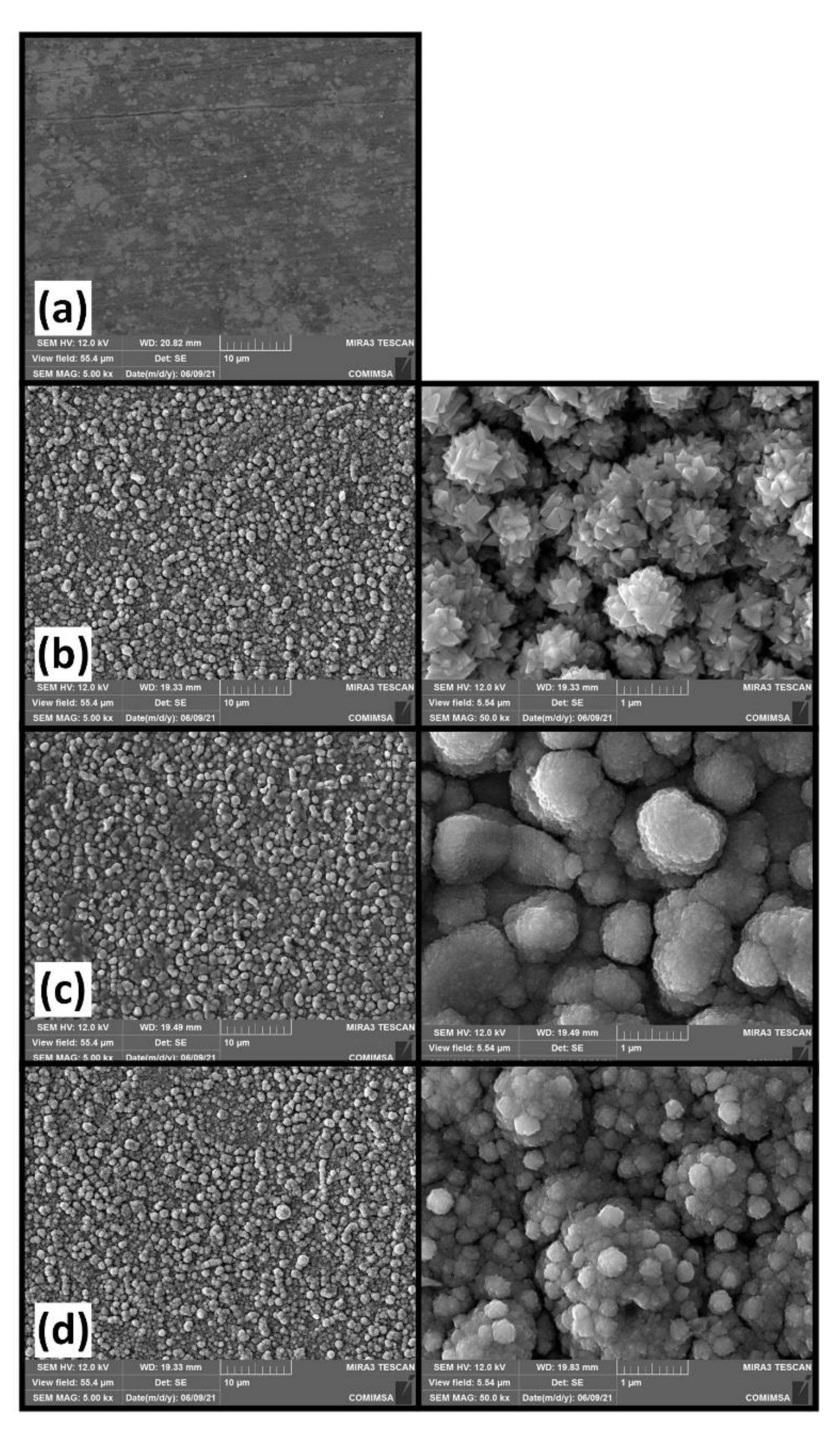
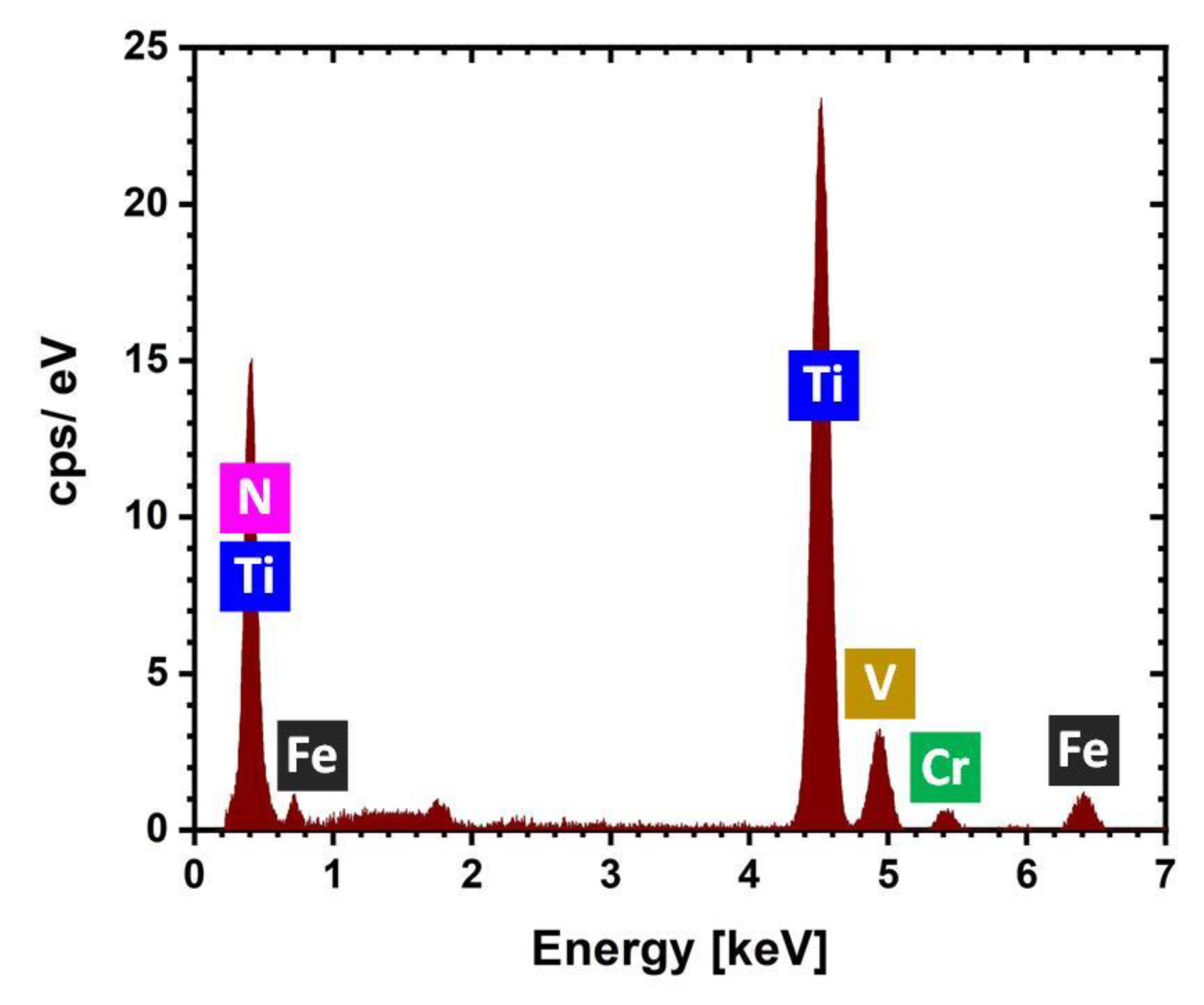
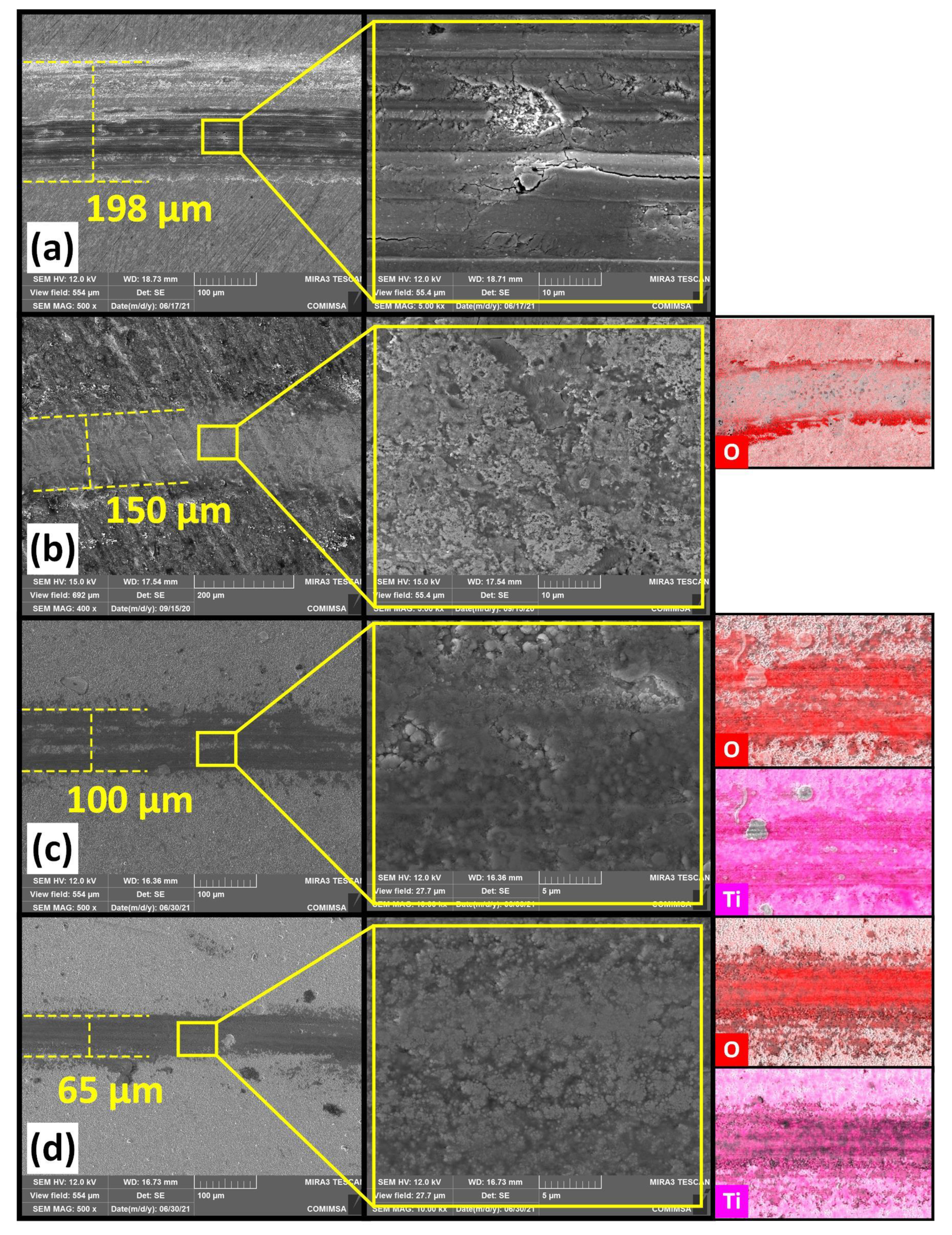
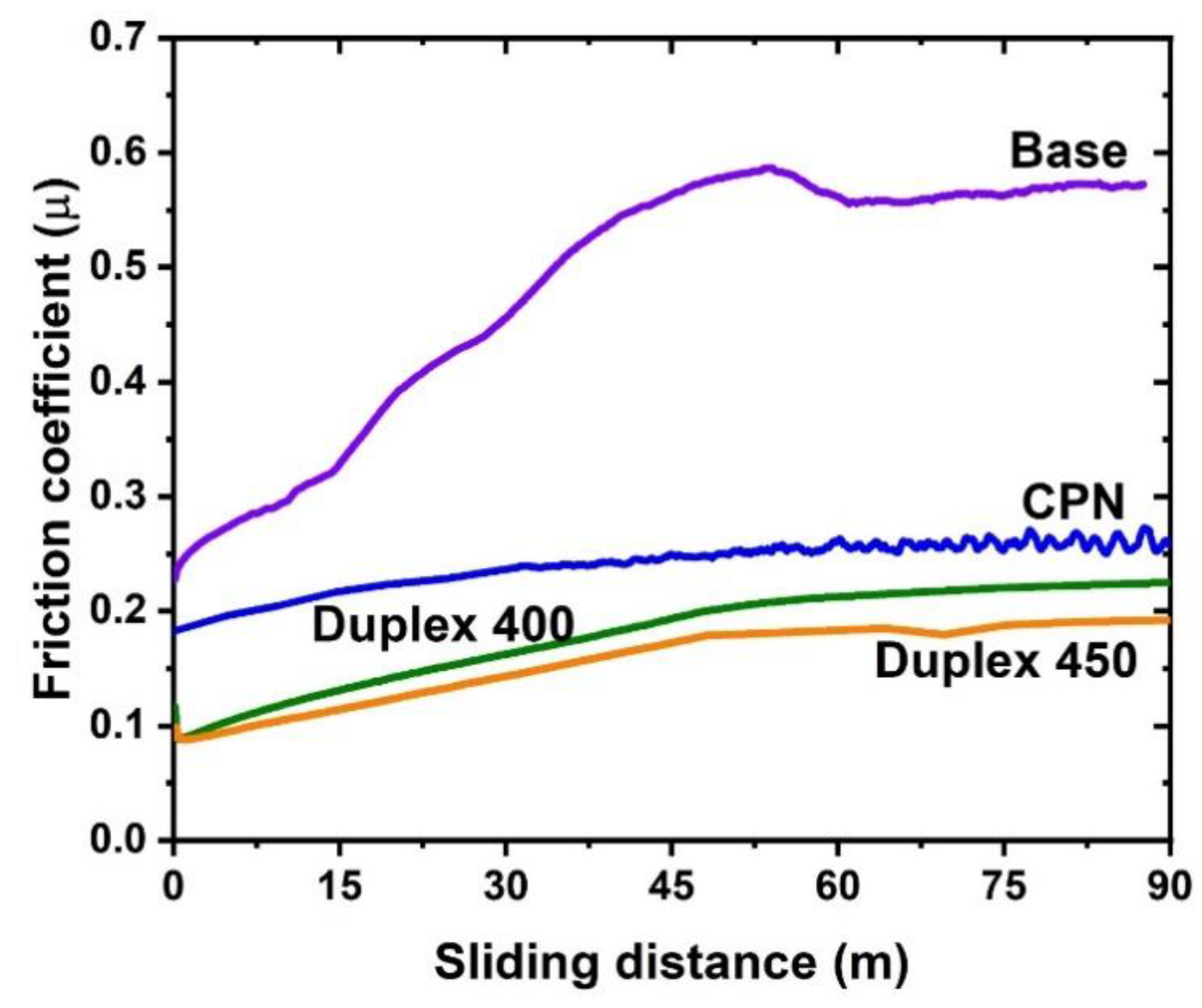
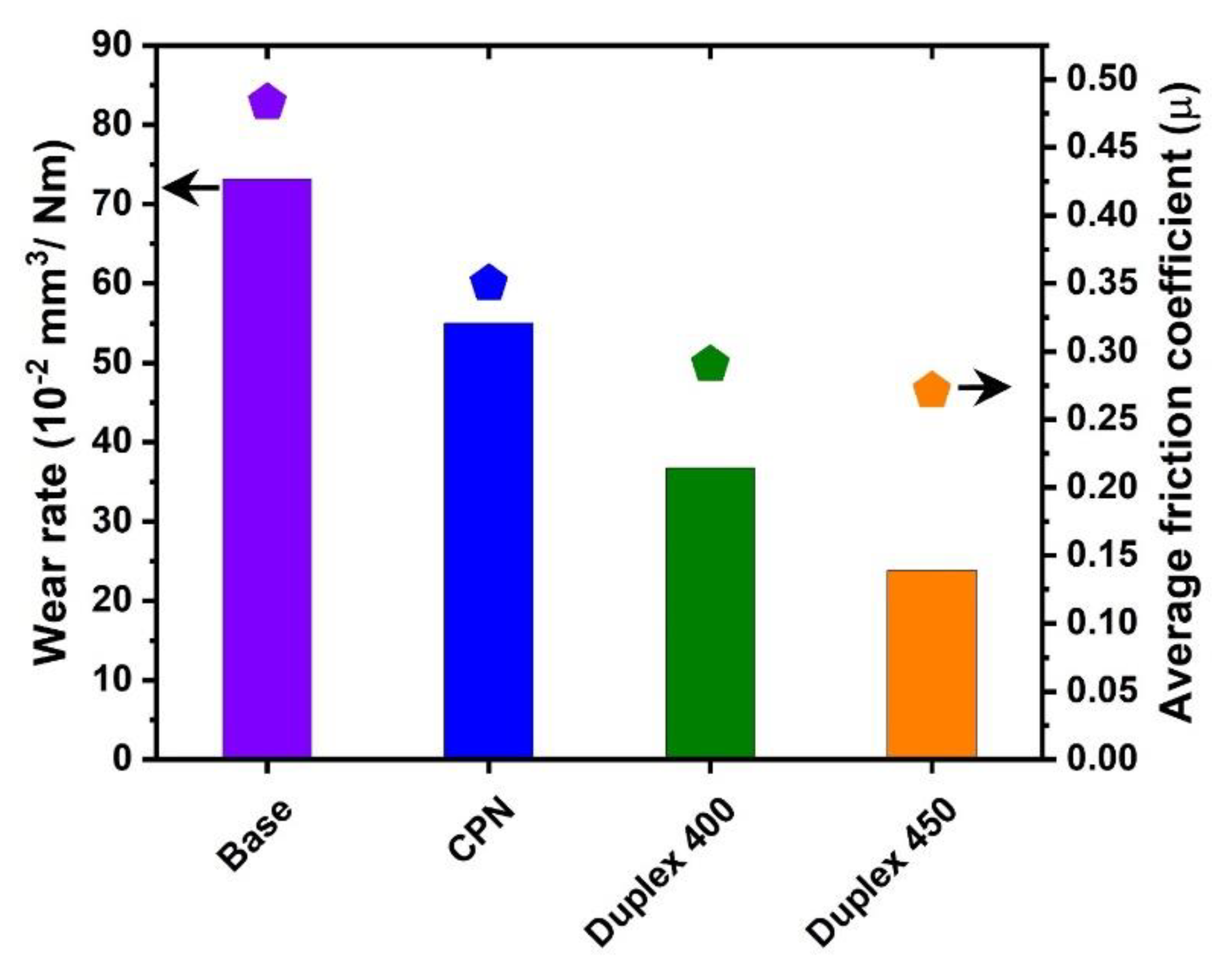
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libório, M.S.; Praxedes, G.B.; Lima, L.L.F.; Nascimento, I.G.; Sousa, R.R.M.; Naeem, M.; Costa, T.H.; Alves, S.M.; Iqbal, J. Surface modification of M2 steel by combination of cathodic cage plasma deposition and magnetron sputtered MoS2-TiN multilayer coatings. Surf. Coat. Technol. 2020, 384, 125327. [Google Scholar] [CrossRef]
- Nascimento, I.O.; Naeem, M.; Freitas, R.S.; Nascimento, R.M.; Viana, B.C.; Sousa, R.R.M.; Feitor, M.C.; Iqbal, J.; Costa, T.H.C. Comparative study of structural and stoichiometric properties of titanium nitride films deposited by cathodic cage plasma deposition and magnetron sputtering. Eur. Phys. J. Plus 2022, 137, 319. [Google Scholar] [CrossRef]
- daS Rocha, A.; Strohaecker, T.; Hirsch, T. Effect of different surface states before plasma nitriding on properties and machining behavior of M2 high-speed steel. Surf. Coat. Technol. 2003, 165, 176–185. [Google Scholar] [CrossRef]
- Bonu, V.; Srinivas, G.; Kumar, V.P.; Joseph, A.; Narayana, C.; Barshilia, H.C. Temperature dependent erosion and Raman analyses of arc-deposited H free thick DLC coating on Cr/CrN coated plasma nitrided steel. Surf. Coat. Technol. 2022, 436, 128308. [Google Scholar] [CrossRef]
