Understanding Fe-Containing Intermetallic Compounds in Al Alloys: An Overview of Recent Advances from the LiME Research Hub
Abstract
1. Introduction
- Controlling the nucleation and growth process so to produce primary FIMCs with a compact morphology and a fine particle size; and
2. Characteristics of and the Relationship between FIMCs
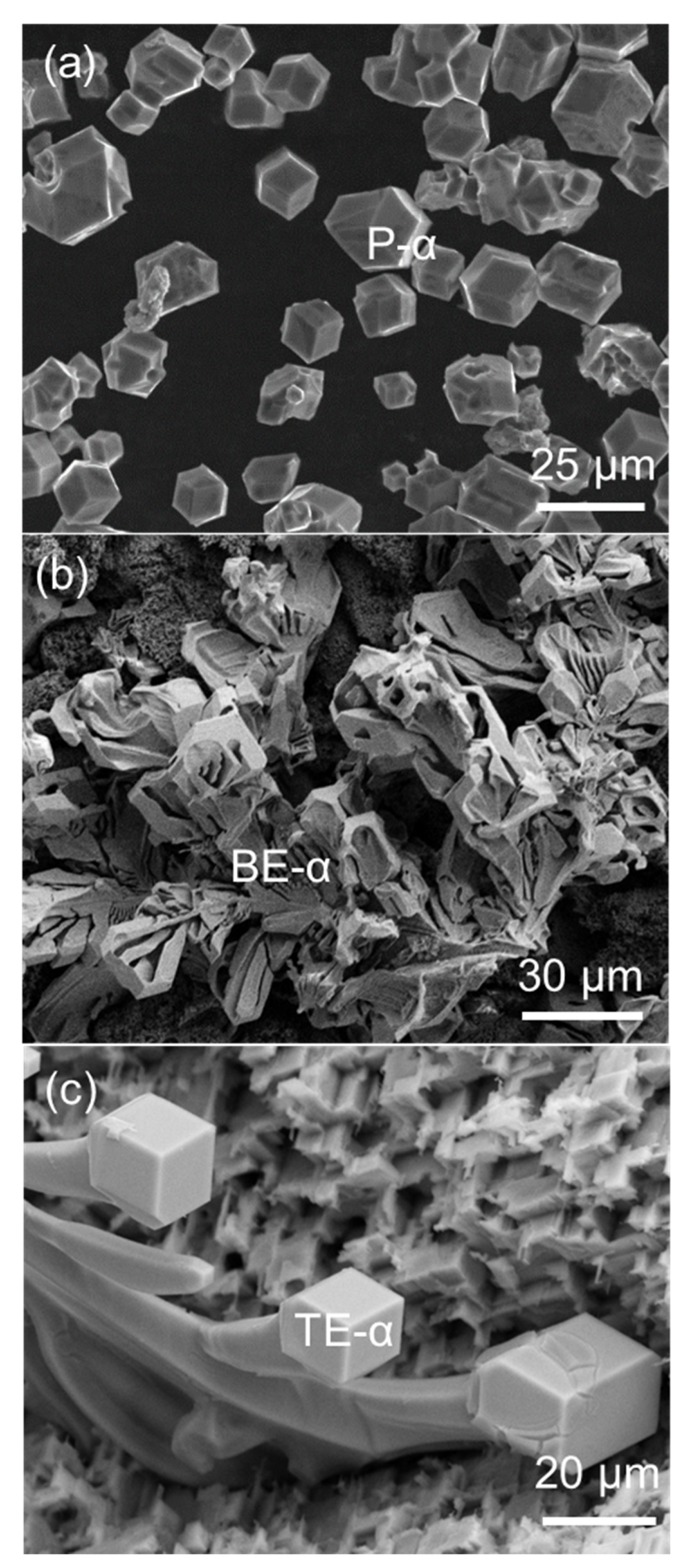
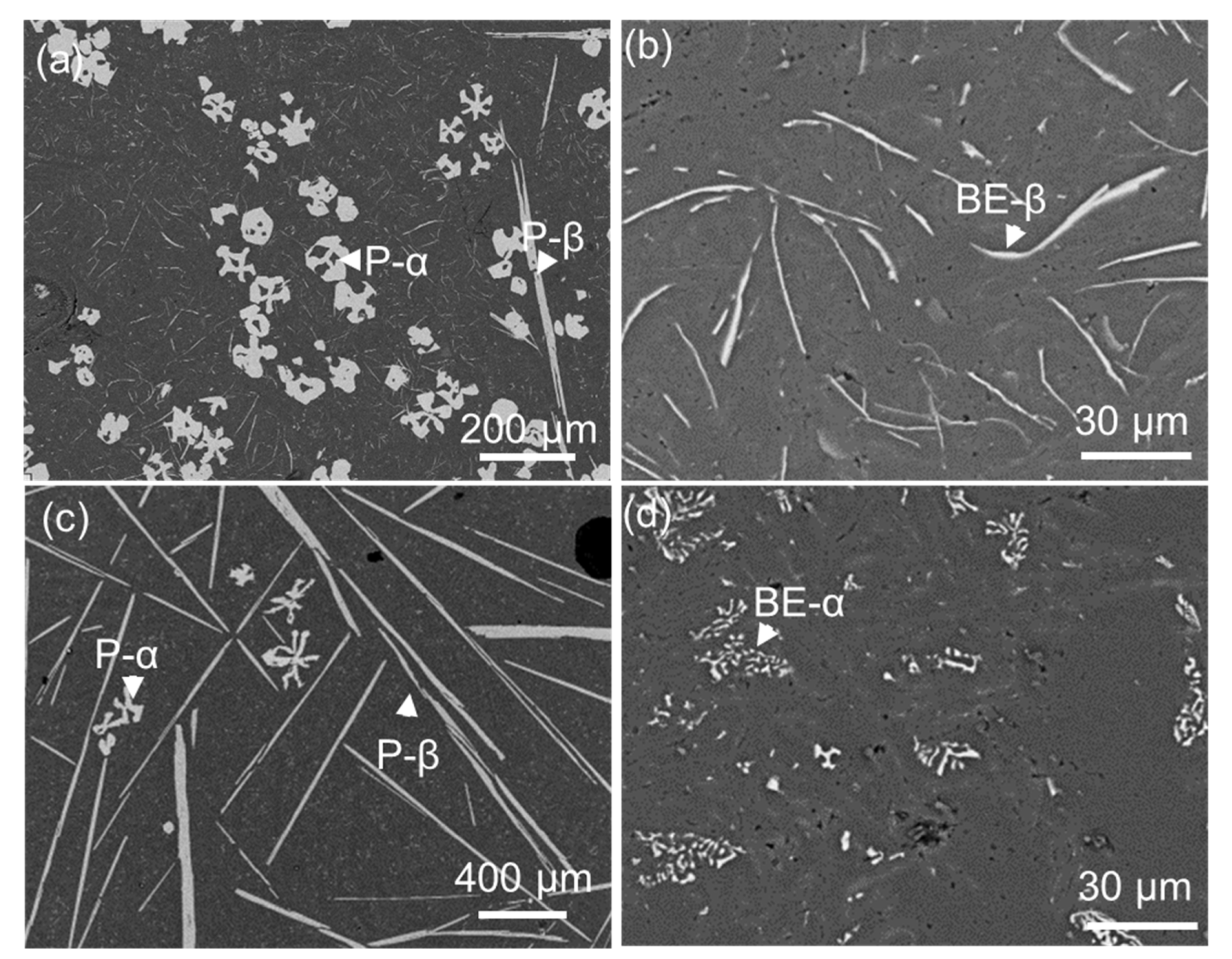
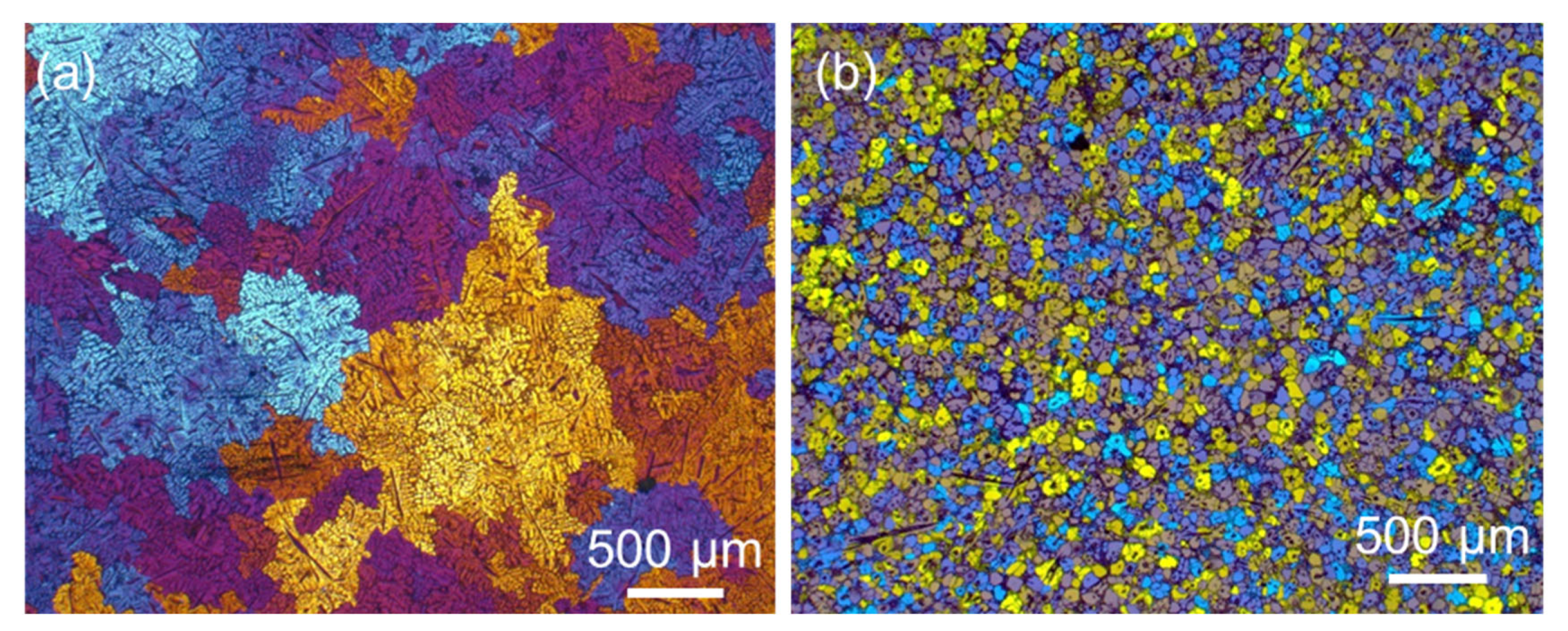
2.1. Morphology of FIMCs
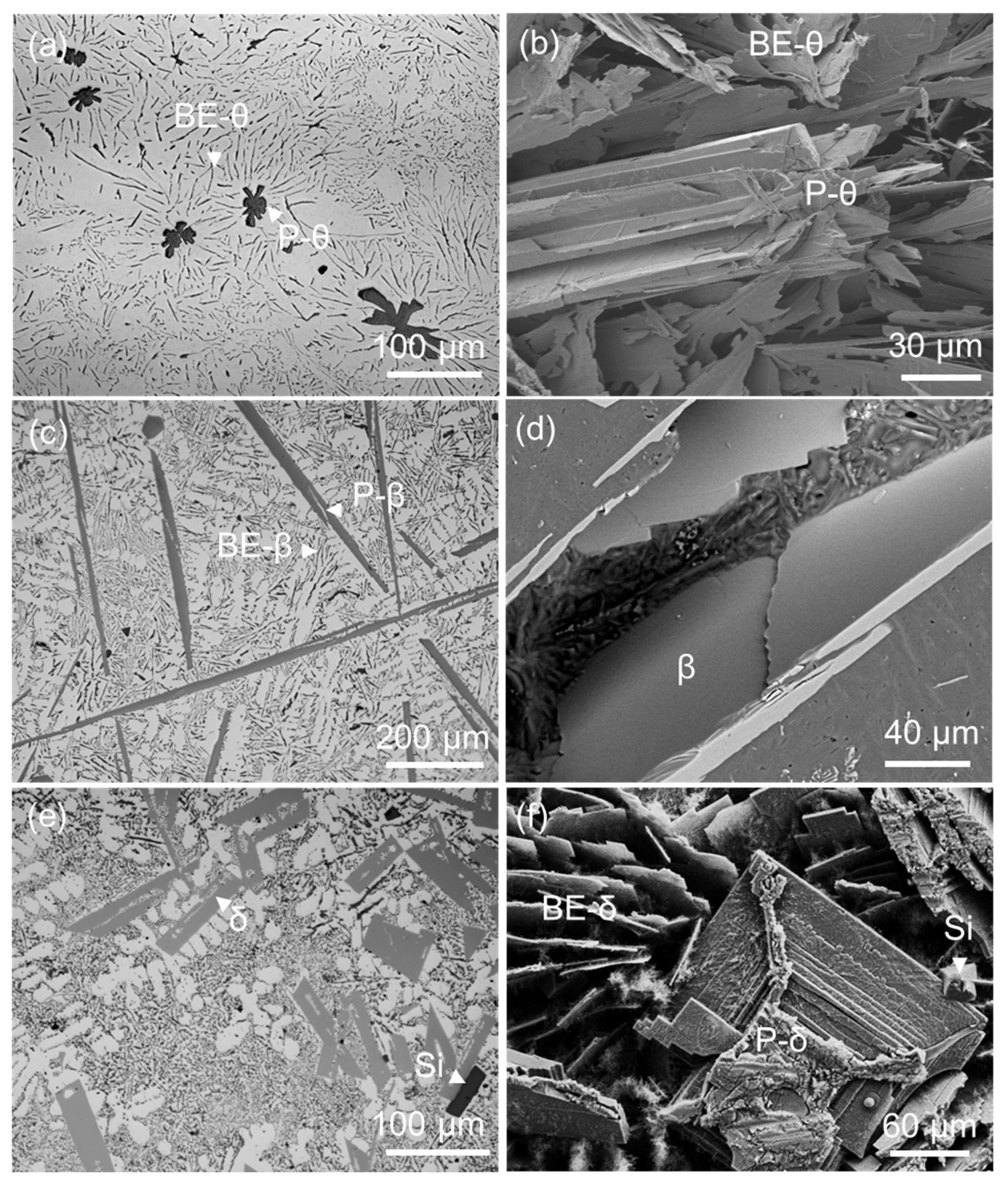
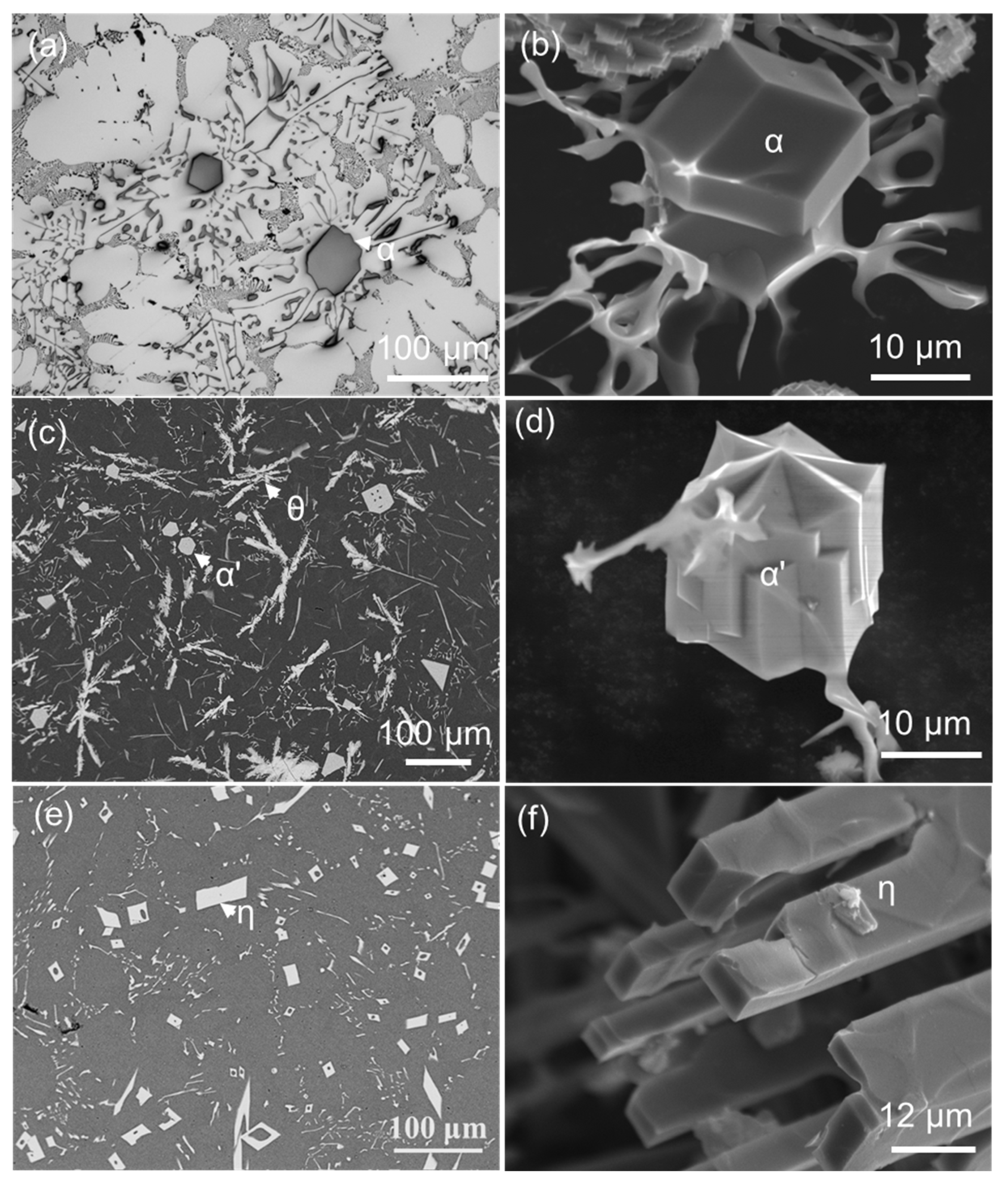
| Alloys | FIMCs | at.% | Technique | |||
|---|---|---|---|---|---|---|
| Al | Fe | Mn | Si | |||
| Al-3Fe (HP Al) | θ-Al13Fe4 | 83.5 ± 0.4 | 16.5 ± 0.1 | - | - | SEM [80] |
| Al-1Fe (CP Al) | P-θ-Al13Fe4 | 80.8 ± 0.4 | 18.9 ± 0.1 | 0.0 | 0.3 ± 0.0 | TEM [80] |
| Al-4Fe-4Si | P- θ-Al13Fe4 | 76.3 ± 0.6 | 20.6 ± 0.5 | 0.4 ± 0.05 | 2.7 ± 0.2 | TEM [80] |
| Al-4Fe-4Si | PT-α’-Al8Fe2Si | 74.4 ± 0.3 | 15.3 ± 0.1 | - | 10.4 ± 0.1 | TEM [80] |
| Al-4Fe-4Si | PT-β-Al5FeSi | 69.2 ± 0.3 | 13.8 ± 0.1 | - | 16.9 ± 0.1 | TEM [80] |
| Al-2Fe-8Si | P-β-Al5FeSi | 68.0 ± 0.4 | 14.8 ± 0.1 | - | 17.3 ± 0.1 | SEM |
