Specific Surface Area Evolution and Shrinkage Control of Pre-Sintered Nickel Clusters
Abstract
1. Introduction
2. Theory
2.1. Atoms Movement on Particle Surfaces
2.2. A Control Method of Linear Sintering Shrinkage Ratio
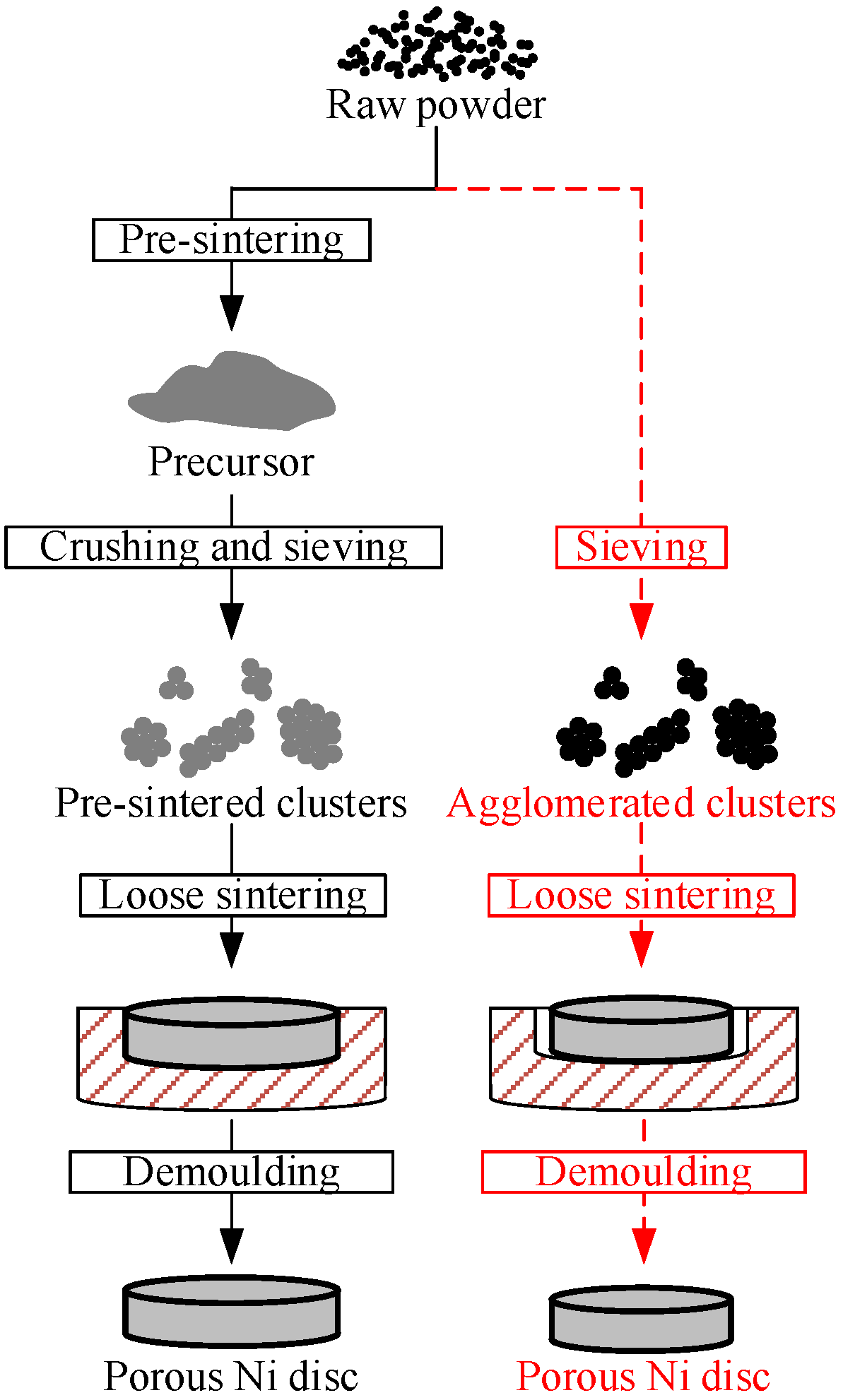
3. Materials and Methods
4. Results and Discussion
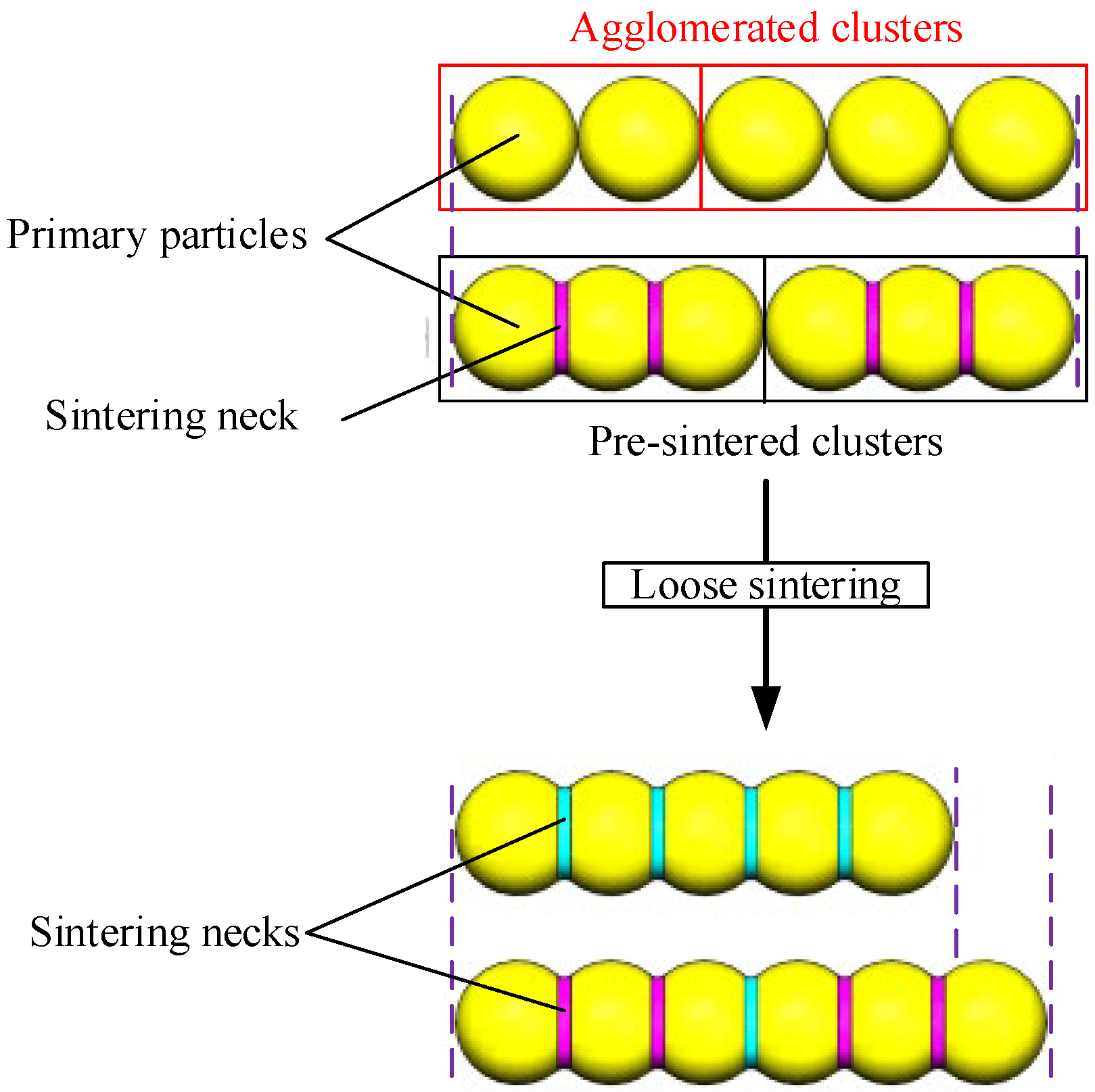
4.1. Morphology of Clusters
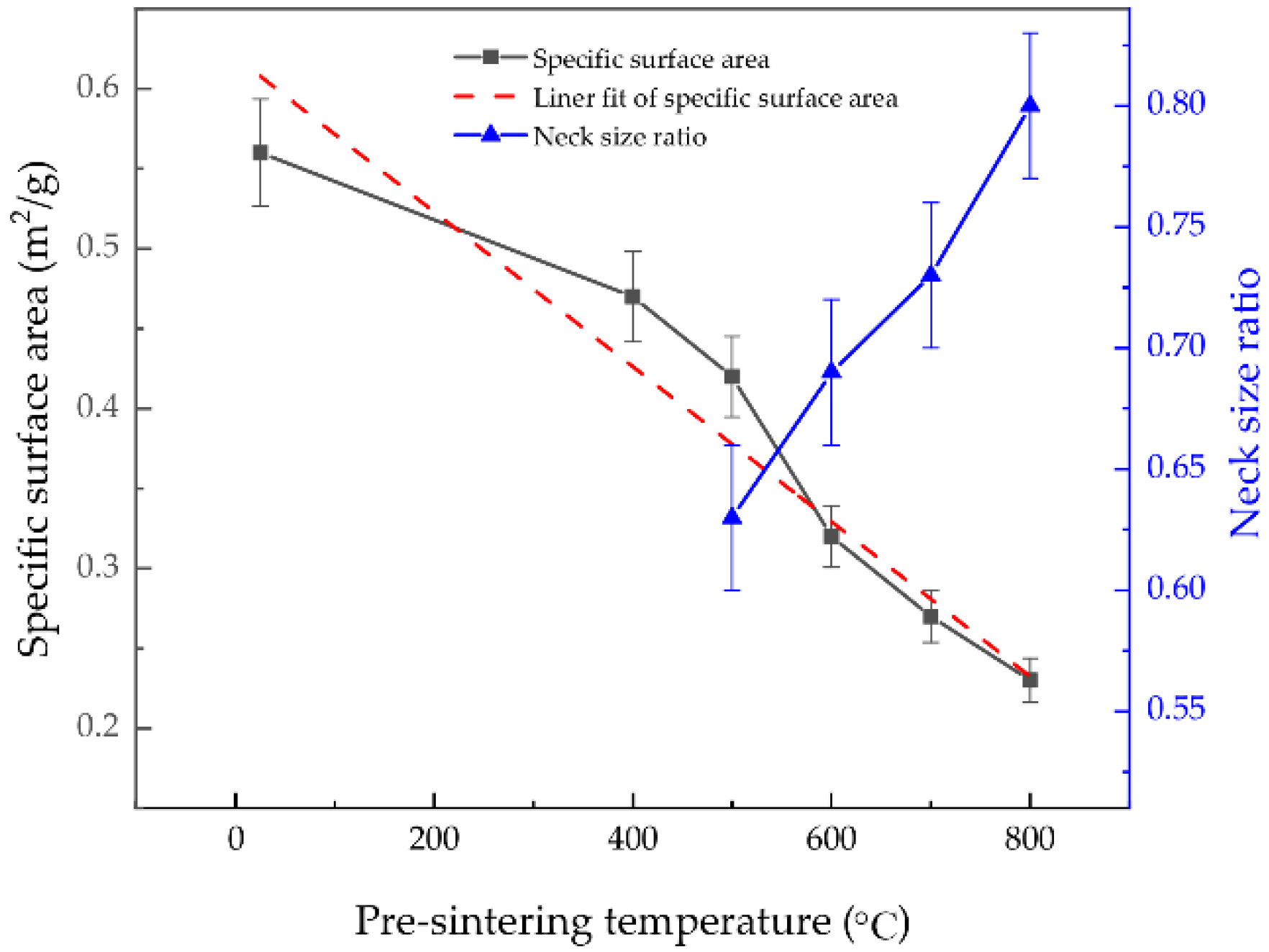
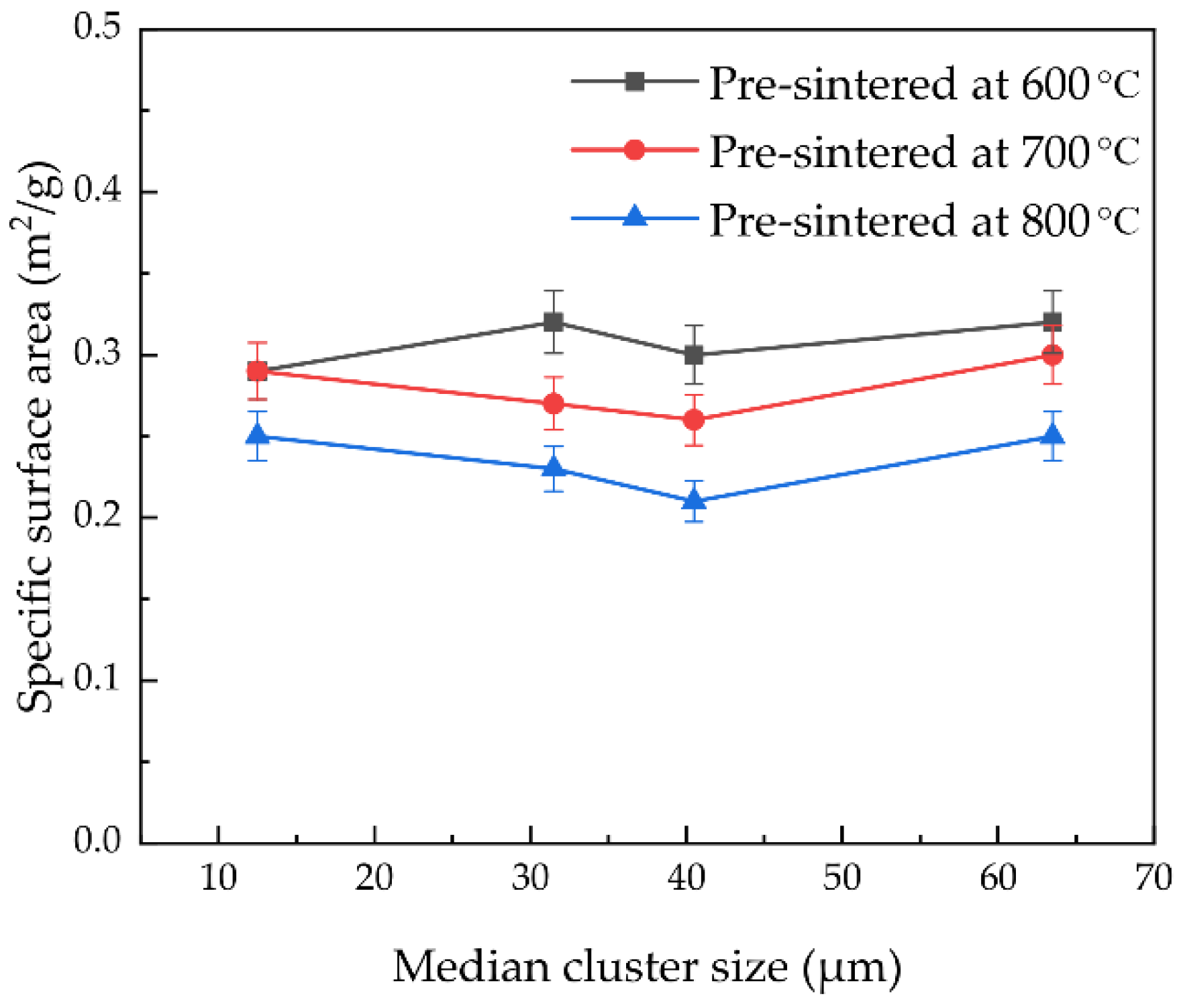
4.2. Specific Surface Area
4.3. Radial Shrinkage Ratio
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, D.K.; Saravanan, T.T.; Khanra, G.P.; Girikumar, S.; Sharma, S.C.; Sreekumar, K.; Sinha, P.P. Studies on the processing of nickel base porous wicks for capillary pumped loop for thermal management of spacecrafts. Adv. Powder. Technol. 2010, 21, 658–662. [Google Scholar] [CrossRef]
- Wang, Z.B.; Chen, J.; Liu, R.T.; Li, H.; Lin, X.Y.; He, D.; Xiong, X. Preparation of an ultrafine nickel powder by solid-phase reduction with a NaCl separator agent. Adv. Powder. Technol. 2020, 31, 3433–3439. [Google Scholar]
- Fedorova, Z.A.; Danilova, M.M.; Zaikovskii, V.I. Porous nickel-based catalysts for tri-reforming of methane to synthesis gas: Catalytic activity. Mater. Lett. 2020, 261, 127087. [Google Scholar] [CrossRef]