- Das, K.; Alphonsa, J.; Ghosh, M.; Ghanshyam, J.; Rane, R.; Mukherjee, S. Influence of pretreatment on surface behavior of duplex plasma treated AISI H13 tool steel. Surf. Interfaces 2017, 8, 206–213. [Google Scholar] [CrossRef]
- Alsaran, A.; Altun, H.; Karakan, M.; Celik, A. Effect of post-oxidizing on tribological and corrosion behaviour of plasma nitrided AISI 5140 steel. Surf. Coat. Technol. 2004, 176, 344–348. [Google Scholar] [CrossRef]
- Díaz-Guillén, J.C.; Naeem, M.; Hdz-García, H.M.; Acevedo-Davila, J.L.; Díaz-Guillén, M.R.; Khan, M.A.; Iqbal, J.; Mtz-Enriquez, A.I. Duplex plasma treatment of AISI D2 tool steel by combining plasma nitriding (with and without white layer) and post-oxidation. Surf. Coat. Technol. 2020, 385, 125420. [Google Scholar] [CrossRef]
- Balashabadi, P.; Larijani, M.M.; Shokri, A.A.; Jafari-Khamse, E.; Seyedi, H.; Eshghi, S. The effect of bias voltage on microstructure and hardness of TiN films grown by ion coating deposition. Eur. Phys. J. Plus 2015, 130, 1–10. [Google Scholar] [CrossRef]
- Guha, S.; Das, S. Investigation over effect of different carbon content on various properties of titanium carbon nitride (TiCN) coating grown on Si (100) substrate by chemical vapor deposition (CVD) process. Eur. Phys. J. Plus 2022, 137, 363. [Google Scholar] [CrossRef]
