Abstract
In this work, selective laser melting (SLM) technology was used to prepare Mg-4Y-3Nd-Zr (WE43) alloy. This alloy and production method are promising for the design of biodegradable implants. The aim of this study was to investigate the chemical composition, microstructure, mechanical properties, corrosion behavior in simulated body fluid (SBF), and cytotoxicity of the alloy produced by SLM method and to compare it with conventionally gravity cast reference alloy. Analysis of the surface of the revealed an oxygen content of 7 wt.%. Undesirable unmelted and only partially adhered spherical particles of the starting powder were also found. The microstructure of the material was very fine and consisted of α-Mg dendritic matrix, β-Mg41(Nd, Y)5 intermetallic phase, Y2O3 inclusions, and 0.6 vol.% of residual porosity. The Vickers hardness, compressive yield strength, compressive strength, and maximum compressive strain were 88 HV0.1, 201 MPa, 394 MPa, and 14%, respectively, which are close to the reference values in as-cast. The in vitro corrosion rates determined by immersion and potentiodynamic tests were 2.6 mm/year and 1.3 mm/year, respectively. Cytotoxicity tests indicated good biocompatibility of the 3D-printed alloy.
1. Introduction
Magnesium and its alloys are interesting technical materials. Thanks to its excellent physical and chemical properties such as low density, high specific strength, good damping performance, it is widely used in the automotive and aerospace industries [1]. Magnesium has also been widely studied for applications in medicine. In the medical field, good biocompatibility of Mg is important, but also good mechanical properties and Young’s modulus, which is very similar to that of human bone [2,3,4,5]. Magnesium is one of the most important elements in the human body, so it is non-toxic to the human body and degrades spontaneously in the body, and it can be used as a biodegradable material for implants, especially for bone fixation, so that reoperation after a fracture is not necessary. It can be also used as a cardiovascular stent [2,6,7,8,9].
One of the commonly used commercial magnesium alloys is an alloy containing rare earth (REE) elements, such as Nd, Y, Dy, Gd, and Zr, referred to as Mg-4Y-3RE-Zr or WE43 alloy. This alloy shows a good combination of strength and plasticity and increased corrosion resistance [10,11]. The corrosion resistance is due to the addition of rare earth elements, especially yttrium, which forms a protective layer of Y2O3 slowing down the diffusion and transport element between the material and the corrosive environment [6,12,13]. However, this protection only works if sufficient Y is present in the alloy, otherwise localized forms of corrosion can occur [12]. In the case of magnesium and magnesium alloys it is very necessary to avoid impurities, especially Fe, Ni, Co, Cu, which significantly worsen the corrosion resistance due to the formation of galvanic microcells [4,11,14]. Another problem with magnesium and its alloys is the excessive amount of hydrogen produced during the corrosion process. Hydrogen gas can cause health complications, slow down the healing process, and reduce mechanical contact between the implant and the tissue [4,15,16]. WE43 alloy is one of the most promising candidates for biodegradable medical implants and is therefore the subject of our study [10,13,17,18].
In the last decades, additive manufacturing (AM) methods have been increasingly used to produce various metal components from stainless steels [1,9,19,20,21,22,23], Al [1,24,25,26], Ti [1,9,26,27,28,29,30,31], Ni [1,26,31], Co [9], alloys, and other [1,9]. These methods allow each product to be designed and adapted to the specific requirements. A large variety of product sizes and shapes can also be achieved with AM. Among additive manufacturing methods, selective laser melting (SLM) is widely used in industry. The SLM method is based on computer-controlled laser melting of powder layers, which gradually form the desired product in layers. Important processing parameters that determine the product properties are laser power, scanning speed, and hatch distance. To prevent oxidation of the material, an inert gas-argon or nitrogen-must be supplied to the working chamber [10,23,32,33].
SLM technology for various industrial materials and components has been well established in the past. However, to the best of our knowledge, the number of reports on SLM produced Mg-based alloys is still limited due to difficulties in handling and processing highly reactive Mg-based powders [3,18,34]. Therefore, a comprehensive study of SLM-prepared WE43 alloy is reported in this work. The study includes structural and chemical characterization, mechanical and corrosion analysis, as well as cytotoxicity investigation.
2. Materials and Methods
2.1. Material Preparation
In this study, WE43 alloy prepared by SLM method was investigated. The input material was WE43 powder atomized with an inert gas (the analysis of this powder is presented in [35,36]. The bulk material was produced from powder by SLM process on an SLM 280HL machine (SLM Solutions Group AG, Lübeck, Germany) equipped with YLR-Laser (IPG Photonics, Oxford, MA, USA) with a wavelength of 1060 nm. The laser beam had a Gaussian profile with a focus of 82 µm. The printing process was carried out under argon atmosphere flow to keep the oxygen value below 0.2%. Cube samples of 8 × 8 × 8 mm in size (Figure 1) were printed with a laser power of 175 W, a scanning speed of 800 mm/s, a hatching distance of 90 µm, and a melted layer thickness of 50 µm. The samples were cut into smaller pieces that were used for microstructural characterization, mechanical, corrosion, and biological testing. As-cast ingot purchased from an industrial supplier was used as a reference material.
Figure 1.
Sample produced by SLM method.
2.2. Microstructure Characterization
A standard metallographic procedure according to EN ISO 10993-18 standard was used to prepare samples for microstructural characterization. The samples were ground on SiC grinding papers of grain size P800-P2500, polished on D2 diamond paste and finally polished on Etosil E silica suspension.
The microstructure was observed using a TESCAN VEGA-3 LMU scanning electron microscope equipped with an EDS analyzer (Aztec-Oxford Instruments, Brno, Czech Republic). Porosity was evaluated on several cross-sections by image analysis in ImageJ software (NIH, Rockville, MD, USA).
More detailed microstructural observation were carried out using a Jeol 2200FS transmission electron microscope (JEOL Ltd., Tokyo, Japan). Samples were cut with a diamond spark (perpendicular to the built-up direction) from the sample of material in thin slices of about 0.5 mm thickness, which were then ground to a thickness of 50 µm. Circular samples of 3 mm diameter were cut from the thinned slices. The samples thus prepared were further thinned in the center to a final thickness of 10 µm using a Gatan Dimple Grinder Model 656 (JEOL Ltd., Tokyo, Japan). These samples were then ion polished using a Gatan PIPS model 691, which allowed areas of the material to be observed by TEM.