- Guo, C.S.; Jiang, C.; Ma, J.; Cao, Y.W.; Zou, Y.; Liu, Y. Experimental study on comprehensive performance of sintering biporous wicks prepared by salt dissolution and cold pressing process. J. Heat Transf. 2020, 142, 011902. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, L.; Zhang, K.F.; Tian, Y. Effects of multiple sintering parameters on the thermal performance of bi-porous nickel wicks in loop heat pipes. Int. J. Heat Mass Transf. 2016, 99, 638–646. [Google Scholar] [CrossRef]
- Cao, Y.W.; Guo, C.S.; Wu, D.T.; Zou, Y. Effects of pore structure characteristics on performance of sintered bi-porous Ti3AlC2 wicks. Mater. Res. Express 2021, 8, 015602. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Zhao, R.Z.; Liu, Z.C.; Liu, W. Application of biporous wick in flat-plate loop heat pipe with long heat transfer distance. Appl. Therm. Eng. 2021, 184, 116283. [Google Scholar] [CrossRef]
- Wang, C.X.; Li, Y.W.; Li, D.T.; Wang, Y.J.; Cheng, Y.J. Miniature vacuum leak element made with porous nickel sheet. Vacuum 2018, 158, 146–151. [Google Scholar] [CrossRef]
- Ryi, S.K.; Park, J.S.; Kim, S.H.; Hong, S.C.; Kim, D.W. Development of porous nickel membrane made by uniaxial pressing for hydrogen separation. Desalination 2006, 200, 213–215. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.C.; Chen, B.B.; Liu, W.; Li, C.; Yang, J.G. Development of biporous wicks for flat-plate loop heat pipe. Exp. Therm. Fluid Sci. 2012, 37, 91–97. [Google Scholar] [CrossRef]