- Smagoń, K.; Stach, S.; Ţălu, Ş.; Arman, A.; Achour, A.; Luna, C.; Ghobadi, N.; Mardani, M.; Hafezi, F.; Ahmadpourian, A.; et al. Studies of the micromorphology of sputtered TiN thin films by autocorrelation techniques. Eur. Phys. J. Plus 2017, 132, 1–15. [Google Scholar] [CrossRef]
- Libório, M.S.; Almeida, E.O.; Alves, S.M.; Costa, T.H.C.; Feitor, M.C.; Nascimento, R.M.; Sousa, R.R.M.; Naeem, M.; Jelani, M. Enhanced surface properties of M2 steel by plasma nitriding pre-treatment and magnetron sputtered TiN coating. Int. J. Surf. Sci. Eng. 2020, 14, 288–306. [Google Scholar] [CrossRef]
- Edenhofer, B. Physical and Metallurgical Aspects of Ionitriding. Pt. 1 and Pt. 2. In Heat Treatment Metals; Wolfson Heat Treatment Centre: Birmingham, UK, 1974. [Google Scholar]
- Li, C.; Georges, J.; Li, X. Active screen plasma nitriding of austenitic stainless steel. Surf. Eng. 2002, 18, 453–457. [Google Scholar] [CrossRef]
- Li, C.; Bell, T. Sliding wear properties of active screen plasma nitrided 316 austenitic stainless steel. Wear 2004, 256, 1144–1152. [Google Scholar] [CrossRef]
- Lin, K.; Li, X.; Tian, L.; Dong, H. Active screen plasma surface co-alloying of 316 austenitic stainless steel with both nitrogen and niobium for the application of bipolar plates in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 10281–10292. [Google Scholar] [CrossRef] [Green Version]
