Effect of Copper on the Formation of L12 Intermetallic Phases in Al–Cu–X (X = Ti, Zr, Hf) Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
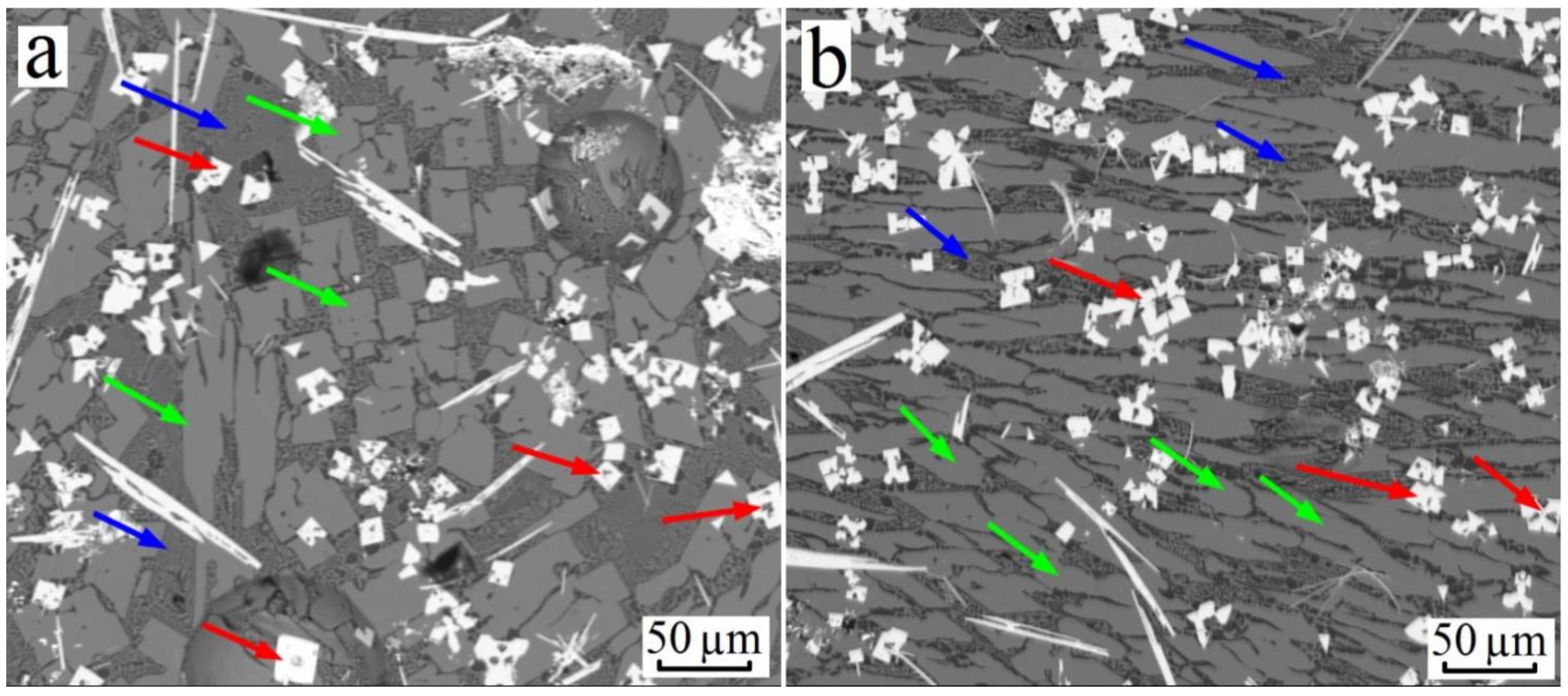
3.1. Al–Cu–Ti System
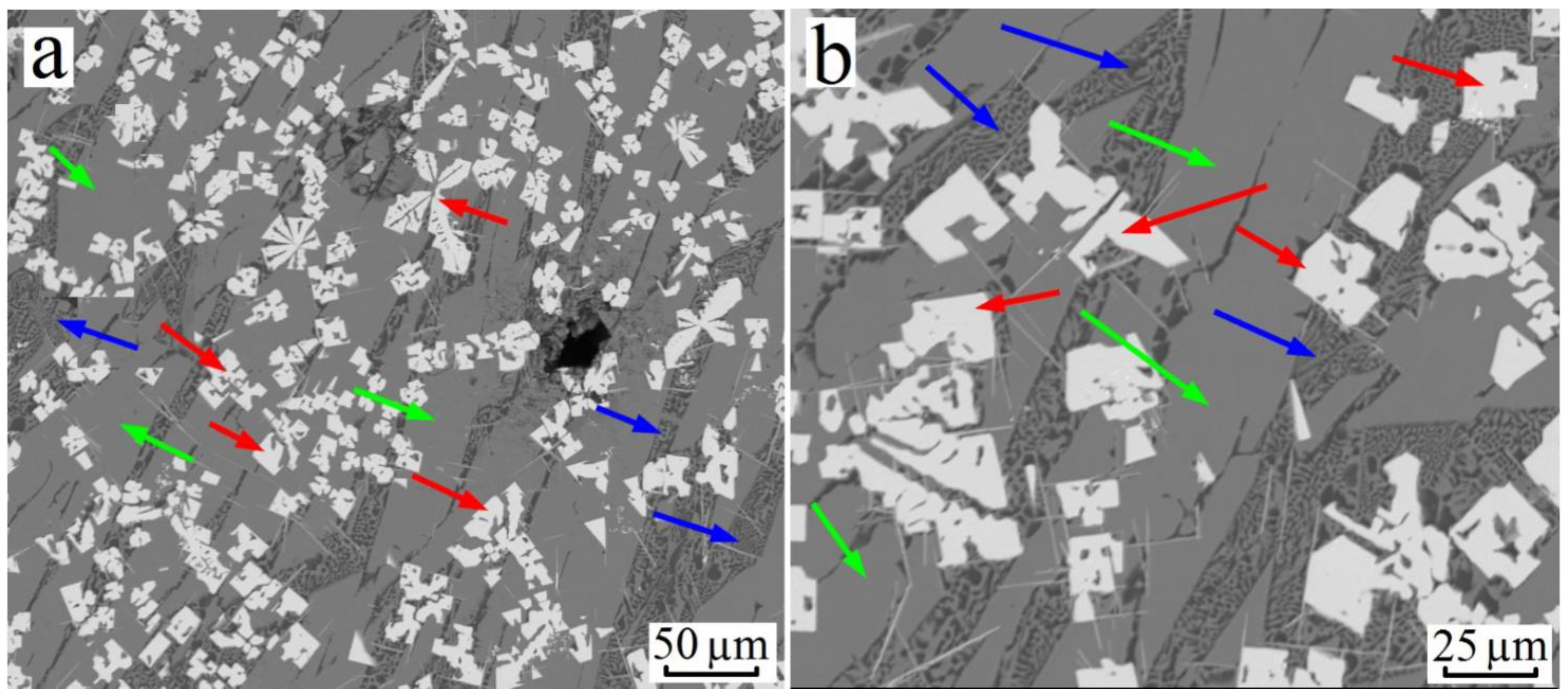
3.2. Al–Cu–Zr System
3.3. Al–Cu–Hf Yystem
3.4. Formation of Trialuminides with a Cubic Lattice in Alloys of the Al–Cu–X (X: Ti, Zr, Hf) System
- 1.
- 2.
- the chemical driving force ();
- 3.
- the elastic strain energy (), which reduces the chemical driving force (), preventing nucleation.
4. Conclusions
- (1)
- One of the necessary conditions for the formation of (Al1-xCux)3Ti, (Al1-xCux)3Zr, (Al1-xCux)3Hf aluminides having L12 structure is the hypereutectic concentration of copper in the alloys. Ceteris paribus, an increase in the copper concentration (above the eutectic) in the alloys rapidly changed the ratio of stable and metastable trialuminides in favor of the formation of a larger amount of the latter. The sequence of dissolution in liquid aluminum of copper and then of TM (Zr or Hf) significantly accelerated the process of formation of metastable aluminides in the alloys.
- (2)
- Under identical synthesis conditions in the crystal lattice of metastable aluminides formed in experimental Al–Cu–Ti, Al–Cu–Zr and Al–Cu–Hf alloys, copper substituted up to 8, 10, and 13 at.% of the aluminum, respectively. The amount of aluminum substituted with copper in the crystal lattice of metastable aluminides was three times higher than in stable ones.
- (3)
- In the studied Al–Cu–Ti alloys, the low volume fraction of precipitating aluminides was the result of insufficient supersaturation of α-Al with titanium at the peritectic temperature (according to calculations based on the classical nucleation theory). The degree of supersaturation of the investigated alloys with titanium was 2 and 3.5 times, with zirconium being in the range of 26 and 53 times, and hafnium 174 to 219 times. As such, the difference in the degree of supersaturation of the alloys was 2–3 orders of magnitude.