- Singh, R.; Nguyen, T.; Mochizuki, M. Capillary evaporator development and qualification for loop heat pipes. Appl. Therm. Eng. 2014, 63, 406–418. [Google Scholar] [CrossRef]
- Shigeno, K.; Tezuka, M. Examination of non-shrinkage firing using low-temperature co-fired alumina containing a small quantity of Cu-Ti-Nb-Ag-O additive. Int. J. Appl. Ceram. Technol. 2020, 17, 713–721. [Google Scholar] [CrossRef]
- Espinosa, F.A.D.; Peters, T.B.; Brisson, J.G. Effect of fabrication parameters on the thermophysical properties of sintered wicks for heat pipe applications. Int. J. Heat Mass Transf. 2012, 55, 7471–7486. [Google Scholar] [CrossRef]
- Jernot, J.P.; Coster, M.; Chermant, J.L. Model of variation of the specific surface area during sintering. Powder Technol. 1981, 30, 21–29. [Google Scholar] [CrossRef]
- Huang, Y.H.; Jiang, D.L.; Zhang, J.X.; Chen, Z.M.; Lin, Q.L.; Huang, Z.R. Sintering kinetics of YAG ceramics. J. Rare Earths 2014, 32, 416–422. [Google Scholar] [CrossRef]
- German, R.M. Sintering: From Empirical Observations to Scientific Principles; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Zeng, L.; Sun, H.J.; Peng, T.J.; Zheng, W.M. The sintering kinetics and properties of sintered glass-ceramics from coal fly ash of different particle size. Results Phys. 2019, 15, 102774. [Google Scholar] [CrossRef]
