Deformation Mechanism Investigation on Low Density 18Mn Steels under Different Solid Solution Treatments
Abstract
:1. Introduction
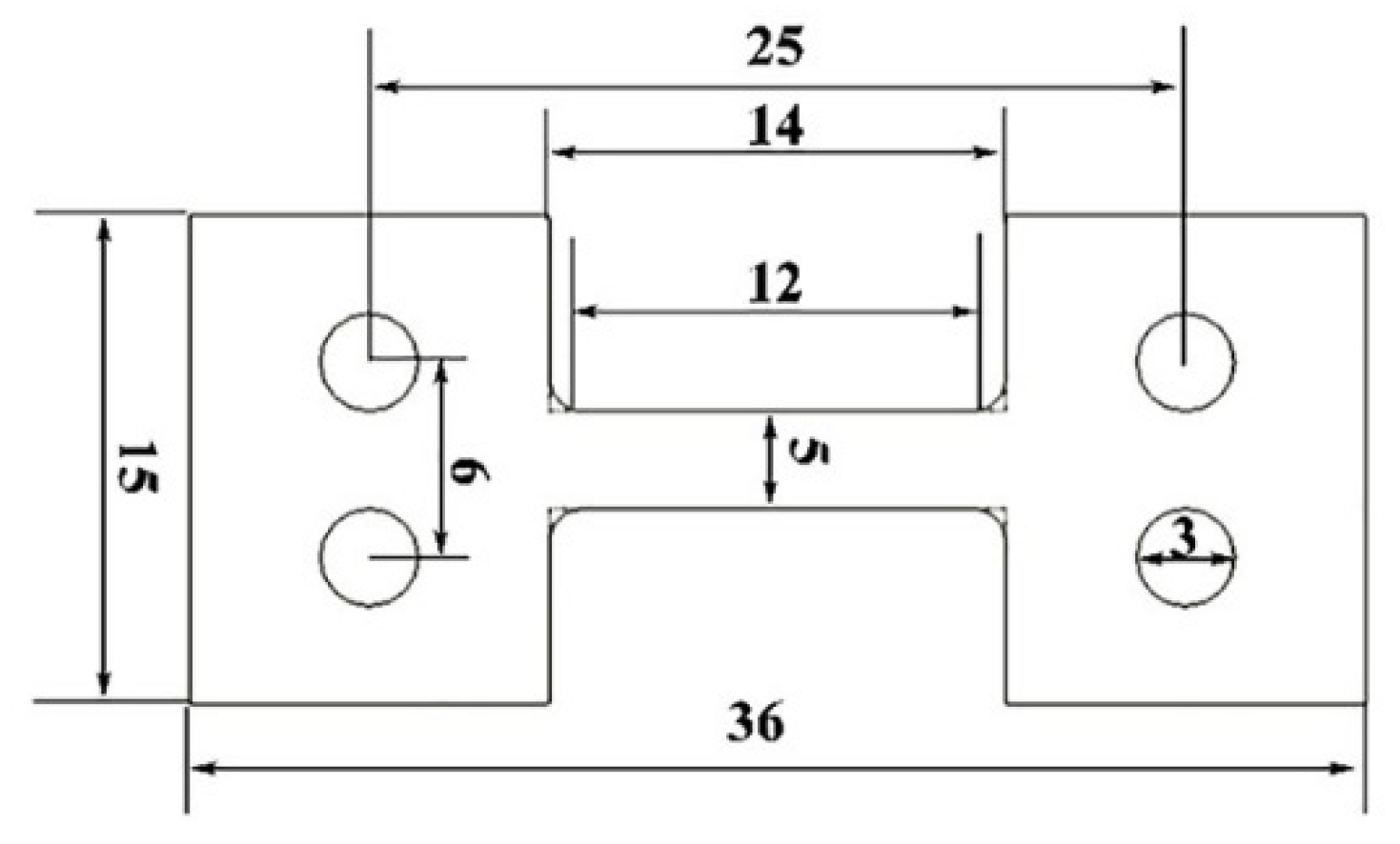
2. Experimental Materials and Methods
3. Experimental Results
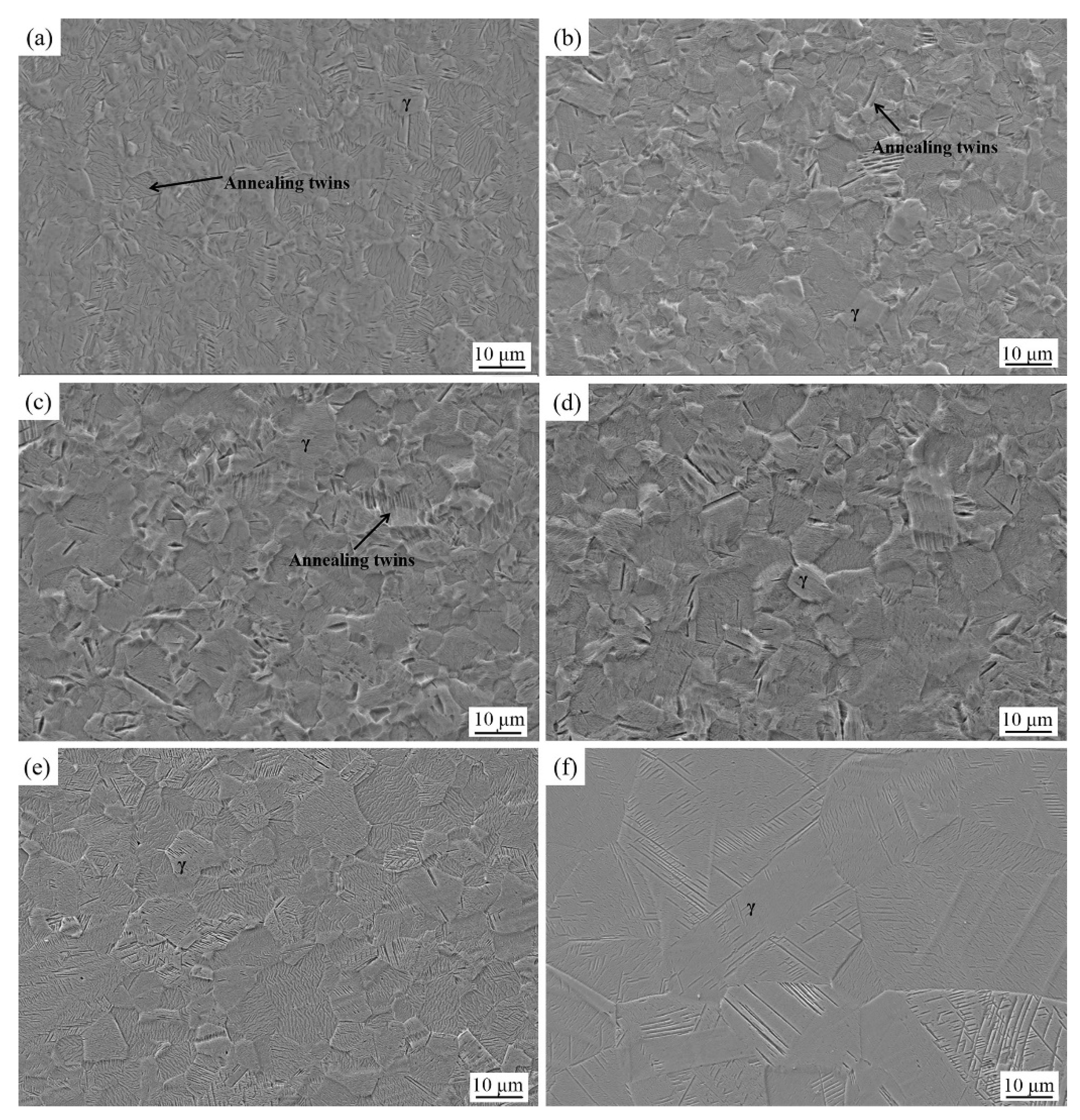
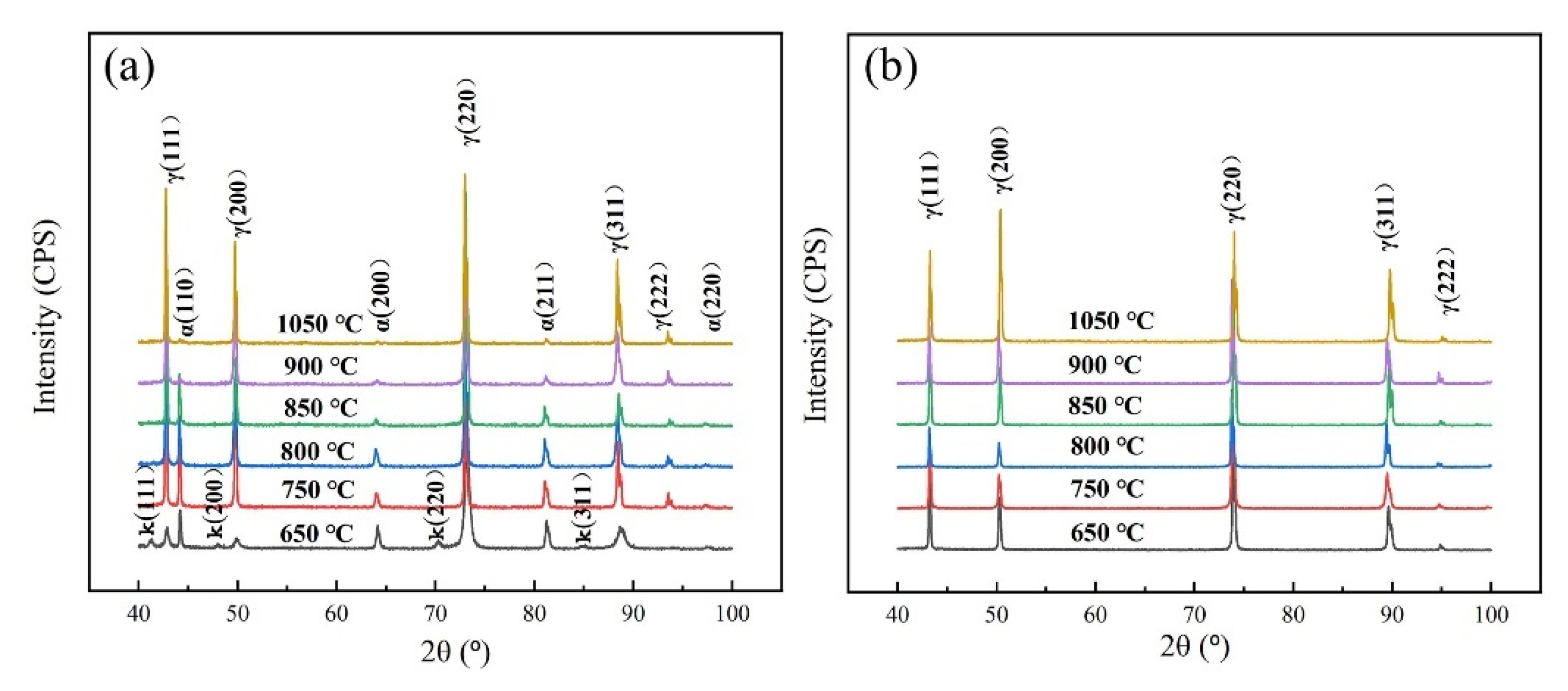
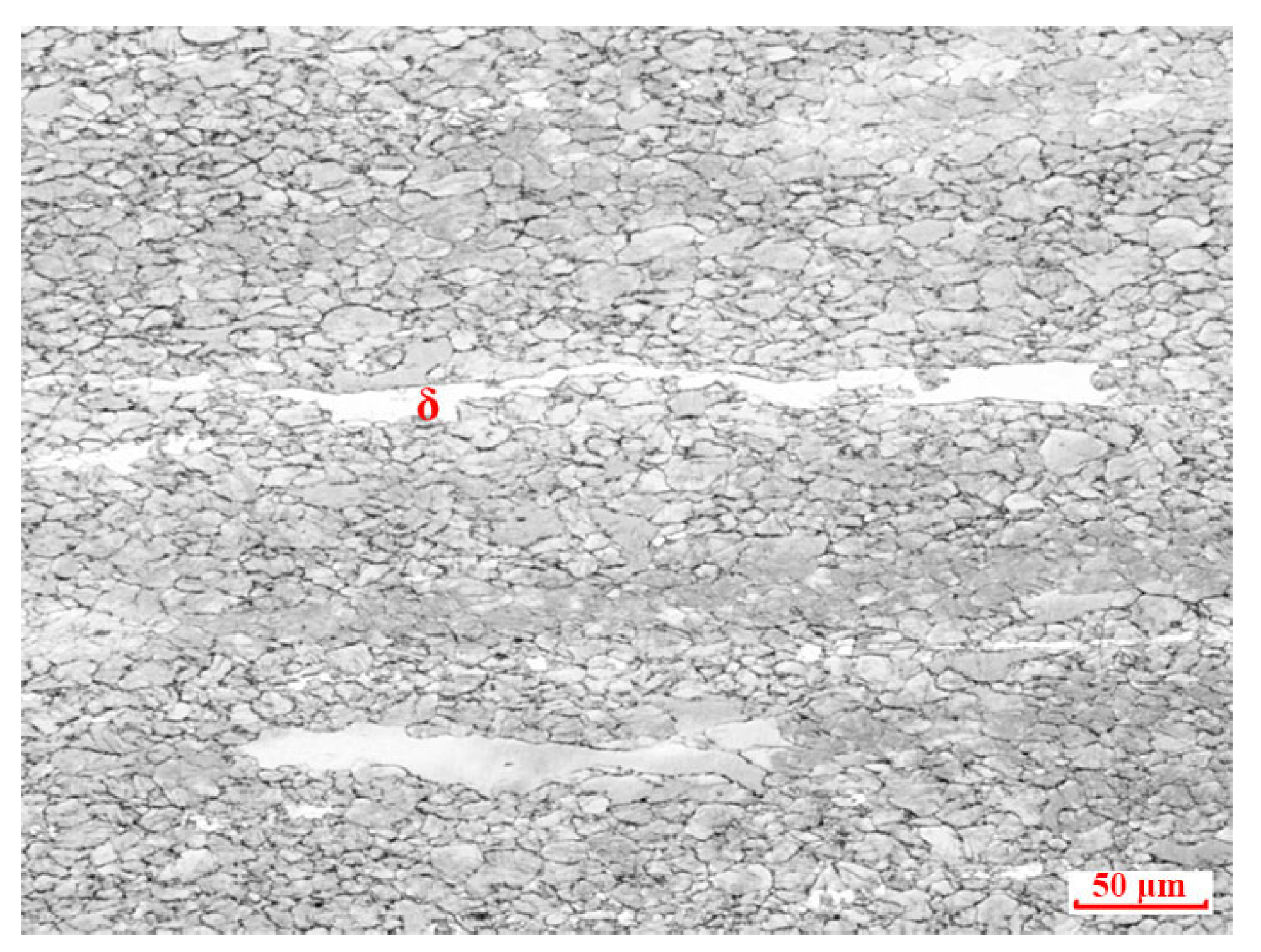
3.1. Microstructure of Experimental Steels after Solid Solution Treatment under Different Temperatures
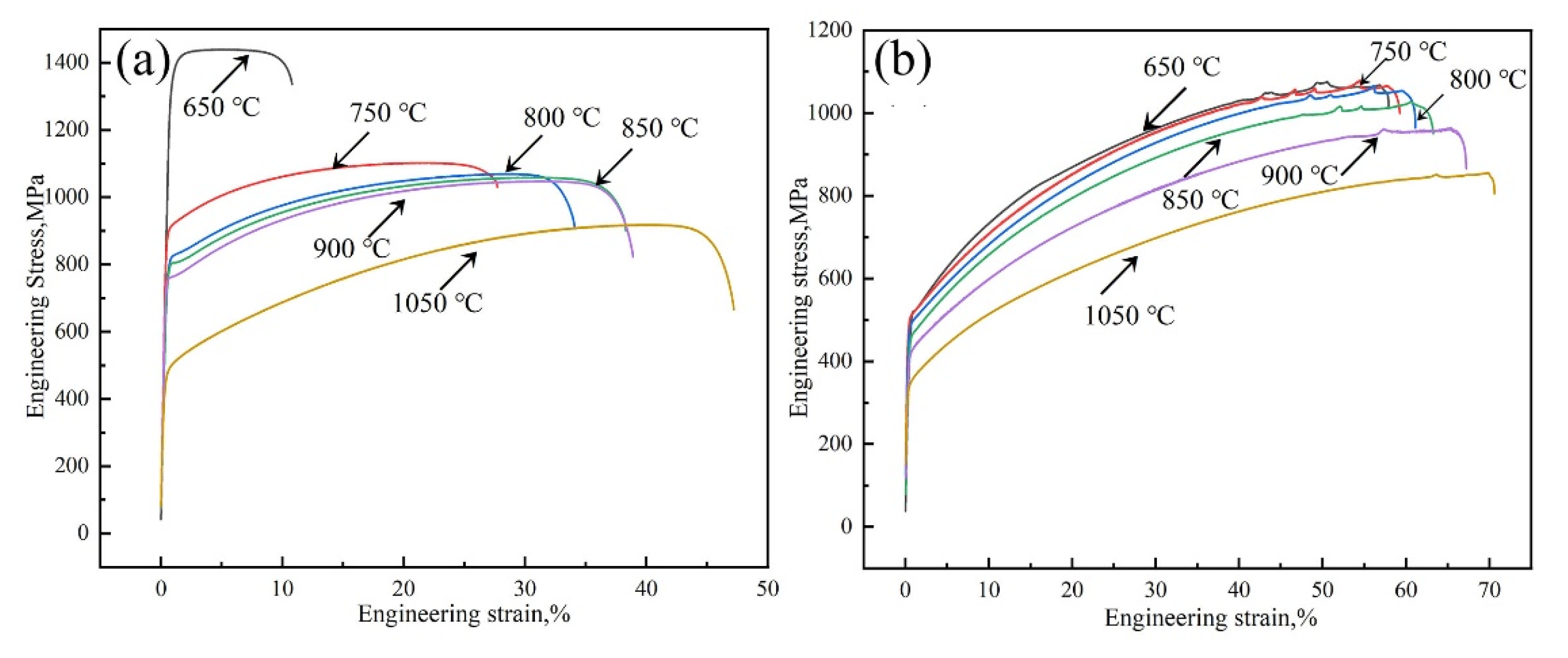
3.2. Tensile Properties at Room Temperature of Experimental Steels under Different Solid Solution Treatments
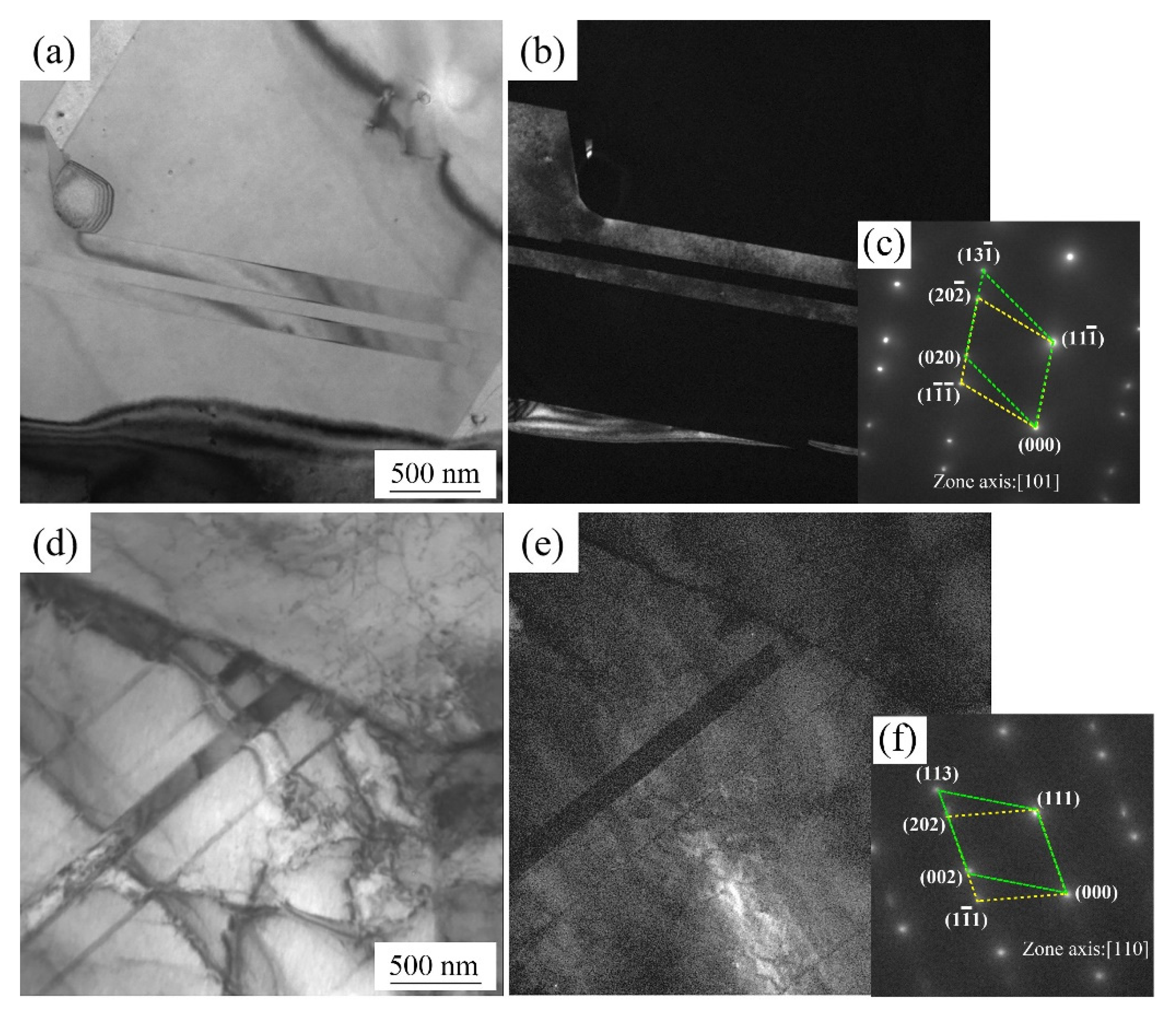
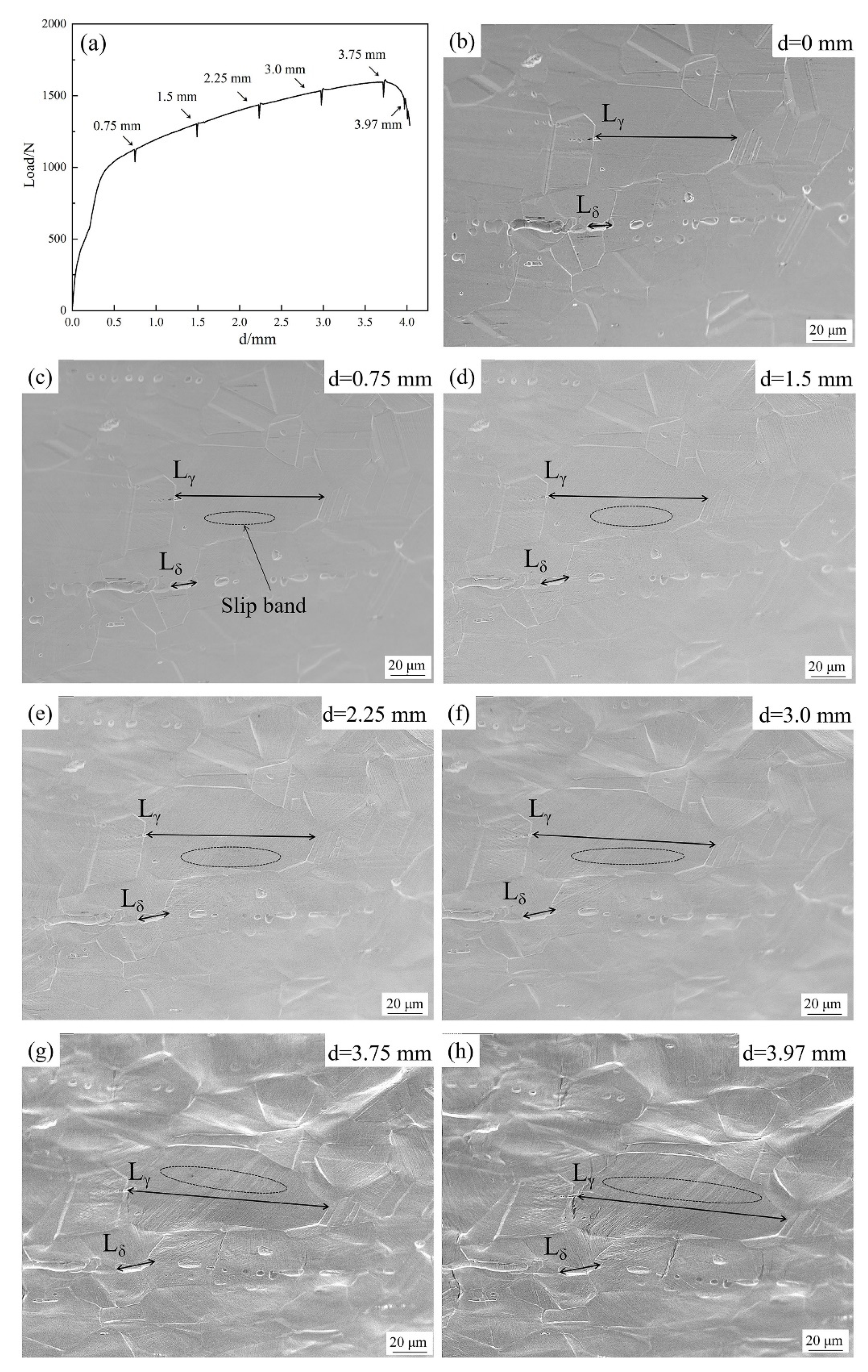
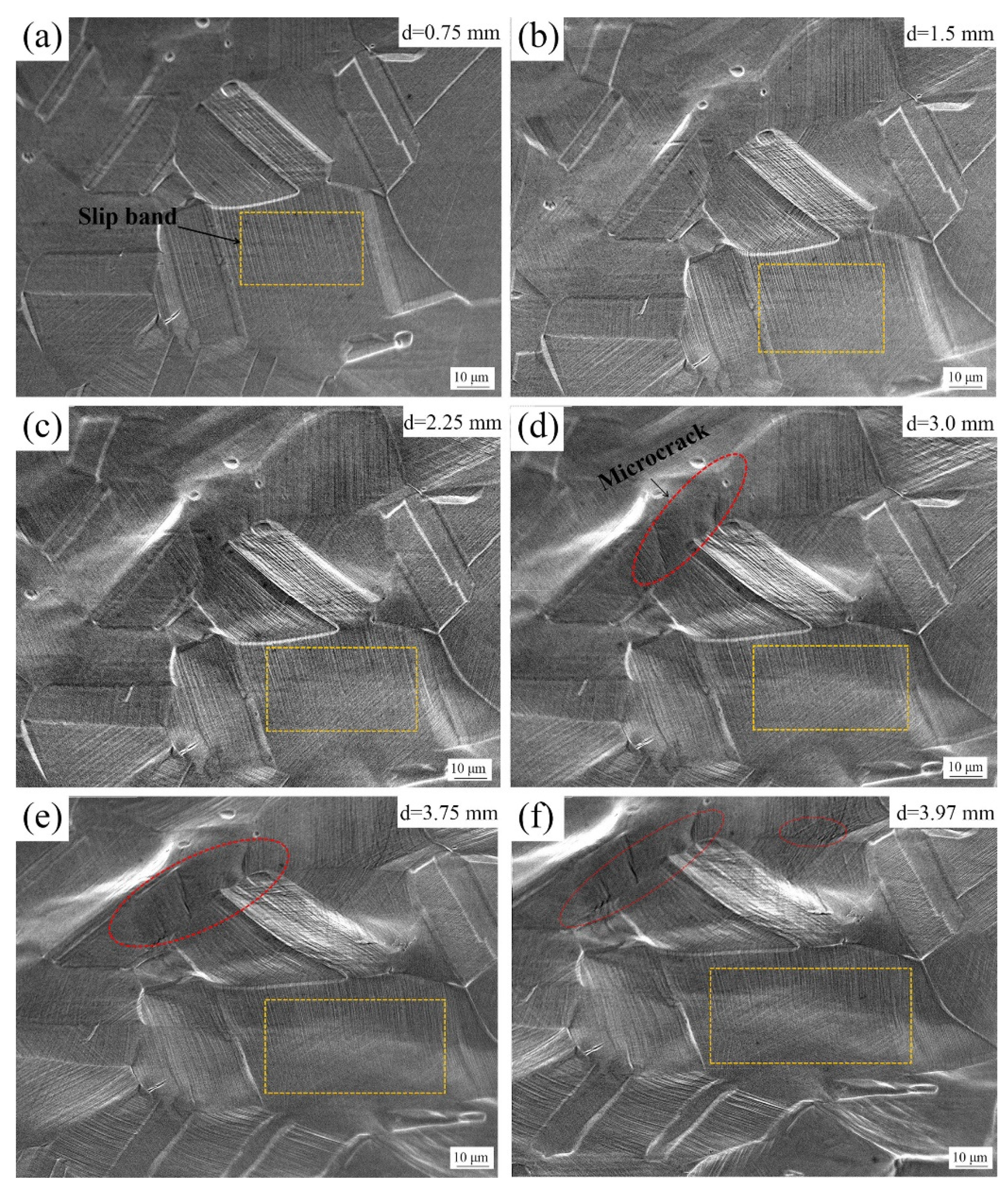
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Cooman, B.C.; Estrin, Y.; Kim, S.K. Twinning-induced plasticity (TWIP) steels. Acta Mater. 2018, 142, 283–362. [Google Scholar] [CrossRef]
- Behm, V.; Höfemann, M.; Hatscher, A.; Springer, A.; Kaierle, S.; Hein, D.; Otto, M.; Overmeyer, L. Investigations on Laser Beam Welding Dissimilar Material Combinations of Austenitic High Manganese (FeMn) and Ferrite Steels. Phys. Procedia 2014, 56, 610–619. [Google Scholar]
