The Microstructure, Mechanical Properties, and Corrosion Resistance of a Novel Extruded Titanium Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
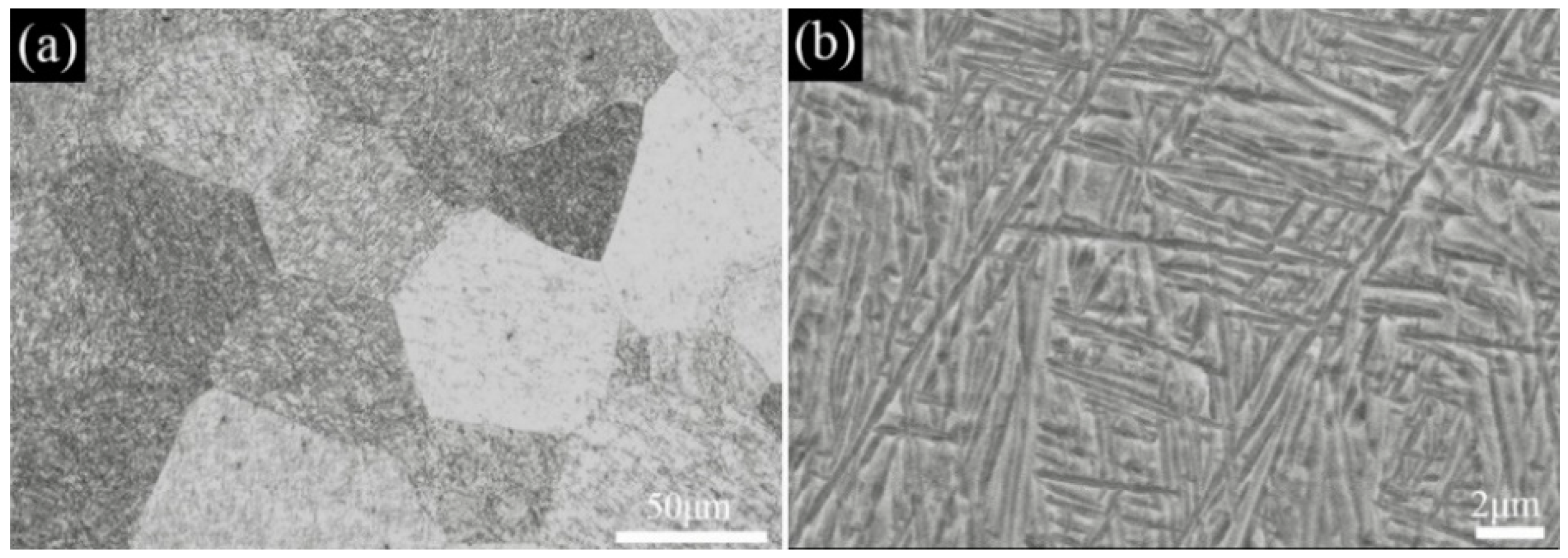