2.3. Mechanical Testing
The hardness was measured by the Vickers method according to EN ISO 6507-1 standard at a load of 100 g (HV 0.1) and a loading time of 10–15 s. Twenty measurements were taken for each sample and the mean values and standard deviations were calculated. The mechanical properties in compression were measured on samples with dimensions 3.5 × 3.5 × 6.5 mm according to EN ISO 604 using the universal LabTest 5.250SP1_VM machine. A constant strain rate of 0.001 s−1 was used for the compression tests.
2.4. Corrosion Properties
Two corrosion tests were performed on the samples—potentiodynamic and immersion test. Both measurements were carried out in SBF (simulated body fluid) prepared according to Müller—Table 1.

Table 1.
Composition of SBF used in corrosion tests.
An SBF volume of 100 mL per 1 cm2 of sample surface was used for the immersion test. The samples were fixed in plastic holders and immersed in SBF for 3 days at 37 °C. The corrosion rate was evaluated based on the weight changes after the removal of corrosion products according to standard EN ISO 10993-15. Four measurements were performed for each material. Potentiodynamic measurements were performed in a corrosion cell using a conventional three-electrode circuit, with the sample used as a working electrode. A platinum wire was used as the counter electrode and a saturated calomel electrode (SCE) was used as the reference electrode. The measurements were carried out at room temperature on a Gamry PC3 instrument. The following electrochemical tests were performed: measurement of the free corrosion potential (Ekorr) for 60 min, measurement of the polarization resistance and measurement of the potentiodynamic curve. The polarization resistance was measured in the range ±20 mV vs. Ekorr at a scan rate of 0.1 mV s−1. Polarization resistance and corrosion rate were calculated according to Equations (1)–(3), where ba and bc represent the anodic and cathodic Tafel slope, respectively [37].
The cathodic part of the curve was measured at the conditions: 0.2 V vs. Ekorr to −2.5 V vs. Ereff. The anodic part of the curve was measured at the conditions: −0.2 V vs. Ekorr to −0.6 V vs. Ereff. The scan rate for both parts of the potentiodynamic curves was 1 mV s−1. Subsequently, the corrosion products after immersion testing were analyzed by SEM/EDS.
2.5. Cytotoxicity Test
The in vitro cytotoxicity test (elution test) was performed accordance to ISO 10993-5. Samples (6 × 7.5 × 7.5 mm) were sterilized in 70% ethanol (2 h) and under UV light (2 h). Two alloy samples were transferred in MEM cultivation medium (Minimal Essential Medium, Sigma, M0446) with 5% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA, F7524) and agitated (130 RPM) at 37 °C in closed vessels for 24 h. The reduced concentration of FBS was used as recommended in the standard (FBS can mask the toxicity). The surface-to-volume ratio was 87.5 mm2 per mL because for degradable alloys, it is advisable [38] to use higher S/V than recommended in the standard.
Murine fibroblasts L929 (ATCC® CCL-1™) were maintained in MEM (Sigma, M0446) medium with 10% FBS at standard conditions. Prior to the exposition, murine fibroblasts L929 were trypsinized and resuspended in MEM + 10% FBS to create a suspension with a concentration of 1 × 105 cells per mL. Thereafter, 100 µL of the cell suspension was seeded into a 96-well plate, which means the seeding density of 1 × 104 cells per well.
After one day, the medium was replaced by the extracts prepared as described above. Extracts were used either in an undiluted form (100% extracts) or diluted with the medium (50 and 25% extracts). Hexaplicates were used for each sample. Sole MEM with 5% FBS served as a control (untreated cells). After one day of incubation with the extracts, cell metabolic activity was evaluated using resazurin assay [39]. Extracts were removed, wells were washed with PBS (phosphate buffered saline), and resazurin solution (final concentration 25 µg/mL) in MEM + 10% FBS without phenol red was added. After 2 h of incubation, fluorescence at 560/590 nm (excitation/emission) was measured. Cytotoxicity of the extracts was depicted as a percentage of metabolic activity of the control. Extracts causing the decrease below 70% of the activity of the control (untreated cells) are considered cytotoxic, as described in the standard ISO 10993-5.
The concentration of released ions was measured by ICP-MS. Ultrapure HNO3 was added to the extracts (10 µL/mL) prior to the measurement.
3. Results and Discussion
3.1. Microstructure and Chemical Composition
The microstructure of the WE43 alloy in the as-cast condition is illustrated in Figure 2. It can be seen that this material has a relatively coarse microstructure composed of α-Mg dendrites (dark) and eutectic mixture (light) in the interdendritic regions. According to EDS and XRD analysis (not shown), the eutectic is mainly enriched in Nd and contains α-Mg + β-Mg41Nd5 phases [36,40]. In the β intermetallic phase, Nd is partially substituted by Y and other RE elements and can thus be written as β-Mg41(Nd, Y)5. The coarse structure results from the slow cooling of the melt during gravity casting. The porosity of the alloy in the as-cast state was not detected.
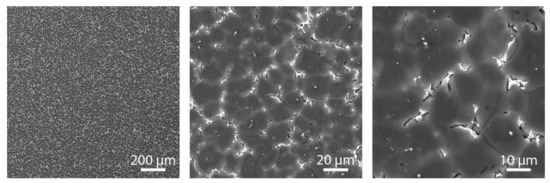
Figure 2.
Microstructure of the reference as-cast WE43 alloy at different magnifications.
Figure 3 shows the surface of the 3D-printed samples studied. It can be seen from the figure that the surface contains unmelted and only partially attached powder particles. Such particles are often found on the surface of alloys produced by SLM [24,41,42,43,44]. In these areas, the powder material was only partially melted by the laser beam and the connection with the previous layer was insufficient. This is the so-called balling effect, which results from (i) discontinuous weld mark, (ii) insufficient heating of the powder at the end of the SLM building process, and (iii) oxide coatings on the powder particles that prevent their melting. The powder particles are prone to release from the surface of the material, which can have detrimental effects on the surrounding tissues. In addition, they can also form stress concentrators and negatively affect the mechanical (especially fatigue) properties of the material. For these reasons, they should always be removed from the implant surface by a mechanical means or chemical etching.