- (4)
- Al2.5Cu0.5Zr and Al2.5Cu0.5Hf aluminides having the L12 structure with lattice parameters a = 0.40662(7) nm (Z3) and a = 0.40441(5) nm (H5), respectively, were synthesized. The high degrees of crystallographic and dimensional similarity between the lattices of aluminides and α-Al (0.4050 nm) suggest the applicability of these intermetallic compounds as modifiers of aluminum alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, T.; Liu, X. Replacement with Each Other of Ti and Zr in the Intermetallics of Al–(Si–)Ti–Zr Alloys. J. Mater. Sci. Technol. 2013, 29, 291–296. [Google Scholar] [CrossRef]
- Karpets, M.V.; Milman, Y.V.; Barabash, O.M.; Korzhova, N.P.; Senkov, O.N.; Miracle, D.B.; Legkaya, T.N.; Voskoboynik, I.V. The influence of Zr alloying on the structure and properties of Al3Ti. Intermetallics 2003, 11, 241–249. [Google Scholar] [CrossRef]
- Nayak, S.S.; Murty, B.S. Synthesis and stability of L12–Al3Ti by mechanical alloying. Mater. Sci. Eng. A 2004, 367, 218–224. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Rocha, L.A.; Ariza, E.; Sequeira, P.D.; Watanabe, Y.; Fernandes, J.C.S. Corrosion behaviour of Al/Al3Ti and Al/Al3Zr functionally graded materials produced by centrifugal solid-particle method: Influence of the intermetallics volume fraction. Corros. Sci. 2011, 53, 2058–2065. [Google Scholar] [CrossRef] [Green Version]
- Kogachi, M.; Minamigawa, S.; Nakahigashi, K. Long-range order in L12 ternary intermetallic compounds Al3Ti-X (X = Fe,Ni,Cu,Ag). Scr. Metall. Mater. 1992, 27, 407–412. [Google Scholar] [CrossRef]
- Milman, Y.V.; Miracle, D.B.; Chugunova, S.I.; Voskoboinik, I.V.; Korzhova, N.P.; Legkaya, T.N.; Podrezov, Y.N. Mechanical behaviour of Al3Ti intermetallic and L12 phases on its basis. Intermetallics 2001, 9, 839–845. [Google Scholar] [CrossRef]
- Mabuchi, H.; Kito, A.; Nakamoto, M.; Tsuda, H.; Nakayama, Y. Effects of manganese on the L12 compound formation in Al3Ti-based alloys. Intermetallics 1996, 4, S193–S199. [Google Scholar] [CrossRef]
