Mechanical and Physical Properties of Differently Alloyed Sintered Steels as a Function of the Sintering Temperature
Abstract
:1. Introduction
- -
- Change of particle surface chemistry, in particular, the reduction of oxide layers covering the powder particles.
- -
- Formation and growth of solid metallic bridges out of pressing contacts.
- -
- Dissolution of alloy elements, in particular, carbon and distribution within the metallic matrix.
2. Materials and Methods
3. Results
3.1. Study of the Degassing and Reduction Behavior
3.2. Influence of the Temperature on Formation of Sintering Contacts
3.3. Metallography and Microstructures
3.4. Dimensional and Mechanical Properties as a Function of the Sintering Temperature
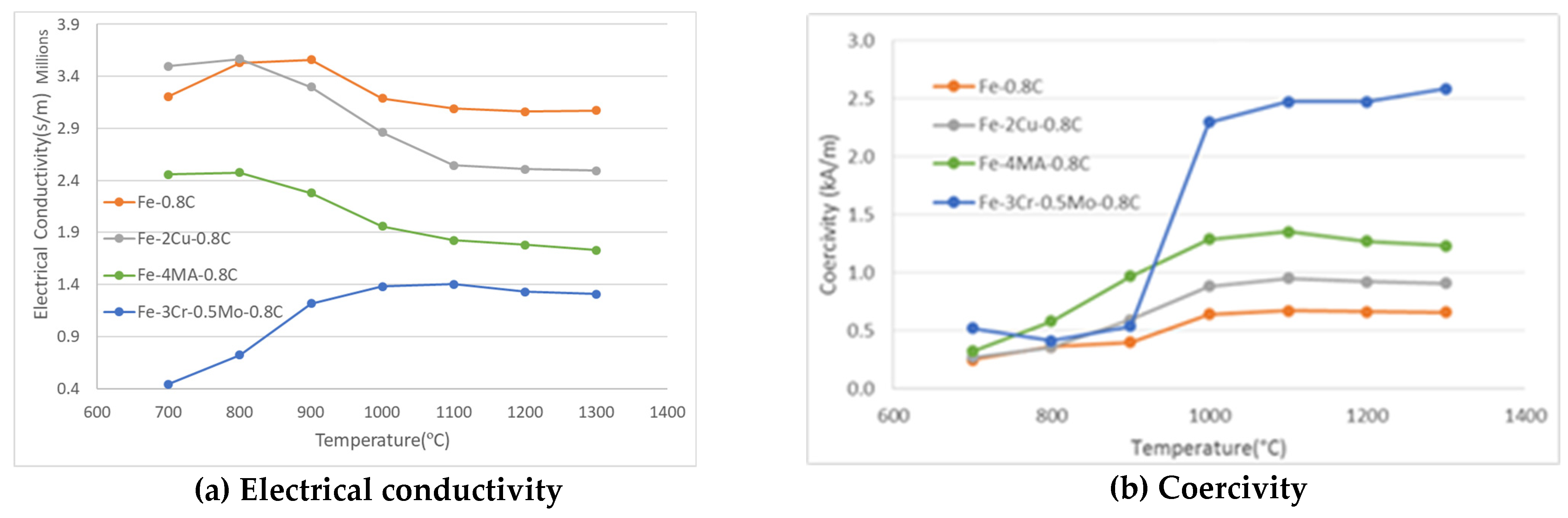
3.5. Electrical and Magnetic Properties as a Function of the Sintering Temperature
4. Summary and Conclusions
- -
- For steel grades that do not contain oxygen-affine alloy elements, the formation and growth of sintering contacts starts already at moderate temperatures. Oxygen removal is typically attained at belt furnace temperatures, as well as satisfactory properties.
- -
- In the case of Fe-C, the dissolution of carbon is the most crucial step that controls the properties.
- -
- For Fe-Cu-C, the formation of transient liquid phase and resulting homogenization of Cu is a further relevant process that improves hardness and strength while slightly lowering the impact energy.
- -
- Adding oxygen-affine elements through the masteralloy route results in the “internal getter” effect, i.e., oxygen transfer from the iron particle surfaces to the masteralloy particles, but this enhances formation of iron-iron sintering contacts. The masteralloy particles are subsequently reduced carbothermally at higher temperatures.