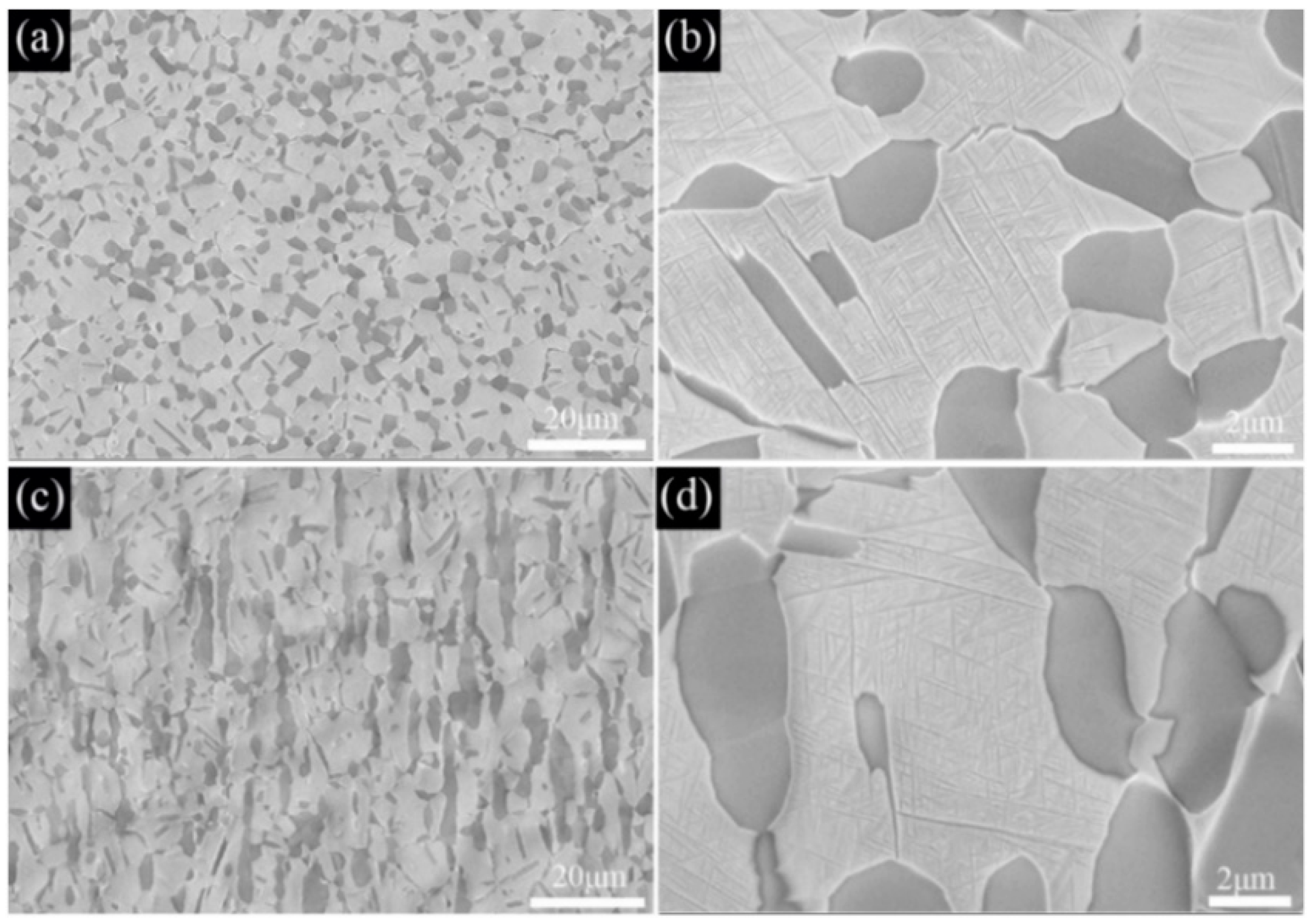
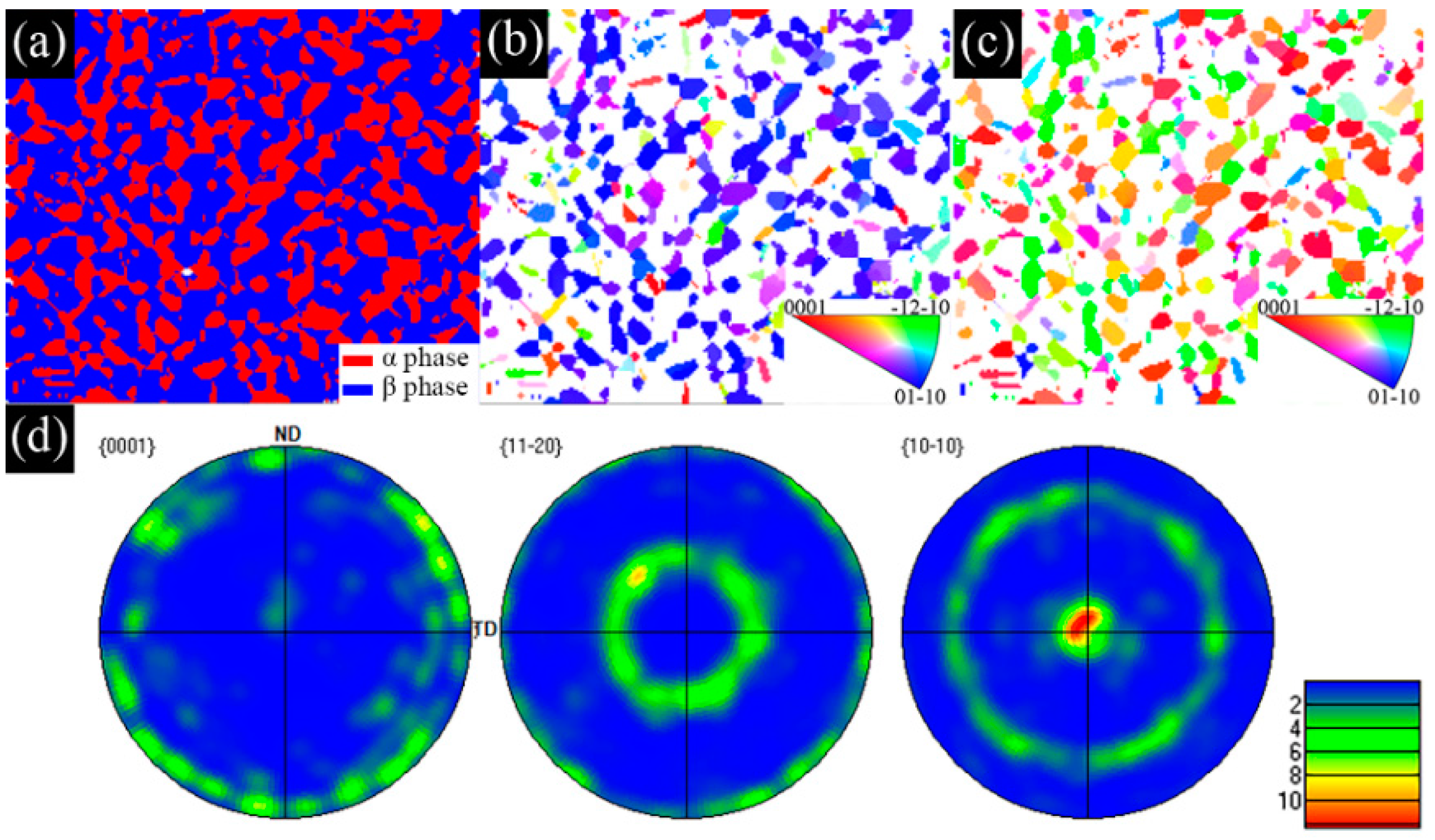
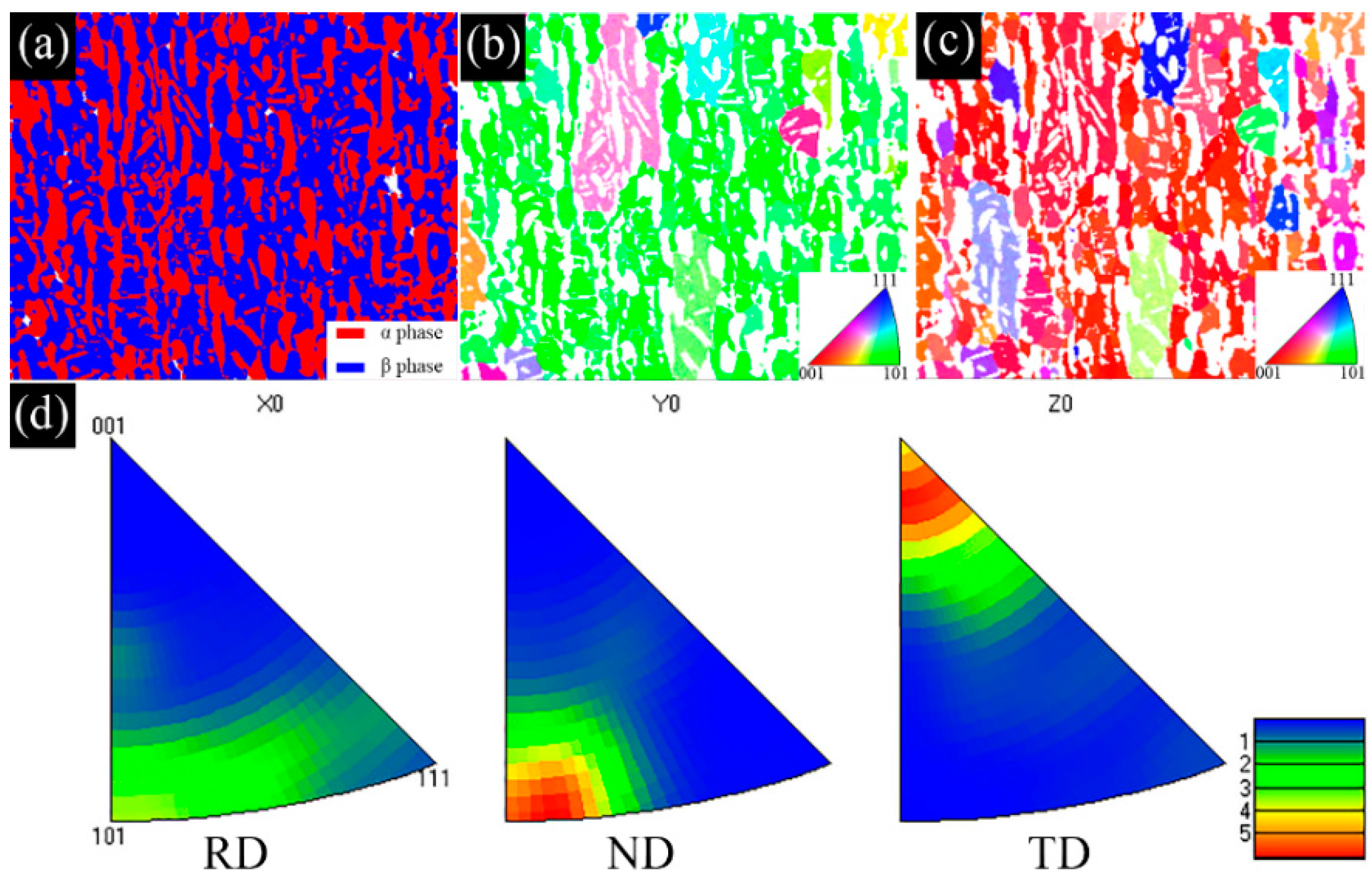
3.1. Microstructural Evolution
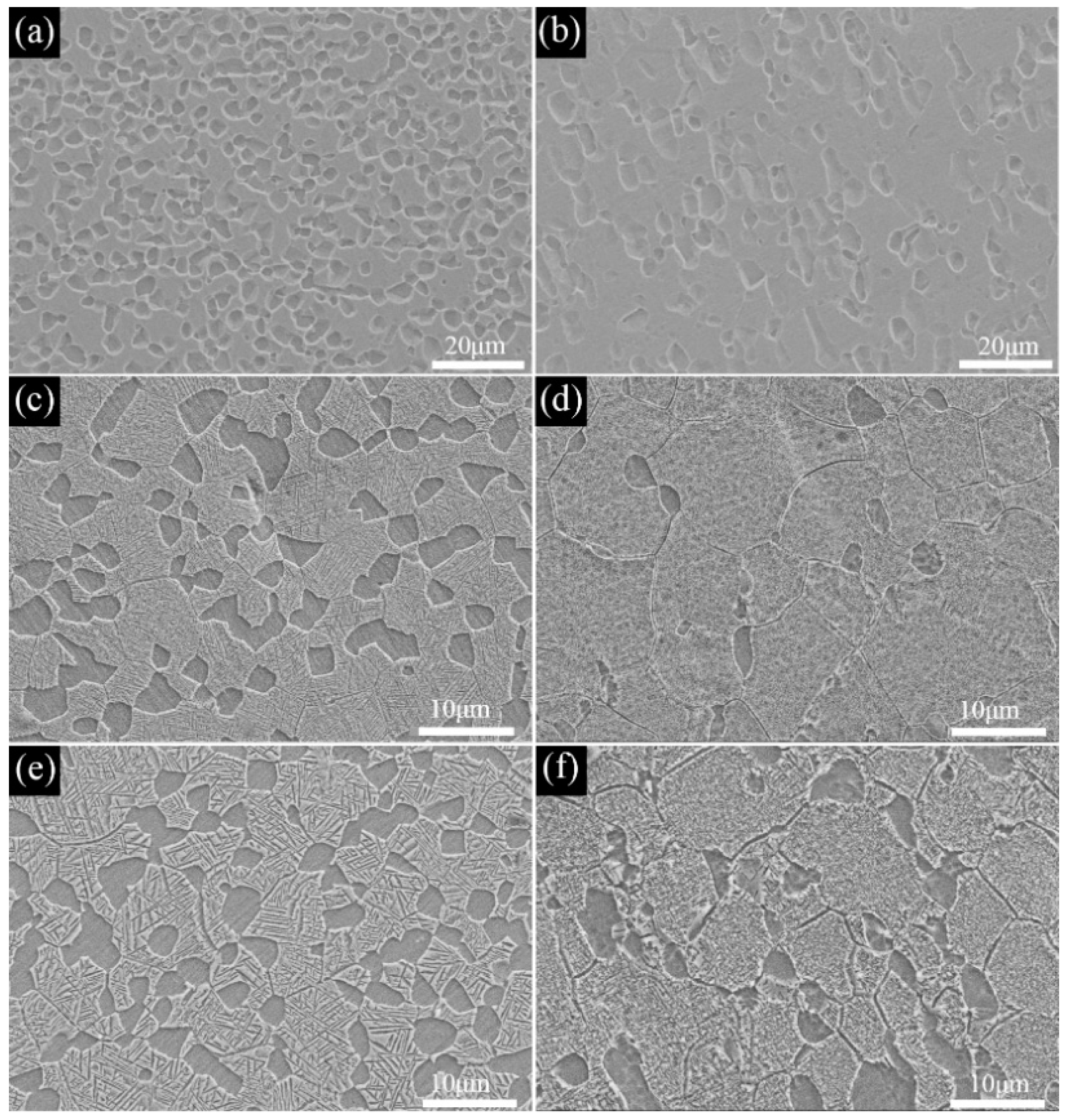