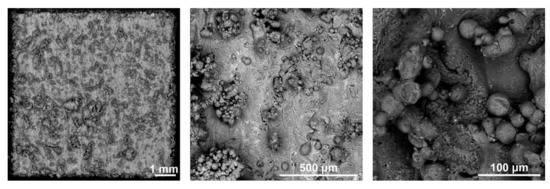
Figure 3.
Surface morphology of the 3D-printed material (SEM).
The element distribution maps in Figure 4 and the EDS analysis of the surface show an increased oxygen concentration (light area) over almost the entire surface of the 3D printed material. Residual oxygen coming from the atmosphere in the 3D printing chamber reacts with both Mg and rare earth metals to form surface oxide skins. EDS analysis showed 7 wt.% O concentration on the surface, but the oxides were not detected by XRD due to their small thickness.
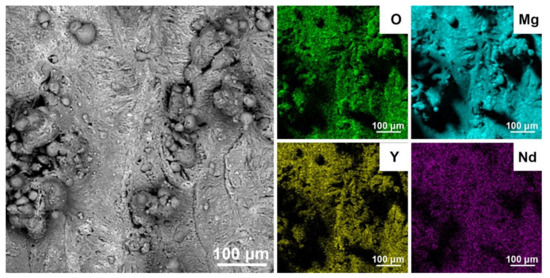
Figure 4.
SEM image and EDS maps of elements distribution on the surface of 3D-printed material.
Figure 5 shows SEM images of a cross-section of the 3D-printed material at different magnifications. The microstructure is very fine and consists of α-Mg matrix (grey), oxide particles (light), and β-Mg41(Nd, Y)5 intermetallic phases (light). In Figure 5, at the highest magnification, the very fine dendritic morphology is visible. The microstructural refinement of the 3D-printed material is a direct consequence of the very high melt cooling rates (up to 106–108 K/s) during the SLM process [32].
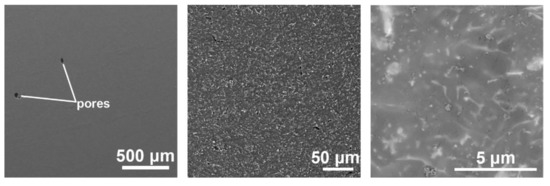
Figure 5.
Microstructure of the 3D-printed material at different magnifications.
The macro view in Figure 5 also shows the presence of residual porosity within the 3D-printed WE43 alloy. The volume fraction of porosity was measured by imaging analysis on several cross-sections of the material and was determined to be 0.6 vol.%. Residual porosity is an intrinsic property of materials produced by SLM and can result from several phenomena such as (i) gas entrapped in the powder during SLM; (ii) local superheating and evaporation of the alloy due to high laser power density; (iii) local imperfect melting and bonding of powder particles due to insufficient laser power density and other factors [10,36,45,46,47,48,49,50]. In our case, the volume of residual porosity is very low, indicating that the laser beam parameters in the SLM process were selected and controlled properly.
A more detailed view and maps of the distribution of elements within the reference as-cast material, where we can see the structure without oxide (Figure 6) and the 3D-printed material (Figure 7) show that there are separate oxide inclusions (light) enriched mainly in Y, which is due to a high affinity of Y for O. The oxides inside the matrix come from (i) the oxidized surface of the starting powder particles and (ii) the reaction of the powder and melt with residual oxygen in the working chamber of the SLM machine. For this reason, the oxide particles have elongated shapes, as will be shown in the detailed TEM view below. The SEM image in Figure 7 also clearly shows the dendritic boundaries with intermetallic phases β-Mg41(Nd, Y)5. In the SEM mode, these boundaries appear lighter than the surrounding Mg matrix due to enrichment of Nd and other RE elements. In addition, very fine light particles of intermetallic phases are observed inside the dendrites as light spots.
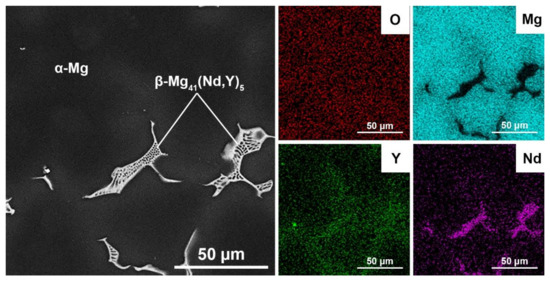
Figure 6.
SEM image and EDS maps of elements distribution inside the reference as-cast material.
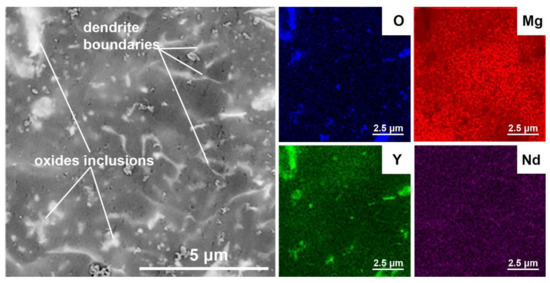
Figure 7.
SEM image and EDS maps of elements distribution inside the 3D-printed material.