- -
- For the Cr-Mo pre-alloyed steel, oxygen removal requires fairly high temperatures; this process also triggers off carbon dissolution and formation of solid interparticle contacts. Therefore, this steel variant requires the highest sintering temperatures.
- -
- Fe-C and Fe-Cu-C are well suited for the cost-effective production of standard powder metallurgy precision parts. The Cr-Mo pre-alloyed grade, in contrast, must be sintered under specific conditions, especially at sufficiently high temperatures, which requires special furnaces [47]. However, if properly sintered, this material offers the best property portfolio. The masteralloy approach finally can be regarded as an attractive compromise between production requirements and properties attained.
- -
- Physical properties are useful to describe the homogenization of alloy elements. The electrical conductivity is mainly sensitive to metallic alloy elements while the coercive force mirrors predominantly the dissolution of carbon.
- -
- Further investigations are required to fully establish the property profiles of both pre-alloyed and masteralloy variants. This holds in particular for the fatigue properties, which are relevant for many applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kremel, S.; Danninger, H.; Yu, Y. Effect of sintering conditions on particle contacts and mechanical properties of PM steels prepared from 3% Cr prealloyed powder. Powder Metall. Prog. 2002, 2, 211–221. [Google Scholar]
- Zapf, G.; Dalal, K. Introduction of high oxygen affinity elements manganese, chromium and vanadium in the powder metallurgy of P/M parts. In Modern Developments in Powder Metallurgy; Metal Powder Industries Federation: Princeton, NJ, USA, 1977; Volume 10, pp. 129–152. [Google Scholar]