| Al-5Mg-2Si-0.7Mn-1.3Fe (0.01 K/s) | P-θ-Al13Fe4 | 77.5 ± 0.4 | 16.2 ± 0.2 | 1.9 ± 0.1 | 2.0 ± 0.1 | TEM |
| Al-5Mg-2Si-0.7Mn-1.1Fe (0.01 K/s) | P-α-Al15(Fe, Mn)3Si2 | 74.3 ± 0.4 | 13.7 ± 0.2 | 2.1 ± 0.1 | 7.7 ± 0.1 | TEM |
| Al-5Mg-2Si-0.7Mn-1.1Fe (3.5 K/s) | P-α-Al15(Fe, Mn)3Si2 | 75.9 ± 0.5 | 11.2 ± 0.2 | 7.4 ± 0.1 | 5.5 ± 0.1 | SEM [31] |
| Al-5Mg-2Si-0.7Mn-1.1Fe (3.5 K/s) | BE-α-Al15(Fe, Mn)3Si2 | 78.8 ± 0.5 | 9.0 ± 0.2 | 5.6 ± 0.1 | 6.6 ± 0.1 | SEM |
| Al-5Mg-2Si-0.7Mn-1.1Fe (3.5 K/s) | TE-α-Al15(Fe, Mn)3Si2 | 85.1 ± 0.5 | 12.9 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.1 | SEM |
| Al-16Si-3Fe | P-δ-Al4FeSi2 | 53.7 ± 0.5 | 15.7 ± 0.2 | - | 30.6 ± 0.2 | SEM |
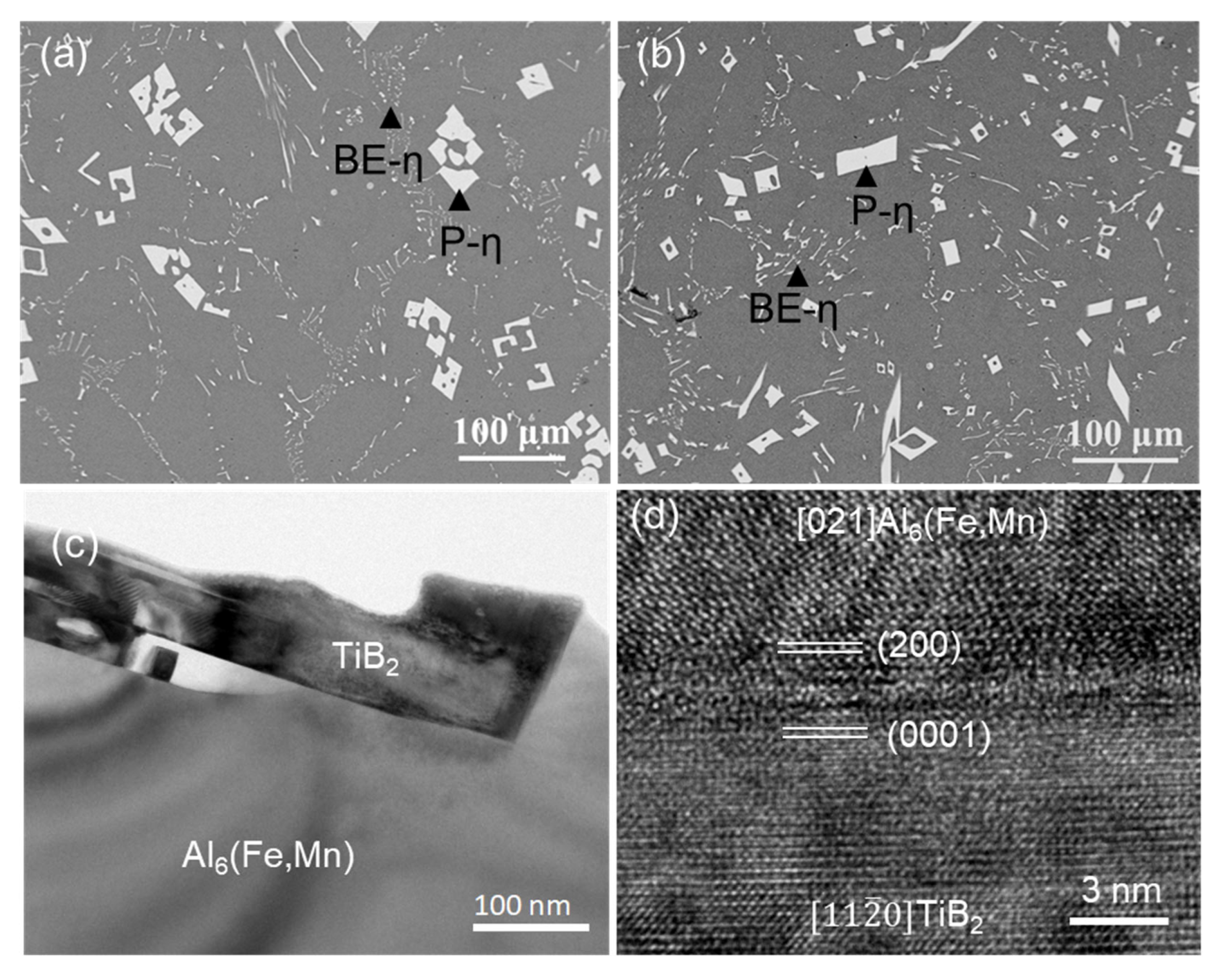
| Al-2Mn-1Fe | P-η-Al6(Fe, Mn) | 84.5 ± 0.5 | 4.9 ± 0.2 | 10.5 ± 0.3 | - | SEM |
| Al-2Mn-1Fe | BE-η-Al6(Fe, Mn) | 87.6 ± 0.5 | 8.3 ± 0.2 | 4.1 ± 0.3 | - | SEM |
| Al-1.5Fe-0.7Mn-0Mg | P-η-Al6(Fe, Mn) | 88.9 ± 0.3 | 9.0 ± 0.4 | 2.3 ± 0.2 | - | TEM [83] |
| Al-1.5Fe-0.7Mn-1Mg | P-η-Al6(Fe, Mn) | 88.8 ± 0.3 | 11.1 ± 0.4 | 2.6 ± 0.08 | - | TEM [83] |
| Al-1.5Fe-0.7Mn-3Mg | P-η-Al6(Fe, Mn) | 88.8 ± 0.2 | 8.2 ± 0.15 | 3.0 ± 0.06 | - | TEM [83] |
| Alloys | Crystals | a(Å) | b(Å) | c(Å) | α(º) | β(º) | γ(º) | Technique |