- Kim, H.; Suh, D.-W.; Kim, N.J. Fe-Al-Mn-C lightweight structural alloys: A review on the microstructures and mechanical properties. Sci. Technol. Adv. Mater. 2013, 14, 014205. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gao, J.X.; Li, H.R.; Li, C.Q.; Zhang, H.C.; Li, Y.G. Microband-Induced Plasticity in a Nb Content Fe-28Mn-10Al-C Low Density Steel. Metals 2021, 11, 345. [Google Scholar] [CrossRef]
- Vidilli, A.L.; Otani, L.B.; Wolf, W.; Kiminami, C.S.; Botta, W.J.; Coury, F.G.; Bolfarini, C. Design of a FeMnAlC steel with TWIP effect and evaluation of its tensile and fatigue properties. J. Alloys Compd. 2020, 831, 154806. [Google Scholar] [CrossRef]
- Zambrano, O.A. A General Perspective of Fe-Mn-Al-C Steels. J. Mater. Sci. 2017, 53, 14003–14062. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-T.; Jin, K.G.; Han, S.H.; Hwang, S.W.; Choi, K.; Lee, C.S. Stacking fault energy and plastic deformation of fully austenitic high manganese steels: Effect of Al addition. Mater. Sci. Eng. A 2010, 527, 3651–3661. [Google Scholar] [CrossRef]
