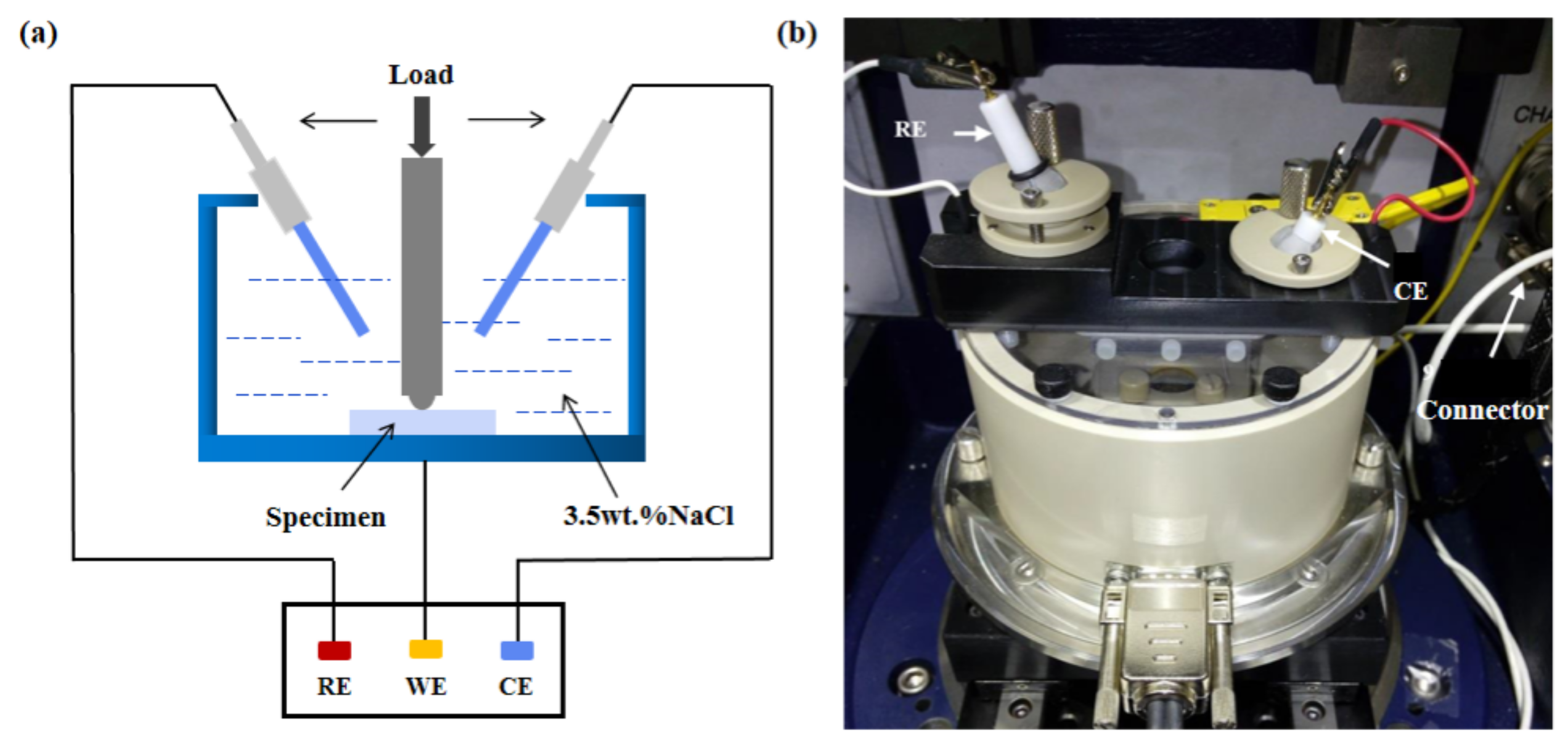
3.1. Electrochemical Behavior
The tribocorrosion experiments of CoCrNi alloy sliding against Si
3N
4 were carried out in 3.5 wt.% NaCl solution under 4 N normal load. The changes of OCP overtime during the whole process are shown in
Figure 2. The OCP remained in the stable range, and the CoCrNi surface was passivated for 10 min before the sliding. OCP rapidly shifts negatively after the slide begins. This is due to the fact that the passive film covering the surface of CoCrNi is destroyed under external load after the friction begins, exposing the fresh metal to the solution and increasing the corrosion tendency of the material [
25,
26]. As a mixed potential, OCP reflects the relative state of the passivated area and the non-passivated area, and its value depends on the integrity of the passivating film on the material surface [
27]. The continuous negative shift of CoCrNi potential indicates that the rupture rate of passive film is greater than the regeneration rate, and depassivation is dominant in this condition. In addition, as can be seen from the time range from the 1800 s to 2000 s in
Figure 2, the potential fluctuates seriously during friction but still keeps a declining trend. This is because, in the process of tribocorroison, the mechanical worn (depassivated) area is served as the anode, while the surrounding unworn (passive) areas are served as the cathode, resulting in galvanic coupling, which greatly increases the corrosion rate. After the sliding is finished, OCP starts to increase abruptly for the first 100 s and then more gradually reaches −0.28 V. As many reports have mentioned [
28,
29], this is a result of the reestablishment of damaged passive film on the worn surface [
30,
31,
32]. In conclusion, the potential transfer of CoCrNi during tribocorroison is closely related to the integrity of the passive film. As a thermodynamic judgment basis for metal stability, the continuous negative potential shift indicates that the corrosion resistance of CoCrNi is reduced, which is a typical wear accelerated corrosion phenomenon.
OCP measurement enables gathering the electrochemical state of wear track and can easily evaluate the change of electrochemical state of the metal surface caused by rubbing. However, this measurement technique is insufficient for investigating corrosion kinetics upon friction, which can otherwise be completed by potentiodynamic polarization [
19].
Then, the potentiodynamic polarization curves of CoCrNi under both static and corrosive wear conditions were measured and compared with commercial 316L stainless (Shanghai Lan Dong Industrial, Shanghai, China) steel and Inconel 600 (Shanghai Lan Dong Industrial, Shanghai, China). The corresponding electrochemical parameters obtained by extrapolation according to the standard Tafel were shown in