|---|---|---|---|---|---|---|---|---|
| Al-3Fe (HP Al) (0.01 K/s) | θ-Al13Fe4 (0at.%Si) | 15.4824 (3) | 8.08146 (15) | 12.4689 (3) | 90 | 107.689 (2) | 90 | SCXC [80] |
| Al-1Fe (CP Al) (0.01 K/s) | θ-Al13Fe4 (0.3at.%Si) | 15.447 (4) | 8.0567 (10) | 12.429 (2) | 90 | 107.83 (2) | 90 | SCXC [80] |
| Al-4Fe-4Si (0.01 K/s) | θ-Al13Fe4 (2.4at.% Si) | 15.4239 (11) | 8.0521 (5) | 12.4040 (8) | 90 | 107.649 (7) | 90 | SCXC [80] |
| Al-5Mg-2Si-0.7Mn-1.3Fe (0.01 K/s) | α-Al15(Fe, Mn)3Si2 | 2.60804 (8) | 12.60804 (8) | 12.60804 (8) | 90 | 90 | 90 | SCXC |
| Al-4Si-4Fe (3.5 K/s) | α’-Al8Fe2Si | 12.13 | 12.13 | 26.68 | 90 | 90 | 120 | TEM [80] |
| Al-4Si-4Fe (3.5 K/s) | β-Al5FeSi | 6.16 | 6.18 | 20.97 | 90 | - | 90 | TEM [80] |
| Al-5Mg-2Si-0.7Mn-1.3Fe (3.5 K/s) | θ-Al13Fe4 | 15.864 | - | 12.571 | - | - | - | TEM [66] |
| Al-5Mg-2Si-0.7Mn-1.1Fe (3.5 K/s) | α-Al15(Fe, Mn)3Si2(P) | 12.70 | 12.70 | 12.70 | 90 | 90 | 90 | TEM [31] |
| Al-5Mg-2Si-0.7Mn-1.1Fe (3.5 K/s) | α-Al15(Fe, Mn)3Si2(BE) | 12.58 | 12.58 | 12.58 | 90 | 90 | 90 | TEM [31] |
| Al-5Mg-2Si-0.7Mn-1.1Fe (3.5 K/s) | α-Al15(Fe, Mn)3Si2(TE) | 12.83 | 12.83 | 12.83 | 90 | 90 | 90 | TEM [31] |