- Deng, S.H.; Li, J.N.; Li, R.D.; Zhao, H.J.; Yuan, T.; Li, L.B.; Zhang, Y.J. The effect of particle size on the densification kinetics of tungsten powder during spark plasma sintering. Int. J. Refract Met. Hard Mater. 2020, 93, 105358. [Google Scholar] [CrossRef]
- Matsui, K.; Matsumoto, A.; Uehara, M.; Enomoto, N.; Hojo, J. Sintering kinetics at isothermal shrinkage: Effect of specific surface area on the initial sintering stage of fine zirconia powder. J. Am. Ceram. Soc. 2007, 90, 44–49. [Google Scholar] [CrossRef]
- Han, Y.; Fan, J.L.; Liu, T.; Cheng, H.C.; Tian, J.M. The effects of ball-milling treatment on the densification behavior of ultra-fine tungsten powder. Int. J. Refract Met. Hard Mater. 2011, 29, 743–750. [Google Scholar] [CrossRef]
- Coble, R.L. Initial sintering of alumina and hematite. J. Am. Ceram. Soc. 1958, 41, 55–62. [Google Scholar] [CrossRef]
- Kothari, N.C. The effect of particle size on sintering kinetics in alumina powder. J. Nucl. Mater. 1965, 17, 43–53. [Google Scholar] [CrossRef]