The most detailed microstructural observation of the 3D-printed WE43 alloy was performed by TEM + EDS (Figure 8). Figure 8a shows a detail of the microstructure in the vicinity of dendrite boundaries. According to the X-ray elemental maps included in this figure, three structural components are identified: (i) α-Mg matrix dendrites (light); (ii) β-Mg41(Nd, Y)5 particles inside the dendrites and at the dendrite boundaries (dark); and (iii) a very fine mixture of α-Mg + β-Mg41(Nd, Y)5 along the dendrite boundaries. The β-Mg41(Nd, Y)5 intermetallic phases are extremely fine (well below 1 μm). The microstructure of the WE43 alloy in Figure 8a is a direct result of the specific temperature regime during the SLM process. It is conducted in the layer-by-layer manner so that the laser beam melts only a small volume of powder each time. As a result, rapid cooling the melt at rates up to 103–106 K/s occurs during SLM [32,51,52,53]. The rapidly solidified microstructure is therefore extremely fine (Figure 5, Figure 7 and Figure 8) and contains dendrites of super-saturated solid solution α-Mg [11,36,54,55] and interdendritic particles β-Mg41(Nd, Y)5. Afterward, very fine intermetallic phases β-Mg41(Nd, Y)5 precipitate in the solid state inside the dendrites and along their boundaries due to the decomposition of the solid solution of α-Mg supersaturated with RE elements. Precipitation is accelerated by the elevated temperature in the SLM working chamber. The finest β-Mg41(Nd, Y)5 phases are located mainly along the dendrite boundaries where α-Mg supersaturation is highest. Figure 8a,b shows details of the oxide inclusions in the 3D-printed material together with EDS maps of the Mg, O, Y, and Nd elements. The elemental maps show that yttrium oxide dominates in the inclusions. Nd is mainly concentrated in the fine intermetallic phases β-Mg41(Nd, Y)5. In cross-section, the yttrium oxide inclusions have a typical elongated “river-like” shape, suggesting that their origin is in the oxide shells on the particles of the input powder.
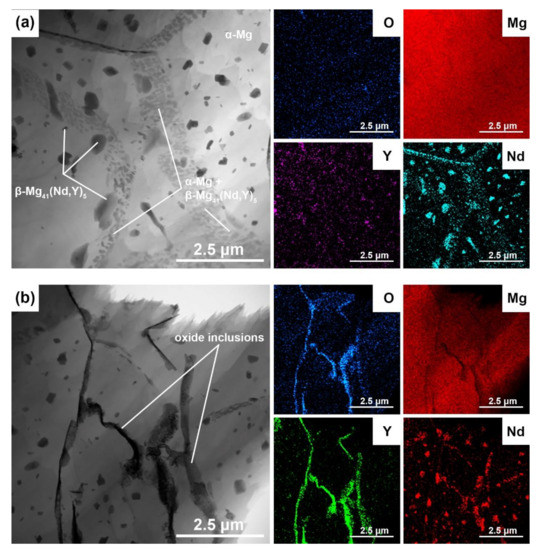
Figure 8.
TEM micrograph and elements distribution maps of the 3D-printed WE43: (a) detail in the vicinity of dendrite boundaries, (b) detail of oxide inclusions.
3.2. Mechanical Properties
Table 2 summarizes the results of the hardness tests. For both materials, 20 measurements were carried out. It can be seen that the hardness of the 3D-printed WE43 alloy is slightly lower compared to the as-cast reference material, despite its refined microstructure (Figure 5, Figure 7 and Figure 8). This difference can be attributed mainly to the presence of oxide particles, which weaken the compactness of the 3D-printed material (Figure 7 and Figure 8b). In addition, the residual porosity observed in the 3D-printed alloy may also play a role (Figure 5).

Table 2.
Vickers hardness HV0.1 (20 measurements) of the 3D-printed material and as-cast reference material.
Figure 9 and Table 3 summarize the results of compression tests. It can be seen that the compressive strength of tested materials shows a similar trend to the hardness, i.e., the 3D-printed material has a slightly lower strength than the reference material as-cast product. From the point of view of compressive plasticity, both materials behave similarly. The decreased compressive strength of the 3D-printed WE43 alloy reflects its microstructural properties, mainly the presence of oxide particles and slight residual porosity (Figure 5, Figure 7 and Figure 8). It is important to note that neither pores nor oxides have a significant effect on the plasticity in compression, as demonstrated by the comparable plasticity of the 3D-printed material to that of the WE43 reference as-cast materials. The 3D-printing SLM technology thus offers a promising alternative to conventional metallurgy in the production of Mg alloy components. 3D printing of Mg alloys has been described in [3,13,45,56]. The authors prepared WE43 alloy by 3D printing and the results of the compression test were comparable to the values reported in this paper. The variation in values depended on the porosity of the material and whether the measurements were taken before or after exposure to the corrosive environment.
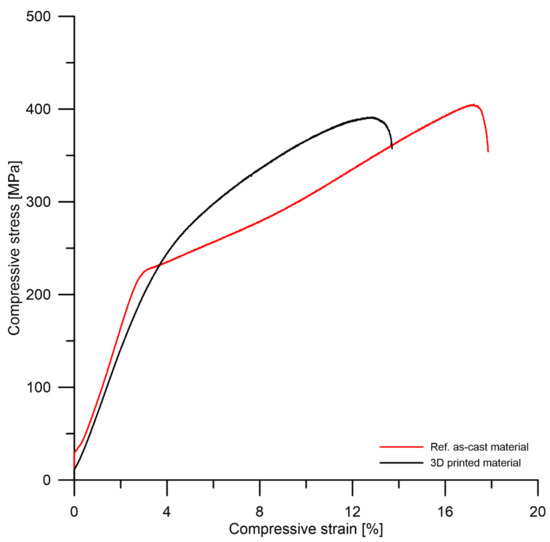
Figure 9.
Compression stress–strain curves of tested materials.

Table 3.
Mechanical compressive properties of the 3D-printed material and as-cast reference material.
3.3. Corrosion Properties
The results of the potentiodynamic measurements are summarized in Figure 10 and Table 4. It can be seen that the corrosion potential of the 3D-printed material is slightly shifted to more positive values compared to the reference as-cast material. This is probably due to the presence of oxides with nobler behavior in the 3D-printed alloy (Figure 5 and Figure 7). The corrosion rates in the SBF solution determined by Tafel extrapolation of the potentiodynamic curves are close to the value 1 mm/year for both tested materials.
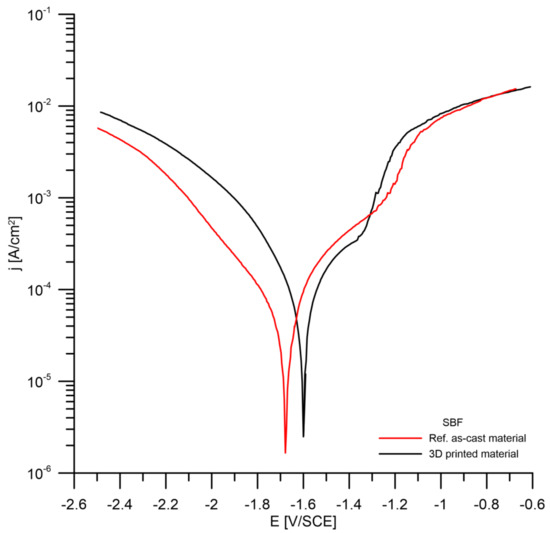
Figure 10.
