Microstructure, Mechanical Properties, and Fish-Scaling Resistance of a Ti-Nb Microalloyed Hot-Rolled Enamel Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
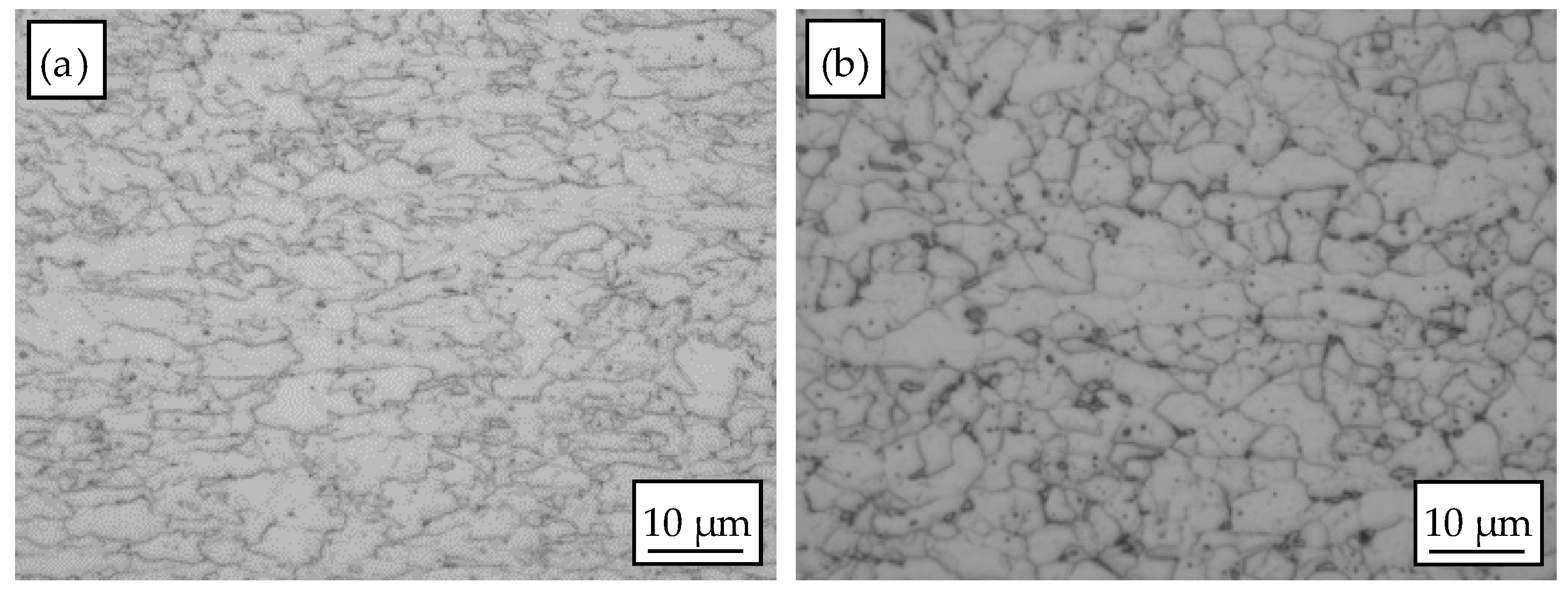
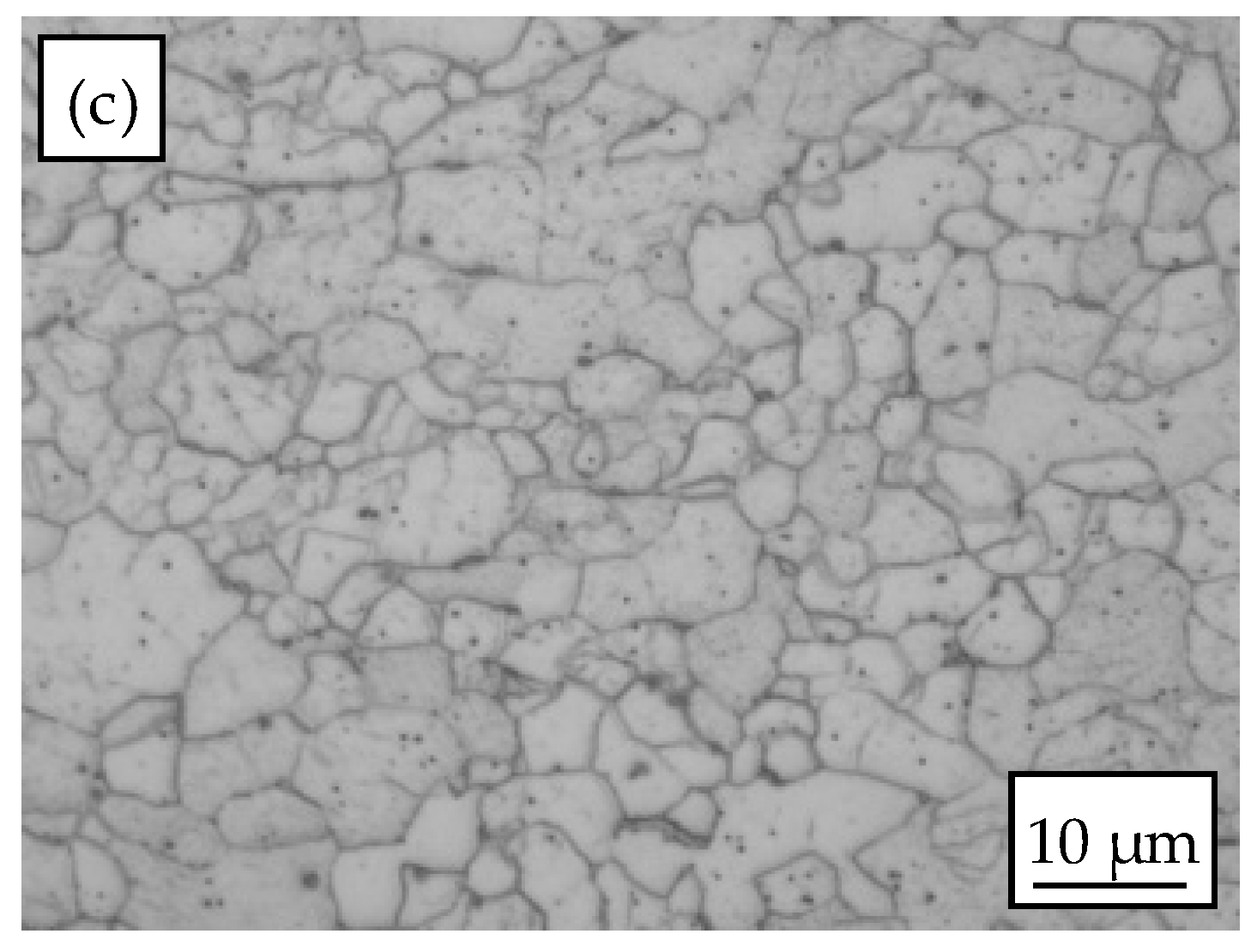
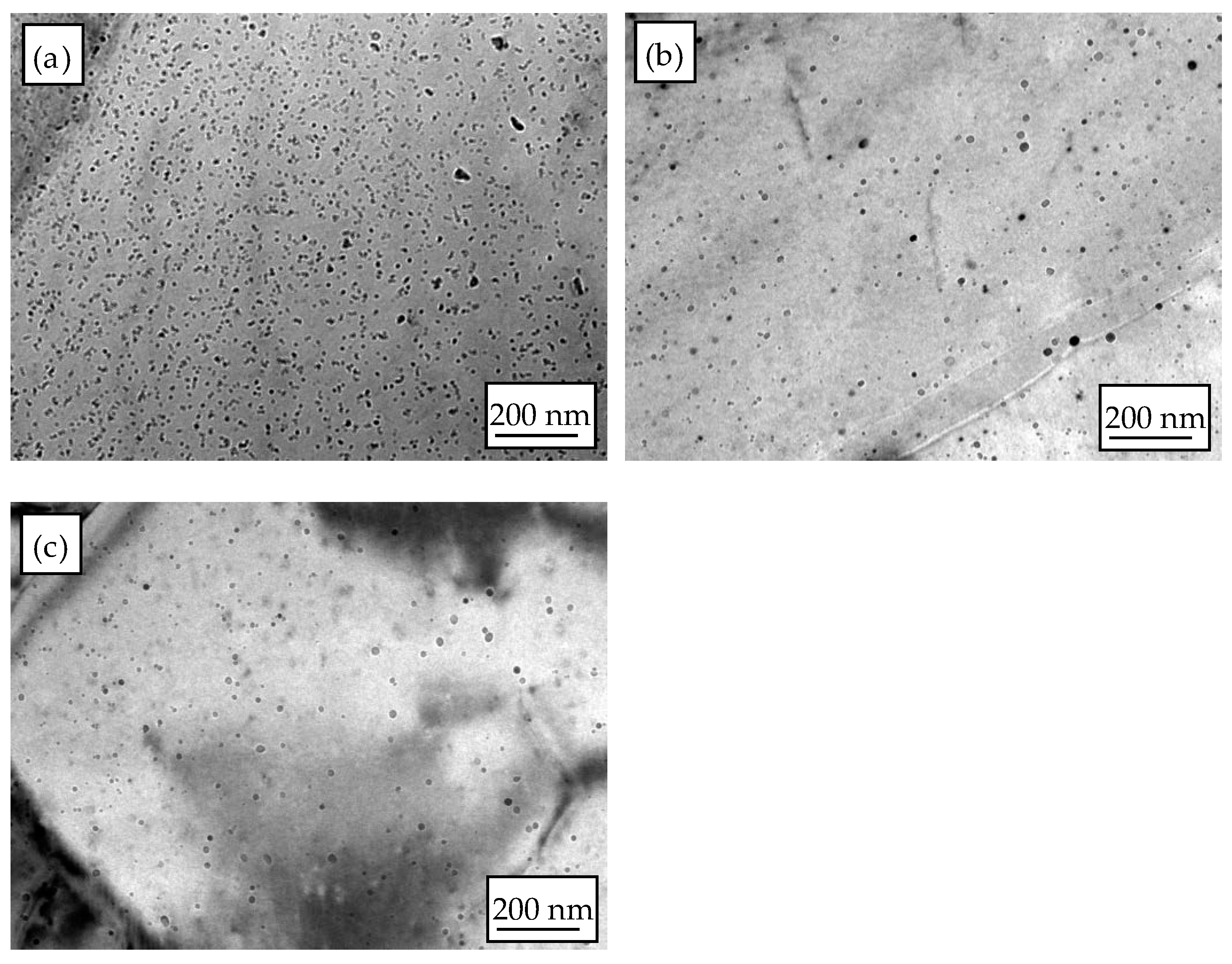
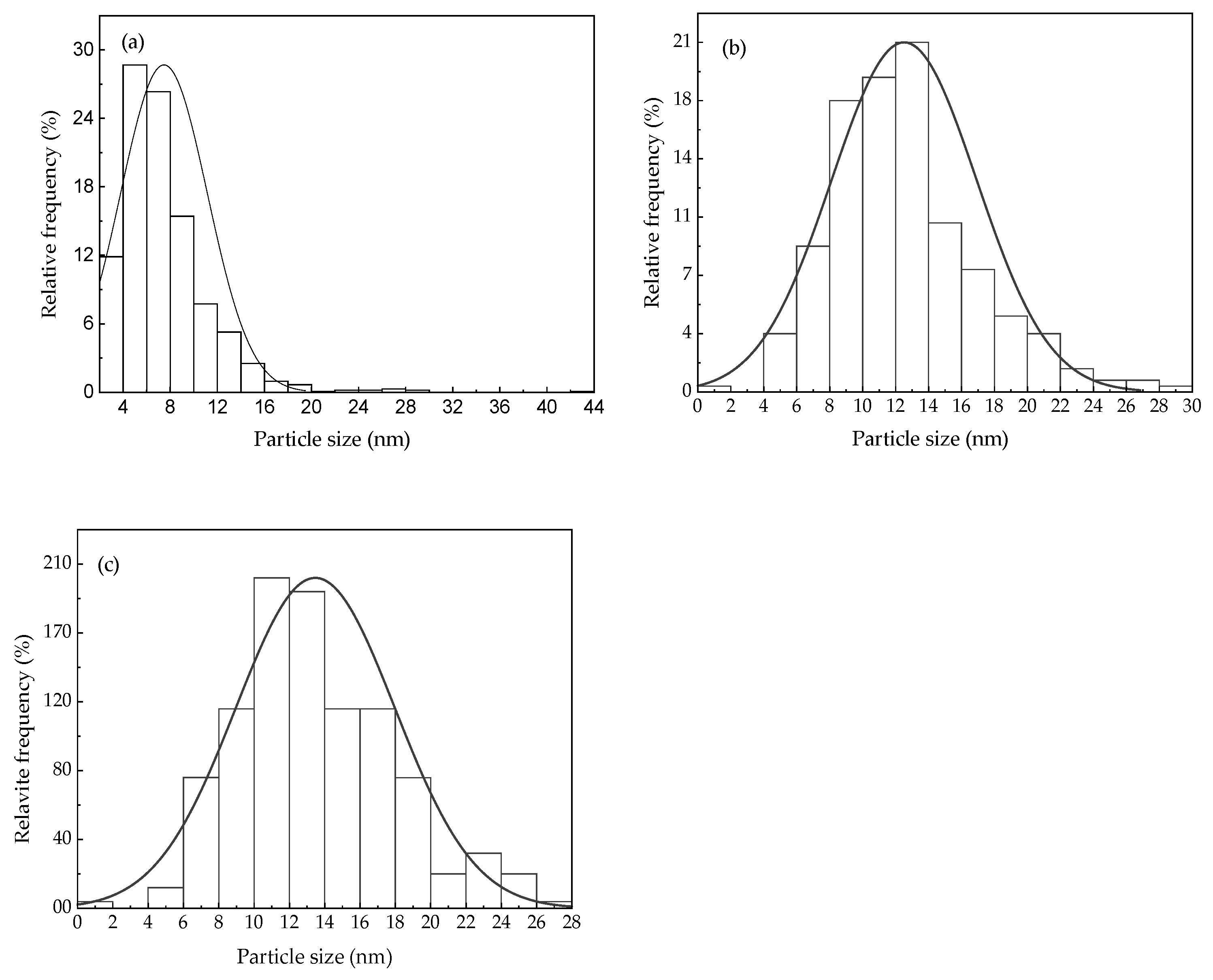
3.1. Microstructure
3.2. Mechanical Properties
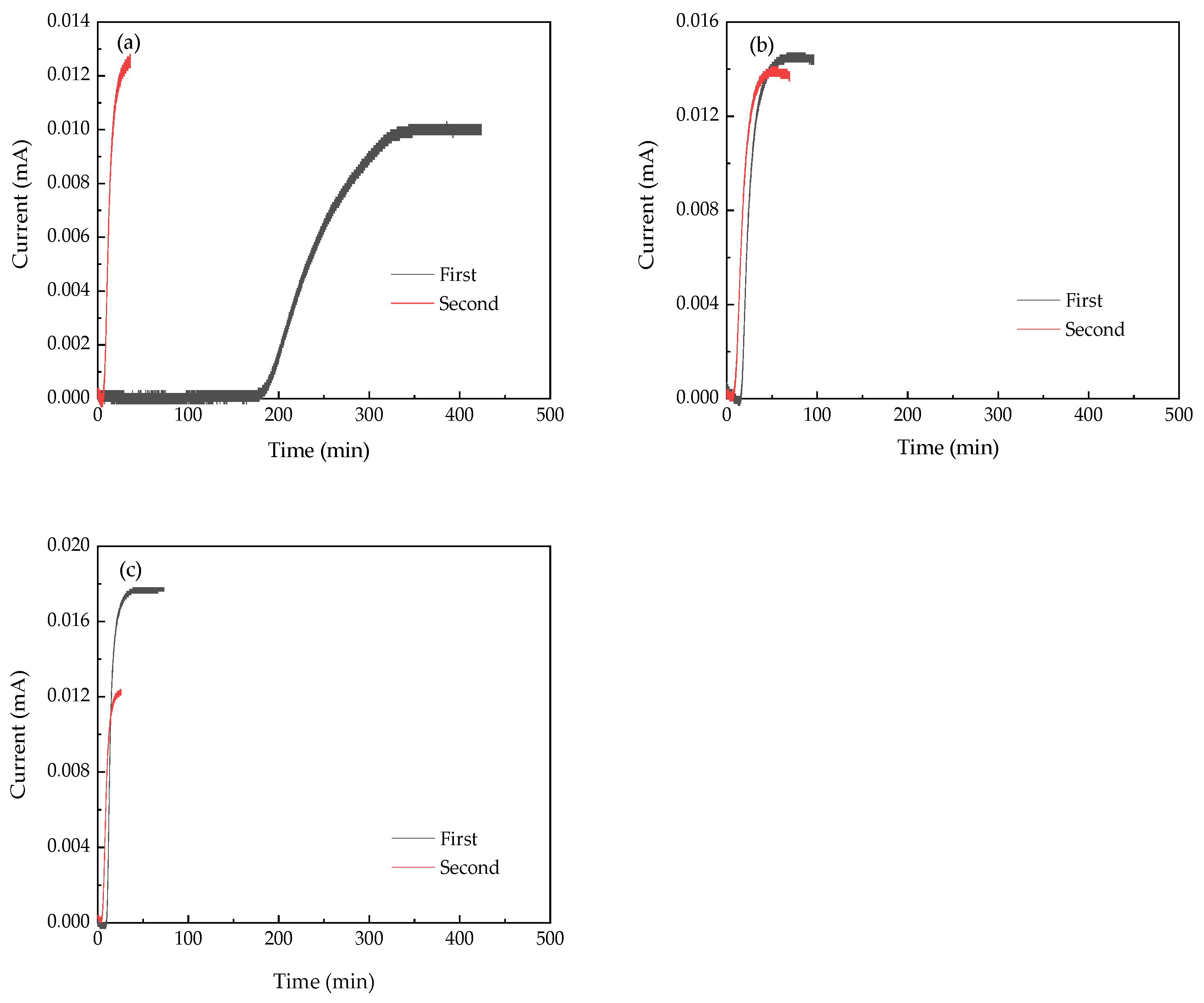
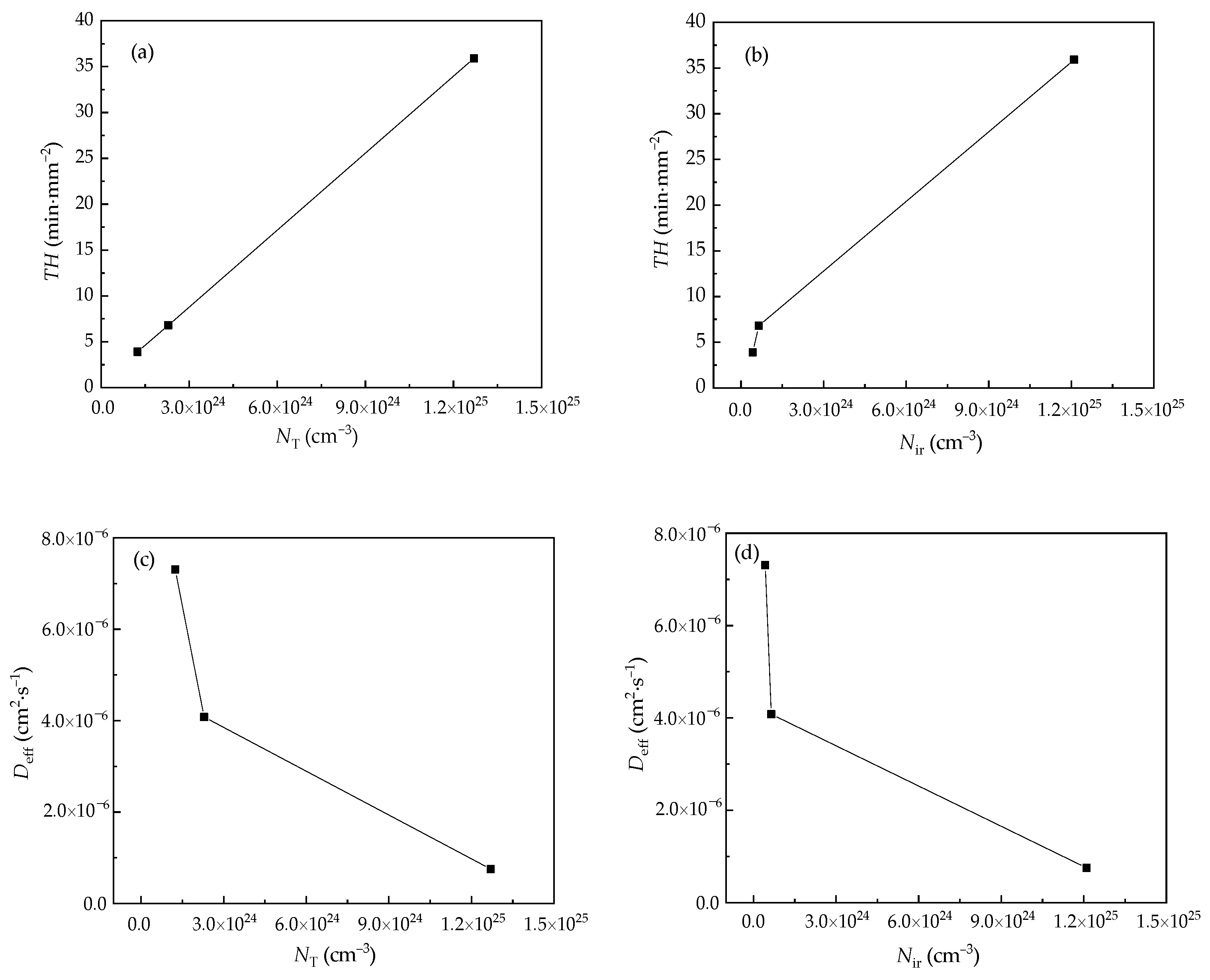
3.3. Hydrogen Permeation Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, H.H.; Wan, S.; Tieu, K.A.; Pham, S.T.; Zhu, H. Tribological behaviour of enamel coatings. Wear 2019, 426–427, 319–329. [Google Scholar] [CrossRef]
- Wang, D. Effect of crystallization on the property of hard enamel coating on steel substrate. Appl. Surf. Sci. 2009, 255, 4640–4645. [Google Scholar] [CrossRef]
- Liu, M.L.; Tang, H.Q.; Yan, W.; Subramanian, S.V. The effect of the second phase on the anti-scaling property of enamel steel DC04EK. Metallogr. Microstruct. Anal. 2022, 11, 595–601. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Liu, X.; Zhao, Y.; Li, X. Variations of microstructure and resistance to fish-scaling of a hot rolled enamel steel before and after enamel firing. J. Mater. Res. Technol. 