- Naeem, M.; Shafiq, M.; Zaka-ul-Islam, M.; Bashir, M.I.; Díaz-Guillén, J.C.; Lopez-Badillo, C.M.; Zakaullah, M. Novel duplex cathodic cage plasma nitriding of non-alloyed steel using aluminum and austenite steel cathodic cages. J. Alloys Compd. 2017, 721, 307–311. [Google Scholar] [CrossRef]
- Fernades, F.; Filho, E.R.; Souza, I.; Nascimento, I.; Sousa, R.; Almeida, E.; Feitor, M.; Costa, T.; Naeem, M.; Iqbal, J. Novel synthesis of copper oxide on fabric samples by cathodic cage plasma deposition. Polym. Adv. Technol. 2020, 31, 520–526. [Google Scholar] [CrossRef]
- Morell-Pacheco, A.; Kim, H.; Wang, T.; Shiau, C.H.; Balerio, R.; Gabriel, A.; Shao, L. Ni coating on 316L stainless steel using cage plasma treatment: Feasibility and swelling studies. J. Nucl. Mater. 2020, 540, 152385. [Google Scholar] [CrossRef]
- Da Silva, S.S.; Bottoni, R.; Gontijo, L.C.; Ferreira, S.O. Plasma deposition of titanium nitride thin films under the effect of hollow cathode length in cathodic cage. Matéria 2017, 22, 1–12. [Google Scholar]
- Podgornik, B.; Vižintin, J.; Wänstrand, O.; Larsson, M.; Hogmark, S.; Ronkainen, H.; Holmberg, K. Tribological properties of plasma nitrided and hard coated AISI 4140 steel. Wear 2001, 249, 254–259. [Google Scholar] [CrossRef]
- Sprute, T.; Tillmann, W.; Grisales, D.; Selvadurai, U.; Fischer, G. Influence of substrate pre-treatments on residual stresses and tribo-mechanical properties of TiAlN-based PVD coatings. Surf. Coat. Technol. 2014, 260, 369–379. [Google Scholar] [CrossRef]