Potentiodynamic curves of the investigated materials (SBF solution).

Table 4.
Results of potentiodynamic test of 3D-printed and reference material (SBF solution).
The corrosion test was also carried out by immersion testing in SBF and the results are summarized in Table 5. For the reference as-cast material, the corrosion rate is similar to that determined by Tafel extrapolation. In contrast, the 3D-printed alloy shows twice the corrosion rate in the immersion test. The enhanced corrosion rate of the 3D printed alloy is related to the existence of a specific microstructure of this alloy. As shown previously (Figure 5, Figure 7 and Figure 8), the alloy contains yttrium oxide inclusions and residual porosity. Both factors facilitate corrosion attack by creating paths for the corrosion environment to penetrate the material more rapidly.

Table 5.
Results of immersion tests in SBF solution at 37 °C.
The SEM view and EDS analysis of the corrosion products on the 3D-printed material after SBF exposure are presented in Figure 11 and Table 6. The corrosion products are relatively uniformly distributed over the entire surface of the material. Due to the inorganic nature of corrosion products and the drying process before the SEM examination, they contain numerous cracks. The main components of corrosion products are Mg, O, P, and Ca. This means that they consist mainly of mixed calcium-magnesium phosphates and carbonates. Similar findings have been reported by other authors [36,57,58,59,60]. The mechanism of alloy corrosion in SBF includes several chemical reactions, which can be expressed by the following simplified equations [4,15,16,61]:
Mg → Mg2+ + 2e− (anodic reaction)
2H2O + 2e− → 2OH− + H2 (cathodic reaction)
3Mg2+ (Ca2+) + 4OH− + 2H2PO4− → (Mg,Ca)3(PO4)2 + 4H2O (formation of insoluble product)
Mg2+ (Ca2+) + OH− + HCO3− → (Mg,Ca)CO3 + H2O (formation of insoluble product)
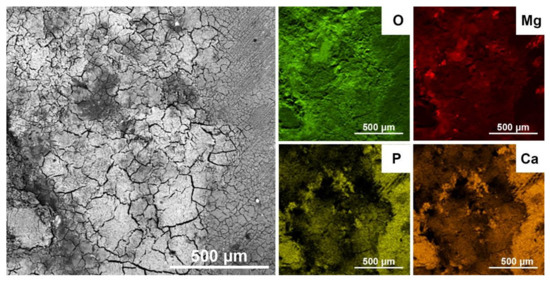
Figure 11.
Surface of the 3D-printed alloy corroded in SBF solution (SEM) and element distribution maps (EDS).

Table 6.
EDS analysis of the corrosion products on the 3D-printed alloy after exposure in SBF EDS analysis of the corrosion products on the 3D-printed alloy after exposure in SBF.
In terms of biocompatibility, the formation of phosphates during corrosion facilitates bone tissue ingrowth and good osseointegration of the 3D-printed implant with bone because of the similarity between the corrosion products and the inorganic part of the human bone [3,5,57,58,62,63]. Also, a corrosion rate of 2.6 mm/year may be acceptable for medical implants such as bone fixation screws. If we consider, for example, a screw with a thickness of 5 mm, its degradation will occur approximately one year after implantation. Therefore, the screw could have a load-bearing capacity for a sufficiently long period of bone tissue healing. The minimum degradation time of a biodegradable implant should not be less than 6–12 months [64]. The biodegradable metallic material should provide sufficient strength to the healing bone for at least 12 weeks, provided, of course, that it is non-toxic and does not have a negative effect on the body [65]. In the case of magnesium alloy implants, the rate of implant degradation should not exceed the rate of healing of the tissue itself, which in an adult is at least 12–18 weeks, to maintain the mechanical integrity of the implant [66].
3.4. In-Vitro Cytotoxicity According to ISO 10993-5
In vitro cytotoxicity of the 3D-printed WE43 alloy was tested according to ISO 10993-5 standard. Results are expressed in Figure 12 as relative metabolic activity of L929 cells after 1 day incubation with 1 day extracts of the alloy. Figure 12 shows that metabolic activity of the cells incubated with 100% extracts of both samples was above the cytotoxicity limit of 70% and, moreover, none of the diluted extracts show any cytotoxicity. Table 7 gives concentrations of released ions in extracts of both samples and pure medium. It is observed that magnesium matrix of the alloy dissolves preferentially in the medium. Concentrations of potentially toxic RE elements is lower than Mg by three orders of magnitude. Magnesium is generally considered as relatively non-toxic element [18,38,60,67]. Cytotoxicity experiments indicate a good biocompatibility of the 3D-printed WE43 alloy.
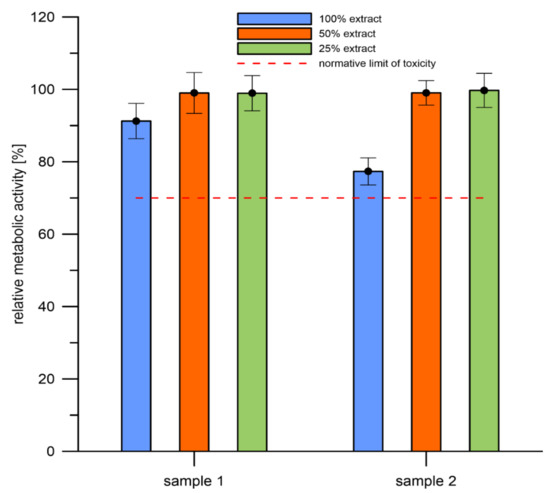
Figure 12.
Relative metabolic activity of the L929 cells (resazurin assay) after 1 day incubation with extracts of the samples. Sole extraction medium served as a control (untreated cells, for which metabolic activity was taken as 100%) (sample 1 and sample 2 are 3D-printed materials).

Table 7.
Concentration of elements in extracts and pure medium (ICP-MS).