2021, 11, 466–473. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, X.; Yu, B.; Yuan, X.; Liu, X. Effect of coiling temperature on microstructure, properties and resistance to fish-scaling of hot rolled enamel steel. Materials 2017, 10, 1012. [Google Scholar] [CrossRef]
- Yang, X.; Jha, A.; Brydson, R.; Cochrane, R.C. The effects of a nickel oxide precoat on the gas bubble structure and fish-scaling resistance in vitreous enamels. Mater. Sci. Eng. A 2004, 366, 254–261. [Google Scholar] [CrossRef]
- Yang, X.; Jha, A.; Brydson, R.; Cochrane, R.C. An analysis of the microstructure and interfacial chemistry of steel–enamel interface. Thin Solid Film. 2003, 443, 33–45. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Shao, X.; Kang, Y.; Li, Y. An ultra-low-carbon steel with outstanding fish-scaling resistance and cold formability for enameling applications. Metall. Mater. Trans. A 2019, 50, 1805–1815. [Google Scholar] [CrossRef]
- Dong, F.; Du, L.; Liu, X.; Xue, F. Optimization of chemical compositions in low-carbon Al-killed enamel steel produced by ultra-fast continuous annealing. Mater. Charact. 2013, 84, 81–87. [Google Scholar] [CrossRef]