- De Las Heras, E.; Egidi, D.A.; Corengia, P.; González-Santamaría, D.; García-Luis, A.; Brizuela, M.; López, G.A.; Martinez, M.F. Duplex surface treatment of an AISI 316L stainless steel; microstructure and tribological behaviour. Surf. Coat. Technol. 2008, 202, 2945–2954. [Google Scholar] [CrossRef]
- Bashir, M.I.; Shafiq, M.; Naeem, M.; Zaka-ul-Islam, M.; Díaz-Guillén, J.C.; Lopez-Badillo, C.M.; Zakaullah, M. Enhanced surface properties of aluminum by PVD-TiN coating combined with cathodic cage plasma nitriding. Surf. Coat. Technol. 2017, 327, 59–65. [Google Scholar] [CrossRef]
- Sousa, R.R.M.D.; Moura, Y.J.L.; Sousa, P.A.O.D.; Medeiros Neto, J.Q.; Costa, T.H.D.C.; Alves Junior, C. Nitriding of AISI 1020 steel: Comparison between conventional nitriding and nitriding with cathodic cage. Mater. Res. 2014, 17, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.G.C.; Viana, B.C.; Santos, F.E.P.; Fernandes, F.; Feitor, M.C.; Costa, T.H.C.; Naeem, M.; Sousa, R.R.M. Surface modification of tool steel by cathodic cage TiN deposition. Surf. Eng. 2021, 37, 334–342. [Google Scholar] [CrossRef]
- Diaz-Guillen, J.C.; Naeem, M.; Acevedo-Davila, J.L.; Hdz-Garcia, H.M.; Iqbal, J.; Khan, M.A.; Mayen, J. Improved Mechanical Properties, Wear and Corrosion Resistance of 316L Steel by Homogeneous Chromium Nitride Layer Synthesis Using Plasma Nitriding. J. Mater. Eng. Perform. 2020, 29, 877–889. [Google Scholar] [CrossRef]