| Al-1.5Fe-0.7Mn-0Mg | η-Al6(Fe, Mn) | 7.57 | 6.63 | 8.37 | - | - | - | TEM [83] |
| Al-Fe-Mn-1Mg | η-Al6(Fe, Mn) | 7.36 | 6.47 | 8.65 | - | - | - | TEM [83] |
| Al-Fe-Mn-3Mg | η-Al6(Fe, Mn) | 7.28 | 6.54 | 8.57 | - | - | - | TEM [83] |
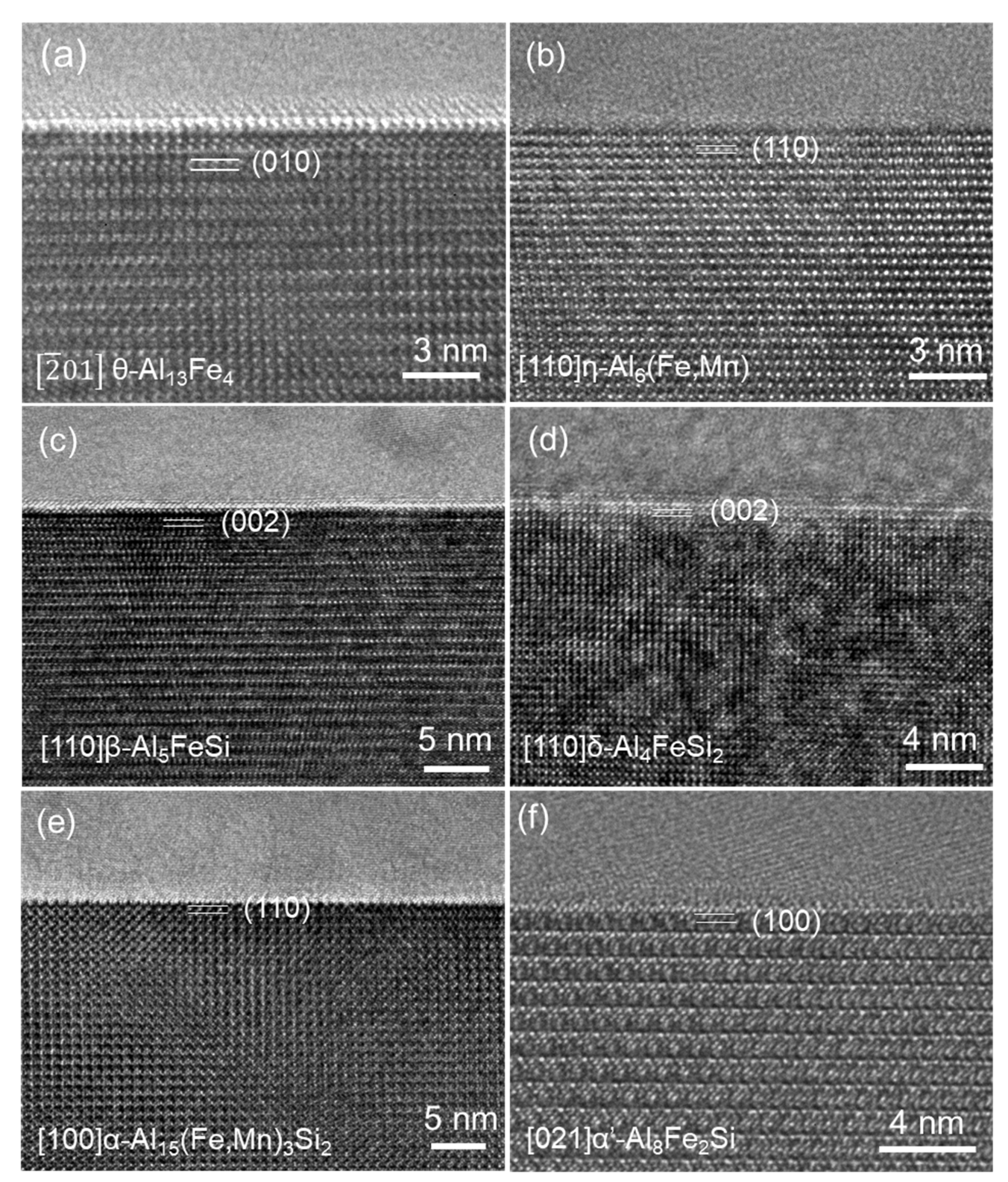
2.2. Composition and Crystallographic Variation of FIMCs
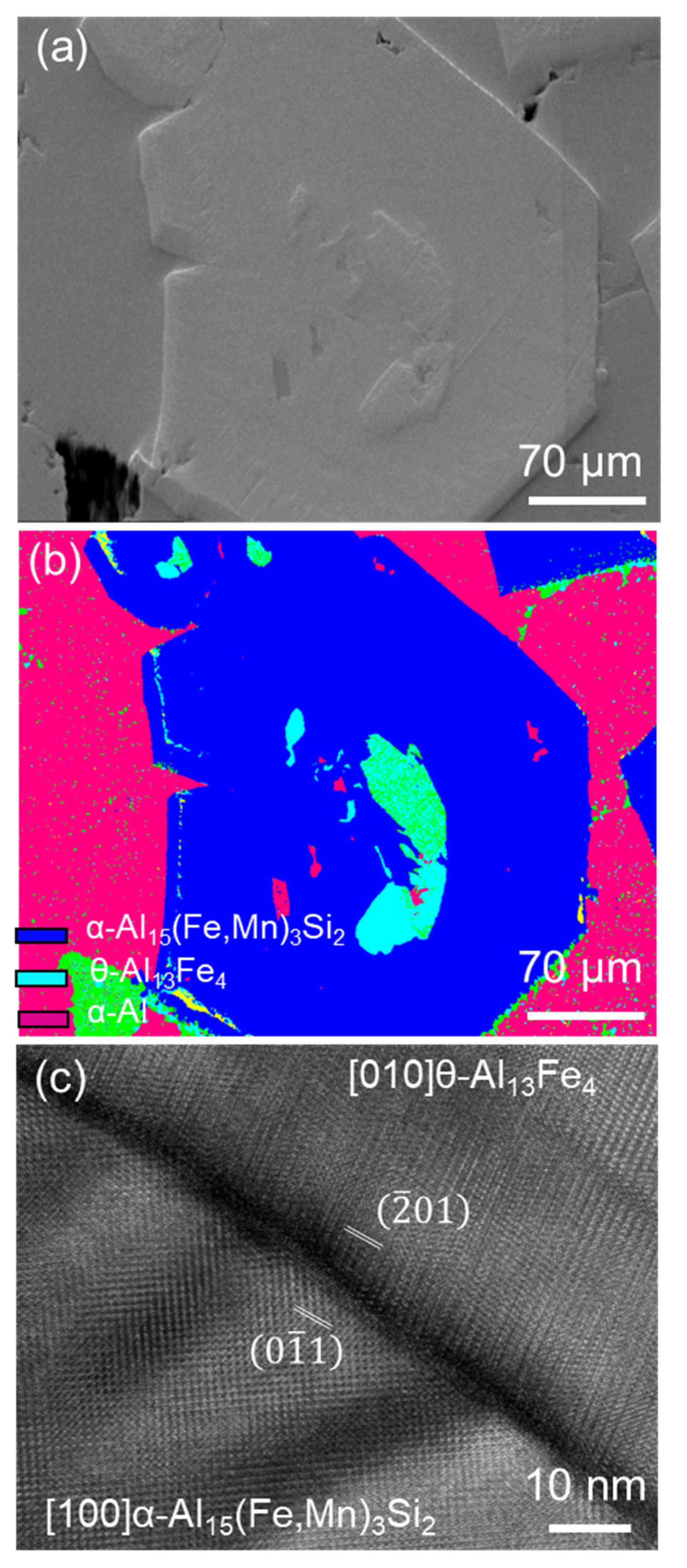
2.3. The Same Intermetallic Compounds with Different Morphologies
3. Competition of Nucleation and Phase Formation of FIMCs
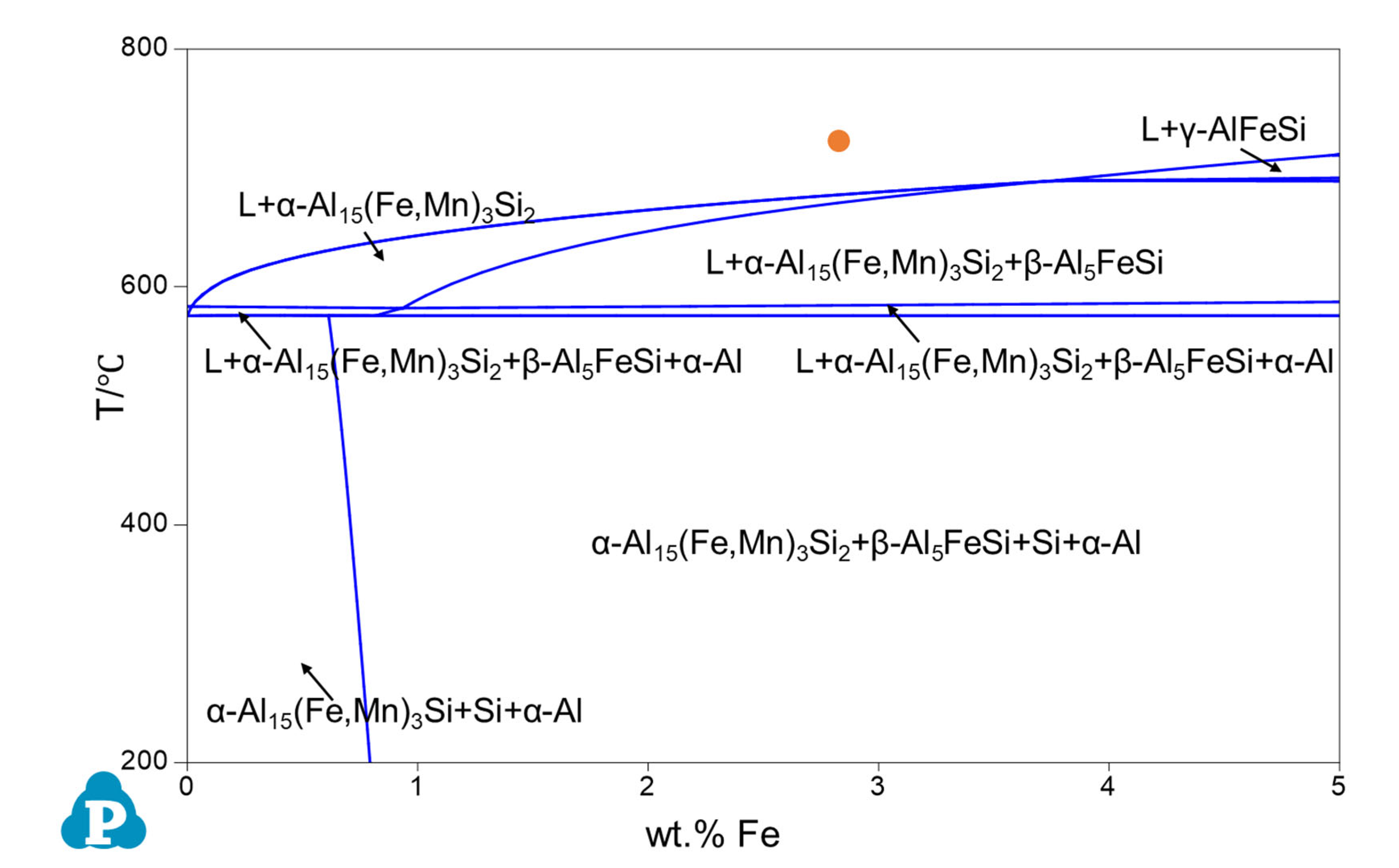
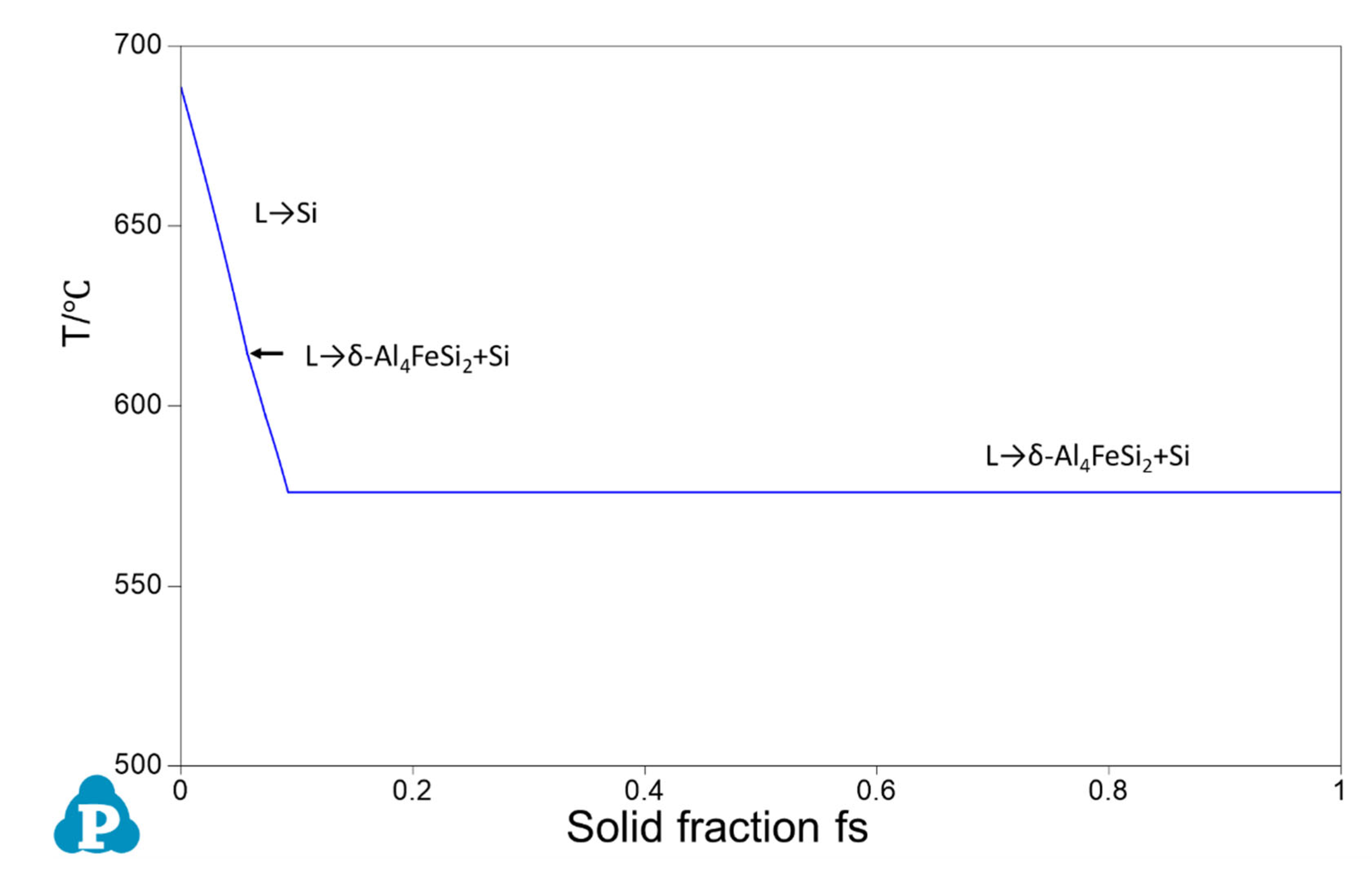
3.1. Phase Competition of P-FIMCs and Its Effect on the Solidification Sequence
3.2. Nucleation Competition between FIMCs
3.3. The Mechanism of Nucleation Competition between FIMCs
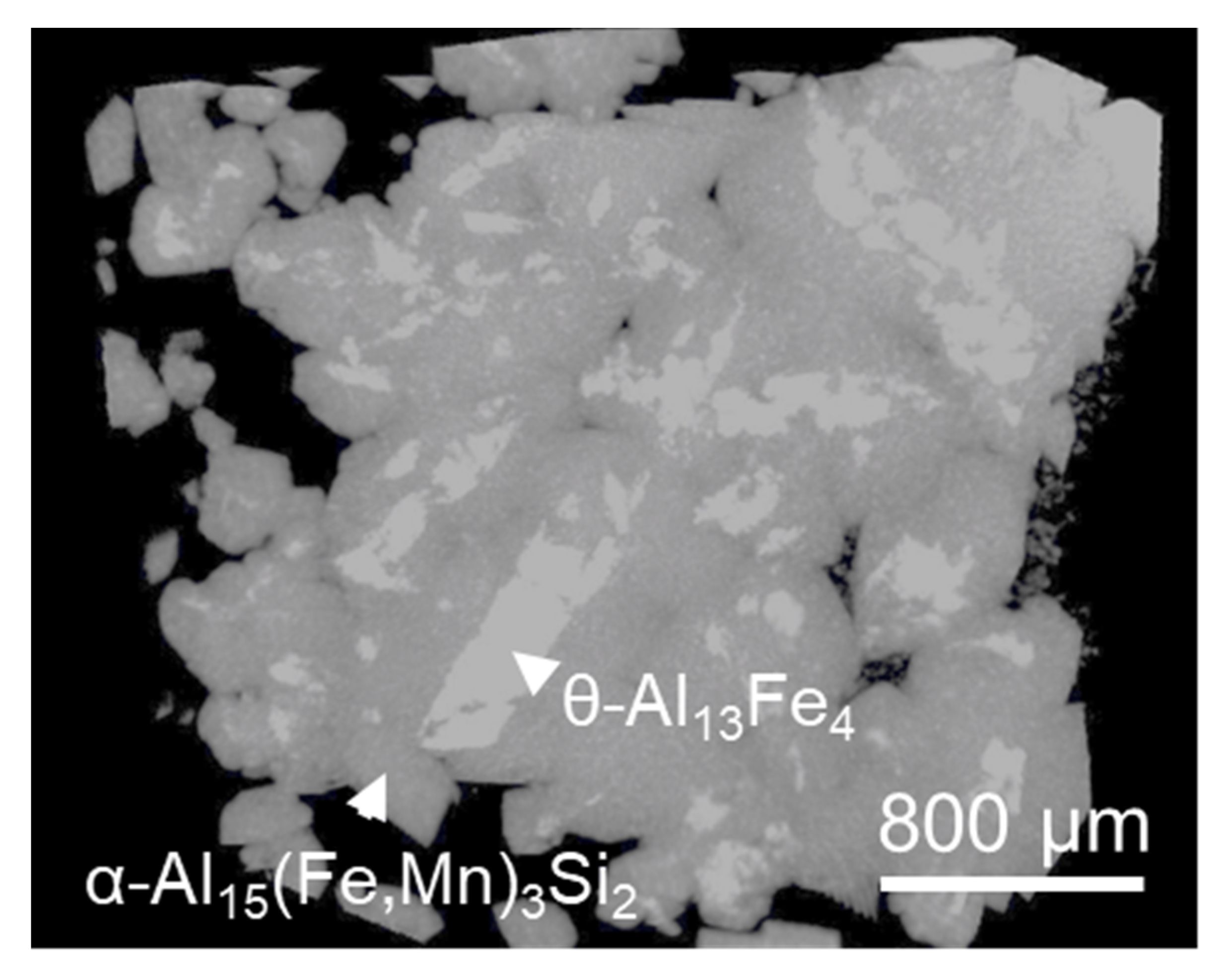
4. Phase Transformation between FIMCs
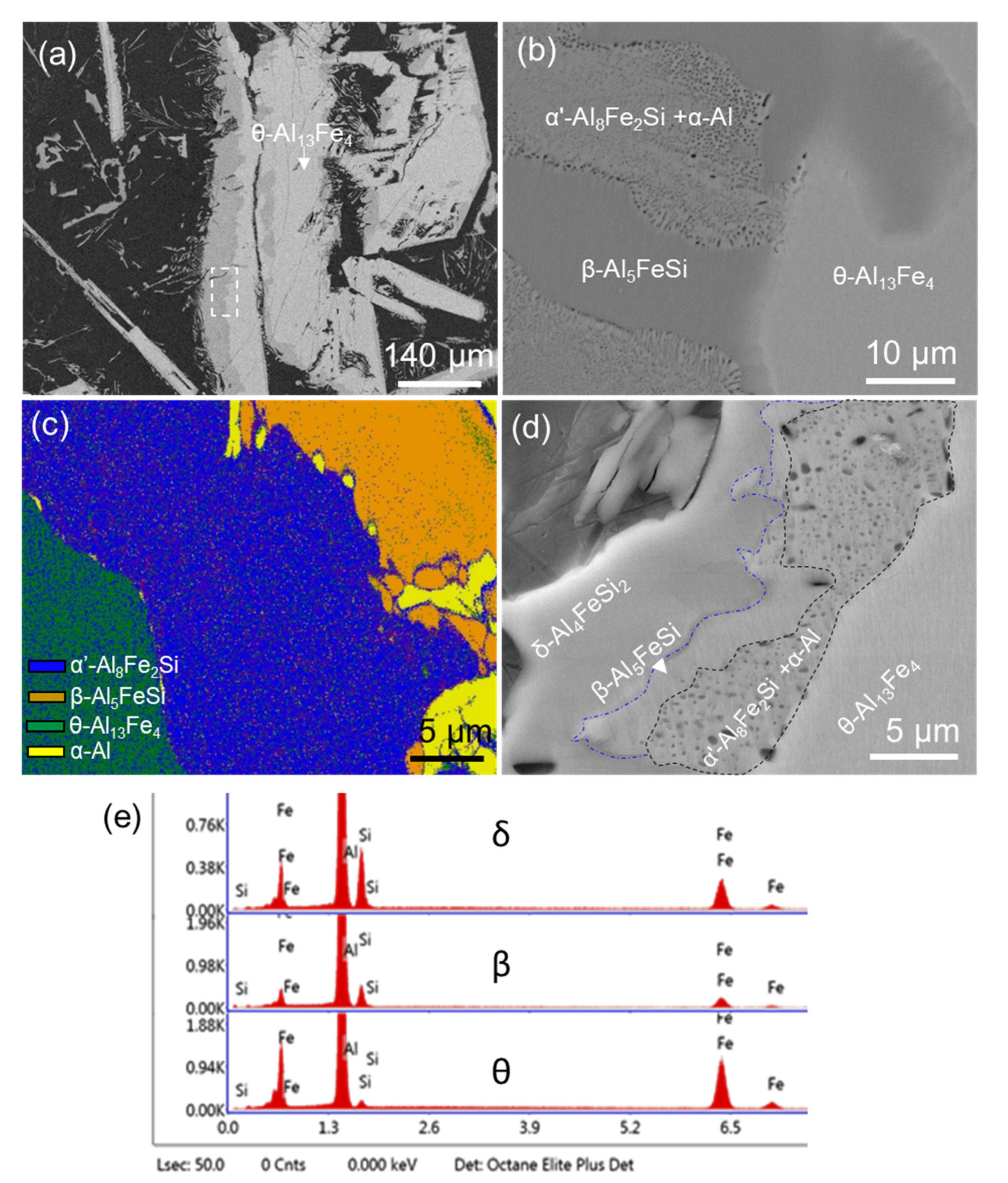
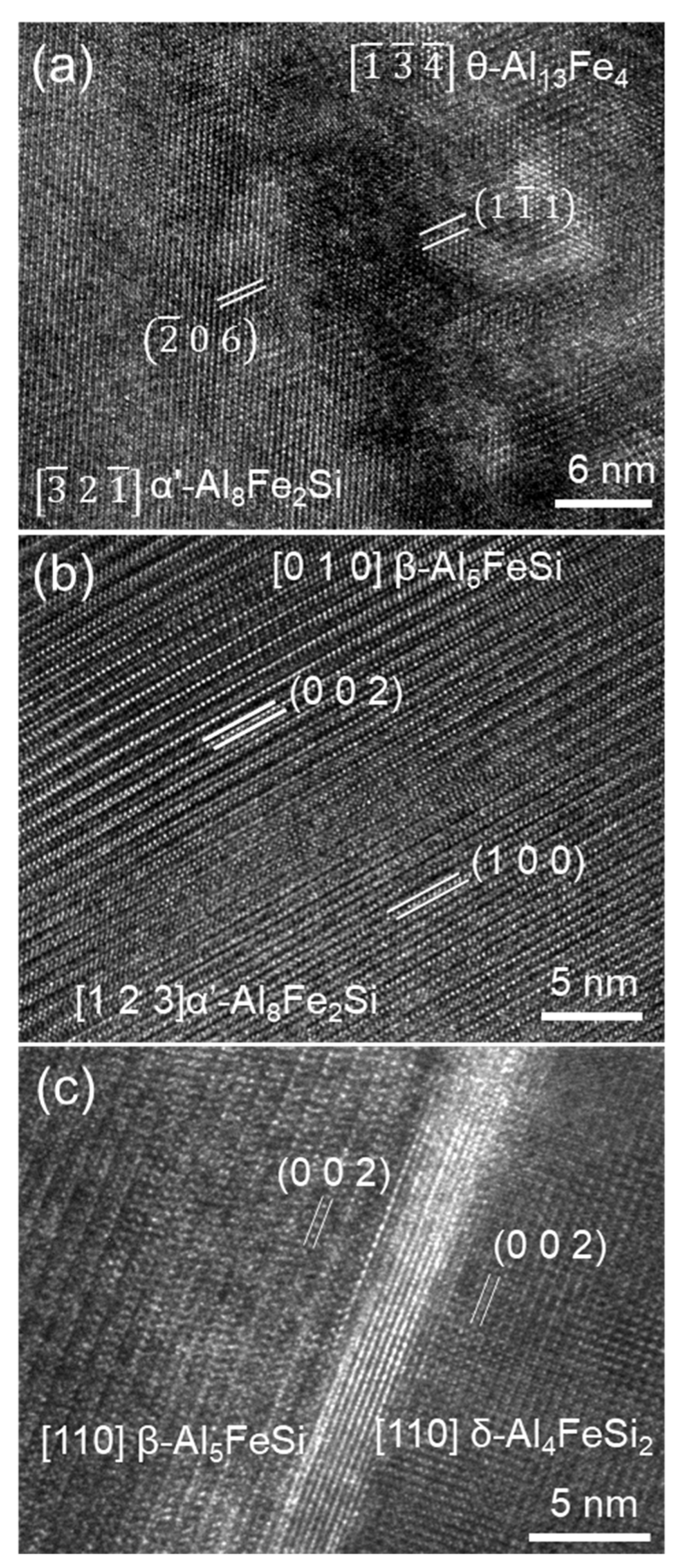
4.1. Multi-Step Transformation from θ to α’, β then δ
| ( 0 3) θ-Al13Fe4//(1 1) α’-Al8Fe2Si and [ ] θ-Al13Fe4//[ 2 ] -Al8Fe2Si | (OR2) |
| (0 0 2) β-Al5FeSi//10.2°(1 0 0) α’-Al8Fe2Si, [0 1 0] β-Al5FeSi//[1 2 3] α’-Al8Fe2Si, | (OR3) |
| And (0 0 2) [1 1 0] β-Al5FeSi//(0 0 2) [1 1 0] δ-Al4FeSi2 | (OR4) |