Table 1. As can be seen from
Figure 3, the three alloys showed a clear active-passive transition in both static and tribocorroison states in NaCl solution, and 316L stainless steel even exhibits secondary passivation under static conditions, The reason is that the passivation layer generated on the surface of 316L was unstable at the beginning, and the dissolution made the corrosion current density rise. The secondary passivation area indicated that the surface of 316L was passivated again. CoCrNi exhibits a wider passivation zone (438 mV) than that of 316L stainless steel (414 mV) and Inconel 600 (371 mV) under static conditions, indicating that the passivating film formed on the CoCrNi surface is more stable. From
Table 1, the corrosion current density of Inconel 600 in the static state is 2.473 μA/cm
2, about twice that in CoCrNi, suggesting that Inconel 600 has a faster corrosion rate and poor corrosion resistance compared with CoCrNi under static conditions.
The polarization curves under corrosive wear conditions can be used to analyze the corrosion rate and repassivation ability of metals after the passive film is mechanically damaged or removed [
33]. It can be seen from
Figure 3b that, compared with static conditions, the self-corrosion potential of the three alloys is shifted towards a cathodic direction under corrosive wear conditions, which is consistent with the results measured under OCP. In addition, the corrosion current density of the three alloys is found to increase significantly under tribocorrosion, indicating that the presence of wear accelerates corrosion to a further extent. When the potential in the anode region is below the pitting potential, metastable pits will be formed on the worn surface during sliding. The study of Burstein et al. [
34,
35] shows that the formation of metastable pits largely depends on the state of perforated cover composed of corrosion products and any remnant passive film over the pit mouth. In the process of tribocorroison, the perforated cover is repeated in a process of removal and regeneration due to mechanical sliding and passivation, making the concentration of chloride less than the required value for the growth of metastable pits (about 75% of saturation). This leads to a fluctuated current as shown in
Figure 3b. By comparing
Figure 3a,b, the potentiodynamic polarization curve of Inconel 600 showed a rapid increase in current density on the right side of
Epit accompanied by violent current fluctuation, indicating the severe anodic dissolution of Inconel 600 in both conditions. It is worth noting that CoCrNi shows a very wide range of passivation zones (719 mV) during tribocorroison compared to 316L stainless steel (467 mV) and Inconel 600 (348 mV), further demonstrating the superior ability to maintain the passivation of CoCrNi.