The line indicates the normative limit of 70% of the metabolic activity of the control. Error bars stand for the standard error deviation of six replicates.
4. Conclusions
The aim of this work was to provide a comprehensive study of 3D-printed biodegradable alloy WE43, including chemical, microstructural, mechanical, corrosion, and cytotoxicity tests. The main conclusions can be summarized as follows:
- Great attention should be paid to the surface state of the 3D-printed alloy. Unmelted and only partially adherent spherical particles of the initial powder alloy were found on the surface. These particles are undesirable because they are detrimental to the surrounding tissue and negatively affect the mechanical (especially fatigue) properties.
- The microstructure of the material is very fine and contains α-Mg dendritic matrix, β-Mg41(Nd, Y)5 intermetallic phase, Y2O3 inclusions, and 0.6 vol.% of residual porosity.
- Thanks to the fine microstructure, the hardness, compressive yield strength, ultimate compressive strength, and maximum compressive strain showed values similar to those of a conventional casting.
- An in vitro corrosion rate of 2.6 mm/year was obtained in the SBF immersion test. This value may be acceptable for some implant types. Despite the satisfactory results of the corrosion experiments, it seems that further improvement of the corrosion properties of the material is necessary for future applications. This improvement can be achieved, for example, by appropriate control of the SLM process, reduction of the oxidation rate, surface treatment, etc.
- Standard in vitro cytotoxicity tests indicated good biocompatibility of the 3D-printed WE43 alloy studied in this work.
Author Contributions
P.L.: conceptualization, investigation, project administration, writing-original draft; T.L.: investigation, formal analysis, conceptualization, writing—original draft; J.K.: investigation, formal analysis; E.J.: investigation, methodology, formal analysis, conceptualization; Š.M.: investigation, formal analysis; A.M.: investigation, writing—review and editing; D.V.: supervision, methodology, writing-review and editing; J.S.: samples fabrication; D.K.: samples fabrication; E.G.H.A.: formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported from the grant of Specific university research—grant No A1_FCHT_2022_007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data will be provided as request.
Acknowledgments
The authors acknowledge the assistance provided by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2018124.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezer, N.; Evis, Z.; Koç, M. Additive manufacturing of biodegradable magnesium implants and scaffolds: Review of the recent advances and research trends. J. Magnes. Alloy. 2021, 9, 392–415. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, I.; Zhou, J.; Zadpoor, A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.S.; Koga, G.Y.; Avila, J.A.; Bittencourt, I.M.; Fernandez, F.; Miyazaki, M.H.; Botta, W.J.; Bose Filho, W.W. Corrosion resistance of WE43 Mg alloy in sodium chloride solution. Mater. Chem. Phys. 2021, 272, 124930. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Zhou, H.; Li, J.; Li, J.; Ruan, Q.; Peng, X.; Li, S.; Jin, W.; Yu, Z.; Chu, P.K.; Li, W. A composite coating with physical interlocking and chemical bonding on WE43 magnesium alloy for corrosion protection and cytocompatibility enhancement. Surf. Coat. Technol. 2021, 412, 127078. [Google Scholar] [CrossRef]
- Velásquez-García, L.F.; Kornbluth, Y. Biomedical Applications of Metal 3D Printing. Annu. Rev. Biomed. Eng. 2021, 23, 307–338. [Google Scholar] [CrossRef]
- Esmaily, M.; Zeng, Z.; Mortazavi, A.N.; Gullino, A.; Choudhary, S.; Derra, T.; Benn, F.; D’Elia, F.; Müther, M.; Thomas, S.; et al. A detailed microstructural and corrosion analysis of magnesium alloy WE43 manufactured by selective laser melting. Addit. Manuf. 2020, 35, 101321. [Google Scholar] [CrossRef]
- Dvorský, D.; Kubásek, J.; Roudnická, M.; Průša, F.; Nečas, D.; Minárik, P.; Stráská, J.; Vojtěch, D. The effect of powder size on the mechanical and corrosion properties and the ignition temperature of WE43 alloy prepared by spark plasma sintering. J. Magnes. Alloy. 2021, 9, 1349–1362. [Google Scholar] [CrossRef]
- He, W.; Zhang, E.; Yang, K. Effect of Y on the bio-corrosion behavior of extruded Mg–Zn–Mn alloy in Hank’s solution. Mater. Sci. Eng. C 2010, 30, 167–174. [Google Scholar] [CrossRef]
- Gangireddy, S.; Gwalani, B.; Liu, K.; Faierson, E.J.; Mishra, R.S. Microstructure and mechanical behavior of an additive manufactured (AM) WE43-Mg alloy. Addit. Manuf. 2019, 26, 53–64. [Google Scholar] [CrossRef]
- Yang, J.; Blawert, C.; Lamaka, S.V.; Yasakau, K.A.; Wang, L.; Laipple, D.; Schieda, M.; Di, S.; Zheludkevich, M.L. Corrosion inhibition of pure Mg containing a high level of iron impurity in pH neutral NaCl solution. Corros. Sci. 2018, 142, 222–237. [Google Scholar] [CrossRef]
- Kalb, H.; Rzany, A.; Hensel, B. Impact of microgalvanic corrosion on the degradation morphology of WE43 and pure magnesium under exposure to simulated body fluid. Corros. Sci. 2012, 57, 122–130. [Google Scholar] [CrossRef]