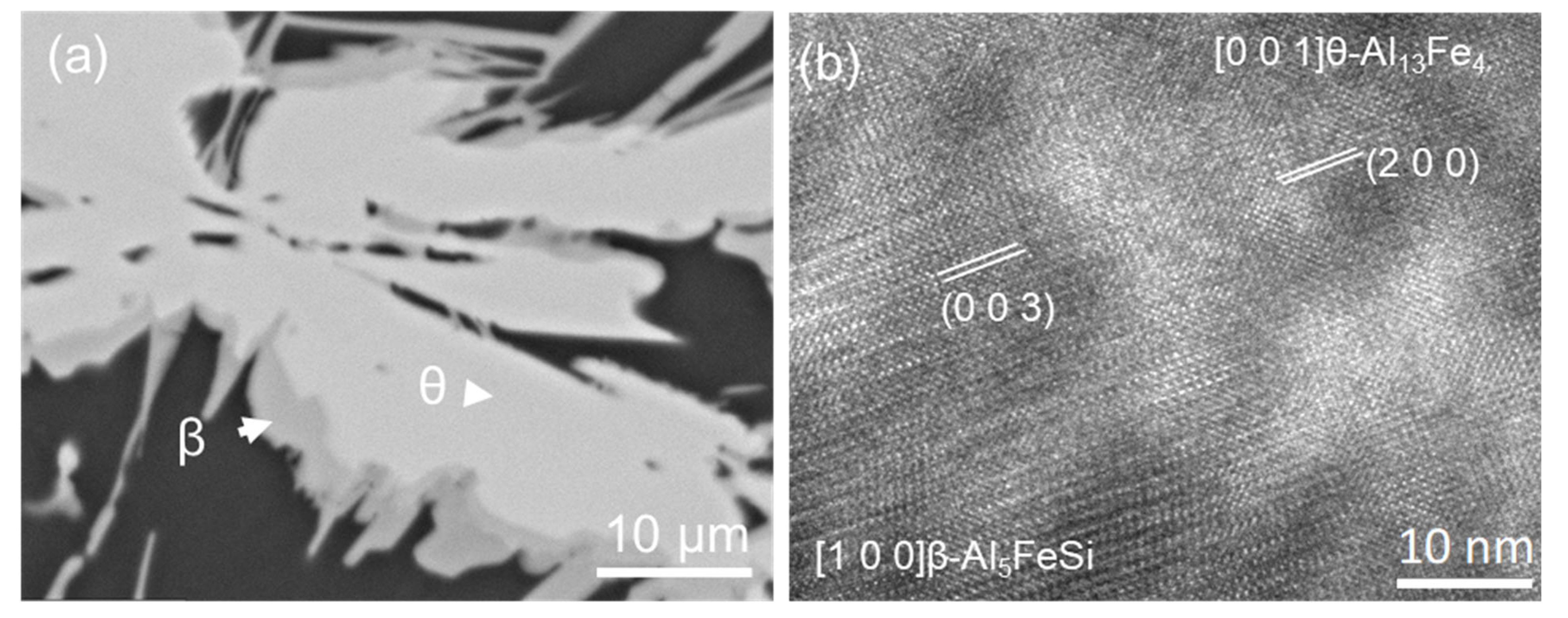
4.2. One Step Phase Transformation from θ to β
| (200) θ-Al13Fe4//(003) β-Al5FeSi, and [1] θ-Al13Fe4//[100] β-Al5FeSi | (OR5) |
5. Heterogeneous Nucleation and Refinement of FIMCs
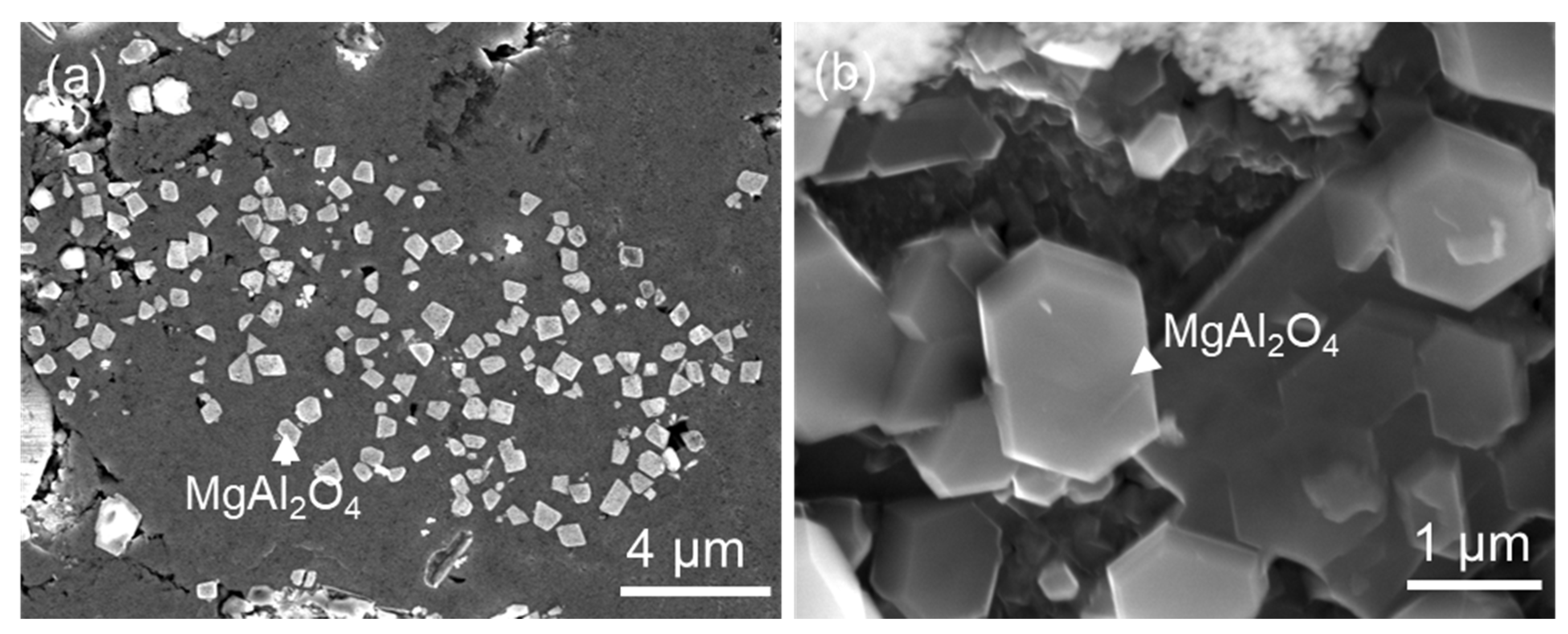
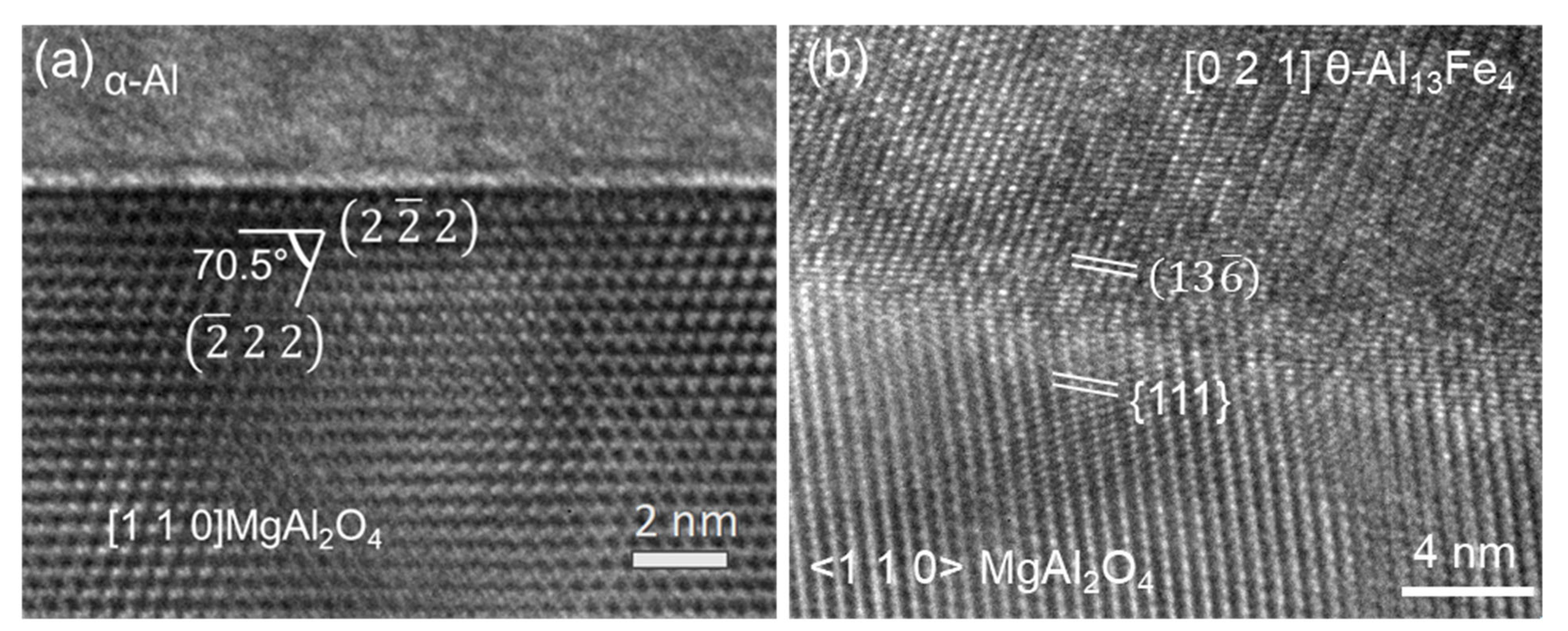
5.1. Heterogeneous Nucleation of FIMCs on Native Oxides
| MgAl2O4//θ-Al13Fe4 and | (OR5) |
| [1 1 0] MgAl2O4//3.4° [0 2 1] θ-Al13Fe4. [66] |
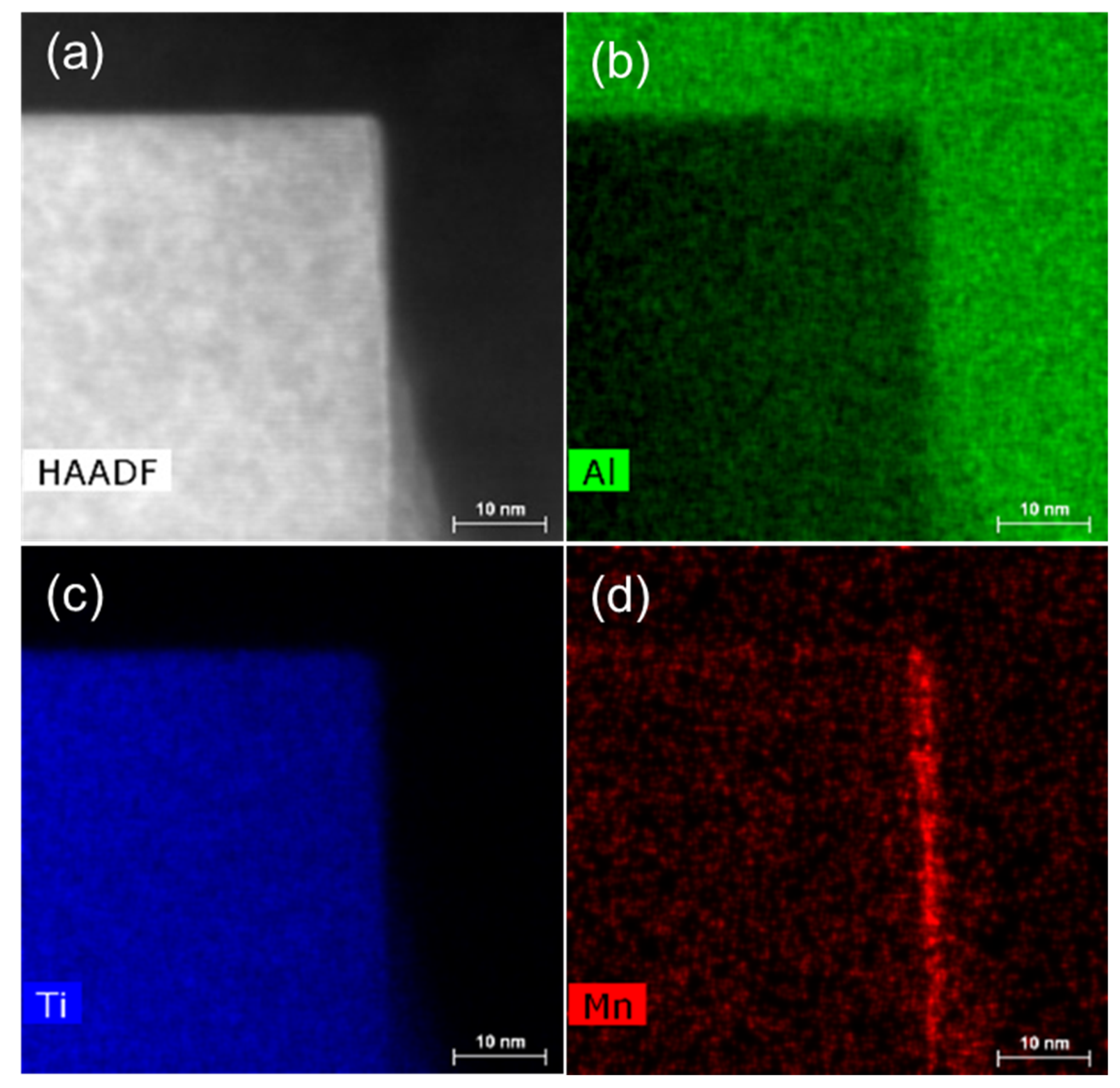
5.2. Heterogeneous Nucleation on the Other IMCs and Refinement of FIMCs
5.3. Compositional Templating for Heterogeneous Nucleation and Refinement of FIMCs
| (OR6) |
6. Challenges/Future Research
7. Summary
- FIMCs in Al alloys are not strictly stoichiometric compounds and hence can accommodate certain levels of alloying elements in their crystal lattice without changing their crystal structure, although this may cause some changes in their lattice parameters. For instance, Si content in the θ phase increases with increasing Si content in Al alloys. The maximum Si concentration in θ is experimentally measured to be 2.7 at.%. The lattice parameters of θ decreased with the increasing Si concentration in θ.
