Achieving a Combination of Higher Strength and Higher Ductility for Enhanced Wear Resistance of AlCrFeNiTi0.5 High-Entropy Alloy by Mo Addition
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Phase Characterization
3.2. Microstructure
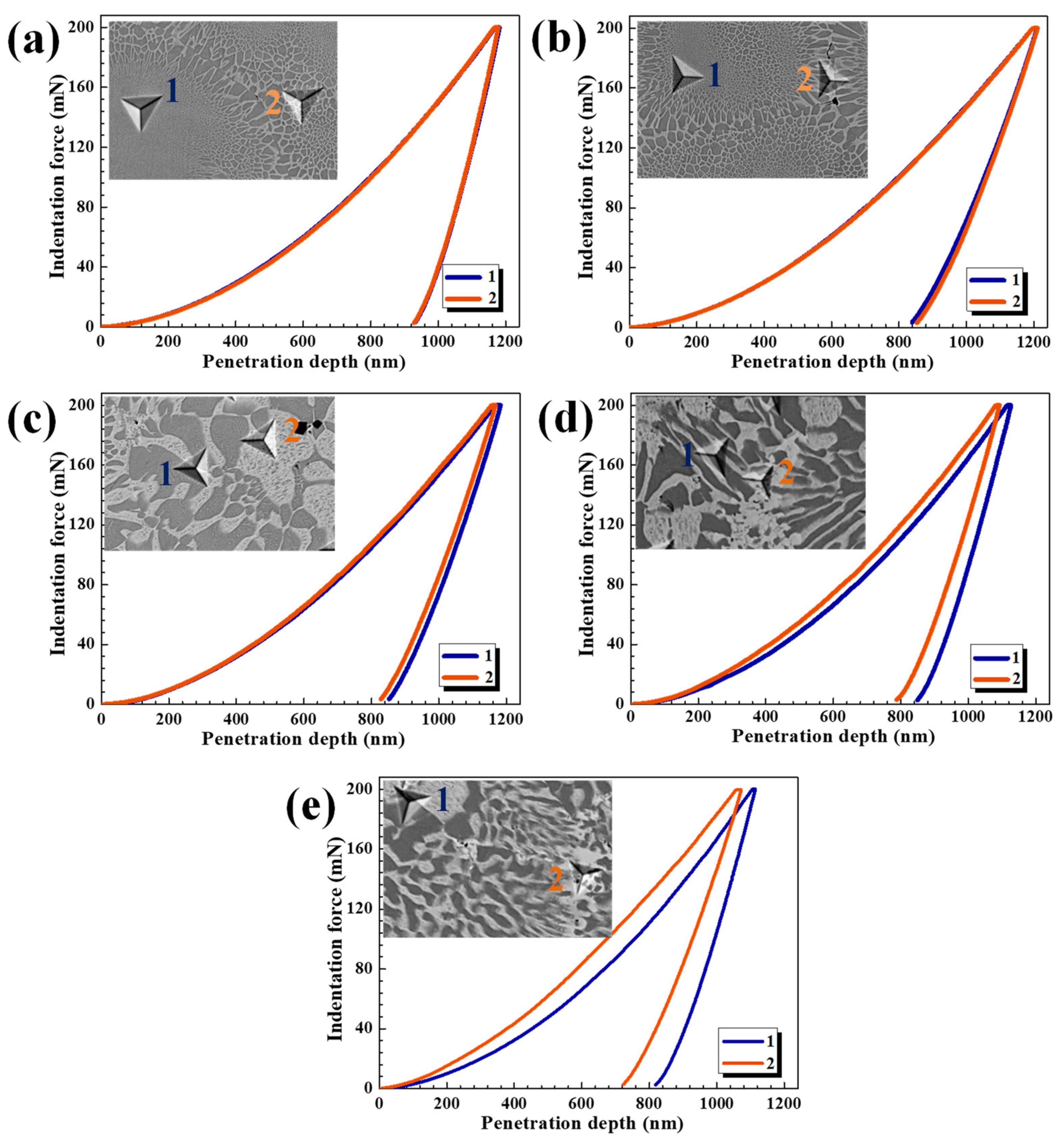
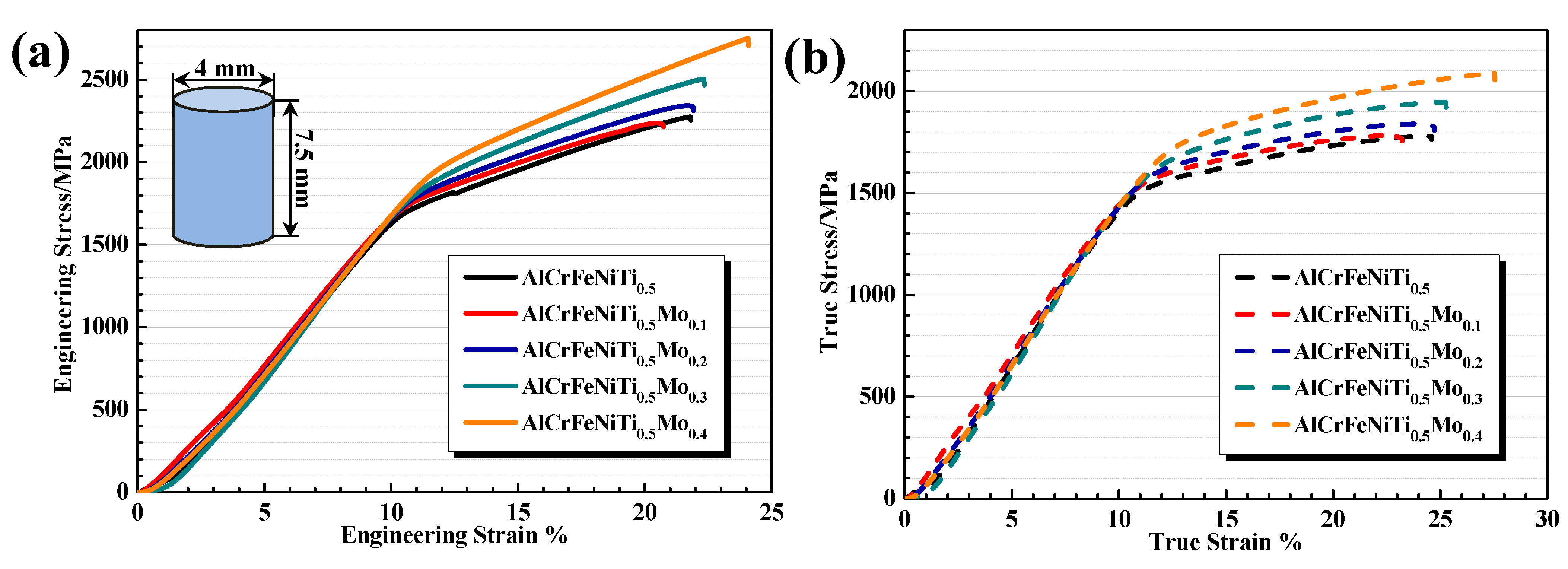
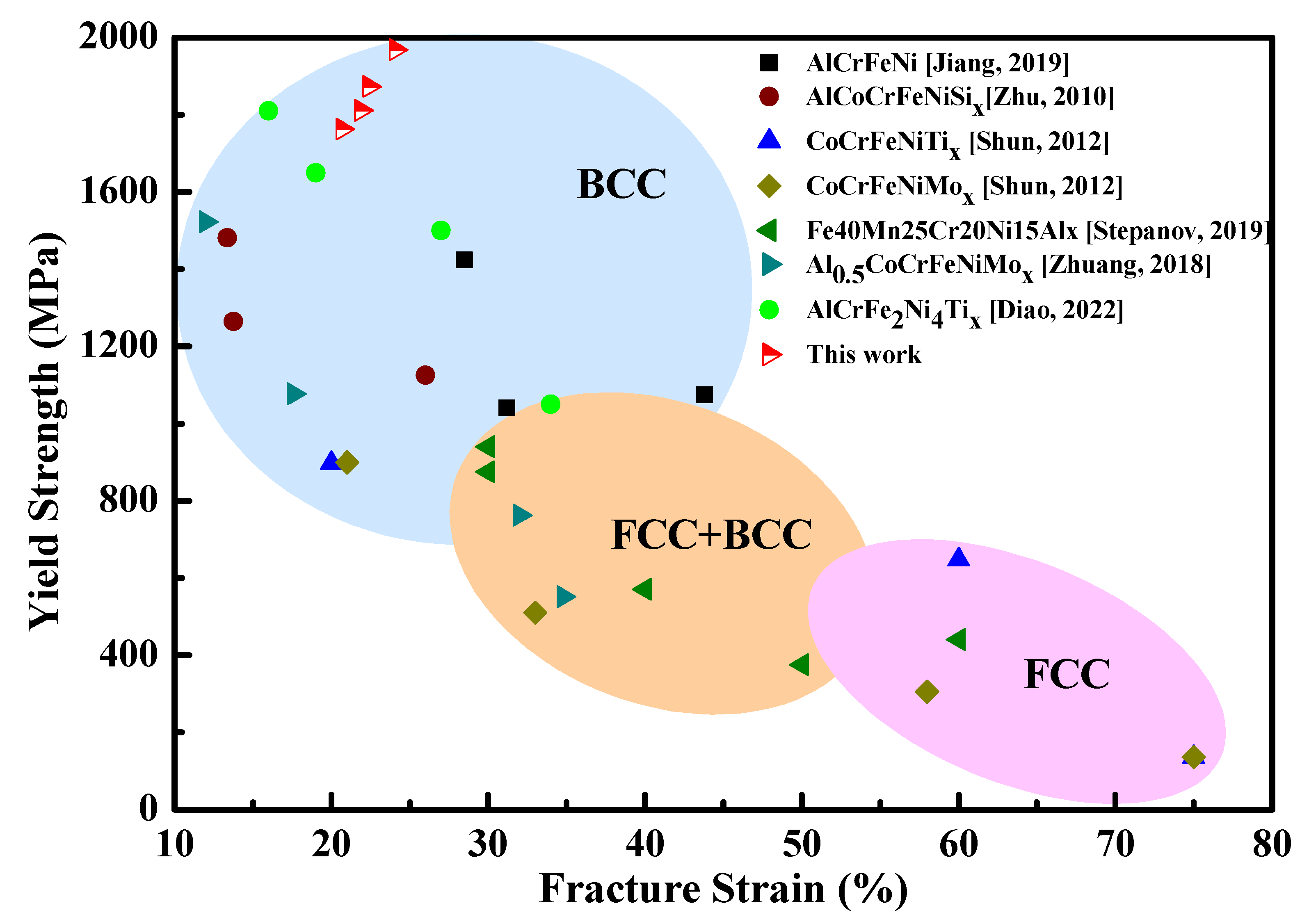
3.3. Mechanical Properties
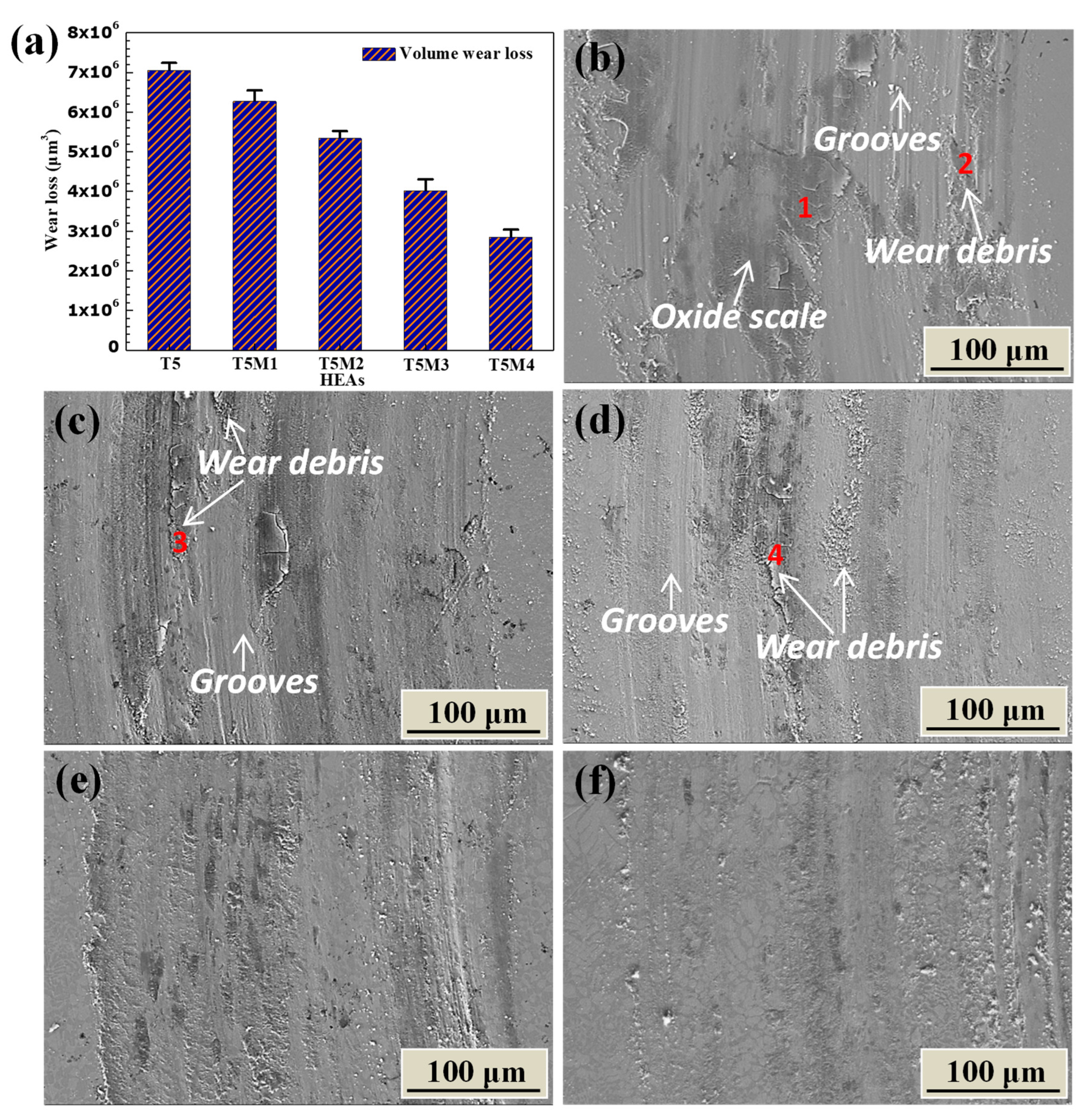
3.4. Wear Resistance
4. Discussion
4.1. Mo Distribution and Phase Prediction
4.2. Further Discussion on Mo Effects Mechanical Properties and Wear Resistance
5. Conclusions
- (1)
- The as-cast AlCrFeNiTi0.5Mox alloys all consisted of a Fe–Cr-rich, disordered BCC phase and a NiAl-rich, ordered BCC phase (B2). The added Mo mainly stayed and acted a solid solute in the BCC phase, strengthening the phase. When Mo molar ratio was more than 0.3, a modified BCC phase formed at the grain boundary, which was enriched with both Mo and Ti.
- (2)
- The Mo addition markedly increased the strength and ductility of AlCrFeNiTi0.5 alloy. The increase in strength was mainly caused by Mo-strengthened disordered BCC phase and precipitation-strengthening by hard (Mo, Ti)-rich BCC precipitates at grain boundaries. The improved ductility benefited from the reduced interfacial lattice mismatch between the disordered BCC phase and B2 phase.