- De Oro Calderon, R.; Gierl-Mayer, C.; Danninger, H. Sintering enhancement by the use of masteralloys: Important concepts and new possibilities. In Proceedings of the World Congress on Powder Metallurgy, Beijing, China, 16–20 September 2018; pp. 462–472. [Google Scholar]
- De Oro Calderon, R.; Gierl-Mayer, C.; Danninger, H. Effect of sintering conditions on the properties of lean pm steels produced through the masteralloy route. In Proceedings of the World Congress on Powder Metallurgy, Beijing, China, 16–20 September 2018; pp. 473–480. [Google Scholar]
- Kulecki, P.; Lichańska, E.; Sułowski, M. The effect of processing parameters on microstructure and mechanical properties of sintered structural steels based on prealloyed powders. Metall. Mater. 2015, 60, 2543–2549. [Google Scholar] [CrossRef]
- Gierl-Mayer, C.; Molnar, C.W.; Danninger, H. Correlations between microstructure, mechanical properties and sintering parameters for PM steels. In Proceedings of the Euro PM Congress and Exhibition, Barcelona, Spain, 9–12 October 2011; pp. 1–7. [Google Scholar]
- Danninger, H.; Gierl, C. Processes in PM steel compacts during the initial stages of sintering. Mater. Chem. Phys. 2001, 67, 49–55. [Google Scholar] [CrossRef]
- Höganäs. Handbook for Sintered Components. Iron and Steel Powders for Sintered Components; Höganäs AB: Hoganas, Sweden, 2017. [Google Scholar]
- De Oro Calderon, R.; Jaliliziyaeian, M.; Dunkley, J.; Gierl-Mayer, C.; Danninger, H. New chances for the masteralloy approach. Powder Metall. Prog. 2018, 18, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Fleck, N.; Smith, R. Effect of density on tensile strength, fracture toughness, and fatigue crack propagation behaviour of sintered steel. Powder Metall. 1981, 24, 121–125. [Google Scholar] [CrossRef]