- The composition of FIMCs changes significantly to adjust the consuming rate of different alloy elements at different stages of solidification. For instance, Mn concentration in α-Al15(Fe, Mn)3Si2 reduced from 7.4 at.% in the primary α, to 5.6 at.% in the binary eutectic α, and then 0.8 at.% in the ternary eutectic α, without changing the crystal structure. In addition, the solubility of Si in FIMCs has a sequence of <<<<<, which contributed to the phase transformation between different types of FIMCs.
- The FIMCs that have a broad plate-like morphology, such as θ, β and δ, may have different morphological features. The θ has up to tenfold twins, which can grow into star-like morphology on its cross-section; the β is thin but coarse; the branches on β are less observed; the δ is shorter than β and grows with spiral traces. The terminating surface planes of the relevant FIMCs were identified. The θ is {010} faceted. The β and δ are both {002} faceted. The α and η are {110} faceted. The α’ is {100} faceted.
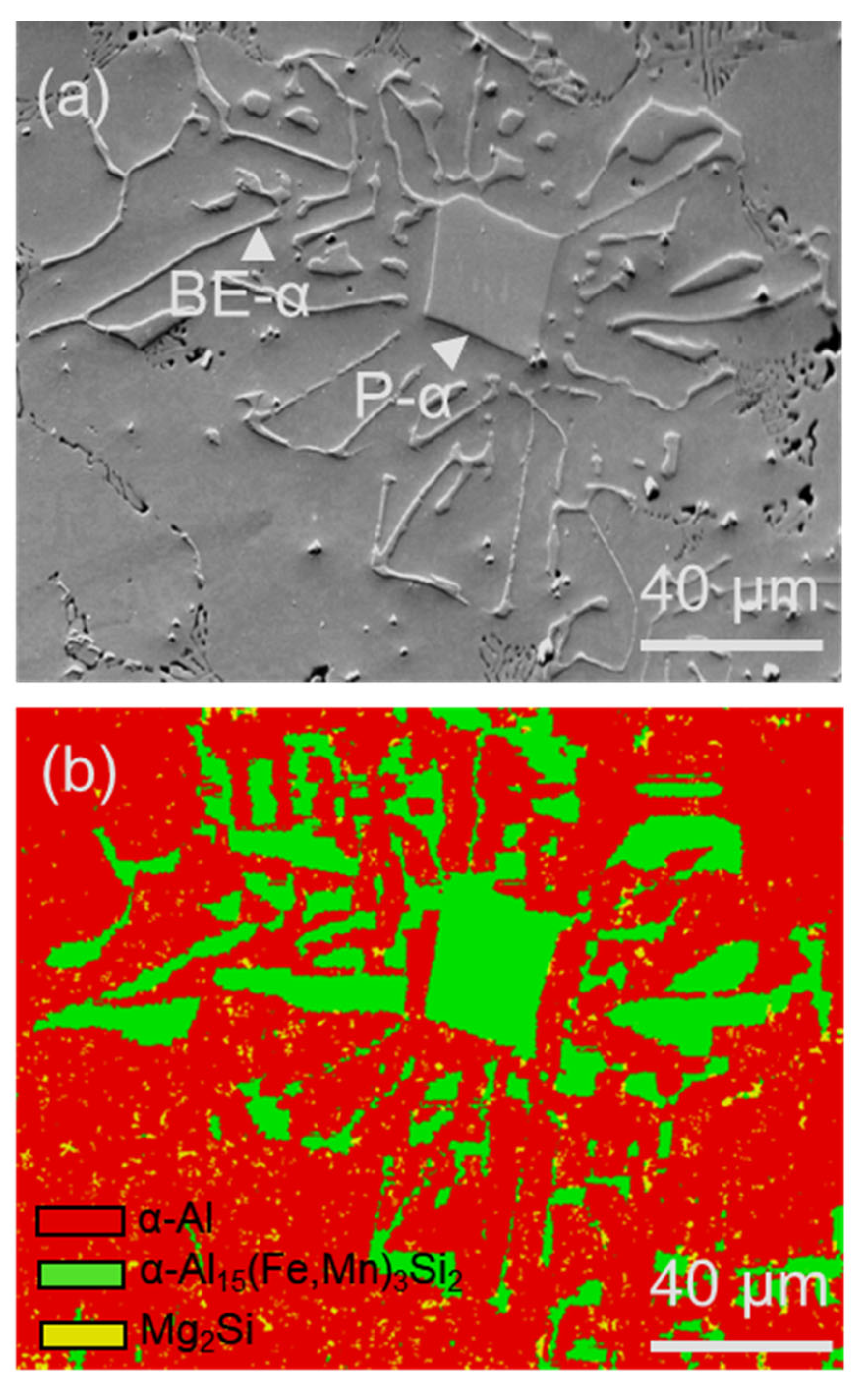
- It has been identified that the α-Al15(Fe, Mn)3Si2 with Chinese script morphology is not the primary α, but belongs to the binary eutectic structure although it may be nucleated on (or grow from) the compacted primary α-Al15(Fe, Mn)3Si2. In addition, the compacted morphology of α-Al15(Fe, Mn)3Si2 in a ternary eutectic was observed at a slow cooling rate—0.01 K/s. It is possible that the compact ternary α-Al15(Fe, Mn)3Si2 was nucleated or grew from the binary eutectic α-Al15(Fe, Mn)3Si2.
- Evidence of the heterogeneous nucleation of θ-Al13Fe4 on native MgAl2O4 particles was observed. A well-defined OR was observed:MgAl2O4//θ-Al13Fe4 and [1 1 0] MgAl2O4//3.4° [0 2 1] θ-Al13Fe4. However, it was found that the primary α-Al15(Fe, Mn)3Si2 in Al-5Mg-2Si-0.6Mn-1.3Fe alloy was not nucleated on the native oxides, but formed through solid-state phase transformation from the previously formed non-equilibrium θ-Al13Fe4 phase. Such transformations can be achieved through either a multi-step transformation or a single-step transformation. For instance, multi-step phase transformation of θ→α’→β→δ was observed in Al-20Si-0.7Fe. The phase transformation was driven by the alloy elements, especially Si diffusion. The intermediate structure (α’-Al8Fe2Si + α-Al) was also observed. The well-defined orientation relationships between θ/α’, α’/β, β/δ, θ/β and θ/α were observed. A single-step phase transformation from θ-Al13Fe4 to β-Al5FeSi was also observed with an OR: (200) θ-Al13Fe4//(003) β-Al5FeSi, and [1] θ-Al13Fe4//[100] β-Al5FeSi.
- A compositional templating concept was proposed. Different types of alloy elements, such as Fe, Mn, and Si, can segregate on AlB2 or TiB2 particle surfaces, providing both the compositional and structural templating required by heterogeneous nucleation of FIMCs. The AlB2 or TiB2 particles with interfacial segregation of alloy elements such as Fe, Mn, and/or Si can grain-refine different types of phases. In addition, it was found that both the primary θ-Al13Fe4 and α-Al grains in Al-4Fe alloys are significantly refined by addition of 0.5 wt.% Zr by enhancing heterogeneous nucleation on the primary Al3Zr particles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Al Institute. Global Al Recycling: A Cornerstone of Sustainable Development; International Al Institute: London, UK, 2009; pp. 6–7. [Google Scholar]
- Eurometax. Our Metals Future: The Metals Industry’s 2050 Vision for a Sustainable Europe; Eurometax: Woluwe-Saint-Pierre, Belgium, 2015. [Google Scholar]
- About Us—The Liquid Metal Engineering Hub. Available online: http://www.lime.ac.uk/about-us/ (accessed on 1 July 2022).
- Foresight Group. The Future of Manufacturing: A New Era of Opportunity and Challenge for the UK; Summary Report; Government Office for Science: London, UK, 2013. [Google Scholar]
- Aluminum Industry Worldwide-Statistics & Facts; Statista Research Department: Hamburg, Germany. 2022. Available online: http://www.statista.com/topics/2072/aluminum/#topicheader_wrapper (accessed on 1 July 2022).
- Aluminium for Future Generations. Available online: https://recycling.world-aluminium.org/home/ (accessed on 1 July 2022).