- Yu, L.; Subodh, D.; Ian, J.; Yu-Lung, C. Biodegradable magnesium alloys for orthopedic applications. Biomater. Transl. 2021, 2, 214–235. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.U.; Mol, J.M.C.; Weinans, H.; et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Zimowska, M.; Ryl, J.; Zieliński, A.; Osipenko, M.A.; Adamiec, J.; Wrzesińska, A.; Claesson, P.M.; Kurilo, I.I. Aqueous molybdate provides effective corrosion inhibition of WE43 magnesium alloy in sodium chloride solutions. Corros. Sci. 2021, 190, 109664. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Lei, X.; Zhang, L.; Man, C.; Yao, J.; Cheng, X.; Li, X. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Suryawanshi, J.; Prashanth, K.G.; Ramamurty, U. Mechanical behavior of selective laser melted 316L stainless steel. Mater. Sci. Eng. A 2017, 696, 113–121. [Google Scholar] [CrossRef]
- Mohd Yusuf, S.; Nie, M.; Chen, Y.; Yang, S.; Gao, N. Microstructure and corrosion performance of 316L stainless steel fabricated by Selective Laser Melting and processed through high-pressure torsion. J. Alloy. Compd. 2018, 763, 360–375. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Z.; Zeng, X. A comparison on metallurgical behaviors of 316L stainless steel by selective laser melting and laser cladding deposition. Mater. Sci. Eng. A 2017, 685, 265–273. [Google Scholar] [CrossRef]
- Ahmed Obeidi, M.; Uí Mhurchadha, S.M.; Raghavendra, R.; Conway, A.; Souto, C.; Tormey, D.; Ahad, I.U.; Brabazon, D. Comparison of the porosity and mechanical performance of 316L stainless steel manufactured on different laser powder bed fusion metal additive manufacturing machines. J. Mater. Res. Technol. 2021, 13, 2361–2374. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Simonelli, M.; Parry, L.; Ashcroft, I.; Tuck, C.; Hague, R. 3D printing of Aluminium alloys: Additive Manufacturing of Aluminium alloys using selective laser melting. Prog. Mater. Sci. 2019, 106, 100578. [Google Scholar] [CrossRef]
- Buttard, M.; Chehab, B.; Shahani, R.; Robaut, F.; Renou, G.; Tassin, C.; Rauch, E.; Donnadieu, P.; Deschamps, A.; Blandin, J.-J.; et al. Multi-scale microstuctural investigation of a new Al-Mn-Ni-Cu-Zr aluminium alloy processed by laser powder bed fusion. Materialia 2021, 18, 101160. [Google Scholar] [CrossRef]
- Moghimian, P.; Poirié, T.; Habibnejad-Korayem, M.; Zavala, J.A.; Kroeger, J.; Marion, F.; Larouche, F. Metal powders in additive manufacturing: A review on reusability and recyclability of common titanium, nickel and aluminum alloys. Addit. Manuf. 2021, 43, 102017. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, X.J.; Saunders, M.; Suvorova, A.; Zhang, L.C.; Liu, Y.J.; Fang, M.H.; Huang, Z.H.; Sercombe, T.B. A selective laser melting and solution heat treatment refined Al–12Si alloy with a controllable ultrafine eutectic microstructure and 25% tensile ductility. Acta Mater. 2015, 95, 74–82. [Google Scholar] [CrossRef]
- Simonelli, M.; Tse, Y.Y.; Tuck, C. Effect of the build orientation on the mechanical properties and fracture modes of SLM Ti–6Al–4V. Mater. Sci. Eng. A 2014, 616, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, G.; Liang, H.; Gao, C.; Peng, S.; Shen, L.; Shuai, C. Additive manufacturing of bone scaffolds. Int. J. Bioprint. 2019, 5, 148. [Google Scholar] [CrossRef]
- Fousová, M.; Stejskalova, T.; Vojtěch, D. Metallographic and Fractographic Evaluation of 3D-Printed Titanium Samples to Eliminate Unsatisfactory Mechanical Properties. Solid State Phenom. 2017, 270, 212–217. [Google Scholar] [CrossRef]
- Khanna, N.; Zadafiya, K.; Patel, T.; Kaynak, Y.; Rahman Rashid, R.A.; Vafadar, A. Review on machining of additively manufactured nickel and titanium alloys. J. Mater. Res. Technol. 2021, 15, 3192–3221. [Google Scholar] [CrossRef]
- Bär, F.; Berger, L.; Jauer, L.; Kurtuldu, G.; Schäublin, R.; Schleifenbaum, J.H.; Löffler, J.F. Laser additive manufacturing of biodegradable magnesium alloy WE43: A detailed microstructure analysis. Acta Biomater. 2019, 98, 36–49. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Farnoush, H.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. An investigation into interaction between magnesium powder and Ar gas: Implications for selective laser melting of magnesium. Powder Technol. 2018, 333, 252–261. [Google Scholar] [CrossRef]
- Kopp, A.; Derra, T.; Müther, M.; Jauer, L.; Schleifenbaum, J.H.; Voshage, M.; Jung, O.; Smeets, R.; Kröger, N. Influence of design and postprocessing parameters on the degradation behavior and mechanical properties of additively manufactured magnesium scaffolds. Acta Biomater. 2019, 98, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Krištofová, P.; Kubásek, J.; Vojtěch, D.; Paloušek, D.; Suchý, J. Microstructure of the Mg-4Y-3RE-Zr (WE43) Magnesium Alloy Produced by 3D Printing. Manuf. Technol. 2019, 19, 89–94. [Google Scholar] [CrossRef]
- Suchý, J.; Klakurková, L.; Man, O.; Remešová, M.; Horynová, M.; Paloušek, D.; Koutný, D.; Krištofová, P.; Vojtěch, D.; Čelko, L. Corrosion behaviour of WE43 magnesium alloy printed using selective laser melting in simulation body fluid solution. J. Manuf. Process. 2021, 69, 556–566. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Fischer, J.; Pröfrock, D.; Hort, N.; Willumeit, R.; Feyerabend, F. Reprint of: Improved cytotoxicity testing of magnesium materials. Mater. Sci. Eng. B 2011, 176, 1773–1777. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Benink, H.A.; Worzella, T.J.; Minor, L. The Assay Guidance Manual; Cell Viability Assays; Eli Lily and Co., National Center for Advancing Translsational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Kubásek, J.; Vojtěch, D.; Martínek, M. Structural characteristics and elevated temperature mechanical properties of AJ62 Mg alloy. Mater. Charact. 2013, 86, 270–282. [Google Scholar] [CrossRef]
- Zhang, J.; Amini, N.; Morton, D.A.V.; Hapgood, K.P. 3D printing with particles as feedstock materials. Adv. Powder Technol. 2021, 32, 3324–3345. [Google Scholar] [CrossRef]