- Liu, Z.; Kang, Y.; Zhang, Z.; Shao, X. Effect of batch annealing temperature on microstructure and resistance to fish scaling of ultra-low carbon enamel steel. Metals 2017, 7, 51. [Google Scholar] [CrossRef]
- Fang, J.; Xu, C.; Li, Y.; Peng, R.; Fu, X. Effect of grain orientation and interface coherency on the hydrogen trapping ability of TiC precipitates in a ferritic steel. Mater. Lett. 2022, 308, 131281. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y.; Tarui, T. The first direct observation of hydrogen trapping sites in TiC precipitation-hardening steel through atom probe tomography. Scr. Mater. 2010, 63, 261–264. [Google Scholar] [CrossRef]
- Wallaert, E.; Depover, T.; Arafin, M.; Verbeken, K. Thermal desorption spectroscopy evaluation of the hydrogen-trapping capacity of NbC and NbN precipitates. Metall. Mater. Trans. A 2014, 45, 2412–2420. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lu, H.; Liang, J.; Rosenthal, A.; Liu, H.; Sneddon, G.; McCarroll, I.; Zhao, Z.; Li, W.; Guo, A.; et al. Observation of hydrogen trapping at dislocations, grain boundaries, and precipitates. Science 2020, 367, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Chen, L.; Wang, Z.; Yang, X.S.; Qiao, L.; Pang, X. Quantitative investigation on deep hydrogen trapping in tempered martensitic steel. J. Alloys Compd. 2021, 854, 157218. [Google Scholar] [CrossRef]
- Wei, F.G.; Hara, T.; Tsuchida, T.; Tsuzaki, K. Hydrogen trapping in quenched and tempered 0.42C-0.30Ti steel containing bimodally dispersed TiC particles. ISIJ Int. 2003, 43, 539–547. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Y.; Wang, H.; Mi, Z.; Liu, Z.; Gao, L.; Yan, Y.; Su, Y.; Qiao, L. A first-principles study on the hydrogen trap characteristics of coherent nano-precipitates in α-Fe. Int. J. Hydrogen Energy 2020, 45, 27941–27949. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y. Origin of hydrogen trapping site in vanadium carbide precipitation strengthening steel. Acta Mater. 2018, 153, 193–204. [Google Scholar] [CrossRef]
- Turk, A.; Martín, D.S.; Rivera-Díaz-del-Castillo, P.E.J.; Galindo-Nava, E.I. Correlation between vanadium carbide size and hydrogen trapping in ferritic steel. Scr. Mater. 2018, 152, 112–116. [Google Scholar] [CrossRef]