- (3)
- The Mo-free AlCrFeNiTi0.5 showed the largest wear volume loss, which was about 2.5 times as large as that of AlCrFeNiTi0.5Mo0.4 due to its lower yield strength, fracture strength, and ductility. The AlCrFeNiTi0.5Mo0.4 alloy showed the lowest wear volume loss, which possessed the highest values of all the above mentioned three mechanical properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Tsai, M.-H. Three Strategies for the Design of Advanced High-Entropy Alloys. Entropy 2016, 18, 252. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Peighambardoust, N.S.; Alamdari, A.A.; Unal, U.; Motallebzadeh, A. In vitro biocompatibility evaluation of Ti1.5ZrTa0.5Nb0.5Hf0.5 refractory high-entropy alloy film for orthopedic implants: Microstructural, mechanical properties and corrosion behavior. J. Alloys Compd. 2021, 883, 160786. [Google Scholar] [CrossRef]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and V on structure and mechanical properties of high-entropy alloys based on CoCrFeNi system. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Owen, L.R.; Jones, N.G. Lattice distortions in high-entropy alloys. J. Mater. Res. 2018, 33, 2954–2969. [Google Scholar] [CrossRef]
- Shim, S.H.; Pouraliakbar, H.; Lee, B.J.; Kim, Y.K.; Rizi, M.S.; Hong, S.I. Strengthening and deformation behavior of as-cast CoCrCu1.5MnNi high entropy alloy with micro-/nanoscale precipitation. Mater. Sci. Eng. A 2022, 853, 143729. [Google Scholar] [CrossRef]
- Saboktakin Rizi, M.; Minouei, H.; Lee, B.J.; Toroghinejad, M.R.; Hong, S.I. Effects of carbon and molybdenum on the nanostructural evolution and strength/ductility trade-off in Fe40Mn40Co10Cr10 high-entropy alloys. J. Alloys Compd. 2022, 911, 165108. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Shim, S.H.; Kim, Y.K.; Rizi, M.S.; Noh, H.; Hong, S.I. Microstructure evolution and mechanical properties of (CoCrNi)90(AlTiZr)5(CuFeMo)5 multicomponent alloy: A pathway through multicomponent alloys toward new superalloys. J. Alloys Compd. 2021, 860, 158412. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Zhang, Z.; Armstrong, D.E.J.; Grant, P.S. The effects of irradiation on CrMnFeCoNi high-entropy alloy and its derivatives. Prog. Mater. Sci. 2022, 123, 100807. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.; Huang, Z.; Zhou, Q.; Ren, Y.; Du, Y.; Wang, H. Mechanical and dry sliding tribological properties of CoCrNiNbx medium-entropy alloys at room temperature. Tribol. Int. 2021, 163, 107160. [Google Scholar] [CrossRef]
- Ye, F.; Jiao, Z.; Yan, S.; Guo, L.; Feng, L.; Yu, J. Microbeam plasma arc remanufacturing: Effects of Al on microstructure, wear resistance, corrosion resistance and high temperature oxidation resistance of AlxCoCrFeMnNi high-entropy alloy cladding layer. Vacuum 2020, 174, 109178. [Google Scholar] [CrossRef]