- Danninger, H.; Xu, C.; Lindqvist, B. Oxygen removal during sintering of steels prepared from Cr-Mo and Mo prealloyed powders. Mater. Sci. Forum 2007, 534, 577–580. [Google Scholar] [CrossRef]
- Cavdar, U. Effect of the copper amount in iron-based powder-metal compacts. Mater. Technol. 2015, 49, 57–62. [Google Scholar]
- Wong-Ángel, W.D.; Téllez-Jurado, L.; Chávez-Alcalá, J.F.; Chavira-Martínez, E.; Verduzco-Cedeño, V.F. Effect of copper on the mechanical properties of alloys formed by powder metallurgy. Mater. Des. 2014, 58, 12–18. [Google Scholar] [CrossRef]
- Pang Jianming, F.J.; Congchao, P.; Yaoxin, S. Effect of copper powder on the properties of iron-based powder metallurgy sintered products. In Proceedings of the World Congress on Powder Metallurgy, Beijing, China, 16–20 September 2018; pp. 98–106. [Google Scholar]
- Hu, H.; Xu, G.; Zhou, M.; Yuan, Q. Effect of Mo content on microstructure and property of low-carbon bainitic steels. Metals 2016, 6, 173. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, Y.; Matlock, D.K.; Krauss, G. Effect of molybdenum on microstructure and mechanical properties of intercritically annealed and isothermally transformed low carbon steel. Mater. Sci. Technol. 1993, 9, 718–724. [Google Scholar] [CrossRef]
- Danninger, H.; De Oro Calderon, R.; Gierl-Mayer, C. Chemical reactions during sintering of PM steel compacts as a function of the alloying route. Powder Metall. 2018, 2018. 61, 241–250. [Google Scholar] [CrossRef]
- Danninger, H.; De Oro Calderon, R.; Gierl-Mayer, C. Oxygen transfer reactions during sintering of ferrous powder compacts. Adv. Eng. Forum 2018, 27, 3–13. [Google Scholar] [CrossRef]
- Danninger, H.; De Oro Calderon, R.; Gierl-Mayer, C. Surface chemistry of metal powders and changes during the sintering process. In Proceedings of the World Congress on Powder Metallurgy, Beijing, China, 16–20 September 2018; pp. 92–97. [Google Scholar]