3.2. Wear Behavior
Generally speaking, applied potential can change the tribological characteristics of the contact surface, which plays an important role in determining the tribocorrosion behavior of metals. Here, we will evaluate the tribocorroison resistance of the three alloys in the passivation state by exploring the wear volume and friction coefficient.
In view of the difference of the polarization curves of the three alloys under static conditions, the potentiostatic friction tests of CoCrNi and Inconel 600 at −0.2 V and CoCrNi and 316L SS at 0.1 V were compared to ensure a passivated surface state for all alloys. As can be seen from
Figure 4a, at −0.2 V, the mass loss of CoCrNi is about 1/5 lower than that of Inconel 600, showing distinguished wear resistance. In addition, the average friction coefficient of CoCrNi (0.26) is also lower than that of Inconel 600 (0.31). Similarly, compared with 316L SS (
Figure 4b), CoCrNi showed lower mass loss and friction coefficient at 0.1 V. By comparing
Figure 4a,b, it can be seen that, as the applied potential changes from −0.2 V to 0.1 V, the total mass loss of CoCrNi increases by about three times. This is related to the formation of thick passive film on the surface of CoCrNi, which is easy to be destroyed or removed under external loading at high potential, resulting in the increased material loss reminiscent of corrosion accelerated wear. In conclusion, the comparison of friction coefficient and wear volume between the three alloys in the passivated state shows that CoCrNi has better tribological properties in 3.5 wt. % NaCl solution over the others.
To further systematically investigate the influence of potential on the tribocorrosion behavior of CoCrNi alloy in 3.5 wt.% NaCl, seven potentials (−0.8 V, −0.42 V, 0.2 V, OCP, 0 V, 0.1 V and 0.25 V
Ag/AgCl) were selected from potentiodynamic polarization curves, as indicated by
Figure 3a. It must be pointed out that the seven potentials correspond to different surface states of CoCrNi, which is crucial to the analysis of tribocorrosion behaviors. To be specific, −0.8 V corresponds to a cathode potential, which inhibits the corrosion process and then the material loss only depends on the pure mechanical wear. In addition, −0.42 V is the self-corrosion potential of CoCrNi. Under this condition, bare metal will not spontaneously form a passive film nor will polarization occur. The other three potentials of −0.2 V, 0 V, and 0.1 V correspond to the three states at the beginning, middle, and end of the passivation process, respectively, and the anodic dissolution will occur on the alloy surface under a potential of 0.25 V.
Figure 5a shows the evolution of wear volume with different potentials in CoCrNi. It is obvious that the applied potential has a significant effect on the total material loss of CoCrNi alloy, and the wear volume increases with the applied potential. At −0.8 V, the CoCrNi wear volume is about 42,879 μm
3, which is much lower than that under other potentials. There is little difference between the wear amount of CoCrNi under self-corrosion potential and OCP conditions. It is worth noting that, when the applied potential is −0.2 V, the total material loss is only 132,736 μm
3, which is much lower than under the other two passive conditions of 0 V and 0.1 V (45,368 μm
3 and 501,461 μm
3). In anodic polarization tests, material loss is enhanced by the corrosive attack, and the synergistic effect between wear and corrosion results in high volume loss.