- Araujo, A.G.F.; Naeem, M.; Araujo, L.N.M.; Costa, T.H.C.; Khan, K.H.; Díaz-Guillén, J.C.; Iqbal, J.; Liborio, M.S.; Sousa, R.R.M. Design, manufacturing and plasma nitriding of AISI-M2 steel forming tool and its performance analysis. J. Mater. Res. Technol. 2020, 9, 14517–14527. [Google Scholar] [CrossRef]
- Moreno-Bárcenas, A.; Alvarado-Orozco, J.M.; Carmona, J.G.; Mondragón-Rodríguez, G.C.; González-Hernández, J.; García-García, A. Synergistic effect of plasma nitriding and bias voltage on the adhesion of diamond-like carbon coatings on M2 steel by PECVD. Surf. Coat. Technol. 2019, 374, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Serna, M.M.; Rossi, J.L. MC complex carbide in AISI M2 high-speed steel. Mater. Lett. 2009, 63, 691–693. [Google Scholar] [CrossRef]
- Alves, C., Jr.; De Araújo, F.O.; Ribeiro, K.J.B.; Da Costa, J.A.P.; Sousa, R.D.; De Sousa, R.S. Use of cathodic cage in plasma nitriding. Surf. Coat. Technol. 2006, 201, 2450–2454. [Google Scholar] [CrossRef]
- Saeed, A.; Khan, A.W.; Jan, F.; Abrar, M.; Khalid, M.; Zakaullah, M. Validity of “sputtering and re-condensation” model in active screen cage plasma nitriding process. Appl. Surf. Sci. 2013, 273, 173–178. [Google Scholar] [CrossRef]
- Hubbard, P.; Partridge, J.G.; Doyle, E.D.; McCulloch, D.G.; Taylor, M.B.; Dowey, S.J. Investigation of nitrogen mass transfer within an industrial plasma nitriding system I: The role of surface deposits. Surf. Coat. Technol. 2010, 204, 1145–1150. [Google Scholar] [CrossRef]