- Sun, S.J.; Tian, Y.Z.; Lin, H.R.; Yang, H.J.; Dong, X.G.; Wang, Y.H.; Zhang, Z.F. Achieving high ductility in the 1.7 GPa grade CoCrFeMnNi high-entropy alloy at 77 K. Mater. Sci. Eng. A 2019, 740–741, 336–341. [Google Scholar] [CrossRef]
- Sun, S.J.; Tian, Y.Z.; Lin, H.R.; Lu, S.; Yang, H.J.; Zhang, Z.F. Modulating the prestrain history to optimize strength and ductility in CoCrFeMnNi high-entropy alloy. Scr. Mater. 2019, 163, 111–115. [Google Scholar] [CrossRef]
- Wang, Z.; Baker, I.; Guo, W.; Poplawsky, J.D. The effect of carbon on the microstructures, mechanical properties, and deformation mechanisms of thermo-mechanically treated Fe40.4Ni11.3Mn34.8Al7.5Cr6 high entropy alloys. Acta Mater. 2017, 126, 346–360. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, Y.J.; Taylor, A.; Styles, M.J.; Marceau, R.K.W.; Ceguerra, A.V.; Gibson, M.A.; Liu, Z.K.; Fraser, H.L.; Birbilis, N. A lightweight single-phase AlTiVCr compositionally complex alloy. Acta Mater. 2017, 123, 115–124. [Google Scholar] [CrossRef]
- Feng, R.; Gao, M.C.; Lee, C.; Mathes, M.; Zuo, T.; Chen, S.; Hawk, J.A.; Zhang, Y.; Liaw, P.K. Design of Light-Weight High-Entropy Alloys. Entropy 2016, 18, 333. [Google Scholar] [CrossRef]
- Du, C.; Hu, L.; Pan, Q.; Chen, K.; Zhou, P.; Wang, G. Effect of Cu on the strengthening and embrittling of an FeCoNiCr-xCu HEA. Mater. Sci. Eng. A 2022, 832, 142413. [Google Scholar] [CrossRef]
- Tsau, C.-H.; Lin, S.-X.; Fang, C.-H. Microstructures and corrosion behaviors of FeCoNi and CrFeCoNi equimolar alloys. Mater. Chem. Phys. 2017, 186, 534–540. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Liu, Y.; Fang, Q.; Wu, Y.; Chen, S.; Liu, C.T. Microstructure and mechanical properties of equimolar FeCoCrNi high entropy alloy prepared via powder extrusion. Intermetallics 2016, 75, 25–30. [Google Scholar] [CrossRef]
- Zeng, Z.; Xiang, M.; Zhang, D.; Shi, J.; Wang, W.; Tang, X.; Tang, W.; Wang, Y.; Ma, X.; Chen, Z.; et al. Mechanical properties of Cantor alloys driven by additional elements: A review. J. Mater. Res. Technol. 2021, 15, 1920–1934. [Google Scholar] [CrossRef]
- Cantor, B. Multicomponent high-entropy Cantor alloys. Prog. Mater. Sci. 2021, 120, 100754. [Google Scholar] [CrossRef]
- Chen, S.; Oh, H.S.; Gludovatz, B.; Kim, S.J.; Park, E.S.; Zhang, Z.; Ritchie, R.O.; Yu, Q. Real-time observations of TRIP-induced ultrahigh strain hardening in a dual-phase CrMnFeCoNi high-entropy alloy. Nat. Commun. 2020, 11, 826. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; An, X.; Zhang, Y.; Sun, S.; Tian, Y.; Zhang, Z.; Wang, A.; Liu, J.; Song, M.; et al. Deformation-induced crystalline-to-amorphous phase transformation in a CrMnFeCoNi high-entropy alloy. Sci. Adv. 2021, 7, eabe3105. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Li, Q.; Wang, S.; Wu, M.; Yang, S.; Xiang, D. Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy. Metals 2022, 12, 191. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Tian, L.; Jiao, Z.M.; Yuan, G.Z.; Ma, S.G.; Wang, Z.H.; Yang, H.J.; Zhang, Y.; Qiao, J.W. Effect of Strain Rate on Deformation Behavior of AlCoCrFeNi High-Entropy Alloy by Nanoindentation. J. Mater. Eng. Perform. 2016, 25, 2255–2260. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, W.; Xia, Z.; Xiong, W.; Fu, Z. Influence of synthesis method on microstructure and mechanical behavior of Co-free AlCrFeNi medium-entropy alloy. Intermetallics 2019, 108, 45–54. [Google Scholar] [CrossRef]