- Wei, F.G.; Tsuzaki, K. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies, 1st ed.; Woodhead Publishing Limited: Oxford, UK, 2012; pp. 493–525. [Google Scholar]
- Zhou, P.; Li, W.; Zhao, H.; Jin, X. Role of microstructure on electrochemical hydrogen permeation properties in advanced high strength steels. Int. J. Hydrogen Energy 2018, 43, 10905–10914. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The adsorption and diffusion of electrolytic hydrogen in palladium. Proc. R. Soc. A 1962, 270, 90–102. [Google Scholar]
- Samanta, S.; Kumari, P.; Mondal, K.; Dutta, M.; Singh, S.B. An alternative and comprehensive approach to estimate trapped hydrogen in steels using electrochemical permeation tests. Int. J. Hydrogen Energy 2020, 45, 26666–26687. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, E.; Wan, J.; Liu, J.; Huang, Y.; Li, X. Effect of Nb on the hydrogen-induced cracking of high-strength low-alloy steel. Corros. Sci. 2018, 139, 83–96. [Google Scholar] [CrossRef]
- Dong, C.F.; Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Effects of hydrogen-charging on the susceptibility of X100 pipeline steel to hydrogen-induced cracking. Int. J. Hydrogen Energy 2009, 34, 9879–9884. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Huang, F.; Cheng, Y.K.; Li, K.; Hu, Q.; Liu, J. The significant effect of tantalum on the hydrogen-induced cracking of pipeline steel: Morphology, hydrogen permeation, and theoretical studies. Corros. Sci. 2022, 200, 110213. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, X.; Pan, S.; Krakauer, B.K.; Zhu, M. Correlation between microstructures and yield strength of a high strength enameling steel. J. Mater. Sci. Technol. 2012, 28, 737–744. [Google Scholar] [CrossRef]
- Okuyamas, T.; Nishimoto, A.; Kurokawa, T. New type cold rolled steel sheet for enamelling produced by the continuous casting method. Vitr. Enamel 1990, 41, 49–60. [Google Scholar]
- Wei, F.G.; Tsuzaki, K. Quantitative analysis on hydrogen trapping of TiC particles in steel. Metall. Mater. Trans A 2006, 37, 331–353. [Google Scholar] [CrossRef]
- Kawakami, K.; Matsumiya, T. Numerical analysis of hydrogen trap state by TiC and V4C3 in bcc-Fe. ISIJ Int. 2012, 52, 1693–1697. [Google Scholar] [CrossRef]