- Ghosh, G.; Vaynman, S.; Asta, M.; Fine, M.E. Stability and elastic properties of L12-(Al,Cu)3(Ti,Zr) phases: Ab initio calculations and experiments. Intermetallics 2007, 15, 44–54. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S. Mechanical properties and fracture of in-situ Al3Ti particulate reinforced A356 composites. Mater. Sci. Eng. A 2019, 754, 46–56. [Google Scholar] [CrossRef]
- Prusov, E.; Deev, V.; Rakhuba, E. Aluminum Matrix In-Situ Composites Reinforced with Mg2Si and Al3Ti. Mater. Today Proc. 2019, 11, 386–391. [Google Scholar] [CrossRef]
- Suzuki, A.; Kosugi, N.; Takata, N.; Kobashi, M. Microstructure and compressive properties of porous hybrid materials consisting of ductile Al/Ti and brittle Al3Ti phases fabricated by reaction sintering with space holder. Mater. Sci. Eng. A 2020, 776, 139000. [Google Scholar] [CrossRef]
- Himmler, D.; Randelzhofer, P.; Körner, C. In-situ Al3Ti particle reinforcement for stiff aluminum die castings. J. Alloys Compd. 2022, 904, 163984. [Google Scholar] [CrossRef]
- Gong, Y.P.; Ma, S.M.; Hei, H.J.; Ma, Y.; Zhou, B.; Wang, Y.S.; Yu, S.W.; Wang, X.; Wu, Y.C. Tailoring microstructure and its effect on wear behavior of an Al–7Si alloy reinforced with in situ formed Al3Ti particulates. J. Mater. Res. Technol. 2020, 9, 7136–7148. [Google Scholar] [CrossRef]
- Dinaharan, I.; Ashok Kumar, G.; Vijay, S.J.; Murugan, N. Development of Al3Ti and Al3Zr intermetallic particulate reinforced aluminum alloy AA6061 in situ composites using friction stir processing. Mater. Des. 2014, 63, 213–222. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Jiang, F.C. Fracture toughness of Ceramic-Fiber-Reinforced Metallic-Intermetallic-Laminate (CFR-MIL) composites. Mater. Sci. Eng. A 2016, 649, 407–416. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Petrov, I.Y.; Mali, V.I.; Esikov, M.A.; Kuzmin, R.I.; Lozanov, V.V.; Pyczak, F.; Stark, A.; Dovzhenko, G.D.; Bataev, I.A.; et al. Ti-Al3Ti metal-intermetallic laminate (MIL) composite with a cubic titanium trialuminide stabilized with silver: Selection of fabrication regimes, structure, and properties. J. Alloys Compd. 2022, 916, 165480. [Google Scholar] [CrossRef]