- Wu, M.; Setiawan, R.C.; Li, D.Y. Benefits of passive element Ti to the resistance of AlCrFeCoNi high-entropy alloy to corrosion and corrosive wear. Wear 2022, 492–493, 204231. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti addition on the sliding wear behavior of AlCrFeCoNi high-entropy alloy. Wear 2020, 462–463, 203493. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, W.; Wu, B.; Cao, X.; Liu, L.; Fu, Z. Effects of Co and Ti on microstructure and mechanical behavior of Al0.75FeNiCrCo high entropy alloy prepared by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2015, 648, 217–224. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Chen, Z.; Wen, H.; Lavernia, E.J. Influence of Ti addition and sintering method on microstructure and mechanical behavior of a medium-entropy Al0.6CoNiFe alloy. Mater. Sci. Eng. A 2014, 619, 137–145. [Google Scholar] [CrossRef]
- Li, S.; Chen, F.; Tang, X.; Ge, G.; Sun, Z.; Geng, Z.; Fan, M.; Huang, P. Effect of Ti on the Microstructure and Mechanical Properties of AlCrFeNiTix Eutectic High-Entropy Alloys. J. Mater. Eng. Perform. 2022. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Influence of Titanium on Microstructure, Phase Formation and Wear Behaviour of AlCoCrFeNiTix High-Entropy Alloy. Entropy 2018, 20, 505. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Quan, H.; Chen, Y.; Wang, S.; Zhang, S. Effect of Mo addition on structures and properties of FeCoNiCrMn high entropy alloy film by direct current magnetron sputtering. J. Alloys Compd. 2022, 895, 162709. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Kao, W.-P.; Yeh, J.-W.; Tsai, C.-W. Effect of Mo on the Mechanical and Corrosion Behaviors in Non-Equal Molar AlCrFeMnNi BCC High-Entropy Alloys. Mater. Sci. Mater. Rev. 2022, 15, 751. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Y.; Cui, S.; Yi, Y.; Xing, X.; Wang, X.; Li, W. Effect of Mo Element on the Mechanical Properties and Tribological Responses of CoCrFeNiMox High-Entropy Alloys. Metals 2021, 11, 468. [Google Scholar] [CrossRef]
- Qin, G.; Chen, R.; Zheng, H.; Fang, H.; Wang, L.; Su, Y.; Guo, J.; Fu, H. Strengthening FCC-CoCrFeMnNi high entropy alloys by Mo addition. J. Mater. Sci. Technol. 2019, 35, 578–583. [Google Scholar] [CrossRef]
- Zhuang, Y.-X.; Zhang, X.-L.; Gu, X.-Y. Effect of Annealing on Microstructure and Mechanical Properties of Al0.5CoCrFeMoxNi High-Entropy Alloys. Entropy 2018, 20, 812. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Li, J.; Kou, H.; Liu, W. Characterization of BCC phases in AlCoCrFeNiTix high entropy alloys. Mater. Lett. 2015, 138, 78–80. [Google Scholar] [CrossRef]