- Chu, F.; Zhang, K.; Shen, H.; Liu, M.; Huang, W.; Zhang, X.; Liang, E.; Zhou, Z.; Lei, L.; Hou, J.; et al. Influence of satellite and agglomeration of powder on the processability of AlSi10Mg powder in Laser Powder Bed Fusion. J. Mater. Res. Technol. 2021, 11, 2059–2073. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Everitt, N.M.; Maskery, I.; Ashcroft, I.; Tuck, C. Selective laser melting of aluminum alloys. MRS Bull. 2017, 42, 311–319. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Hyer, H.; Zhou, L.; Benson, G.; McWilliams, B.; Cho, K.; Sohn, Y. Additive manufacturing of dense WE43 Mg alloy by laser powder bed fusion. Addit. Manuf. 2020, 33, 101123. [Google Scholar] [CrossRef]
- Zou, J.; Zhu, B.; Zhang, G.; Guo, S.; Xiao, R. Power density effect on the laser beam-induced eruption of spatters in fiber laser keyhole welding. Opt. Laser Technol. 2022, 147, 107651. [Google Scholar] [CrossRef]
- Lazov, L.; Teirumnieks, E.; Karadzhov, T.; Angelov, N. Influence of power density and frequency of the process of laser marking of steel products. Infrared Phys. Technol. 2021, 116, 103783. [Google Scholar] [CrossRef]
- Majeed, A.; Zhang, Y.; Lv, J.; Peng, T.; Atta, Z.; Ahmed, A. Investigation of T4 and T6 heat treatment influences on relative density and porosity of AlSi10Mg alloy components manufactured by SLM. Comput. Ind. Eng. 2020, 139, 106194. [Google Scholar] [CrossRef]
- Tian, C.; Li, X.; Li, H.; Guo, G.; Wang, L.; Rong, Y. The effect of porosity on the mechanical property of metal-bonded diamond grinding wheel fabricated by selective laser melting (SLM). Mater. Sci. Eng. A 2019, 743, 697–706. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.M.; Lau, M.L.; Man, H.C. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Menshikova, S.G.; Shirinkina, I.G.; Brodova, I.G.; Brazhkin, V.V.; Lad’yanov, V.I.; Pushkarev, B.E. Morphological features of crystal growth in the Al87Ni8Y5 alloy on rapid cooling of the melt under high pressure. J. Cryst. Growth 2019, 525, 125206. [Google Scholar] [CrossRef]
- Lejček, P.; Roudnická, M.; Čapek, J.; Dvorský, D.; Drahokoupil, J.; Šimek, D.; Čížek, J.; Svora, P.; Molnárová, O.; Vojtěch, D. Selective laser melting of pure iron: Multiscale characterization of hierarchical microstructure. Mater. Charact. 2019, 154, 222–232. [Google Scholar] [CrossRef]
- Bendoumi, A.; Makuch, N.; Chegroune, R.; Kulka, M.; Keddam, M.; Dziarski, P.; Przestacki, D. The effect of temperature distribution and cooling rate on microstructure and microhardness of laser re-melted and laser-borided carbon steels with various carbon concentrations. Surf. Coat. Technol. 2020, 387, 125541. [Google Scholar] [CrossRef]
- Liu, W.; Ren, X.; Li, N.; Gao, C.; Xiong, H. Rapid directionally solidified microstructure characteristic and fracture behaviour of laser melting deposited Nb–Si–Ti alloy. Prog. Nat. Sci. Mater. Int. 2021, 31, 113–120. [Google Scholar] [CrossRef]
- Fousová, M.; Dvorský, D.; Michalcová, A.; Vojtěch, D. Changes in the microstructure and mechanical properties of additively manufactured AlSi10Mg alloy after exposure to elevated temperatures. Mater. Charact. 2018, 137, 119–126. [Google Scholar] [CrossRef]
- Li, M.; Benn, F.; Derra, T.; Kröger, N.; Zinser, M.; Smeets, R.; Molina-Aldareguia, J.M.; Kopp, A.; Llorca, J. Microstructure, mechanical properties, corrosion resistance and cytocompatibility of WE43 Mg alloy scaffolds fabricated by laser powder bed fusion for biomedical applications. Mater. Sci. Eng. C 2021, 119, 111623. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H.; et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan Sathyaraj, P.; Ravichandran, K.; Sankara Narayanan, T.S.N. Controlling the rate of degradation of Mg using magnesium fluoride and magnesium fluoride-magnesium phosphate duplex coatings. J. Magnes. Alloy. 2021, 10, 295–312. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Mena-Morcillo, E.; Veleva, L.P.; Wipf, D.O. Multi-Scale Monitoring the First Stages of Electrochemical Behavior of AZ31B Magnesium Alloy in Simulated Body Fluid. J. Electrochem. Soc. 2018, 165, C749–C755. [Google Scholar] [CrossRef]
- Bütev Öcal, E.; Esen, Z.; Aydınol, K.; Dericioğlu, A.F. Comparison of the short and long-term degradation behaviors of as-cast pure Mg, AZ91 and WE43 alloys. Mater. Chem. Phys. 2020, 241, 122350. [Google Scholar] [CrossRef]
- Galli, S.; Stocchero, M.; Andersson, M.; Karlsson, J.; He, W.; Lilin, T.; Wennerberg, A.; Jimbo, R. The effect of magnesium on early osseointegration in osteoporotic bone: A histological and gene expression investigation. Osteoporos. Int. 2017, 28, 2195–2205. [Google Scholar] [CrossRef] [Green Version]
- Kowalewicz, K.; Vorndran, E.; Feichtner, F.; Waselau, A.-C.; Brueckner, M.; Meyer-Lindenberg, A. In-Vivo Degradation Behavior and Osseointegration of 3D Powder-Printed Calcium Magnesium Phosphate Cement Scaffolds. Materials 2021, 14, 946. [Google Scholar] [CrossRef] [PubMed]
- Vojtěch, D.; Kubásek, J.; Čapek, J.; Pospíšilová, I. Magnesium zinc and iron alloys for medical applications in biodegradable implants. In Proceedings of the 23rd International Conference on Metallurgy and Materials, Brno, Czech Republic, 21–23 May 2014. [Google Scholar]
- Charyeva, O.; Dakischew, O.; Sommer, U.; Heiss, C.; Schnettler, R.; Lips, K.S. Biocompatibility of magnesium implants in primary human reaming debris-derived cells stem cells in vitro. J. Orthop. Traumatol. 2016, 17, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).