3.2. Solution and Aging Treatment
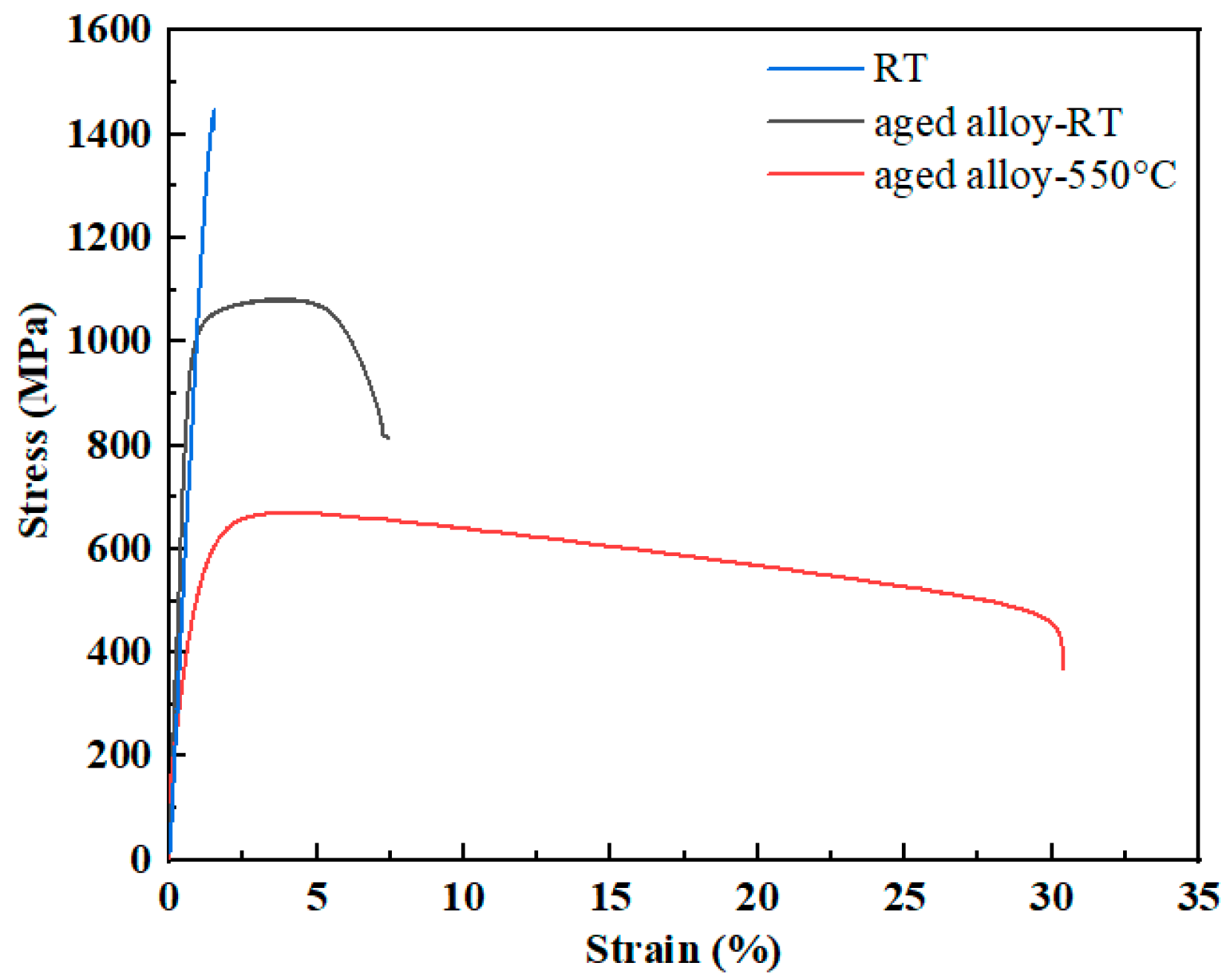
3.3. Tensile Properties
3.4. Corrosion Resistance
4. Conclusions
- (1)
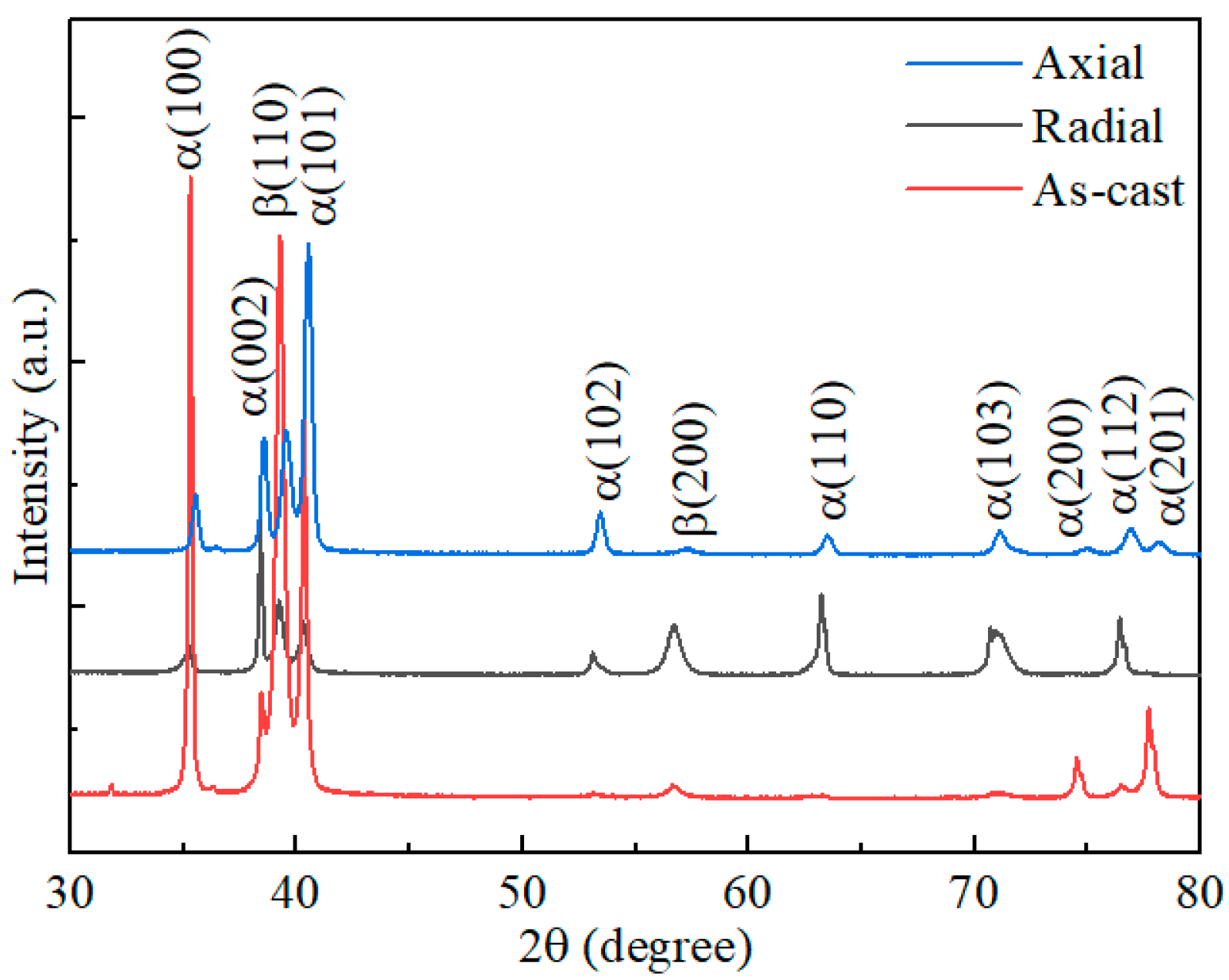
- The as−extruded Ti−6Al−3Mo−2Zr−2Fe alloy exhibited a typical (α + β) duplex microstructure. The α + β phases exhibited a flat strip shape in the axial direction due to the effect of the external force during the extrusion process. The strong <> fiber texture of the α phase was formed in the radial direction. The mixed texture of the <101>//ND and <001>//TD of the β phase was formed in the axial direction.