- Yeh, J.-W. Recent progress in high-entropy alloys. Eur. J. Control 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys. Mater. Sci. Eng. A 2010, 527, 7210–7214. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructures and mechanical properties of multiprincipal component CoCrFeNiTix alloys. Mater. Sci. Eng. A 2012, 556, 170–174. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Chernichenko, R.S.; Tikhonovsky, M.A.; Zherebtsov, S.V. Effect of Al on structure and mechanical properties of Fe-Mn-Cr-Ni-Al non-equiatomic high entropy alloys with high Fe content. J. Alloys Compd. 2019, 770, 194–203. [Google Scholar] [CrossRef]
- Diao, G.; He, A.; Li, D.Y.; Wu, M.; Xu, Z.; Li, W.; Li, Q.Y. Tune a highly ductile AlCrFe2Ni4 alloy by Ti addition for desired high mechanical strength. Mater. Sci. Eng. A 2022, 856, 143910. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Yang, Y.; Wang, J.; Liu, C.T. Atomic-size effect and solid solubility of multicomponent alloys. Scr. Mater. 2015, 94, 28–31. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, N.; Dwivedi, A.; Subramaniam, A. A geometrical parameter for the formation of disordered solid solutions in multi-component alloys. Intermetallics 2014, 53, 112–119. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Fan, L.; Yang, T.; Zhao, Y.; Luan, J.; Zhou, G.; Wang, H.; Jiao, Z.; Liu, C.-T. Ultrahigh strength and ductility in newly developed materials with coherent nanolamellar architectures. Nat. Commun. 2020, 11, 6240. [Google Scholar] [CrossRef]
- Wang, S.D.; Wu, M.Y.; Xu, D.K.; Han, E.-h. Improving corrosive wear resistance of Mg-Zn-Y-Zr alloys through heat treatment. J. Magnes. Alloys 2021. [Google Scholar] [CrossRef]

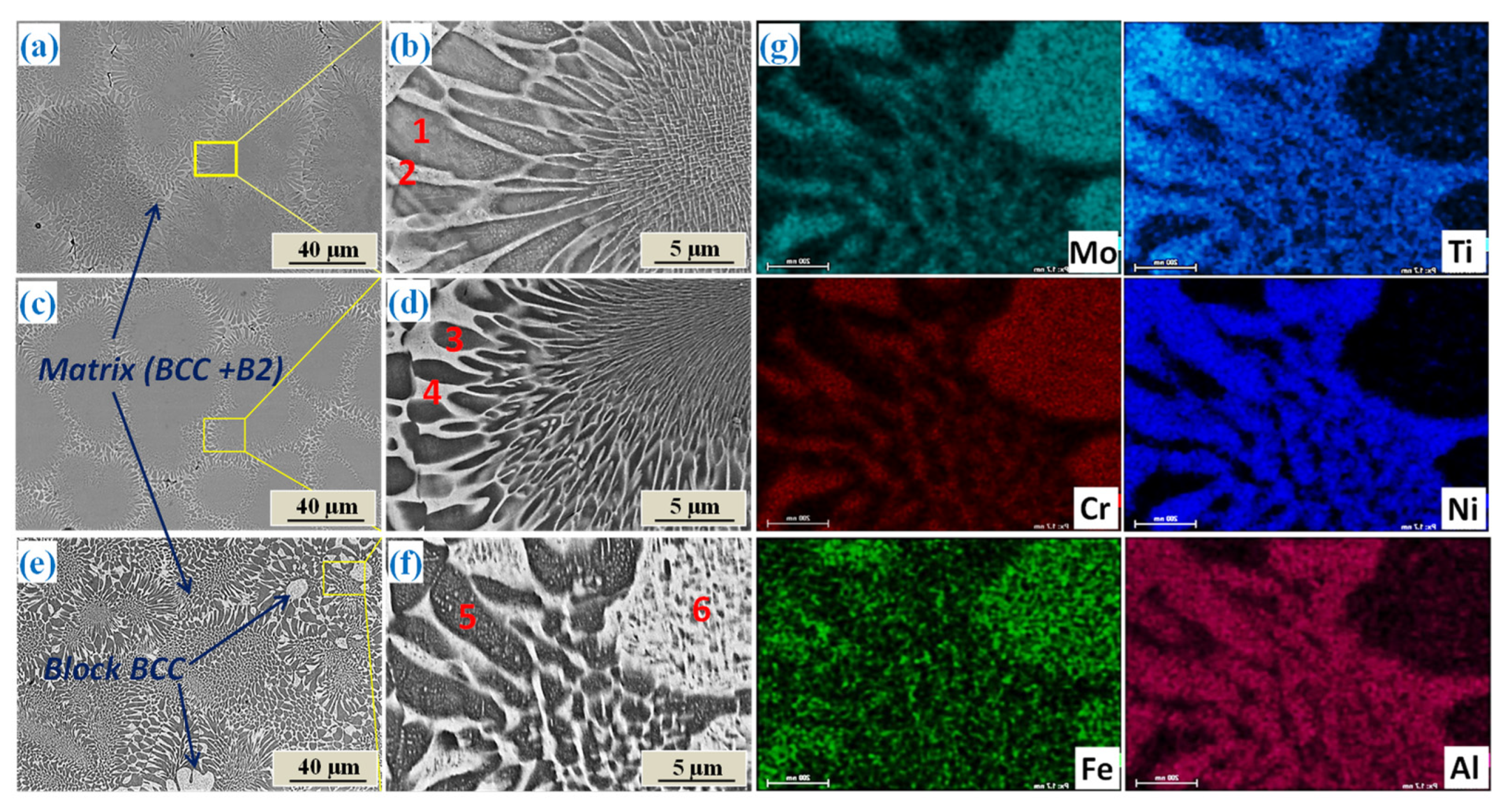
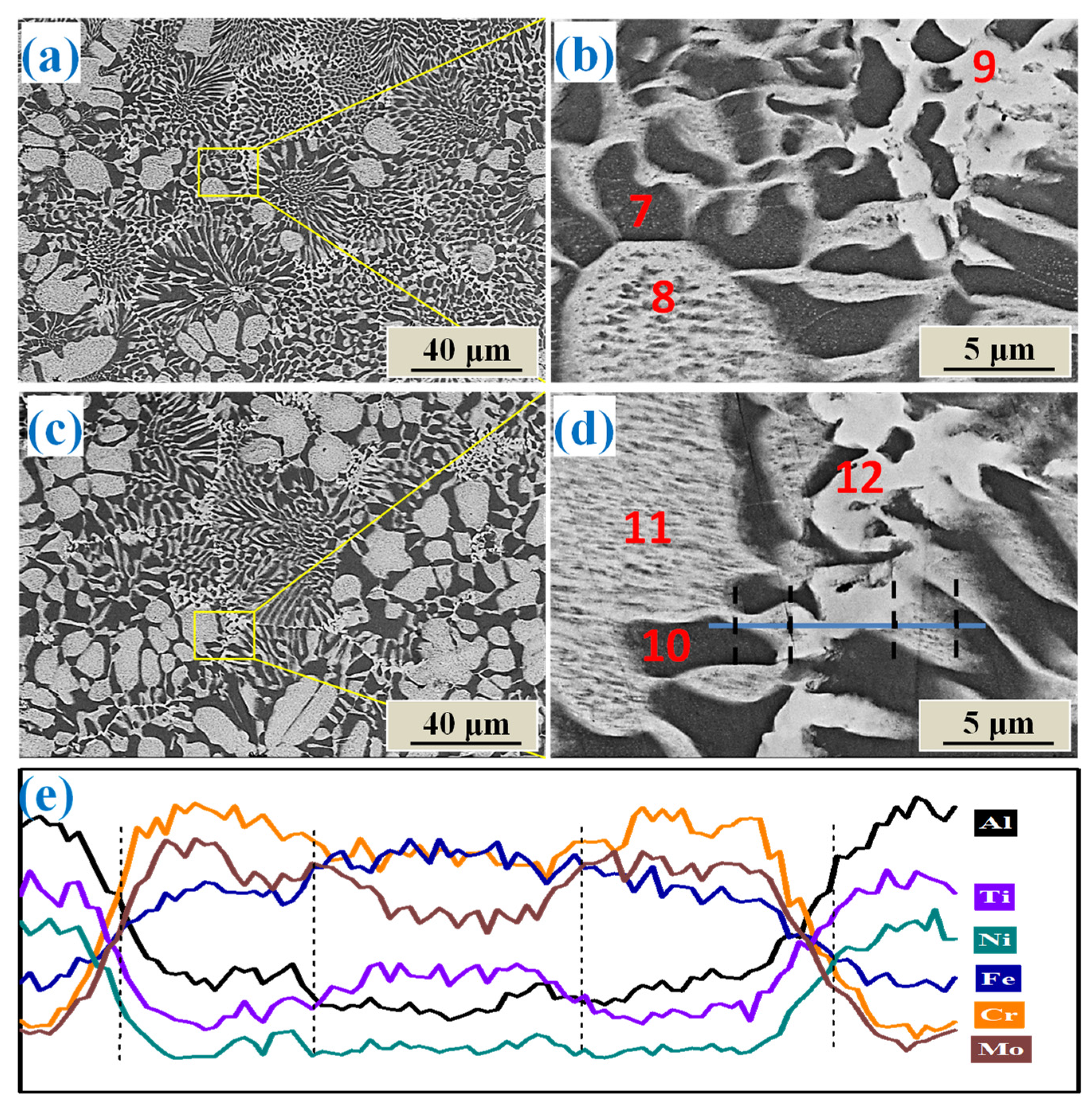


| Alloys | Areas | Al | Cr | Fe | Ni | Ti | Mo |
|---|---|---|---|---|---|---|---|
| AlCrFeNiTi0.5 | 1 | 26.0 | 6.1 | 17.1 | 28.6 | 22.2 | / |
| 2 | 9.6 | 39.9 | 37.5 | 5.9 | 7.1 | / | |
| AlCrFeNiTi0.5Mo0.1 | 3 | 24.6 | 5.4 | 17.0 | 29.5 | 22.9 | 0.5 |
| 4 | 5.9 | 40.8 | 41.9 | 2.3 | 4.8 | 4.3 | |
| AlCrFeNiTi0.5Mo0.2 | 5 | 28.8 | 10.8 | 17.6 | 27.5 | 13.6 | 1.7 |
| 6 | 11.0 | 41.0 | 29.0 | 6.2 | 4.4 | 10.4 | |
| AlCrFeNiTi0.5Mo0.3 | 7 | 24.8 | 4.9 | 14.9 | 33.2 | 21.2 | 1.1 |
| 8 | 11.2 | 36.1 | 26.5 | 6.6 | 6.7 | 12.7 | |
| 9 | 5.9 | 26.8 | 39.2 | 6.9 | 12.7 | 8.4 | |
| AlCrFeNiTi0.5Mo0.4 | 10 | 25.1 | 4.1 | 14.1 | 33.9 | 21.9 | 0.9 |
| 11 | 13.4 | 31.3 | 24.0 | 10.3 | 7.0 | 13.9 | |
| 12 | 6.3 | 21.6 | 36.1 | 9.1 | 15.9 | 11.1 |
| Alloys | Regions | Micro-Hardness (MPa) | Overall Hardness (HV) |
|---|---|---|---|
| AlCrFeNiTi0.5 | 1 (BCC + B2) | 7557.4 | 752.5 |
| 2 (BCC + B2) | 7624.5 | ||
| AlCrFeNiTi0.5Mo0.1 | 1 (BCC + B2) | 8336.1 | 829.2 |
| 2 (BCC + B2) | 8320.5 | ||