The evolutions COF with time are shown in
Figure 5b for CoCrNi at different electrochemical states. The COF reaches a steady-state value exhibiting some peaks at fairly regular time intervals, especially at −0.8 V. Such fluctuations can be attributed to the formation and ejection of debris under pure mechanical wear. In addition, there is a general trend that COF decreases with the increasing potential. At −0.8 V, CoCrNi has a high friction coefficient of about 0.505 reflecting a mechanical wear-dominated friction process [
36]. When the potential is −0.42 V, the friction coefficient decreases first and then stabilizes after the run-in stage, while under OCP, the friction coefficient increases gradually and then stabilizes. This difference of friction coefficient may be related to the passive film formed spontaneously on the CoCrNi surface under OCP conditions. At −0.2 V, the friction coefficient shows obvious undulation in the whole sliding process when compared with conditions at 0 V. This is probably associated with the insufficient passivation on CoCrNi at −0.2 V, but a continuous passive film can be formed at a higher potential. In the friction process, the passive films are easily removed at –0.2 V, and the recovery rate for the passive film is relatively slow (but still greater than that at OCP). The varied area ratio between the depassivated zone and the surrounding passivated zone in the wear track results in the undulation of the friction coefficient.
The morphologies of the wear track under seven applied potential conditions were further obtained via the 3D profilometer(NPFLEX, Bruker, Billerica, MA, USA). The depth and width of the wear track were shown in
Figure 6 and summarized in
Table 2. It has been found that the width and depth of wear track showed an increasing trend with the applied potential, which was consistent with the material loss as shown in
Figure 5a. It is also worth noting that the wear depth marks increase significantly from −0.1 V to 0 V (from 1.99 μm to 3.34 μm), and the wear depth was five times larger for CoCrNi tested at 0.25 V when compared to the values at −0.8 V.
3.3. Morphologies and Component Analysis of the Worn Surfaces
The above results indicate that the deterioration of CoCrNi can be significantly affected by the surface chemical state. To further clarify the effect of potential, surface micrographs were explored after tribocorrosion tests.
Figure 7a shows the overall morphology of the wear track of CoCrNi alloy at −0.8 V potential. It can be seen that CoCrNi forms a thin oxide layer on the wear mark under the condition of cathode polarization and distinct spallation is found as shown in the magnified images (
Figure 7b–d). Some grooves and microcracks parallel to the sliding direction and a small amount of grinding debris can be observed on the surface (
Figure 7e), which indicates that the wear mechanism under this condition mainly includes abrasive wear and oxidation wear. Grooves and spalling are caused by plastic deformation of CoCrNi during tribological load. Under the condition of strong cathodic polarization, corrosion is inhibited, and no protective passive film is formed on the CoCrNi surface. The formation of oxide film in this condition may be caused by the large amount of friction heat generated on the friction surface. The corresponding EDS of
Figure 7a shows that the tribo-film is rich in O, Cr, and Si but depleted with Co and Ni, which indicates that the generated oxide film may be Cr oxide. In addition, the enrichment of Si in the oxides proves that the material transfer from Si
3N
4 counterpart occurs during the sliding process, which also indicates an adhesive wear and the impressive wear resistance of CoCrNi at this condition. XPS test results will be used to analyze the specific composition of the oxide layer.
The worn surface of CoCrNi tested at the self-corrosive potential is shown in
Figure 8. By analyzing the characteristic areas in the wear tracks (
Figure 8b,c), a large number of grooves parallel to the sliding direction are detected, indicating that abrasive wear is the main wear mechanism. In addition, driven by the shear stress upon periodic sliding, spalling caused by micro-cracks propagation and coalesce on the surface can also be observed. EDS results show that all elements in the wear track are evenly distributed without obvious segregation.
The worn surface of CoCrNi tested at OCP is shown in
Figure 9. It can be seen from
Figure 9b,c that a large number of furrows and a small amount of debris accumulation are found inside the wear track, indicating significant ductile deformation along with the grooves which were formed by counterface asperities plowing the surface. This suggested that abrasion was prevailing throughout sliding wear in the OCP condition. Obvious delamination was also found on the end face of the wear track (
Figure 9d), which was a result of severe plastic deformation [
37,
38]. EDS mapping is performed on the wear track as shown in
Figure 9d, and it found that the substrate material is rich in Co and Cr, while the surface film is rich in O, Si, and Ni. Under OCP conditions, the activation of surface metal elements will spontaneously produce a passive film, which is generally composed of metal oxide. According to EDS results, the passive film produced may be Ni oxide, which is different from the passive film produced under cathode polarization conditions (
Figure 7). The Si element accumulated on the surface is a result of material transfer from Si
3N
4, indicating that adhesive wear is also one of the wear mechanisms of CoCrNi in this condition.