- Hwang, S.W.; Ji, J.H.; Lee, E.G.; Park, K.T. Tensile deformation of a duplex Fe–20Mn–9Al–0.6C steel having the reduced specific weight. Mater. Sci. Eng. A 2011, 528, 5196–5203. [Google Scholar]
- Yoo, J.D.; Park, K.T. Microband-induced plasticity in a high Mn-Al-C light steel. Mater. Sci. Eng. A 2008, 496, 417–424. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brüx, U. Microstructures and mechanical properties of high-strength Fe–Mn–Al–C light-weight TRIPLEX steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Kang, S.; Jung, Y.S.; Jun, J.H.; Lee, Y.K. Effects of recrystallization annealing temperature on carbide precipitation, microstructure, and mechanical properties in Fe–18Mn–06C–1.5Al TWIP steel. Mater. Sci. Eng. A 2010, 527, 745–751. [Google Scholar] [CrossRef]
- Chung, K.; Ahn, K.; Yoo, D.H.; Chung, K.H.; Seo, M.H.; Park, S.H. Formability of TWIP (twinning induced plasticity) automotive sheets. Int. J. Plast. 2011, 27, 52–81. [Google Scholar] [CrossRef]
- Busch, C.; Hatscher, A.; Otto, M.; Huinink, S.; Vucetic, M.; Bonk, C.; Bouguecha, A.; Behrens, B.A. Properties and application of high-manganese TWIP-steels in sheet metal forming. Procedia Eng. 2014, 81, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Thermo-Calc Software 2020b; TCFE9 Steels/Fe-alloys database; Thermo-Calc Software AB: Solna, Sweden, 2020.
- Curtze, S.; Kuokkala, V.T.; Oikari, A.; Talonen, J.; Hänninen, H. Thermodynamic modeling of the stacking fault energy of austenitic steels. Acta Mater. 2011, 59, 1068–1076. [Google Scholar] [CrossRef]
- Curtze, S.; Kuokkala, V.T. Dependence of tensile deformation behavior of TWIP steels on stacking fault energy, temperature and strain rate. Acta Mater. 2010, 58, 5129–5141. [Google Scholar] [CrossRef]
- Hillert, M.; Jarl, M. A model for alloying in ferromagnetic metal. Calphad 1978, 2, 227–238. [Google Scholar] [CrossRef]
- Pierce, D.T.; Jiménez, J.A.; Bentley, J.; Raabe, D.; Oskay, C.; Wittig, J.E. The influence of manganese content on the stacking fault and austenite/ε-martensite interfacial energies in Fe–Mn–(Al–Si) steels investigated by experiment and theory. Acta Mater. 2014, 68, 238–253. [Google Scholar] [CrossRef]