- Shi, R.; Ma, Y.; Wang, Z.; Gao, L.; Yang, X.S.; Qiao, L.; Pang, X. Atomic-scale investigation of deep hydrogen trapping in NbC/α-Fe semi-coherent interfaces. Acta Mater. 2020, 200, 686–698. [Google Scholar] [CrossRef]
- Cui, Q.; Wu, J.; Xie, D.; Wu, X.; Huang, Y.; Li, X. Effect of nanosized NbC precipitates on hydrogen diffusion in X80 pipeline steel. Materials 2017, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chiang, L.J.; Lin, Y.C.; Yen, H.W. New approaches in understanding the effects of hydrogen trapping and the fishscaling resistance of enameled steels. Surf. Coat. Technol. 2020, 399, 126135. [Google Scholar] [CrossRef]

| C | Si | Mn | P | S | Als | Ti | Nb | N |
|---|---|---|---|---|---|---|---|---|
| 0.042 | 0.0085 | 1.30 | 0.042 | 0.018 | 0.035 | 0.126 | 0.016 | 0.0017 |
| Sample | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| HR | 711 ± 9 | 761 ± 7 | 21.0 ± 2.8 |
| EF1 | 471 ± 17 | 524 ± 15 | 27.8 ± 1.8 |
| EF2 | 409 ± 8 | 490 ± 12 | 33.9 ± 1.1 |
| Sample | Deff (cm2·s−1) | NT (cm−3) | Nir (cm−3) | Nir/Nr | TH (min·mm−2) |
|---|---|---|---|---|---|
| HR | 7.52 × 10−7 | 1.27 × 1025 | 1.21 × 1025 | 0.95 | 35.9 |
| EF1 | 4.08 × 10−6 | 2.29 × 1024 | 6.50 × 1023 | 0.28 | 6.8 |