When the applied potential is −0.2 V, the surface of CoCrNi alloy has the same state as the initial stage of passivation. The metal anode loses its electrochemical activity and the anodic dissolution rate decreases. From the SEM image (
Figure 10), it can be seen that delamination at the end of the wear scar was also examined, indicating that delamination wear was another primary mechanism for CoCrNi during sliding in 3.5wt. % NaCl solution. Combined with EDS results, it can also be seen that oxidation is dominated on the wear surface. In addition, there are a large number of corrosion products and wear debris in the inner area of the wear track (
Figure 10c–e), which is due to the poor bearing capacity of the passive film formed on the CoCrNi surface, which breaks easily under the action of mechanical sliding and then accumulates inside the wear track.
Figure 11 shows the wear morphology of CoCrNi at 0.1 V applied potential. Under such conditions, the surface state of CoCrNi is in the transition from passivation to anodic dissolution. A large number of grooves and a few corrosion pits can be seen inside the wear track (
Figure 11b). The EDS mapping analysis of the characteristic area of the wear track shows that the metal elements are evenly distributed, and there is obvious Si enrichment in the corrosion pits (
Figure 11d). It should be noted that this Si aggregation is not a result of adhesive wear from the counter-body as mentioned earlier, but a phenomenon intimately related to the corrosion behavior. The large number of grooves produced at 0.1 V potential indicates that the wear mechanism is mainly abrasive wear.
Figure 12 shows the wear morphology of CoCrNi at 0.25 V applied potential. A large number of grooves and corrosion pits are the main features of wear scar under this condition (
Figure 12b). When the applied potential was more positive than the pitting potential, a thick passive film is formed on the surface of CoCrNi, which reduces the shear stress during friction between the fiction pair and the metal matrix, leading to a reduced COF. However, this passive film is easily destroyed under the action of load and then dissolved in the corrosive medium in the form of wear debris, thereby accelerating the material loss. Under the high potential condition, the aggressive Cl
− in the solution will erode the passive film, resulting in many corrosion pits as shown in
Figure 12c. These corrosion pits were scattered on CoCrNi surface and growing deeper into the inner material. EDS mapping of
Figure 12d shows that the corrosion pits are rich in O and Cr while depleted in Co and Ni. Some Si elements are also attached to the surface of CoCrNi, which is consistent with the conclusion obtained under the condition of 0.1 V. The “cover” over the pit mouth, consisting of a remnant of the passive film and corrosion products, was very perforated and had poor adhesion, which would be peeled off readily when hard Si
3N
4 counterpart passed by. Thus, the occurrence of pitting corrosion must facilitate wear of CoCrNi although a low COF is reached in this condition.
Figure 13 presents the high-resolution XPS spectra of Ni 2p, Cr 2p, Co 2p, and O 1s recorded from the worn surface of CoCrNi alloy at the different potentials in 3.5 wt.% NaCl solution. After deconvolution analysis of each core-level spectrum, the valence states of concerned elements were identified. From
Figure 13, similar patterns of these four elements can be found at different potentials. After friction, visible Co, Cr, and Ni elements were oxidized [
39]. This is confirmed by the O 1s spectra, in that the curve can be divided into three peaks ascribed to oxide, hydroxide, and chemisorbed water, respectively. Therefore, the passive films formed at different potentials are composed of oxides, including NiO, Cr
2O
3, and CoO. For relative intensities of compounds from the high-resolution core-level spectrum of each element, several interesting results can be further discussed as follows. Firstly, the passive film at higher potential shows higher oxide and hydroxide content but chemisorbed water content, illustrating that a higher potential obviously enhances the oxidation and corrosion processes. Secondly, at the higher potential, the stable valence CoO amount decreases, and the intermediate valence Co
3O
4 amount increases. Thus, the electrochemical stability of passive film decreases. Thirdly, the Cr
2O
3 amount at 0.25 V increases significantly when compared with that of OCP. This suggests the acceleration of selective dissolution of Cr at higher potential, which may provide the channel for the permeation of Cl
− into the passive film. In summary, the stability of the passive film becomes weak at a higher potential, leading to a deteriorated corrosion resistance of CoCrNi.