- Dumay, A.; Chateau, J.P.; Allain, S.; Migot, S.; Bouaziz, O. Influence of addition elements on the stacking-fault energy and mechanical properties of an austenitic Fe–Mn–C steel. Mater. Sci. Eng. A 2008, 483–484, 184–187. [Google Scholar] [CrossRef]
- Zambrano, O.A. Stacking Fault Energy Maps of Fe–Mn–Al–C–Si Steels: Effect of Temperature, Grain Size, and Variations in Compositions. J. Eng. Mater. Technol. 2016, 138, 041010.1–0410109. [Google Scholar] [CrossRef]
- Holloman, J.H. Tensile deformation. Trans. Metall. Soc. AIME 1945, 162, 268–290. [Google Scholar]
- Lan, P.; Zhang, J. Twinning and Dynamic Strain Aging Behavior during Tensile Deformation of Fe–Mn–C TWIP Steel. Mater. Sci. Eng. A 2017, 700, 250–258. [Google Scholar] [CrossRef]
- Kim, J.G.; Hong, S.; Anjabin, N.; Park, B.H.; Kim, S.K.; Chin, K.G.; Lee, S.; Kim, H.S. Dynamic strain aging of twinning-induced plasticity (TWIP) steel in tensile testing and deep drawing. Mater. Sci. Eng. A 2015, 633, 136–143. [Google Scholar]