- Gierl-Mayer, C.; Danninger, H. Dilatometry coupled with mass spectrometry as instrument for process control in sintering of powder metallurgy steels. Mater. Sci. Forum 2016, 835, 106–115. [Google Scholar] [CrossRef]
- Karamchedu, S.; Hryha, E.; Nyborg, L. Changes in the surface chemistry of chromium-alloyed powder metallurgical steel during delubrication and their impact on sintering. J. Mater. Process. Technol. 2015, 223, 171–185. [Google Scholar] [CrossRef]
- Danninger, H.; De Oro Calderon, R.; Gierl-Mayer, C. Powder metallurgy and sintered materials. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2017; pp. 1–57. [Google Scholar]
- Dudrova, E.; Kabátova, M. A review of failure of sintered steels: Fractography of static and dynamic crack nucleation, coalescence, growth and propagation. Powder Metall. 2016, 59, 148–167. [Google Scholar] [CrossRef]
- Danninger, H. Pore formation during sintering of Fe–Cu and its effects on mechanical properties. Powder Metall. Int. 1987, 19, 19–23. [Google Scholar]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Danninger, H.; Gierl-Mayer, C.; Strobl, S. Evolution of microstructure in ferrous and non-ferrous materials. In Advances in Powder Metallurgy; Chang, I., Zhao, Y., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 308–357. [Google Scholar]
- Lawcock, R.L.; Davies, T.J. Effect of carbon on dimensional and microstructural characteristics of Fe-Cu compacts during sintering. Powder Metall. 1990, 33, 147–149. [Google Scholar] [CrossRef]
- Fredriksson, H.; Hansson, K.; Olsson, A. On the mechanism of liquid copper penetration into iron grain boundaries. Scand. J. Metall. 2001, 30, 41–50. [Google Scholar] [CrossRef]
- Jamil, S.J.; Chadwick, G.A. Investigation and analysis of liquid phase sintering of Fe–Cu and Fe–Cu–C compacts. Powder Metall. 1985, 28, 65–71. [Google Scholar] [CrossRef]