- Raabe, D.; Ponge, D.; Uggowitzer, P.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making sustainable aluminium by recycling scrap: The science of “dirty” alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminium Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Brough, D.; Jouhara, H. The Al industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Das, S.K. Designing Aluminum Alloys for a Recycling Friendly World. Mater. Sci. Forum 2006, 519–521, 1239–1244. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Nana, L.; Damoah, W.; Robertson, D.G. Removal of Fe from Al: A Review. Min. Proc. Ext. Met. Rev. 2012, 33, 99–157. [Google Scholar] [CrossRef]
- Mbuya, T.O.; Odera, B.O.; Ng’ang’a, S.P. Influence of Fe on castability and properties of Al silicon alloys: Literature review. Int. J. Cast Metals Res. 2003, 16, 451–465. [Google Scholar] [CrossRef]
- Yi, J.Z.; Gao, Y.X.; Lee, P.D.; Lindley, T.C. Effect of Fe-content on fatigue crack initiation and propagation in a cast aluminum-silicon alloy (A356–T6). Mater. Sci. Eng. A 2004, 386, 396–407. [Google Scholar] [CrossRef]
- Yang, H.; Ji, S.; Fan, Z. Effect of heat treatment and Fe content on the microstructure and mechanical properties of die-cast Al-Si-Cu alloys. Mater. Des. 2015, 85, 823–832. [Google Scholar] [CrossRef]
- Shen, H.; Yang, W.D.; Liang, H.; Yao, G.C. Research advance in harmful effects and removal of impurity Fe from Al and Al alloys. Adv. Mater. Res. 2011, 295–297, 751–759. [Google Scholar] [CrossRef]
- Nakajima, K.; Takeda, O.; Miki, T.; Matsubae, K.; Nakamura, S.; Nagasaka, T. Thermodynamic analysis of contamination by alloying elements in aluminum recycling. Environ. Sci. Technol. 2010, 44, 5594–5600. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.B. Physical metallurgy of recycling wrought aluminum alloys. Metall. Trans. A 1983, 14, 323–327. [Google Scholar] [CrossRef]
- Dewan, M.A.; Rhamdhani, M.A.; Mitchell, J.B.; Davidson, C.J.; Brooks, G.A.; Easton, M.; Grandfield, J.F. Control and removal of impurities from Al melts: A review. Mater. Sci. Forum 2011, 693, 149–160. [Google Scholar] [CrossRef]
- Taylor, J.A. Iron-containing intermetallic phases in Al-Si based casting alloys. Procedia. Mater. Sci. 2012, 1, 19–33. [Google Scholar] [CrossRef]
- Romming, C.; Hansen, V.; Gjonnes, J. Crystal structure of β-Al4.5FeSi. Acta Crystallogr. Sect. B Struct. Sci. 1994, 50, 307–312. [Google Scholar] [CrossRef]
- Grin, J.; Burkhardt, U.; Peters, K. Refinement of the Fe4Al13 structure and its relationship to the quasihomological homeotypical structures, Z. Kristallogr. Cryst. Mater. 1994, 209, 479–487. [Google Scholar]
- Cao, X.; Campbell, J. Morphology of β-Al5FeSi phase in Al-Si cast alloys. Mater. Trans. 2006, 47, 1303–1312. [Google Scholar] [CrossRef]
- Bacaicoa, I.; Luetje, M.; Wicke, M.; Geisert, A.; Zeismann, F.; Fehlbier, M.A. Brueckner-Foit, 3D morphology of Al5FeSi inclusions in high Fe-content Al-Si-Cu alloys. Procedia Struct. Integr. 2016, 2, 2269–2276. [Google Scholar] [CrossRef]
- Ma, X.L.; Liebertz, H.; Koster, U. Multiple Twins of Monoclinic Al13Fe4 Showing Pseudo-Orthorhombic and Fivefold Symmetries. Physica. Status Solidi. A 1996, 158, 359–367. [Google Scholar] [CrossRef]
- Fung, K.K.; Zou, X.D.; Yang, C.Y. Transmission electron microscopy study of Al13Fe4 tenfold twins in rapidly cooled Al–Fe alloys. Philos. Mag. Lett. 1987, 55, 27–32. [Google Scholar] [CrossRef]
- Song, Z.; Magdysyuk, O.V.; Tang, L.; Sparks, T.; Cai, B. Growth dynamics of faceted Al13Fe4 intermetallic revealed by high-speed synchrotron X-ray quantification. J. Alloys Compd. 2021, 861, 158604. [Google Scholar] [CrossRef]
- Barlock, J.G.; Mondolfo, L.F. Structure of some Al-iron-magnesium-manganese-silicon alloys. Z. Metallkde. 1975, 66, 605–611. [Google Scholar]
- Zhu, X.Z.; Blake, P.; Dou, K.; Ji, S.X. Strengthening die-cast Al-Mg and Al-Mg-Mn alloys with Fe as a beneficial Element. Mater. Sci. Eng. A 2018, 732, 240–250. [Google Scholar] [CrossRef]
- Que, Z.; Zhou, Y.P.; Wang, Y.; Fan, Z. Effect of MgO on Phase Selection in Al–Mg–Si–Fe–Mn Alloys. Trans. Indian Inst. Met. 2015, 68, 1167–1172. [Google Scholar] [CrossRef]
- Cooper, M. The crystal structure of the tenary alloy α(AlFeSi). Acta Crystall. 1967, 23, 1106–1107. [Google Scholar] [CrossRef]
- Que, Z.; Wang, Y.; Fan, Z. Formation of the Fe-Containing Intermetallic Compounds during Solidification of Al-5Mg-2Si-0.7Mn-1.1Fe Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 2173–2181. [Google Scholar] [CrossRef]
- Que, Z.; Wang, Y.; Fan, Z. Heterogeneous Nucleation of Eutectic Structure in Al-Mg-Si Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 2697–2702. [Google Scholar] [CrossRef]
- Song, D.F.; Zhao, Y.L.; Wang, Z.; Jia, Y.W.; Li, D.X.; Fu, Y.N.; Zhang, D.T.; Zhang, W.W. 3D Fe-Rich Phases Evolution and Its Effects on the Fracture Behavior of Al–7.0Si–1.2Fe Alloys by Mn Neutralization. Acta Metall. Sin.-Engl. 2022, 35, 163–175. [Google Scholar] [CrossRef]
- Corby, R.N.; Blabk, P.J. The structure of alpha-(Al Fe Si) by anomalous dispersion methods. Acta Crystall. Section B: Structural Crystallography and Crystal Chemistry 1977, 33, 3468–3475. [Google Scholar] [CrossRef]
- Gueneau, C.; Servant, C.; d’Yvoire, F.; Rodier, N. Fe Al3Si2. Acta Crystall. Sect. C Cryst. Struct. Commun. 1995, 51, 177–179. [Google Scholar] [CrossRef]
- Becker, H.; Bergh, T.; Vullum, P.E.; Leineweber, A.; Li, Y. β- and δ-Al-Fe-Si intermetallic phase, their intergrowth and polytype formation. J. Alloy Compd. 2019, 780, 917–929. [Google Scholar] [CrossRef]
- Shabestari, S.G.; Ghanbari, M. Effect of plastic deformation and semisolid forming on iron–manganese rich intermetallics in Al–8Si–3Cu–4Fe–2Mn alloy. J. Alloys Compd. 2010, 508, 315–319. [Google Scholar] [CrossRef]
- Farshidi, M.H.; Rifai, M.; Miyamoto, H. Microstructure evolution of a recycled Al–Fe–Si–Cu alloy processed by tube channel pressing. Int. J. Miner. Metall. Mater. 2018, 25, 1166–1172. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Jie, J.C.; Gao, Y.; Lu, Y.P.; Li, T.J. Effects of ultrasonic treatment on the formation of iron-containing intermetallic compounds in Al-12% Si-2% Fe alloys. Intermetallics 2013, 42, 120–125. [Google Scholar] [CrossRef]
- Khalifa, W.; Tsunekawa, Y.; Okumiya, M. Effect of ultrasonic treatment on the Fe-intermetallic phases in ADC12 die cast alloy. J. Mater. Process. Technol. 2010, 210, 2178–2187. [Google Scholar] [CrossRef]
- Nafisi, S.; Emadi, D.; Shehata, M.T.; Ghomashchi, R. Effects of electromagnetic stirring and superheat on the microstructural characteristics of Al–Si–Fe alloy. Mater. Sci. Eng. A 2006, 432, 71–83. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Z.; Meng, L.; Guo, Z. Removal of Fe for aluminum recovery from scrap aluminum alloy by supergravity separation with manganese addition. Chem. Eng. Process. Process Intensif. 2022, 173, 108841. [Google Scholar] [CrossRef]
- Khan, M.H.; Das, A.; Li, Z.; Kotadia, H.R. Effects of Fe, Mn, chemical grain refinement and cooling rate on the evolution of Fe intermetallics in a model 6082 Al-alloy. Intermetallics 2021, 132, 107132. [Google Scholar] [CrossRef]
- Huang, H.J.; Cai, Y.H.; Cui, H.; Huang, J.F.; He, J.P.; Zhang, J.S. Influence of Mn addition on microstructure and phase formation of spray deposited Al–25Si–xFe–yMn alloy. Mater. Sci. Eng. A 2009, 502, 118–125. [Google Scholar] [CrossRef]
- Sha, M.; Wu, S.S.; Wang, X.T.; Wan, L. Effects of Co addition on Fe-bearing intermetallic compounds and mechanical properties of AlSi20Cu2Ni1Fe0. 7–1 alloy. J. Alloys Compd. 2013, 551, 468–474. [Google Scholar] [CrossRef]
- Timpel, M.; Wanderka, N.; Grothausmann, R.; Banhart, J. Distribution of Fe-rich phases in eutectic grains of Sr-modified Al–10 wt.% Si–0.1 wt.% Fe casting alloy. J. Alloys Compd. 2013, 558, 18–25. [Google Scholar] [CrossRef]
- Ashtari, P.; Tezuka, H.; Sato, T. Influence of Li addition on intermetallic compound morphologies in Al–Si–Cu–Fe cast alloys. Scr. Mater. 2004, 51, 43–46. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Chen, X.G. Effect of Mn, Si, and cooling rate on the formation of iron-rich intermetallics in 206 Al-Cu cast alloys. Metall. Mater. Trans. B 2012, 43, 1231–1240. [Google Scholar] [CrossRef]
- Kim, H.Y.; Han, S.W.; Lee, H.M. The influence of Mn and Cr on the tensile properties of A356–0.20Fe alloy. Mater. Lett. 2006, 60, 1880–1883. [Google Scholar] [CrossRef]
- Jin, L.; Liu, K.; Chen, X.G. Evolution of Fe-rich intermetallics in Al-Si-Cu 319 cast alloy with various Fe, Mo, and Mn contents. Metall. Mater. Trans. B 2019, 50, 1896–1907. [Google Scholar] [CrossRef]
- Mahta, M.; Emamy, M.; Daman, A.; Keyvani, A.; Campbell, J. Precipitation of Fe rich intermetallics in Cr- and Co-modified A413 alloy. Int. J. Cast Met. Res. 2005, 18, 73–79. [Google Scholar] [CrossRef]
- Sweet, L.; Zhu, S.M.; Gao, S.X.; Taylor, J.A.; Easton, M.A. The effect of iron content on the iron-containing intermetallic phases in a cast 6060 aluminum alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 42, 1737–1749. [Google Scholar] [CrossRef]
- Basak, C.B.; Meduri, A.; Babu, N.H. Influence of Ni in high Fe containing recyclable Al-Si cast alloys. Mater. Des. 2019, 182, 108017. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Chen, X.G. Effect of nickel and vanadium on iron bearing intermetallic phases in AA 5657 simulated DC castings. Mater. Sci. Technol. 2014, 30, 951–961. [Google Scholar] [CrossRef]
- Tzeng, Y.C.; Wu, C.T.; Bor, H.Y.; Horng, J.L.; Tsai, M.L.; Lee, S.L. Effects of scandium addition on iron-bearing phases and tensile properties of Al-7Si-0.6Mg alloys. Mater. Sci. Eng. A 2014, 593, 103–110. [Google Scholar] [CrossRef]
- Basak, C.B.; Babu, N.H. Influence of Cu on modifying the beta phase and enhancing the mechanical properties of recycled Al-Si-Fe cast alloys. Sci. Rep. 2017, 7, 5779. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Chen, W.; Liu, L.; Cao, X.; Zhou, L.; Fu, Z. Effect of trace yttrium addition on the microstructure and tensile properties of recycled Al-7Si-0.3Mg-1.0Fe casting alloys. Mater. Sci. Eng. A 2016, 666, 165–175. [Google Scholar] [CrossRef]
- Song, D.F.; Jia, Y.W.; Li, Q.; Zhao, Y.L.; Zhang, W.W. Effect of initial Fe content on Microstructure and Mechanical Properties of Recycled Al-7.0Si-Fe-Mn alloys with Constant Mn/Fe ratio. Materials 2022, 15, 1618. [Google Scholar] [CrossRef] [PubMed]
- Baldan, R.; Malavazi, J.; Couto, A.A. Microstructure and mechanical behavior of Al9Si0.8Fe alloy with different Mn contents. Mater. Sci. Technol. 2017, 33, 1192–1199. [Google Scholar] [CrossRef]
- Ceschini, L.; Boromei, I.; Morri, A.; Seifeddine, S.; Svensson, I.L. Microstructure, tensile and fatigue properties of the Al-10%Si-2%Cu alloy with different Fe and Mn content cast under controlled conditions. J. Mater. Process Technol. 2009, 209, 5669–5679. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Doty, H.W.; Kaufman, M.J. The effects of Mn additions on the microstructure and mechanical properties of Al-Si-Cu casting alloys. Mater. Sci. Eng. A 2008, 488, 496–504. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. The nucleation of Fe-rich phases on oxide films in Al-11.5Si-0.4Mg cast alloys. Metall. Mater. Trans. A 2003, 34, 1409–1420. [Google Scholar] [CrossRef]
- Que, Z.P.; Wang, Y.; Zhou, Y.P.; Fan, Z. Effect of Al-5Ti-1B Grain Refiner Addition on the Formation of Intermetallic Compounds in Al-Mg-Si-Mn-Fe Alloys. Mater. Sci. Forum 2015, 828–829, 53–57. [Google Scholar] [CrossRef]
- Khalifa, W.; Samuel, F.H.; Gruzleski, J.E.; Doty, H.W.; Valtierra, S. Nucleation of Fe-intermetallic phases in the Al-Si-Fe alloys. Metall. Mater. Trans. A 2005, 36, 1017–1032. [Google Scholar] [CrossRef]
- Lui, A.; Grant, P.S.; Stone, I.C.; O’Reilly, K.A.Q. The role of grain refiner in the nucleation of AlFeSi intermetallic phases during solidification of a 6xxx Aluminum alloy. Metall. Mater. Trans. A 2019, 50, 5242–5252. [Google Scholar] [CrossRef]
- Que, Z.; Mendis, C.L. Heterogeneous nucleation and phase transformation of Fe-rich intermetallic compounds in Al–Mg–Si alloys. J. Alloys Compd. 2020, 836, 155515. [Google Scholar] [CrossRef]
- Que, Z.; Mendis, C.L. Formation of θ-Al13Fe4 and the multi-step phase transformations to α-Al8Fe2Si, β-Al5FeSi and δ-Al4FeSi2 in Al–20Si-0.7Fe alloy. Intermetallics 2020, 127, 106960. [Google Scholar] [CrossRef]
- Sha, G.; O’Reilly, K.A.Q.; Cantor, B.; Titchmarsh, J.M.; Hamerton, R.G. Quasi-peritectic solidification reactions in 6xxx series wrought Al-alloys. Acta Mater. 2003, 51, 1883–1897. [Google Scholar] [CrossRef]
- Gorny, A.; Manickaraj, J.; Cai, Z.; Shankar, S. Evolution of Fe based intermetallic phases in Al–Si hypoeutectic casting alloys: Influence of the Si and Fe concentrations, and solidification rate. J. Alloys Compd. 2013, 577, 103–124. [Google Scholar] [CrossRef]
- Liu, P.; Dunlop, G.L. Crystallographic orientation relationships for Al–Fe and Al–Fe–Si precipitates in aluminium. Acta Metall. 1988, 36, 1481–1489. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, J.S.; Kim, W.T.; Ra, H.Y. Solidification behavior of Al-Si-Fe alloys and phase transformation of metastable intermetallic compound by heat treatment. J. Mater. Sci. 1999, 34, 2163–2168. [Google Scholar] [CrossRef]
- Ashtari, P.; Gatenby, K. Reheating of solid shell during twin belt-casting of an Al–Fe–Si alloy. Scr. Mater. 2008, 58, 150–153. [Google Scholar] [CrossRef]
- Pereira, L.H.; Asato, G.H.; Otani, L.B.; Jorge, A.M.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Changing the solidification sequence and the morphology of iron-containing intermetallic phases in AA6061 Al-alloy processed by spray forming. Mater. Charact. 2018, 145, 507–515. [Google Scholar] [CrossRef]
- Al-Helal, K.; Lazaro-Nebreda, J.; Patel, J.B.; Scamans, G.M. High-shear De-Gassing and de-ironing of an Aluminum casting alloy made directly from aluminum end-of-life vehicle scrap. Recycling 2021, 6, 66. [Google Scholar] [CrossRef]
- Lazaro-Nebreda, J.; Patel, J.B.; Chang, I.T.H.; Stone, I.C.; Fan, Z. Solidification processing of scrap Al-alloys containing high levels of Fe. IOP Conf. Ser. Mater. Sci. Eng. 2019, 529, 012059. [Google Scholar] [CrossRef]
- Li, H.-T.; Zhao, P.; Yang, R.; Patel, J.B.; Chen, X.; Fan, Z. Grain Refinement and Improvement of Solidification Defects in Direct-Chill Cast Billets of A4032 Alloy by Melt Conditioning. Metall. Mater. Trans. B 2017, 48, 2481–2492. [Google Scholar] [CrossRef]
- Sree Manu, K.M.; Barekar, N.S.; Lazaro-Nebreda, J.; Patel, J.B.; Fan, Z. In-situ microstructural control of A6082 alloy to modify second phase particles by melt conditioned direct chill (MC-DC) casting process—A novel approach. J. Mater. Process. Technol. 2021, 295, 117170. [Google Scholar]
- Available online: https://computherm.com/panaluminum (accessed on 1 July 2022).