- Madivala, M.; Schwedt, A.; Prahl, U.; Bleck, W. Strain Hardening, Damage and Fracture Behavior of Al-Added High Mn TWIP Steels. Metals 2019, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Meyers, M.A.; Vöhringer, O.; Lubarda, V.A. The onset of twinning in metals: A constitutive description. Acta Mater. 2001, 49, 4025–4039. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhou, K.K.; Lu, J.; Xu, X.J.; Jiang, Y.F. Influence of Stacking Fault Energy on Grain-refining during Severe Shear Deforming (in Chinese). Chin. J. Mech. Eng. 2008, 44, 126–131. [Google Scholar] [CrossRef]






| Steel | Mn | Si | Al | C | Fe |
|---|---|---|---|---|---|
| 1# | 17.48 | 0.40 | 7.36 | 0.75 | Bal. |
| 2# | 18.40 | 0.46 | 1.50 | 0.70 | Bal. |
| Temperature/℃ | Sample | YS/MPa | TS/MPa | TE/% | TS × TE/GPa·% |
|---|---|---|---|---|---|
| 650 | 1# | 1319 ± 10 | 1439 ± 16 | 10.8 ± 0.7 | 15.5 |
| 2# | 519 ± 8 | 1107 ± 10 | 57.9 ± 1.5 | 64.1 | |
| 750 | 1# | 901 ± 11 | 1106 ± 10 | 27.7 ± 0.5 | 30.6 |
| 2# | 506 ± 10 | 1079 ± 9 | 59.2 ± 0.6 | 63.9 | |
| 800 | 1# | 813 ± 11 | 1069 ± 19 | 34.1 ± 0.7 | 36.5 |