- Heydecke, G.; Butz, F.; Binder, J.R.; Strub, J.R. Material characteristics of a novel shrinkage-free ZrSiO4 ceramic for the fabrication of posterior crowns. Dent. Mater. 2007, 23, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, B.S.B.; Kim, H.D. Fabrication of low cost shirinkage-free porous sialon ceramics. J. Ceram. Process. Res. 2009, 10, 581–588. [Google Scholar]
- Lv, L.; Lu, Y.J.; Zhang, X.Y.; Chen, Y.G.; Huo, W.L.; Liu, W.; Yang, J.L. Preparation of low-shrinkage and high-performance alumina ceramics via incorporation of pre-sintered alumina powder based on Isobam gelcasting. Ceram. Int. 2019, 45, 11654–11659. [Google Scholar] [CrossRef]
- Cao, X.L.; Cheng, P.; Zhao, T.S. Experimental study of evaporative heat transfer in sintered copper bidispersed wick structures. J. Thermophys. Heat Transf. 2002, 16, 547–552. [Google Scholar] [CrossRef]
- Semenic, T.; Lin, Y.Y.; Catton, I. Thermophysical properties of biporous heat pipe evaporators. J. Heat Transf. 2008, 130, 022602. [Google Scholar] [CrossRef]
- Byon, C.; Kim, S.J. Capillary performance of bi-porous sintered metal wicks. Int. J. Heat Mass Transf. 2012, 55, 4096–4103. [Google Scholar] [CrossRef]
- Jost, W. Diffusion in Solids, Liquids, Gases; Academic Press: New York, NY, USA, 1952. [Google Scholar]
- Ehrlich, G.; Stolt, K. Surface diffusion. Annu. Rev. Phys. Chem. 1980, 31, 603–637. [Google Scholar] [CrossRef]
- Skorokhod, V.V.; Ranneva, G.O. Study of the sintering of nickel powders produced by different methods. Powder Metall. Met. Ceram. 1963, 2, 194–198. [Google Scholar] [CrossRef]