- Vecchio, K.S. Synthetic multifunctional metallic-intermetallic laminate composites. JOM 2005, 57, 25–31. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Liu, Y.; Ren, X. A comprehensive DFT study on the thermodynamic and mechanical properties of L12-Al3Ti/Al interface. Vacuum 2021, 183, 109858. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, D.; Liu, Z.; Taylor, J.; Easton, M.; Zhang, M.X. Crystallographic study of Al3Zr and Al3Nb as grain refiners for Al alloys. Trans. Nonferrous Met. Soc. China 2014, 24, 2034–2040. [Google Scholar] [CrossRef]
- Yang, T.; Wei, M.; Ding, Z.; Han, X.; Li, J. First-principle calculations on the Al/L12-Al3Zr heterogeneous nucleation interface. Calphad 2020, 69, 101768. [Google Scholar] [CrossRef]
- Cui, Y.; King, D.J.M.; Horsfield, A.P.; Gourlay, C.M. Solidification orientation relationships between Al3Ti and TiB2. Acta Mater. 2020, 186, 149–161. [Google Scholar] [CrossRef]
- Popova, E.A.; Kotenkov, P.V.; Pastukhov, E.A.; Shubin, A.B. Master alloys Al-Sc-Zr, Al-Sc-Ti, and Al-Ti-Zr: Their manufacture, composition, and structure. Russ. Metall. 2013, 2013, 590–594. [Google Scholar] [CrossRef]
- Moon, K.I.; Chang, K.Y.; Lee, K.S. The effect of ternary addition on the formation and the thermal stability of L12 Al-Zr alloy with nanocrystalline structure by mechanical alloying. J. Alloys Compd. 2000, 312, 273–283. [Google Scholar] [CrossRef]
- Moon, K.I.; Lee, S.H.; Kim, S.J. The effect of Cu and Zn on the phase stability of L12 Al3Hf intermetallic compound synthesized by mechanical alloying. Intermetallics 2002, 10, 793–800. [Google Scholar] [CrossRef]
- Desch, P.B.; Schwarz, R.B.; Nash, P. Formation of metastable L12 phases in Al3Zr and Al-12.5%X-25%Zr (X = Li, Cr, Fe, Ni, Cu). J. Less Common Met. 1991, 168, 69–80. [Google Scholar] [CrossRef]
- Lee, S.H.; Moon, K.I.; Lee, K.S. Enhancement of the fracture toughness of bulk L12-based (Al+12.5at.%M)3Zr(M=Cu,Mn) intermetallics synthesized by mechanical alloying. Intermetallics 2006, 14, 1–8. [Google Scholar] [CrossRef]
- Srinivasan, S.; Desch, P.B.; Schwarz, R.B. Metastable phases in the Al3X (X = Ti, Zr, and Hf) intermetallic system. Scr. Metall. Mater. 1991, 25, 2513–2516. [Google Scholar] [CrossRef]
- Pandey, S.K.; Suryanarayana, C. Structure and transformation behavior of a rapidly solidified Al-6.4wt.%Hf alloy. Mater. Sci. Eng. A 1989, 111, 181–187. [Google Scholar] [CrossRef]