| 2# | 489 ± 14 | 1051 ± 19 | 61.1 ± 0.9 | 64.2 | |
| 850 | 1# | 805 ± 14 | 1061 ± 16 | 38.3 ± 0.8 | 40.6 |
| 2# | 473 ± 10 | 1049 ± 13 | 63.1 ± 1.4 | 66.2 | |
| 900 | 1# | 757 ± 11 | 1047 ± 19 | 38.9 ± 0.7 | 40.7 |
| 2# | 413 ± 14 | 964 ± 14 | 67.2 ± 1.2 | 64.8 | |
| 1050 | 1# | 494 ± 9 | 919 ± 14 | 47.2 ± 0.9 | 43.4 |
| 2# | 363 ± 8 | 855 ± 10 | 70.6 ± 1.4 | 60.4 |
| Parameters | Calculation Formula and Value |
|---|---|
| Steel | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 650 | 0.170 | 0.022 | 0.128 | 0.008 | 0.672 | 0.415 | 0.017 | 153.0 | 98.61 | 1.948 | −42.22 | 770 |
| 750 | 0.168 | 0.033 | 0.130 | 0.008 | 0.661 | 0.364 | −0.028 | 152.8 | 97.44 | 1.951 | −43.25 | 850 | |
| 850 | 0.165 | 0.033 | 0.132 | 0.007 | 0.663 | 0.364 | −0.030 | 152.7 | 95.72 | 1.951 | −43.39 | 864 | |
| 1050 | 0.163 | 0.033 | 0.134 | 0.007 | 0.663 | 0.364 | −0.031 | 152.7 | 94.54 | 1.951 | −43.55 | 877 | |
| 2# | - | 0.179 | 0.029 | 0.030 | 0.008 | 0.753 | 0.434 | −0.005 | 153.6 | 103.8 | 1.939 | −39.91 | 99.6 |
| Steel | 650 °C | 750 °C | 850 °C | 1050 °C |
|---|---|---|---|---|
| 1# | 60.56 | 64.18 | 64.94 | 65.61 |