- Luo, W.D.; Pan, J.Z. Effects of surface diffusion and heating rate on first-stage sintering that densifies by grain-boundary diffusion. J. Am. Ceram. Soc. 2015, 98, 3483–3489. [Google Scholar] [CrossRef]
- Bross, P.; Exner, H.E. Computer simulation of sintering processes. Acta Metall. 1979, 27, 1013–1020. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Wang, L.; Zhang, S.; Zhang, L.; Hu, Q.; Wang, L. Specific Surface Area Evolution and Shrinkage Control of Pre-Sintered Nickel Clusters. Metals 2022, 12, 1693. https://doi.org/10.3390/met12101693
Zheng F, Wang L, Zhang S, Zhang L, Hu Q, Wang L. Specific Surface Area Evolution and Shrinkage Control of Pre-Sintered Nickel Clusters. Metals. 2022; 12(10):1693. https://doi.org/10.3390/met12101693
Chicago/Turabian StyleZheng, Fengshi, Linshan Wang, Shaoming Zhang, Lin Zhang, Qiang Hu, and Limin Wang. 2022. "Specific Surface Area Evolution and Shrinkage Control of Pre-Sintered Nickel Clusters" Metals 12, no. 10: 1693. https://doi.org/10.3390/met12101693
APA StyleZheng, F., Wang, L., Zhang, S., Zhang, L., Hu, Q., & Wang, L. (2022). Specific Surface Area Evolution and Shrinkage Control of Pre-Sintered Nickel Clusters. Metals, 12(10), 1693. https://doi.org/10.3390/met12101693