- (2)
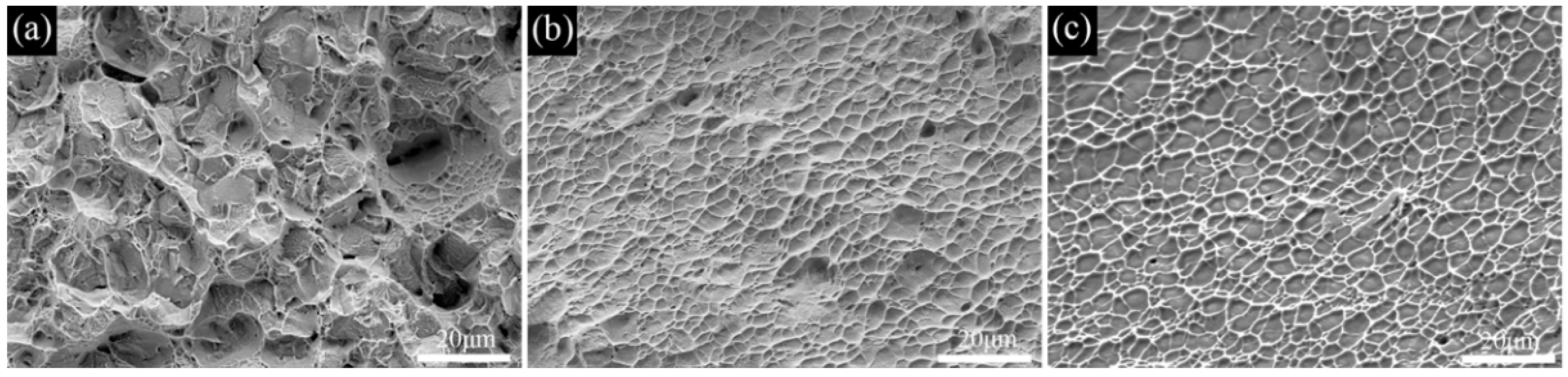
- The transformation temperature Tβ of the Ti−6Al−3Mo−2Zr−2Fe alloy was determined to be 880–890 °C using the metallographic method. When the alloy was solution−treated at 850 °C for 1h and underwent an aging treatment at 550 °C for 6 h, the alloy exhibited excellent mechanical properties. The room−temperature tensile strength and elongation of the alloy were 1081.5 MPa and 6.5%, respectively. The fracture morphology showed a classic dimple shape.
- (3)
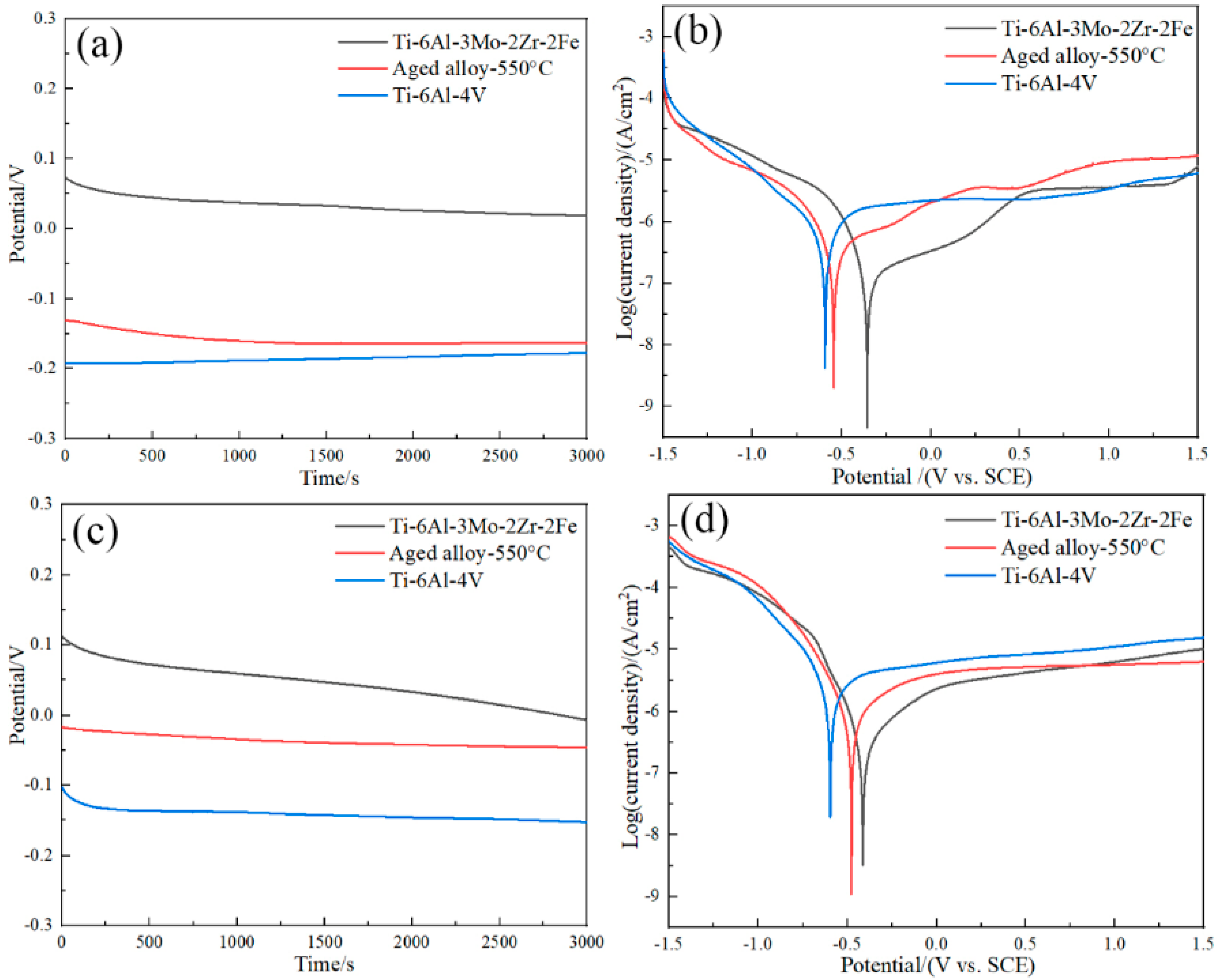
- The corrosion current densities of the aged Ti−6Al−3Mo−2Zr−2Fe alloy in a 3.5% NaCl solution and an acidic solution were 1.7 × 10−7 A/cm2 and 6.7 × 10−7 A/cm2, respectively, which were lower than those of the TC4 alloy. This indicated that the corrosion resistance of the Ti−6Al−3Mo−2Zr−2Fe alloy was better than that of TC4 in both the 3.5%NaCl and acidic solutions, which was related to the alloying elements. The addition of Mo promoted the formation of a passivation film on the alloy surface. The addition of Zr improved the chemical dissolution resistance of the TiO2 passivation film.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pushp, P.; Dasharath, S.; Arati, C. Classification and applications of titanium and its alloys. Mater. Today Proc. 2022, 54, 537–542. [Google Scholar] [CrossRef]
- Liu, S.; Song, X.; Xue, T.; Ma, N.; Wang, Y.; Wang, L. Application and development of titanium alloy and titanium matrix composites in aerospace field. J. Aeronaut. Mater. 2020, 40, 77–94. [Google Scholar] [CrossRef]
- Williams, J.C.; Boyer, R.R. Opportunities and issues in the application of titanium alloys for aerospace components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Tshephe, T.S.; Akinwamide, S.O.; Olevsky, E.; Olubambi, P.A. Additive manufacturing of titanium-based alloys—A review of methods, properties, challenges, and prospects. Heliyon 2022, 8, e09041. [Google Scholar] [CrossRef]
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium alloys for aerospace applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Yingli, Y.; Yuanyuan, L.; Hengzhang, Z.; Dizi, G.; Jinping, W.; Hangbiao, S.; Bin, Z. Research and application status of titanium alloys for marine engineering. Titan. Ind. Prog. 2022, 39, 43–48. [Google Scholar] [CrossRef]
- Yan, S.; Song, G.L.; Li, Z.; Wang, H.; Zheng, D.; Cao, F.; Horynova, M.; Dargusch, M.; Zhou, L. A state-of-the-art review on passivation and biofouling of Ti and its alloys in marine environments. J. Mater. Sci. Technol. 2018, 34, 421–435. [Google Scholar] [CrossRef]
- Li, R.Z.; Feng, C.; Jiang, L.; Cao, Y.Q. Research status and development of titanium alloy drill pipes. Mater. Sci. Fourm 2019, 944, 903–909. [Google Scholar] [CrossRef]
- Wang, C.P.; Wang, H.Z.; Ruan, G.L.; Wang, S.H.; Xiao, Y.X.; Jiang, L.D. Applications and prospects of titanium and its alloys in seawater desalination industry. IOP Conf. Ser. Mater. Sci. Eng. A 2019, 688, 033036. [Google Scholar] [CrossRef]
- Nasad, T.G.; Kochetkov, A.V. Highly Efficient Methods of Machining Titanium for Oil-Gas Drilling Equipment. Chem. Pet. Eng. 2016, 52, 227–230. [Google Scholar] [CrossRef]
- Gorynin, I.V. Titanium alloys for marine application. Mater. Sci. Eng. A 1999, 263, 112–116. [Google Scholar] [CrossRef]