- Popova, E.; Kotenkov, P.; Shubin, A.; Gilev, I. Formation of Metastable Aluminides in Al–Sc–Ti (Zr, Hf) Cast Alloys. Met. Mater. Int. 2020, 26, 1515–1523. [Google Scholar] [CrossRef]
- Popova, E.A.; Kotenkov, P.V.; Gilev, I.O.; Melchakov, S.Y.; Shubin, A.B. Effect of Temperature on the Formation of Stable and Metastable Aluminide Phases in Al–Zr–Nb Alloys. Russ. J. Non-Ferr. Met. 2020, 61, 319–324. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamazaki, R.; Yamanaka, K.; Sato, H. Grain refinement of pure Al using Al2.5Cu0.5Ti particles with an L12 structure. J. Mater. Process. Technol. 2018, 255, 400–410. [Google Scholar] [CrossRef]
- Popova, E.A.; Shubin, A.B.; Kotenkov, P.V.; Bodrova, L.E.; Dolmatov, A.V.; Pastukhov, E.A.; Vatolin, N.A. Al-Sc-Zr master alloy and estimation of its modifying capacity. Russ. Metall. 2011, 8, 715–718. [Google Scholar] [CrossRef]
- Russell, K.C. Ch. 6: Nucleation in Solids. In Phase Transformations; Aaronson, H.I., Ed.; American Society for Metals: Metals Park, OH, USA, 1970. [Google Scholar]
- Wagner, R.; Kampmann, R.; Voorhees, P.W. Ch. 5: Homogeneous Second-Phase Precipitation. In Phase Transformations in Materials; Kostorz, G., Ed.; Wiley-VCH: New York, NY, USA, 2001; pp. 309–407. [Google Scholar]
- Doherty, D. Ch. 14: Diffusive Phase Tranformations in the Solid State. In Physical Metallurgy, 3rd ed.; Cahn, R.W., Haasen, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 933–1030. [Google Scholar]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for developing castable, creep-resistant aluminum-based alloys—A review. Mat. Res. Adv. Tech. 2006, 97, 246–265. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z. Thermodynamics of Nano-Scale Precipitate-Strengthened Fe-Cu and Al-Transition-Metal Systems from First-Principles Calculations. Ph.D. Thesis, Northwestern University, Evanston, IL, USA, 2006. [Google Scholar]
- Frost, H.J.; Ashby, M.F. Deformation-Mechanism Maps: The Plasticity and Creep of Metals and Ceramics; Pergamon Press: New York, NY, USA, 1982. [Google Scholar]
- Meyers, M.A.; Chawla, K.K. Mechanical Metallurgy: Principles and Applications; Prentice-Hall: Hoboken, NJ, USA, 1984. [Google Scholar]