- Kieback, B.; Schatt, W. Anwendung eines kurzzeitigen Flüssigphasensinterns für die Herstellung von Fe-Ti-Sinterlegierungen. Planseeberichte für Pulvermetallurgie 1980, 28, 204–215. [Google Scholar]
- Šalak, A.; Selecká, M. Alloying and sintering of manganese steels by manganese vapour. In Manganese in Powder Metallurgy Steels; Cambridge International Science Publishing: Cambridge, UK, 2012; pp. 39–72. [Google Scholar]
- Baselli, S.; Molinari, A. Sintering shrinkage of cold compacted iron—Effect of thermodynamic driving force, structural and geometrical activity. Powder Metall. 2021, 64, 126–133. [Google Scholar] [CrossRef]
- Dautzenberg, N.; Dorweiler, H.J. Dimensional behaviour of copper—Carbon sintered steels. Powder Metall. Int. 1985, 17, 279–282. [Google Scholar]
- Danninger, H.; Frauendienst, G.; Streb, K.-D.; Ratzi, R. Dissolution of different graphite grades during sintering of PM steels. Mater. Chem. Phy. 2001, 67, 72–77. [Google Scholar] [CrossRef]
- Zhang, Z.; Sandström, R.; Wang, L. Modelling of swelling of Fe–Cu compacts sintered at temperatures above the copper melting point. J. Mater. Process. Technol. 2004, 152, 131–135. [Google Scholar] [CrossRef]
- Magee, B.E.; Lund, J. Mechanisms of liquid-phase sintering in iron-copper powder compacts. Zeitschrift für Metallkunde 1976, 67, 596–602. [Google Scholar] [CrossRef]
- Wanibe, Y.; Yokoyama, H.; Itoh, T. Expansion during liquid phase sintering of iron-copper compacts. Powder Metall. 1990, 33, 65–69. [Google Scholar] [CrossRef]
- Berner, D.; Exner, H.E.; Petzow, G. Swelling of iron-copper mixtures during sintering and infiltration. Mod. Dev. Powder Metall. 1974, 6, 237–250. [Google Scholar]
- Šalak, A.; Selecka, M.; Bures, R. Manganese in ferrous powder metallurgy. Powder Metall. Prog. 2001, 1, 41–58. [Google Scholar]