3.4. Tribocorrosion Mechanism
The tribocorrosion mechanism of CoCrNi in 3.5 wt. % NaCl solution was investigated through SEM, EDS, and XPS analysis of wear track, and was combined with three kinds of electrochemical states, as shown in
Figure 14. In
Figure 14a, cathode polarization is achieved by applying cathode current in a three-electrode system and the cathodic reaction is dominated by the reduction of dissolved oxygen as [
40,
41]:
Under the condition of cathodic polarization, the corrosion is inhibited, and mechanical wear dominates the tribocorrosion process with no synergistic effect between corrosion and wear. In fact, under the condition of cathode polarization, there is no passive film involved in the tribocorroison process, and the contact between the pin and the metal substrate is not uniform due to the difference of surface roughness, which leads to a higher friction coefficient and larger shear stress. Upon sliding, the heavy plastic deformation and fatigue loading on the surface produce a large number of micro-cracks, which then extend to the surface, causing a certain degree of spalling. Once the protective tribo-layer is detached from the contact surface, scratches and tearing of base material can also be examined (
Figure 14a).
As shown in
Figure 14b, in OCP conditions, the potentiostat only plays a role of monitoring the electrochemical signal in the process of tribocorroison. Similar to most passivated metals, CoCrNi exposed to 3.5 wt. % NaCl forms a passive film on the metal surface under the action of dissolved oxygen. Since the passivation and depassivation processes are dynamic, the removal and regeneration of passive film occur repeatedly during rubbing. As described by Celis et al. [
40], the areas where the passive film is constantly destroyed and removed (worn area) act as the anode while the surrounding passivated areas (unworn area) act as a cathode, which forms a macroscopic galvanic cell and results in groove erosion. This will accelerate the corrosion of the wear zone and cause the mechanical properties of CoCrNi to deteriorate when compared with the case in the cathodic polarization condition. On the other hand, different from the tribocorrosion process in
Figure 13a, there is no direct contact between the pin and the metal base in the OCP condition. The passive film formed on the surface of CoCrNi plays a certain role for lubrication here, which can be confirmed by the difference of friction coefficient between the cathodic polarization and OCP conditions in
Figure 5b.
In
Figure 14c, at anode polarization, CoCrNi surface forms a thicker passive film, which can greatly reduce the COF in the process of mechanical sliding (
Figure 5b). The tribocorrosion process is similar to that in the OCP condition, where the passive film can be removed and regenerated quickly. However, driven by the high potential, the passivated CoCrNi alloy may still have a reaction with the aggressive Cl
− contained in the corrosion medium (reactions are displayed below). As shown in
Figure 14c, the Cl
− ion with a small radius can more easily penetrate the passivation film to form chloride on CoCrNi, which is soluble in water. As a result, the passive film is partially dissolved, and the growth of passive film will be inhibited [
42,
43]. This process will destroy the dynamic equilibrium (e.g., dissolution and repassivation) at the surface, leading to pitting corrosion occurring inside the wear track (
Figure 13):
Corrosion pits inside the wear track can therefore promote crack initiation and propagation resulting in accelerated wear.
In summary, at the cathodic potential, the corrosion dissolution of metal ions is negligible, and CoCrNi degradation is mainly through mechanical wear, which results in low volume loss. Under open circuit and anodic polarizing conditions, due to the occurrence of corrosion, the material loss of CoCrNi increased obviously compared with that under pure mechanical wear (cathodic polarization condition), demonstrating that the rate of material degradation could be controlled by external potentials. Furthermore, at 0 V potential, volume loss increased sharply and approached 448,934 um
3 (
Figure 5a), a rise of nearly 10 times that under pure mechanical wear (−0.8 V) due to the beginning of pitting corrosion. In this case, the synergistic effect between wear and corrosion results in high volume loss (
Supplementary Material Figure S2). Clearly, corrosion has a significant effect on the degradation of CoCrNi during tribocorrosion, although it has excellent corrosion resistance in static conditions.