- Ohashi, T.; Ichikawa, R. Grain Refinement in Aluminium-Zirconium and Aluminium-Titanium Alloys by Metastable Phases. Mat. Res. Adv. Tech. 1973, 64, 517–521. [Google Scholar]
- Kerr, H.W.; Cisse, J.; Bolling, G.F. Equilibrium and Nonequilibrium Peritectic Transformations. Acta Metall. 1974, 22, 677–686. [Google Scholar] [CrossRef]
- Fujikawa, S.I. Solid State Diffusion in Light Metals. J. Jpn. Inst. Light Met. 1996, 46, 202–215. [Google Scholar] [CrossRef]
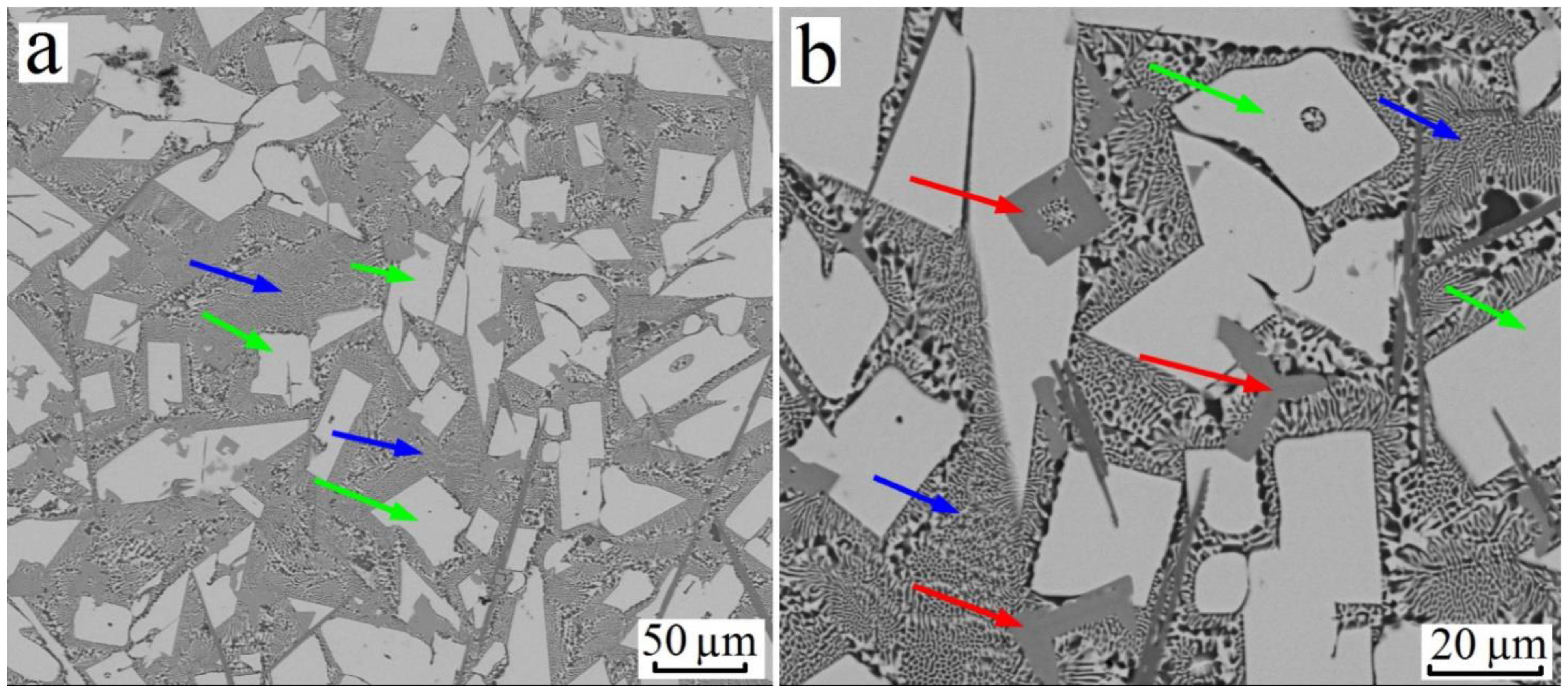
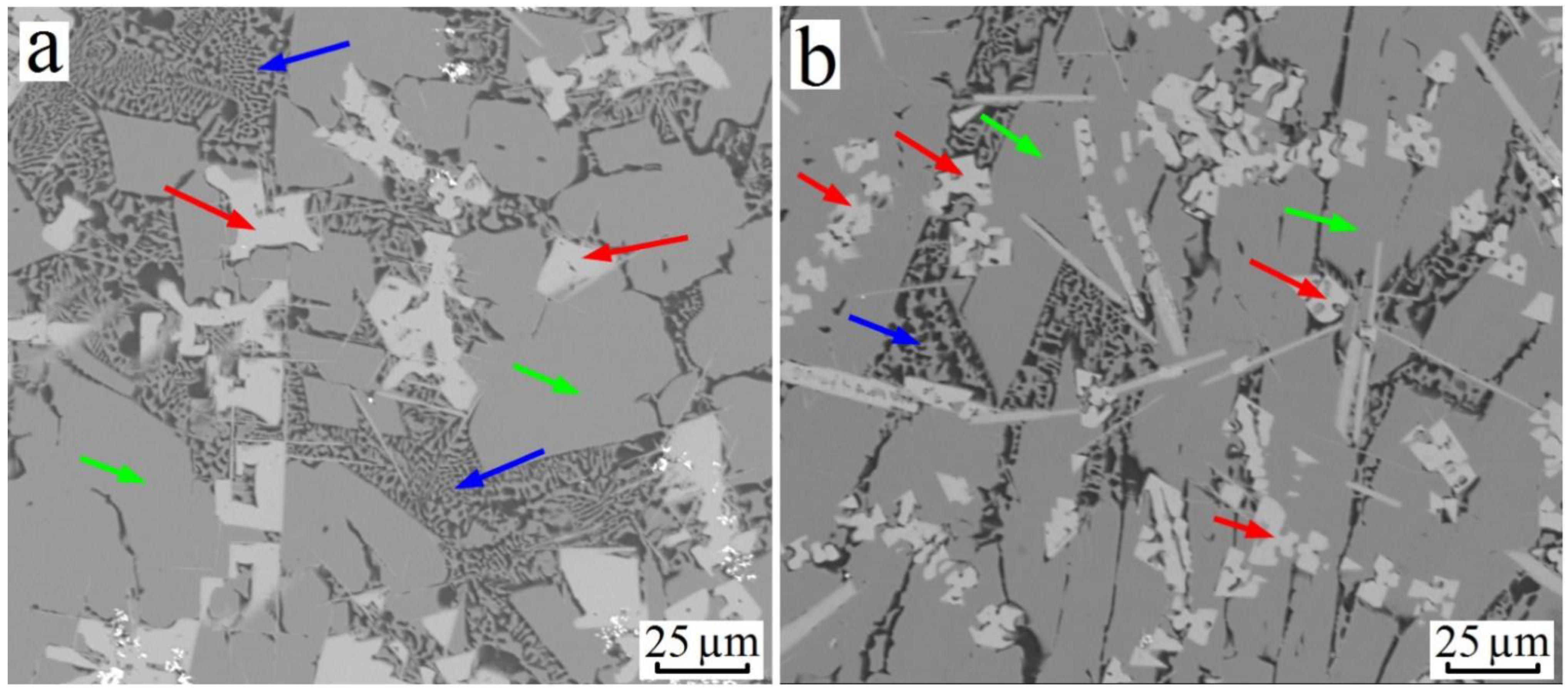
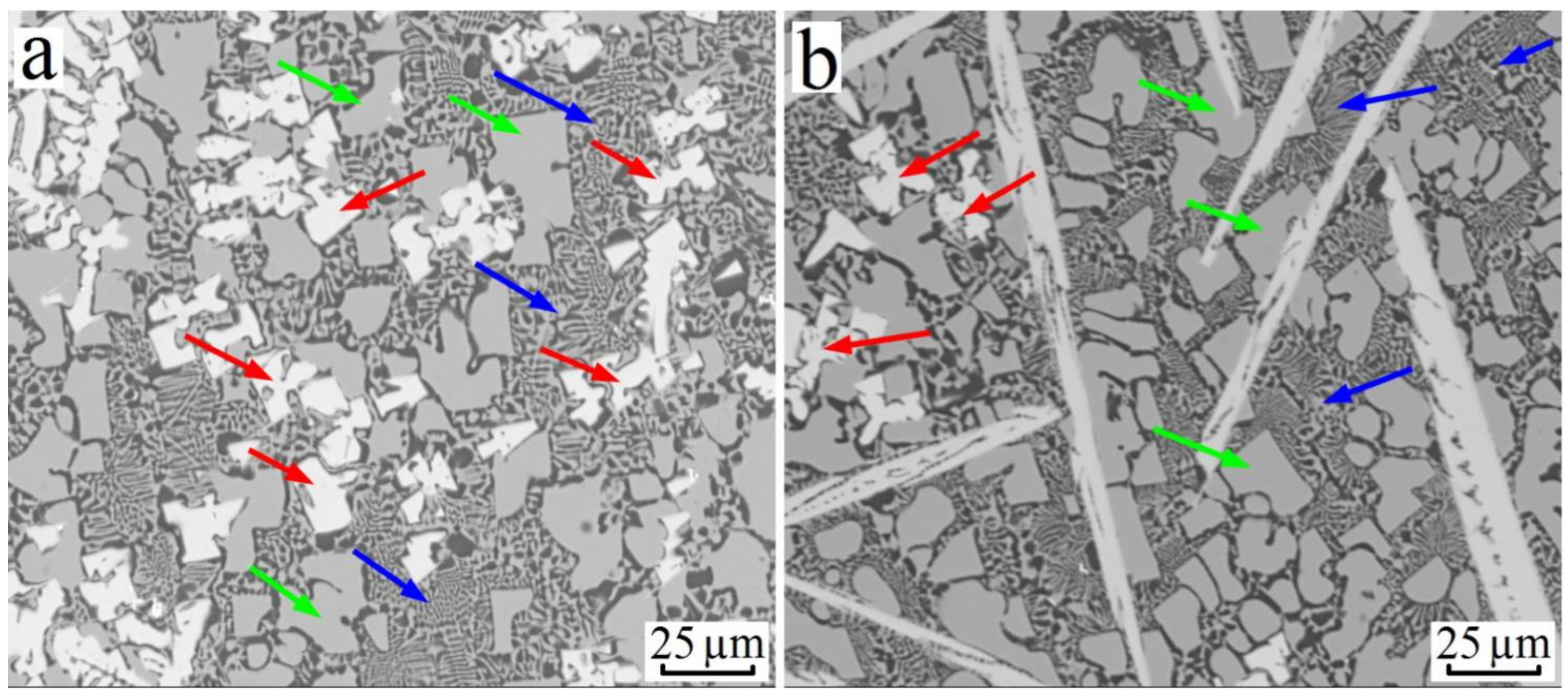
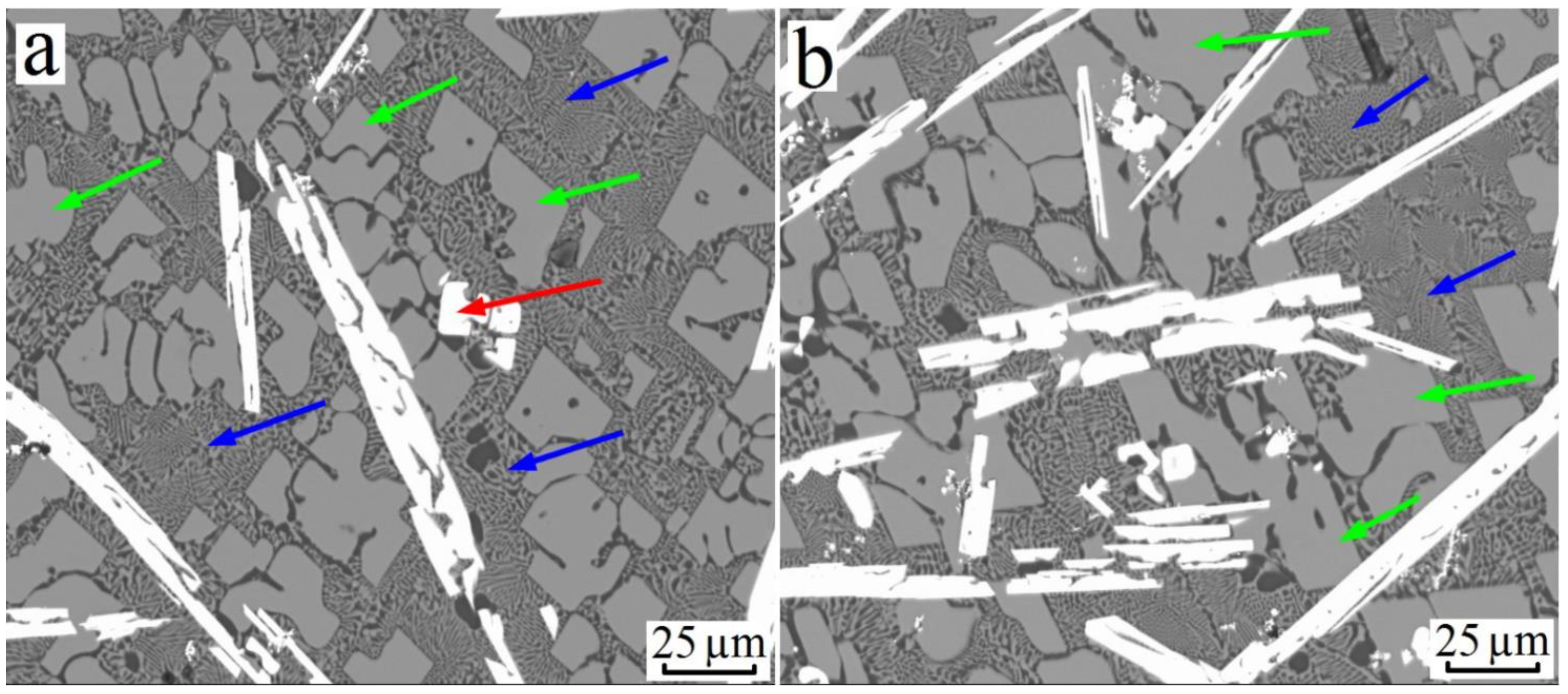

| Alloy№ | Alloys Composition, wt.% (at.%), Degree of Supersaturation with TM. | Synthesis Temperature, °C | Composition of Sluminides Having the L12 Crystal Structure | Stable Aluminides Having the D023 and D022 Crystal Structure |
|---|---|---|---|---|
| T1 | Al–41.91Cu–1.64Ti (Al–23.70Cu–1.23Ti), 2 times | 1250 | Cuboids and dendrites 70.68Al–6.36Cu–22.96Ti or (Al0.92Cu0.08)nTi * | Needles and plates containing 5.71 at.%Cu |
| T2 | Al–40.33Cu–2.75Ti (Al–22.68Cu–2.05Ti), 3.4 times | 1300 | Cuboids and dendrites 70.68Al–6.36Cu–22.96Ti or (Al0.92Cu0.08)nTi * | Needles and plates containing 5.71 at.%Cu |
| Z1 | Al–38.78Cu–4.56Zr (Al–22.13Cu–1.81Zr), 26 times | 1200 | Cuboids and dendrites 68.34Al–6.27Cu–25.39Zr or (Al0.92Cu0.08)3Zr | There are no particles having this crystal structure |
| Z2 | Al–31.47Cu–8.63Zr (Al–17.64Cu–3.37Zr) 48 times | 1300 | Cuboids and dendrites 67.08Al–7.27Cu–25.65Zr or (Al0.90Cu0.10)3Zr | There are no particles having this crystal structure |
| Z3 | Al–36.28Cu–9.02Zr (Al–21.19Cu–3.67Zr) 52 times | 1200 | Cuboids and dendrites 65.39Al–9.05Cu–25.56Zr or (Al0.88Cu0.12)3Zr | Needles and plates |
| Z4 | Al–31.25Cu–8.37Zr (Al–17.45Cu–3.26Zr) 47 times | 1300 | Single cuboids, not analyzed | Plates (Al0.97Cu0.03)3Zr |
| H1 | Al–29.73Cu–13.24Hf) (Al–17.63Cu–2.79Hf 174 times | 1250 | Single cuboids, not analyzed | Plates containing 3.80 at.%Cu |
| H2 | Al–29.47Cu–13.69Hf (Al–17.54Cu–2.91Hf) 182 times | 1300 | Single cuboids, not analyzed | Plates containing 3.80 at.%Cu |