| EF2 | 7.31 × 10−6 | 1.24 × 1024 | 4.27 × 1023 | 0.34 | 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, B.; Zhang, J.; Du, Y.; Wang, X.; Wu, H.; Gao, X.; Du, L. Microstructure, Mechanical Properties, and Fish-Scaling Resistance of a Ti-Nb Microalloyed Hot-Rolled Enamel Steel. Metals 2022, 12, 1970. https://doi.org/10.3390/met12111970
Zhang Y, Yu B, Zhang J, Du Y, Wang X, Wu H, Gao X, Du L. Microstructure, Mechanical Properties, and Fish-Scaling Resistance of a Ti-Nb Microalloyed Hot-Rolled Enamel Steel. Metals. 2022; 12(11):1970. https://doi.org/10.3390/met12111970
Chicago/Turabian StyleZhang, Yi, Bo Yu, Jian Zhang, Yu Du, Xiaonan Wang, Hongyan Wu, Xiuhua Gao, and Linxiu Du. 2022. "Microstructure, Mechanical Properties, and Fish-Scaling Resistance of a Ti-Nb Microalloyed Hot-Rolled Enamel Steel" Metals 12, no. 11: 1970. https://doi.org/10.3390/met12111970
APA StyleZhang, Y., Yu, B., Zhang, J., Du, Y., Wang, X., Wu, H., Gao, X., & Du, L. (2022). Microstructure, Mechanical Properties, and Fish-Scaling Resistance of a Ti-Nb Microalloyed Hot-Rolled Enamel Steel. Metals, 12(11), 1970. https://doi.org/10.3390/met12111970