- Bania, P.J. Beta titanium alloys and their role in the titanium industry. JOM 1994, 46, 16–19. [Google Scholar] [CrossRef]
- Rosenberg, H.W. Titanium alloying in theory and practice. In The Science, Technology and Application of Titanium; Elsevier: Amsterdam, The Netherlands, 1970; pp. 851–859. [Google Scholar]
- Xu, W.; Chen, M.; Lu, X.; Zhang, D.W.; Singh, H.P.; Jian Shu, Y.; Pan, Y.; Qu, X.-H.; Liu, C.Z. Effects of Mo content on corrosion and tribocorrosion behaviours of Ti-Mo orthopaedic alloys fabricated by powder metallurgy. Corros. Sci. 2020, 168, 108557. [Google Scholar] [CrossRef]
- Yu, S.Y.; Brodrick, C.W.; Ryan, M.P.; Scully, J.R. Effects of Nb and Zr Alloying Additions on the Activation Behavior of Ti in Hydrochloric Acid. J. Electrochem. Soc. 1999, 146, 4429–4438. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.; Yang, R.; Li, D.; Wu, W.; Guo, Z. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Kuroda, D.; Kawasaki, H.; Yamamoto, A.; Hiromoto, S.; Hanawa, T. Mechanical properties and microstructures of new Ti-Fe-Ta and Ti-Fe-Ta-Zr system alloys. Mater. Sci. Eng. C 2005, 25, 312–320. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.F.; Tang, H.P.; Liu, C.T.; Liu, B.; Huang, B.Y. Design of powder metallurgy titanium alloys and composites. Mater. Sci. Eng. A 2006, 418, 25–35. [Google Scholar] [CrossRef]
- Ma, X.; Xiang, Z.; Ma, M.; Cui, Y.; Ren, W.; Wang, Z.; Huang, J.; Chen, Z. Investigation of microstructures, textures, mechanical properties and fracture behaviors of a newly developed near α titanium alloy. Mater. Sci. Eng. A 2020, 775, 138996. [Google Scholar] [CrossRef]
- Ding, C.; Liu, C.; Zhang, L.; Deng, Y.; Liu, H.; Wu, D.; Liu, L. Microstructure and tensile properties of a cost-affordable and ultrahigh-strength metastable β titanium alloy with a composition of Ti-6Al-1Mo-1Fe-6.9Cr. J. Alloys Compd. 2022, 901, 163476. [Google Scholar] [CrossRef]
- Wen, X.; Wan, M.; Huang, C.; Lei, M. Strength and fracture toughness of TC21 alloy with multi-level lamellar microstructure. Mater. Sci. Eng. A 2018, 740, 121–129. [Google Scholar] [CrossRef]
- Wang, T.; Guo, H.; Wang, Y.; Yao, Z. Influence of processing parameters on microstructure and tensile properties of TG6 titanium alloy. Mater. Sci. Eng. A 2010, 528, 736–744. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, W.; Mao, X. Effect of microstructure on the tensile properties and impact toughness of TC21G titanium alloy. Mater. Charact. 2022, 185, 111752. [Google Scholar] [CrossRef]
- Li, C.L.; Mi, X.J.; Ye, W.J.; Hui, S.X.; Yu, Y.; Wang, W.Q. A study on the microstructures and tensile properties of new beta high strength titanium alloy. J. Alloys Compd. 2013, 550, 23–30. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, L.; Liang, Z.; Cao, S.; Yang, J.; Xiao, S.; Tian, J.; Chen, Y. Effect of solution treatment and aging on microstructure, tensile properties and creep behavior of a hot-rolled β high strength titanium alloy with a composition of Ti-3.5Al-5Mo-6V-3Cr-2Sn-0.5Fe-0.1B-0.1C. Mater. Sci. Eng. A 2021, 823, 141728. [Google Scholar] [CrossRef]
- Du, Z.; Guo, H.; Liu, J.; Cheng, J.; Zhao, X.; Wang, X.; Liu, F.; Cui, X. Microstructure evolution during aging heat treatment and its effects on tensile properties and dynamic Young’s modulus of a biomedical β titanium alloy. Mater. Sci. Eng. A 2020, 791, 139677. [Google Scholar] [CrossRef]