| H3 | Al–32.83Cu–13.04Hf (Al–19.92Cu–2.82Hf) 176 times | 1300 | 69.03Al–7.67Cu–23.30Hf or (Al0.90Cu0.10)nHf * | Single plates and needles Al3Hf |
| H4 | Al–34.09Cu–13.04Hf (Al–20.91Cu–2.84Hf) 178 times | 1250 | 69.90Al–7.39Cu–22.71Hf or (Al0.90Cu0.10)nHf * | Plates containing 3.55 at.%Cu and needles Al3Hf |
| H5 | Al–35.66Cu–15.36Hf (Al–22.81Cu–3.50Hf) 219 times | 1200 | 67.09Al–10.03Cu–22.88Hf or (Al0.87Cu0.13)nHf * | There are no particles having this crystal structure |
| System | eV/atom | eV/atom | eV/atom |
|---|---|---|---|
| L12 | Impurity | L12 | |
| Al-Ti | −1.576 | −1.197 | −0.379 |
| Al-Zr | −1.964 | −1.250 | −0.714 |
| Al-Hf | −1.609 | −0.954 | −0.655 |
| System | k/atom | k/atom | k/atom |
|---|---|---|---|
| L12 | Impurity | L12 | |
| Al-Ti | −3.365 | −2.518 | 0.847 |
| Al-Zr | −3.354 | −1.000 | 2.354 |
| Al-Hf | −2.989 | −0.837 | 2.152 |
| Al3X (L12) | Atomic Fraction (T = 698 K) | Atomic Fraction | (Peritectic Temperature) | |
|---|---|---|---|---|
| Al3Ti | 0.94 × 10–5 | 0.02151 | 0.25 | 193.7 × ln(46.5 × C0) |
| Al3Zr | 1.02 × 10–5 | 0.00148 | 0.25 | 189.3 × ln(675.7 × C0) |
| Al3Hf | 0.99 × 10–5 | 0.00228 | 0.25 | 192.4 × ln(438.6 × C0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, E.; Kotenkov, P.; Gilev, I.; Pryanichnikov, S.; Shubin, A. Effect of Copper on the Formation of L12 Intermetallic Phases in Al–Cu–X (X = Ti, Zr, Hf) Alloys. Metals 2022, 12, 2067. https://doi.org/10.3390/met12122067
Popova E, Kotenkov P, Gilev I, Pryanichnikov S, Shubin A. Effect of Copper on the Formation of L12 Intermetallic Phases in Al–Cu–X (X = Ti, Zr, Hf) Alloys. Metals. 2022; 12(12):2067. https://doi.org/10.3390/met12122067
Chicago/Turabian StylePopova, Elvira, Pavel Kotenkov, Ivan Gilev, Stepan Pryanichnikov, and Alexey Shubin. 2022. "Effect of Copper on the Formation of L12 Intermetallic Phases in Al–Cu–X (X = Ti, Zr, Hf) Alloys" Metals 12, no. 12: 2067. https://doi.org/10.3390/met12122067
APA StylePopova, E., Kotenkov, P., Gilev, I., Pryanichnikov, S., & Shubin, A. (2022). Effect of Copper on the Formation of L12 Intermetallic Phases in Al–Cu–X (X = Ti, Zr, Hf) Alloys. Metals, 12(12), 2067. https://doi.org/10.3390/met12122067