| 2# | 24.70 | 24.70 | 24.70 | 24.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Y.-T.; He, Y.-L.; Zhu, N.-Q.; Ding, M.-L.; Liu, R.-D.; Zhang, Y. Deformation Mechanism Investigation on Low Density 18Mn Steels under Different Solid Solution Treatments. Metals 2021, 11, 1497. https://doi.org/10.3390/met11091497
Huo Y-T, He Y-L, Zhu N-Q, Ding M-L, Liu R-D, Zhang Y. Deformation Mechanism Investigation on Low Density 18Mn Steels under Different Solid Solution Treatments. Metals. 2021; 11(9):1497. https://doi.org/10.3390/met11091497
Chicago/Turabian StyleHuo, Yong-Tao, Yan-Lin He, Na-Qiong Zhu, Min-Long Ding, Ren-Dong Liu, and Yu Zhang. 2021. "Deformation Mechanism Investigation on Low Density 18Mn Steels under Different Solid Solution Treatments" Metals 11, no. 9: 1497. https://doi.org/10.3390/met11091497
APA StyleHuo, Y.-T., He, Y.-L., Zhu, N.-Q., Ding, M.-L., Liu, R.-D., & Zhang, Y. (2021). Deformation Mechanism Investigation on Low Density 18Mn Steels under Different Solid Solution Treatments. Metals, 11(9), 1497. https://doi.org/10.3390/met11091497