- Gallo, S.C.; Dong, H. On the fundamental mechanisms of active screen plasma nitriding. Vacuum 2009, 84, 321–325. [Google Scholar] [CrossRef]
- Fraczek, T.; Ogorek, M.; Skuza, Z.; Prusak, R. Mechanism of ion nitriding of 316L austenitic steel by active screen method in a hydrogen-nitrogen atmosphere. Int. J. Adv. Manuf. Technol. 2020, 109, 1357–1368. [Google Scholar] [CrossRef]
- Rad, H.F.; Amadeh, A.; Moradi, H. Wear assessment of plasma nitrided AISI H11 steel. Mater. Des. 2011, 32, 2635–2643. [Google Scholar]
- Hoshiyama, Y.; Chiba, K.; Maruoka, T. Effect of Active Screen Plasma Nitriding on Mechanical Properties of Spheroidal Graphite Cast Iron. Metals 2021, 11, 412. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, M. Microstructure and wear resistance of in situ formed duplex coating fabricated by plasma nitriding Ti coated 2024 Al alloy. J. Mater. Sci. Technol. 2014, 30, 1278–1283. [Google Scholar] [CrossRef]



| Si | Mn | Co | W | V | Mo | Cr | C | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.45 | 0.3 | 1 | 6.1 | 1.9 | 5 | 4.1 | 0.8 | Balance |
| Conventional Plasma Nitriding | Cathodic Cage Plasma Deposition | Sample Labeling | ||||||
|---|---|---|---|---|---|---|---|---|
| Gases (sccm) | Pressure (Pa) | Time (h) | Temperature (°C) | Gases (sccm) | Pressure (Pa) | Time (h) | Temperature (°C) | |
| Quenching and tempering | Base | |||||||
| 30 H2−10N2 | 350 | 4 | 400 | CPN | ||||