- Scheil, E. Bemerkungen zur schichtkristallbildung. Z. Metallkd. 1942, 34, 70–72. [Google Scholar] [CrossRef]
- Que, Z.; Fang, C.M.; Chamini, L.; Mendis, C.L.; Wang, Y.; Fan, Z. Effects of Si solution in Al13Fe4 on phase transformation between Fe-containing intermetallic compounds in Al-alloys. J. Alloys Compd. 2022. Revised. [Google Scholar]
- Fang, C.M.; Que, Z.; Dinsdale, A.; Fan, Z. Si solution in θ-Al13Fe4 from first-principles. Intermetallics 2020, 126, 106939. [Google Scholar] [CrossRef]
- Que, Z.; Wang, Y.; Fan, Z.; Hashimoto, T.; Zhou, X. Enhanced heterogeneous nucleation of Al6(Fe,Mn) compound in Al-alloys by interfacial segregation of Mn on TiB2 particles surface. Mater. Lett. 2022, 323, 132570. [Google Scholar] [CrossRef]
- Que, Z.; Zhou, Y.; Wang, Y.; Mendis, C.L.; Fan, Z. Effects of Mg addition on the Al6(Fe,Mn) intermetallic compounds and the grain refinement of α-Al in Al-Fe-Mn alloys. Mater. Charact. 2020, 171, 110758. [Google Scholar] [CrossRef]
- Que, Z.; Zhou, Y.; Wang, Y.; Fan, Z. Proceedings of the 6th Decennial Conference on Solidification Processing 2017. Old Windsor, UK, 25–28 July 2017; pp. 158–161. [Google Scholar]
- Gao, F.; Fan, Z. Competition for nucleation and grain initiation during solidification. Metals 2022, 12, 1512. [Google Scholar] [CrossRef]
- Fang, C.; Dinsdale, A.; Que, Z.; Fan, Z. Intrinsic defects in and electronic properties of θ-Al13Fe4: An ab initio DFT study. J. Phys. Mater. 2019, 2, 015004. [Google Scholar] [CrossRef]
- Fang, C.; Que, Z.; Fan, Z. Crystal Chemistry and Electronic Structure of the β-AlFeSi Phase from First-Principles. J. Solid State Chem. 2021, 299, 122199. [Google Scholar] [CrossRef]
- Dinsdale, A.; Fang, C.; Que, Z.; Fan, Z. Understanding the Thermodynamics and Crystal Structure of Complex Fe Containing Intermetallic Phases Formed on Solidification of Al-alloys. JOM 2019, 71, 1731–1736. [Google Scholar] [CrossRef]
- Fan, Z. An Epitaxial Model for Heterogeneous Nucleation on Potent Substrates. Metall. Mater. Trans. A 2013, 44, 1409–1418. [Google Scholar] [CrossRef]
- Easton, M.A.; Qian, M.; Prasad, A.; Stjohn, D.H. Recent advances in grain refinement of light metals and alloys. Curr. Opin. Solid State Mater. Sci. 2016, 20, 13–24. [Google Scholar] [CrossRef]
- Vainik, R.; Courtenay, J.; Byrant, M. Optimum grain refining with a high performance master alloy. Al Today Int. 2009, 21, 25–26, 28. [Google Scholar]
- Greer, A.L.; Cooper, P.S.; Meredith, M.W.; Schneider, W.; Schumacher, P.; Spittle, J.A.; Tronche, A. Grain refinement of Al-alloys by inoculation. Adv. Eng. Mater. 2003, 5, 81–91. [Google Scholar] [CrossRef]
- Murty, B.S.; Kori, S.A.; Chakraborty, M. Grain refinement of Al and its alloys by heterogeneous nucleation and alloying. Int. Mater. Rev. 2002, 47, 3–29. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al–Ti–B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, C.M.; Zhou, L.; Hashimoto, T.; Zhou, X.; Ramasse, Q.M.; Fan, Z. Mechanism for Zr poisoning of Al-Ti-B based grain refiners. Acta Mater. 2019, 164, 428–439. [Google Scholar] [CrossRef]
- Wang, Y.; Que, Z.; Hashimoto, T.; Zhou, X.; Fan, Z. Mechanism for Si Poisoning of Al-Ti-B Grain Refiners in Al-alloys. Metall. Mater. Trans. A: Phy. Metall. Mater. Sci. 2020, 51, 5743–5757. [Google Scholar]
- Que, Z.; Wang, Y.; Mendis, C.L. Heterogeneous nucleation of α-Al on naturally formed MgAl2O4 particles during solidification of Al–Mg–Si–Fe–Mn alloys. Materialia 2020, 14, 100900. [Google Scholar] [CrossRef]
- Que, Z.; Mendis, C.L. Effects of native AlN particles on heterogeneous nucleation in an Al-3Fe Alloy. Metall. Mater. Trans. A Phy. Metall. Mater. Sci. 2020, 52, 553–559. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, Y.; Ramasse, Q.M.; Schmid-Fetzer, R.; Fan, Z. Segregation of yttrium at Mg/MgO interface in Mg-Y alloy. Acta Mater, 2022; Submitted. [Google Scholar]
- Rosenhain, W.; Grogan, J.D.; Schofield, T.H. Gas removal and grain refinement in Al-alloys. J. Inst. Met. 1930, 44, 305–318. [Google Scholar]
- McCartney, D.G. Grain refining of Al and its alloys using inoculants. Int. Mater. Rev. 1989, 34, 247–260. [Google Scholar] [CrossRef]
- Que, Z.; Xia, J.; Fan, Z. Effects of Zr addition on the grain refinement of α-Al and Al13Fe4 in Al-4Fe alloy. Unpublished.
- Cibula, A. The mechanism of grain refinement of sand castings in Al-alloys. J. Inst. Met. 1949, 76, 321–360. [Google Scholar]
- Guan, R.G.; Tie, D. A Review on Grain Refinement of Aluminum Alloys: Progresses, Challenges and Prospects. Acta Metall. Sin. 2017, 30, 409–432. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, F.; Jiang, B.; Que, Z. Impeding Nucleation for More Significant Grain Refinement. Sci. Rep. 2020, 10, 9448. [Google Scholar]
- Fan, Z.; Men, H.; Wang, Y.; Que, Z. A New Atomistic Mechanism for Heterogeneous Nucleation in the Systems with Negative Lattice Misfit: Creating a 2D Template for Crystal Growth. Metals 2021, 11, 478. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Zhang, S.Y.; Song, J.W.; Zhou, X.L.; Zha, M.; Wang, H.Y. Fe-bearing phase formation, microstructure evolution, and mechanical properties of Al-Mg-Si-Fe alloy fabricated by the twin-roll casting process. J. Alloys Compd. 2021, 886, 161202. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Li, X.; Zhang, H.; Nagaumi, H. Understanding crystal structure and morphology evolution of Fe, Mn, Cr-containing phases in Al-Si cast alloy. Intermetallics 2021, 131, 107103. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zhou, Y.; Chong, X.; Li, X.; Zhang, H.; Nagaumi, H. Exploring crystal structures, stability and mechanical properties of Fe, Mn-containing intermetallics in Al-Si alloy by experiments and first-principles calculations. J. Alloys Compd. 2021, 876, 160022. [Google Scholar] [CrossRef]

| Alloys | a (Å) | b (Å) | c (Å) | α(º) | β(º) | γ(º) | ICSD Collect Code | References |
|---|---|---|---|---|---|---|---|---|
| θ-Al13Fe4 | 15.492 | 8.078 | 12.471 | 90 | 107.69 | 90 | 151129 | [21] |
| α’- Al8Fe2Si | 12.404 | 12.404 | 26.234 | 90 | 90 | 120 | 1293 | [34] |
| β-Al5FeSi | 6.161 | 6.175 | 20.813 | 90 | 90.42 | 90 | 74569 | [20] |
| α-Al15(Fe, Mn)3Si2 | 12.56 | 12.56 | 12.56 | 90 | 90 | 90 | 52623 | [30] |
| δ-Al4FeSi2 | 6.061 | 6.061 | 9.525 | 90 | 90 | 90 | 79710 | [35] |
| η-Al6(Fe, Mn) | 7.498 | 6.495 | 8.837 | 90 | 90 | 90 | 607582 | [27] |
| Alloys | TL (°C) | PE | Tp (°C) | Wt.% | |||
|---|---|---|---|---|---|---|---|
| Fe | Mn | Si | Mg | ||||
| Al-3Fe | 805 | θ-Al13Fe4 | 860 | 3.13 ± 0.06 | 0.01 ± 0.00 | - | - |
| Al-4Si-4Fe | 715 | θ-Al13Fe4 | 760 | 4.10 ± 0.05 | 0.05 ± 0.00 | 4.21 ± 0.06 | 0.003 ± 0.00 |
| Al-5Mg-2Si-0.7Mn-1.3Fe | 668 | α-Al15(Fe, Mn)3Si2 | 720 | 1.29 ± 0.03 | 0.68 ± 0.02 | 2.11 ± 0.03 | 5.43 ± 0.05 |
| Al-3Mg-2Si-0.7Mn-1.3Fe | 662 | α-Al15(Fe, Mn)3Si2 | 720 | 1.25 ± 0.06 | 0.64 ± 0.02 | 2.15 ± 0.03 | 3.05 ± 0.05 |
| Al-5Mg-2Si-0.4Mn-0.7Fe | 638 | α-Al15(Fe, Mn)3Si2 | 690 | 0.67 ± 0.2 | 0.44 ± 0.2 | 2.65 ± 0.2 | 5.66 ± 0.6 |
| Al-12Si-0.6Mn-2.8Fe | 648 | α-Al15(Fe, Mn)3Si2 | 700 | 2.8 ± 0.05 | 0.6 ± 0.01 | 11.7 ± 0.2 | - |
| Al-20Si-0.7Fe | 688 | Si | 750 | 0.65 ± 0.05 | - | 20.5 ± 0.5 | - |
| Al-16Si-3Fe | 670 | β-Al5FeSi | 720 | 3.3 ± 0.05 | 13 ± 0.3 | ||
| Al-2Mn-1Fe | 664 | η-Al6(Fe, Mn) | 720 | 1.0 ± 0.02 | 2.2 ± 0.02 | - | - |
| Al-4Fe | 850 | θ-Al13Fe4 | 900 | 4.37 ± 0.05 | - | - | - |
| Grain Size (μm) | CP-Al (α-Al) | Al-5Mg-2Si-0.7Mn-1.3Fe (α-Al15(Fe, Mn)3Si2) | Al-12Si-0.7Mn-2.8Fe (β-Al5FeSi) | Al-27Si (Si) | Al-2Mn-1Fe (η-Al6(Fe, Mn)) |
|---|---|---|---|---|---|
| No grain refiner | Fully columnar | 38.7 ± 6.8μm | 1178.4 ± 135 μm | 461 ± 51 μm | 26.0 ± 2.1 μm |
| AlB2(Fe, Si) | - | 19.2 ± 5.6 μm | - | - | - |
| TiB2(Mn) | - | - | - | - | 9.5 ± 0.6 μm |
| TiB2(Fe, Si) | - | 11.1 ± 4.4 μm | 425.2 ± 61 μm | 39 ± 5.5 μm | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Que, Z.; Wang, Y.; Mendis, C.L.; Fang, C.; Xia, J.; Zhou, X.; Fan, Z. Understanding Fe-Containing Intermetallic Compounds in Al Alloys: An Overview of Recent Advances from the LiME Research Hub. Metals 2022, 12, 1677. https://doi.org/10.3390/met12101677
Que Z, Wang Y, Mendis CL, Fang C, Xia J, Zhou X, Fan Z. Understanding Fe-Containing Intermetallic Compounds in Al Alloys: An Overview of Recent Advances from the LiME Research Hub. Metals. 2022; 12(10):1677. https://doi.org/10.3390/met12101677
Chicago/Turabian StyleQue, Zhongping, Yun Wang, Chamini L. Mendis, Changming Fang, Junhai Xia, Xiaorong Zhou, and Zhongyun Fan. 2022. "Understanding Fe-Containing Intermetallic Compounds in Al Alloys: An Overview of Recent Advances from the LiME Research Hub" Metals 12, no. 10: 1677. https://doi.org/10.3390/met12101677
APA StyleQue, Z., Wang, Y., Mendis, C. L., Fang, C., Xia, J., Zhou, X., & Fan, Z. (2022). Understanding Fe-Containing Intermetallic Compounds in Al Alloys: An Overview of Recent Advances from the LiME Research Hub. Metals, 12(10), 1677. https://doi.org/10.3390/met12101677