- Momeni, M. Temperature and Interstitial Effect on Physical and Chemical Processes During Sintering of Ferrous Powder Compacts. Ph.D. Thesis, Technische Universität Wien, Vienna, Austria, 2010. [Google Scholar]
- Momeni, M.; Gierl, C.; Danninger, H. Electrical conductivity and microstructural changes of sintered plain carbon steels prepared from different base powders. In Proceedings of the Euro PM Congress and Exhibition, Copenhagen, Denmark, 12–14 October 2009; pp. 1–6. [Google Scholar]
- Simchi, A.; Danninger, H.; Gierl, C. Electrical conductivity and microstructure of sintered ferrous materials: Iron–graphite compacts. Powder Metall. 2001, 44, 148–156. [Google Scholar] [CrossRef]
- Simchi, A.; Danninger, H. Electrical conductivity and microstructure of sintered ferrous materials: Sintered iron. Powder Metall. 2000, 43, 209–218. [Google Scholar] [CrossRef]
- Jangg, G.; Drozda, M.; Eder, G.; Danninger, H. Magnetic properties of sintered iron—Influence of the microstructure of sintered iron materials on the coercive force. Powder Metall. Int. 1984, 16, 60–64. [Google Scholar]
- Jangg, G.; Drozda, M.; Danninger, H.; Nad, R.E. Magnetic properties of sintered iron—The influence of porosity on the magnetic properties of sintered iron. Powder Metall. Int. 1983, 15, 173–177. [Google Scholar]
- Jiles, D.C. Magnetic properties and microstructure of AISI 1000 series carbon steels. J. Phys. D: Appl. Phys. 1988, 21, 1186–1195. [Google Scholar] [CrossRef]
- Arnhold, V.; Kruzhanov, V. Hochtemperatursintern von niedriglegierten PM—Stählen (High temperature sintering of low alloy PM steels). In Pulvermetallurgie in Wissenschaft und Praxis, Proceedings of Hagen PM Symposium, Hagen, Germany, 25–26 November 2021; Fachverband Pulvermetallurgie: Hagen, Germany, 2021; Volume 36, pp. 233–252. (In German) [Google Scholar]