| 30 H2−10N2 | 350 | 4 | 400 | 30 H2 − 10N2 | 350 | 4 | 400 | Duplex 400 |
| 30 H2−10N2 | 350 | 4 | 400 | 30 H2 − 10N2 | 350 | 4 | 450 | Duplex 450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Abreu, L.H.P.; Naeem, M.; Monção, R.M.; Costa, T.H.C.; Díaz-Guillén, J.C.; Iqbal, J.; Sousa, R.R.M. The Effect of Cathodic Cage Plasma TiN Deposition on Surface Properties of Conventional Plasma Nitrided AISI-M2 Steel. Metals 2022, 12, 961. https://doi.org/10.3390/met12060961
de Abreu LHP, Naeem M, Monção RM, Costa THC, Díaz-Guillén JC, Iqbal J, Sousa RRM. The Effect of Cathodic Cage Plasma TiN Deposition on Surface Properties of Conventional Plasma Nitrided AISI-M2 Steel. Metals. 2022; 12(6):961. https://doi.org/10.3390/met12060961
Chicago/Turabian Stylede Abreu, Luiz Henrique Portela, Muhammad Naeem, Renan Matos Monção, Thercio H. C. Costa, Juan C. Díaz-Guillén, Javed Iqbal, and Rômulo Ribeiro Magalhães Sousa. 2022. "The Effect of Cathodic Cage Plasma TiN Deposition on Surface Properties of Conventional Plasma Nitrided AISI-M2 Steel" Metals 12, no. 6: 961. https://doi.org/10.3390/met12060961
APA Stylede Abreu, L. H. P., Naeem, M., Monção, R. M., Costa, T. H. C., Díaz-Guillén, J. C., Iqbal, J., & Sousa, R. R. M. (2022). The Effect of Cathodic Cage Plasma TiN Deposition on Surface Properties of Conventional Plasma Nitrided AISI-M2 Steel. Metals, 12(6), 961. https://doi.org/10.3390/met12060961