| AlCrFeNiTi0.5Mo0.2 | 1 (BCC + B2) | 8571.8 | 876.3 |
| 2 Block BCC | 8831.8 | ||
| AlCrFeNiTi0.5Mo0.3 | 1 (BCC + B2) | 9015.1 | 943.6 |
| 2 (Mo, Ti)-rich BCC | 9830.0 | ||
| AlCrFeNiTi0.5Mo0.4 | 1 (BCC + B2) | 9288.6 | 989.7 |
| 2 (Mo, Ti)-rich BCC | 10,493.0 |
| Types | Alloys | T5 | T5M1 | T5M2 | T5M3 | T5M4 |
|---|---|---|---|---|---|---|
| Engineering | Yield strength (MPa) | 1709.3 | 1763.2 | 1810.9 | 1873.3 | 1968.5 |
| Fracture strength (MPa) | 2274.7 | 2236.1 | 2342.5 | 2504.4 | 2750.9 | |
| Fracture strain (%) | 21.8 | 20.7 | 21.9 | 22.4 | 24.1 | |
| True | Yield strength (MPa) | 1486.7 | 1557.2 | 1612.6 | 1662.1 | 1731.6 |
| Fracture strength (MPa) | 1773.2 | 1780.9 | 1836.6 | 1948.6 | 2088.4 | |
| Plastic strain (%) | 12.5 | 11.3 | 12.6 | 13.1 | 15.1 |
| Alloys | Symbols | O | Si | Al | Cr | Fe | Ni | Ti | Mo |
|---|---|---|---|---|---|---|---|---|---|
| AlCrFeNiTi0.5 | 1 | 62.1 | 2.7 | 10.7 | 7.0 | 6.2 | 6.8 | 4.5 | - |
| 2 | 44.2 | 2.8 | 12.0 | 13.3 | 11.1 | 10.3 | 6.2 | - | |
| AlCrFeNiTi0.5Mo0.1 | 3 | 36.7 | 2.1 | 14.2 | 12.8 | 12.4 | 13.9 | 6.9 | 1 |
| AlCrFeNiTi0.5Mo0.2 | 4 | 32.3 | 2.4 | 15.3 | 12.7 | 13.6 | 14.2 | 7.7 | 1.8 |
| Elements | Enthalpy of Mixing (KJ/mol) | VECi | Atomic Radius (pm) | |||||
|---|---|---|---|---|---|---|---|---|
| Al | Cr | Fe | Ni | Ti | Mo | |||
| Al | 0 | 3 | 118 | |||||
| Cr | −10 | 0 | 6 | 166 | ||||
| Fe | −11 | −1 | 0 | 8 | 156 | |||
| Ni | −22 | −7 | −2 | 0 | 10 | 149 | ||
| Ti | −30 | −7 | −17 | −35 | 0 | 4 | 176 | |
| Mo | −5 | 0 | −2 | −7 | −4 | 0 | 6 | 190 |
| HEAs | ΔHmix(kJ/mol) | δ (%) | VEC |
|---|---|---|---|
| AlCrFeNiTi0.5 | −19.22 | 7.11 | 6.4425 |
| AlCrFeNiTi0.5Mo0.1 | −18.73 | 7.04 | 6.4348 |
| AlCrFeNiTi0.5Mo0.2 | −18.24 | 6.98 | 6.4268 |
| AlCrFeNiTi0.5Mo0.3 | −17.76 | 6.93 | 6.4159 |
| AlCrFeNiTi0.5Mo0.4 | −17.31 | 6.87 | 6.4056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Yuan, J.; Diao, G.; Li, D. Achieving a Combination of Higher Strength and Higher Ductility for Enhanced Wear Resistance of AlCrFeNiTi0.5 High-Entropy Alloy by Mo Addition. Metals 2022, 12, 1910. https://doi.org/10.3390/met12111910
Wu M, Yuan J, Diao G, Li D. Achieving a Combination of Higher Strength and Higher Ductility for Enhanced Wear Resistance of AlCrFeNiTi0.5 High-Entropy Alloy by Mo Addition. Metals. 2022; 12(11):1910. https://doi.org/10.3390/met12111910
Chicago/Turabian StyleWu, Mingyu, Junfeng Yuan, Guijiang Diao, and Dongyang Li. 2022. "Achieving a Combination of Higher Strength and Higher Ductility for Enhanced Wear Resistance of AlCrFeNiTi0.5 High-Entropy Alloy by Mo Addition" Metals 12, no. 11: 1910. https://doi.org/10.3390/met12111910
APA StyleWu, M., Yuan, J., Diao, G., & Li, D. (2022). Achieving a Combination of Higher Strength and Higher Ductility for Enhanced Wear Resistance of AlCrFeNiTi0.5 High-Entropy Alloy by Mo Addition. Metals, 12(11), 1910. https://doi.org/10.3390/met12111910