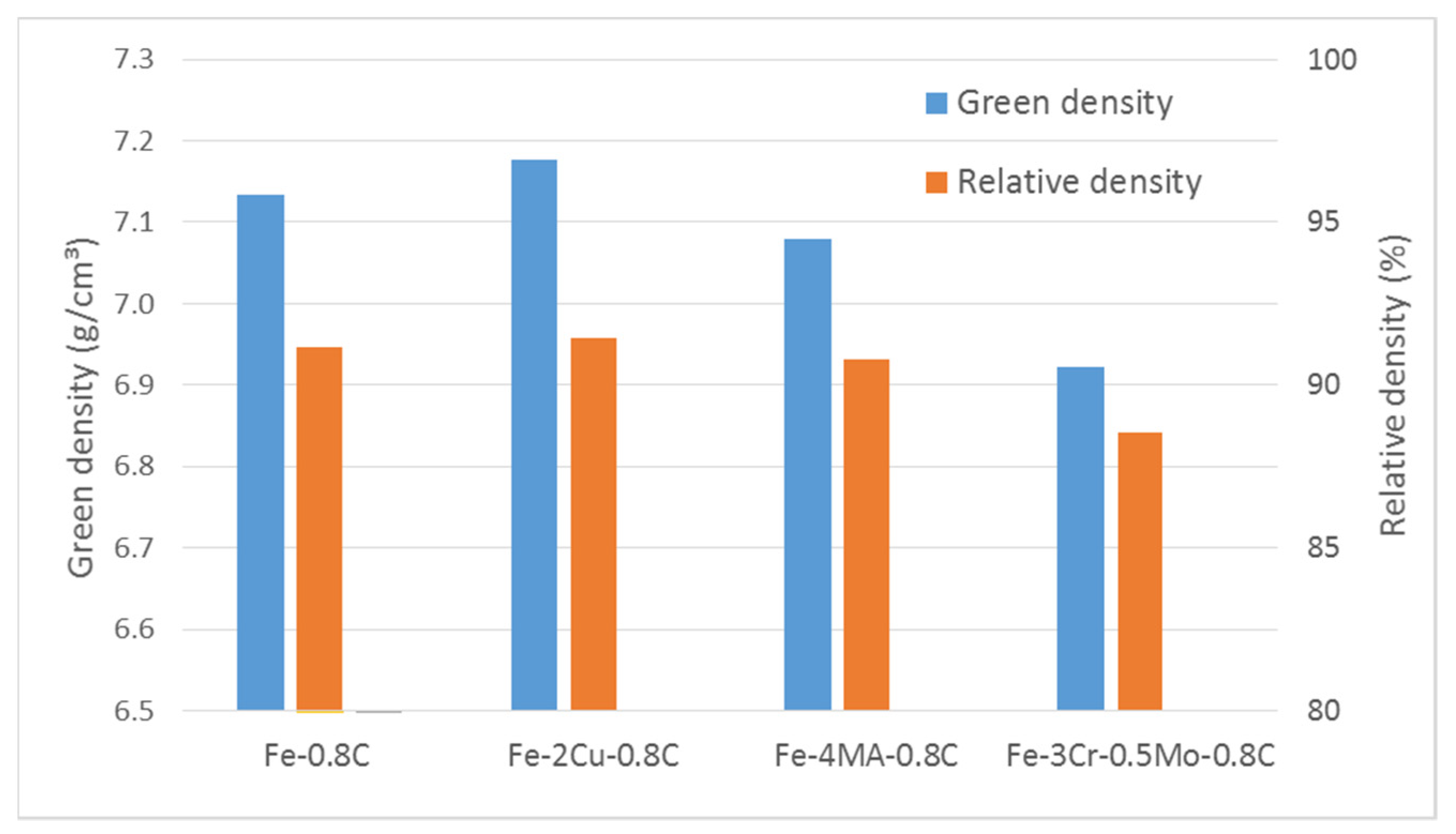
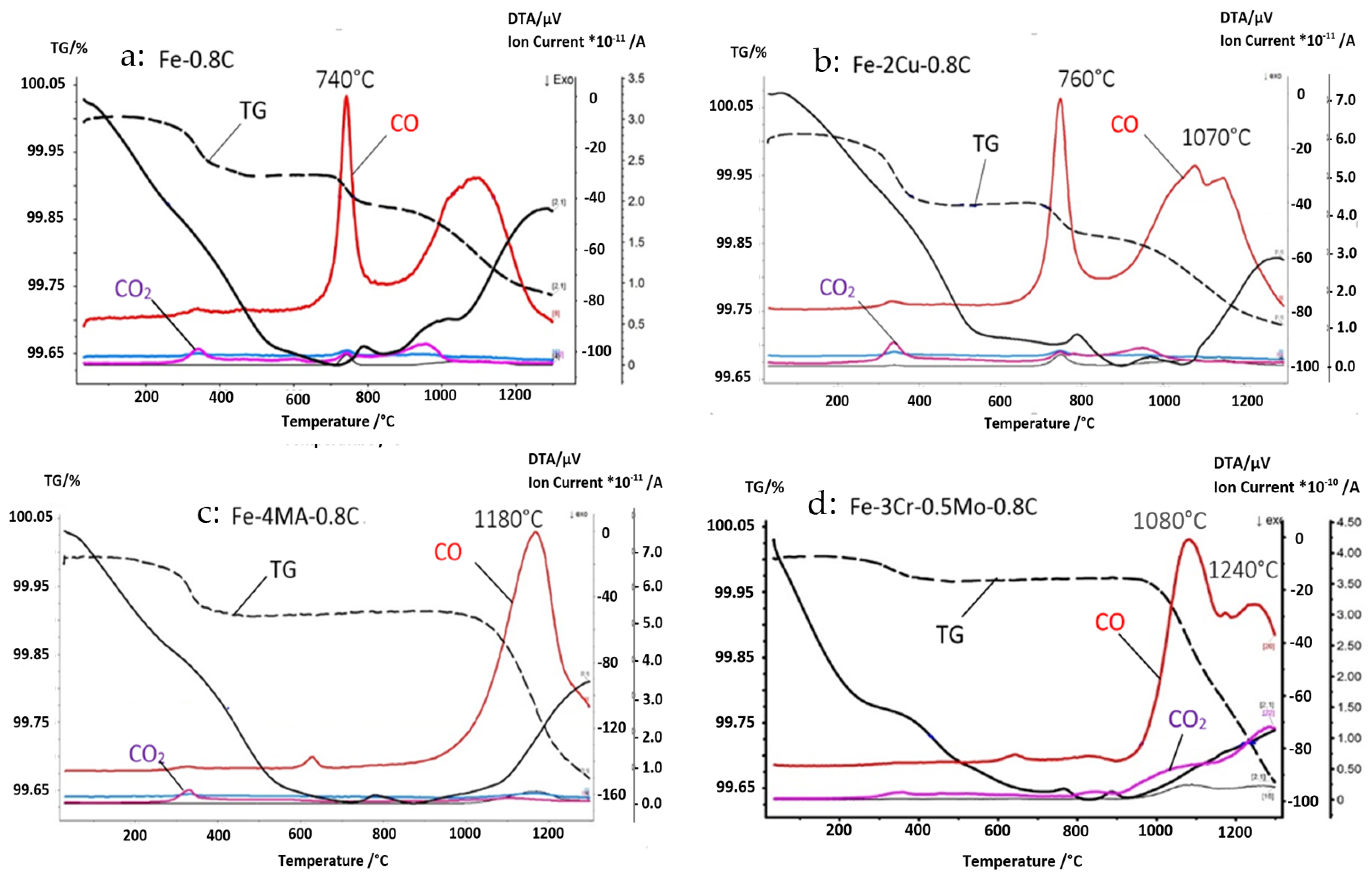
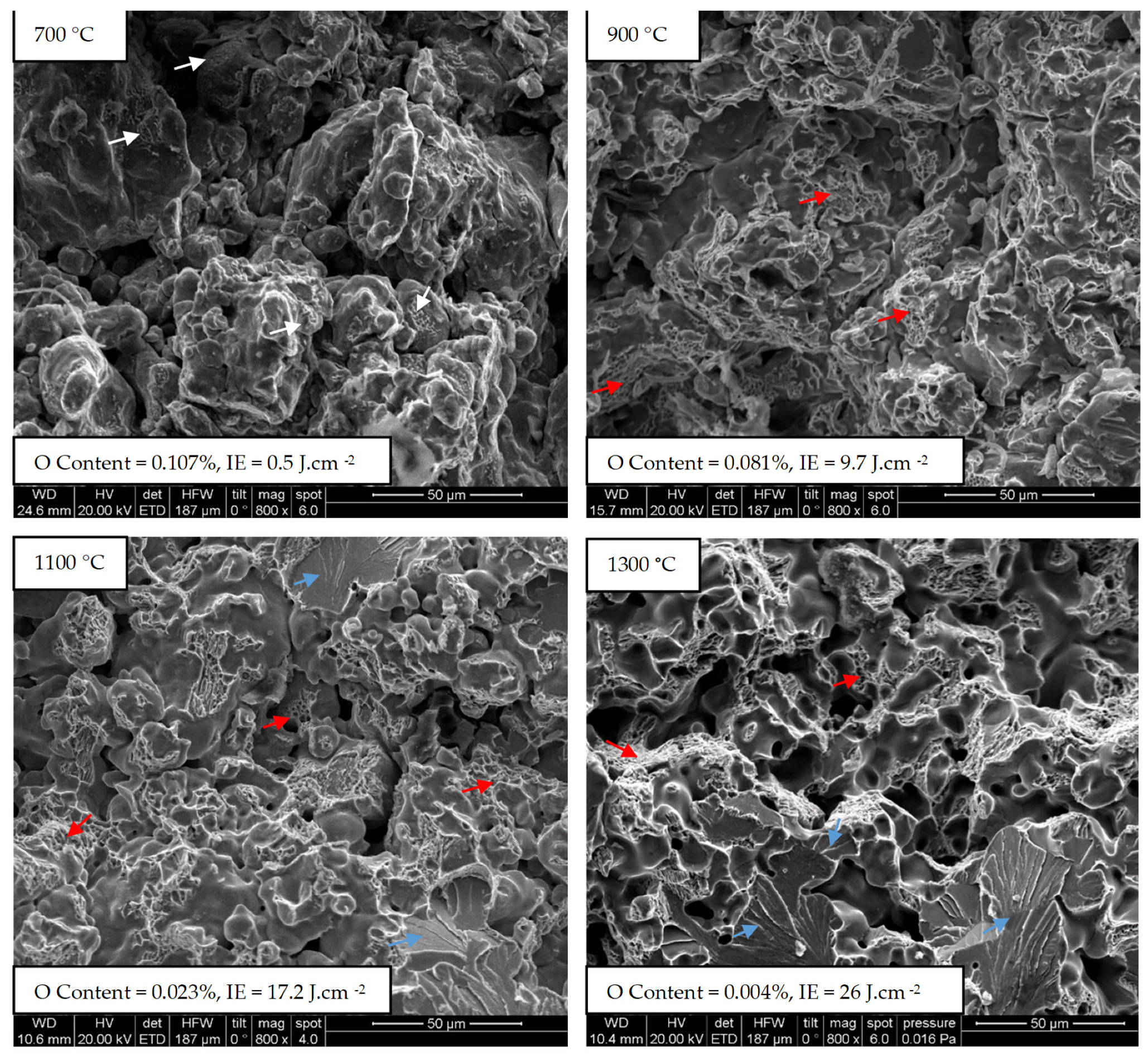




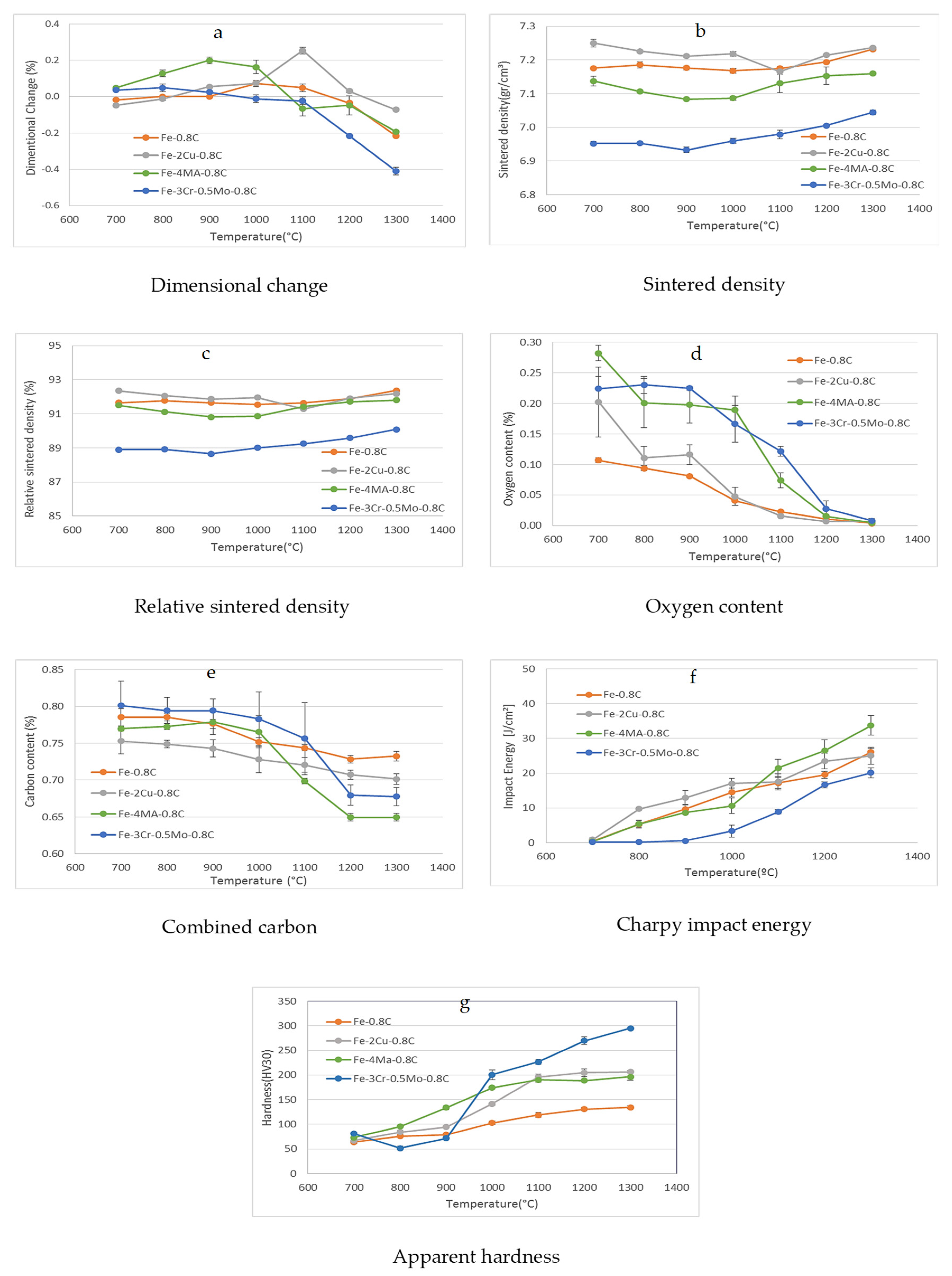
| Fe | Cr | Mo | C | O-Total | |
|---|---|---|---|---|---|
| ASC100.29 | balance | <0.01 | 0.08 | ||
| Astaloy CrM | balance | 3.00 | 0.50 | <0.01 | 0.15 |
| Name | Fe | Mn | Si | Cr | Cu | Ni | C | O |
|---|---|---|---|---|---|---|---|---|
| H46 | balance | 39.9 | 7.6 | 0.7 | 0.2 | 0.1 | 0.48 | 2.18 |
| Name | d10 | d50 | d90 |
|---|---|---|---|
| H46 | 2.33 | 6.7 | 16.1 |
| Designation | Mix Composition | |
|---|---|---|
| 1 | Fe-0.8C | ASC100.29 + 0.8%C (0%) |
| 2 | Fe-2Cu-0.8C | ASC100.29 + 2% Cu + 0.8% C (2%) |
| 3 | Fe-4MA-0.8C | ASC100.29 + 4% MA+ 0.8% C (1.94%) |
| 4 | Fe-3Cr-0.5Mo-0.8C | Astaloy CrM + 0.8%C (3.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojati, M.; Danninger, H.; Gierl-Mayer, C. Mechanical and Physical Properties of Differently Alloyed Sintered Steels as a Function of the Sintering Temperature. Metals 2022, 12, 13. https://doi.org/10.3390/met12010013
Hojati M, Danninger H, Gierl-Mayer C. Mechanical and Physical Properties of Differently Alloyed Sintered Steels as a Function of the Sintering Temperature. Metals. 2022; 12(1):13. https://doi.org/10.3390/met12010013
Chicago/Turabian StyleHojati, Milad, Herbert Danninger, and Christian Gierl-Mayer. 2022. "Mechanical and Physical Properties of Differently Alloyed Sintered Steels as a Function of the Sintering Temperature" Metals 12, no. 1: 13. https://doi.org/10.3390/met12010013
APA StyleHojati, M., Danninger, H., & Gierl-Mayer, C. (2022). Mechanical and Physical Properties of Differently Alloyed Sintered Steels as a Function of the Sintering Temperature. Metals, 12(1), 13. https://doi.org/10.3390/met12010013