- Narayana, P.; Kim, S.W.; Hong, J.K.; Reddy, N.; Yeom, J.T. Tensile properties of a newly developed high−temperature titanium alloy at room temperature and 650 °C. Mater. Sci. Eng. A 2018, 718, 287–291. [Google Scholar] [CrossRef]
- Jin, W.; Feng, K.; Li, Z.; Cai, X.; Yu, L.; Zhou, D. Improvement of corrosion resistance and electrical conductivity of 304 stainless steel using close field unbalanced magnetron sputtered carbon film. Power Sources 2011, 196, 10032–10037. [Google Scholar] [CrossRef]
- Li, Q.; Chen, K.; Xia, C.; Chen, B.; Liu, S.; Yang, T.; Liu, D.; Wang, Y.; Zhang, X. Microstructure evolution, mechanical properties, and corrosion behavior of novel Zr-Ti-V alloys. Mater. Sci. Eng. A 2021, 817, 141358. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, L.; Lin, G.; Shao, Z. Honeycomb−like nanocomposite Ti-Ag-N films prepared by pulsed bias arc ion plating on titanium as bipolar plates for unitized regenerative fuel cells. Power Sources 2012, 198, 196–202. [Google Scholar] [CrossRef]
- Chui, P.; Jing, R.; Zhang, F.; Li, J.; Feng, T. Mechanical properties and corrosion behavior of β-type Ti-Zr-Nb-Mo alloys for biomedical application. J. Alloys Compd. 2020, 842, 155693. [Google Scholar] [CrossRef]
- Su, B.; Luo, L.; Wang, B.; Su, Y.; Wang, L.; Ritchie, R.O.; Guo, E.; Li, T.; Yang, H.; Huang, H.; et al. Annealed microstructure dependent corrosion behavior of Ti-6Al-3Nb-2Zr-1Mo alloy. J. Mater. Sci. Technol. 2021, 62, 234–248. [Google Scholar] [CrossRef]
- Kong, D.S.; Feng, Y.Y. Electrochemical Anodic Dissolution Kinetics of Titanium in Fluoride-Containing Perchloric Acid Solutions at Open-Circuit Potentials. J. Electrochem. Soc. 2009, 156, C283. [Google Scholar] [CrossRef]
- Atapour, M.; Pilchak, A.; Frankel, G.; Williams, J. Corrosion behavior of β titanium alloys for biomedical applications. Mater. Sci. Eng. C 2011, 31, 885–891. [Google Scholar] [CrossRef]
| Solution | Parameters | TAMZF | 550 °C | TC4 |
|---|---|---|---|---|
| 3.5%NaCl | Ecorr (V) | −0.35 | −0.55 | −0.60 |
| icorr (A/cm2) | 1.4 × 10−7 | 1.7 × 10−7 | 5.3 × 10−7 | |
| H2SO4 + HF | Ecorr (V) | −0.42 | −0.48 | −0.61 |
| icorr (A/cm2) | 2.7 × 10−7 | 6.7 × 10−7 | 1.8 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, N.; Chen, S.; Xu, T.; Sun, W.; Lv, B.; Zhang, S.; Niu, H.; Kong, F. The Microstructure, Mechanical Properties, and Corrosion Resistance of a Novel Extruded Titanium Alloy. Metals 2022, 12, 1564. https://doi.org/10.3390/met12101564
Cui N, Chen S, Xu T, Sun W, Lv B, Zhang S, Niu H, Kong F. The Microstructure, Mechanical Properties, and Corrosion Resistance of a Novel Extruded Titanium Alloy. Metals. 2022; 12(10):1564. https://doi.org/10.3390/met12101564
Chicago/Turabian StyleCui, Ning, Saihao Chen, Tiewei Xu, Wei Sun, Binjiang Lv, Shuling Zhang, Hongzhi Niu, and Fantao Kong. 2022. "The Microstructure, Mechanical Properties, and Corrosion Resistance of a Novel Extruded Titanium Alloy" Metals 12, no. 10: 1564. https://doi.org/10.3390/met12101564
APA StyleCui, N., Chen, S., Xu, T., Sun, W., Lv, B., Zhang, S., Niu, H., & Kong, F. (2022). The Microstructure, Mechanical Properties, and Corrosion Resistance of a Novel Extruded Titanium Alloy. Metals, 12(10), 1564. https://doi.org/10.3390/met12101564








