The Influence of Varying Aluminium and Manganese Content on the Corrosion Resistance and Mechanical Properties of High Strength Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
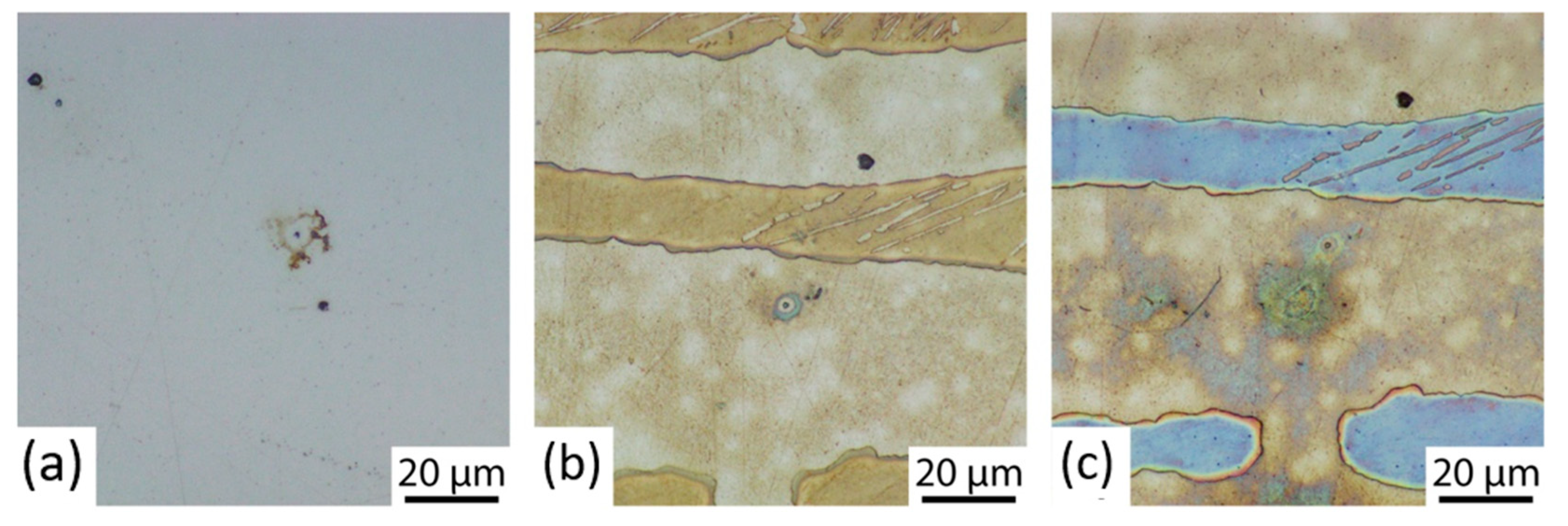
3.1. Microstructure
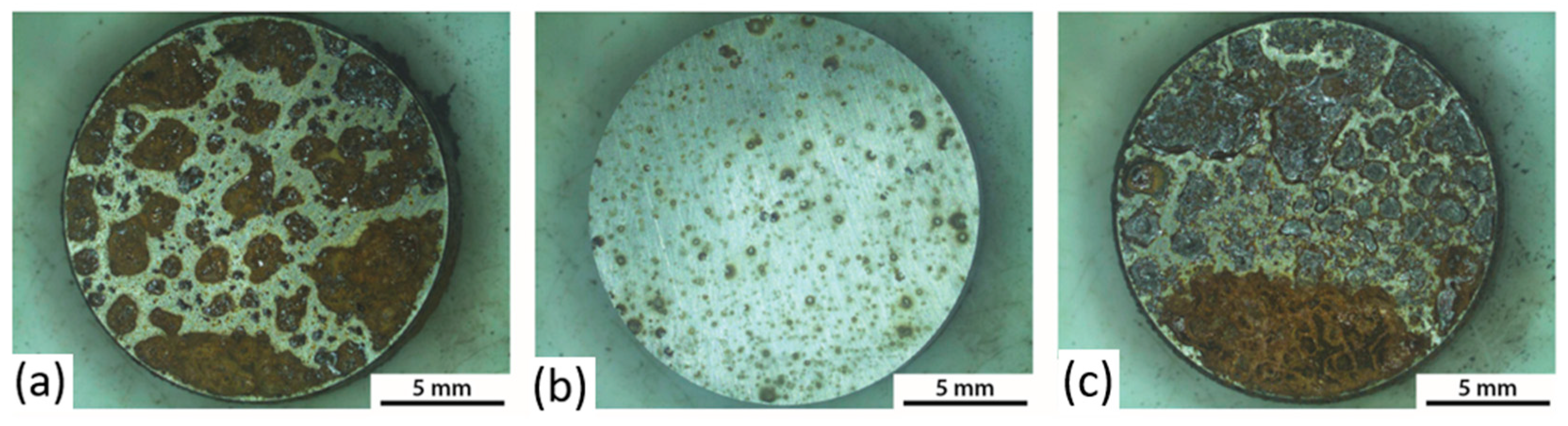
3.2. Corrosion Tests
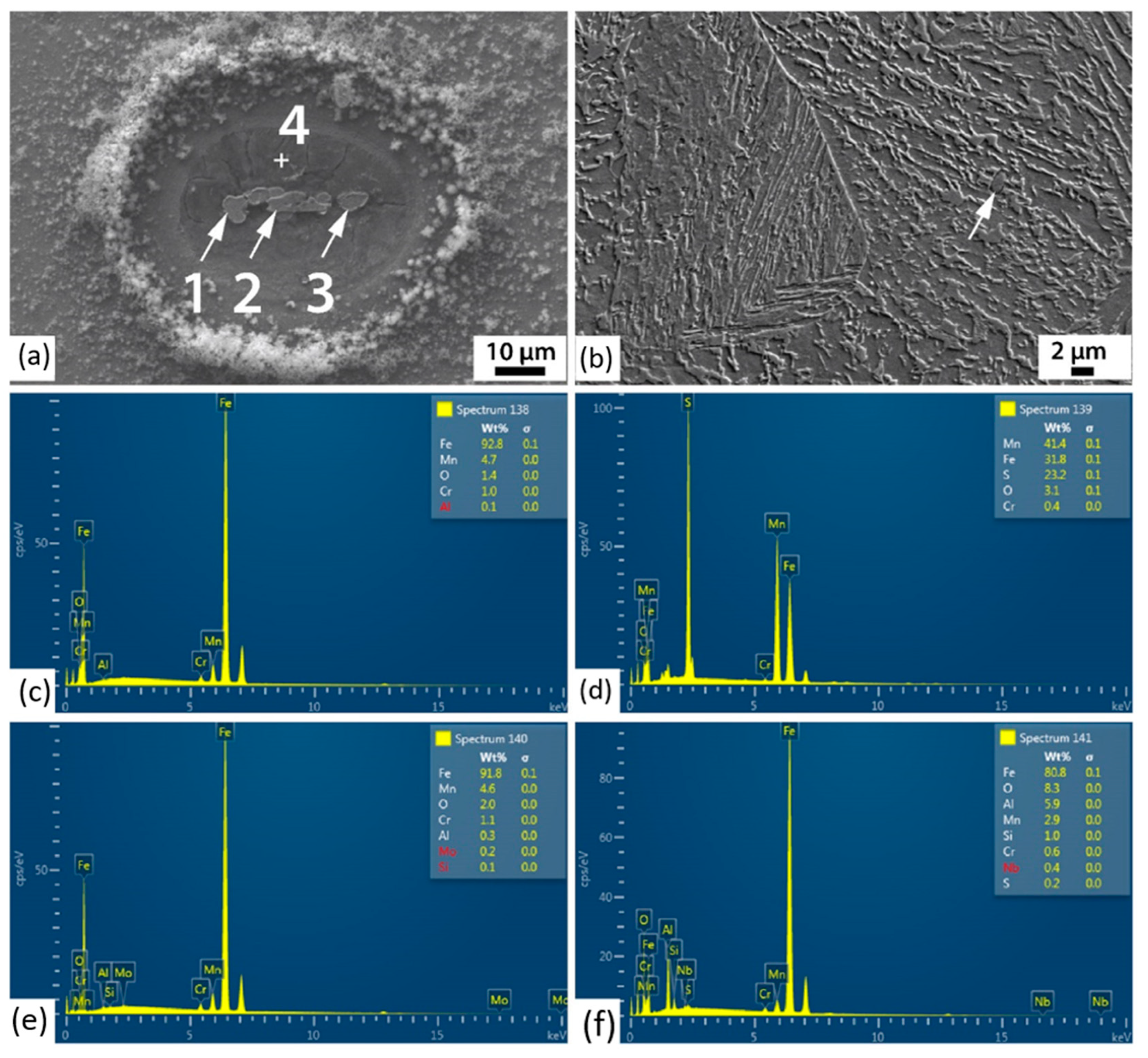
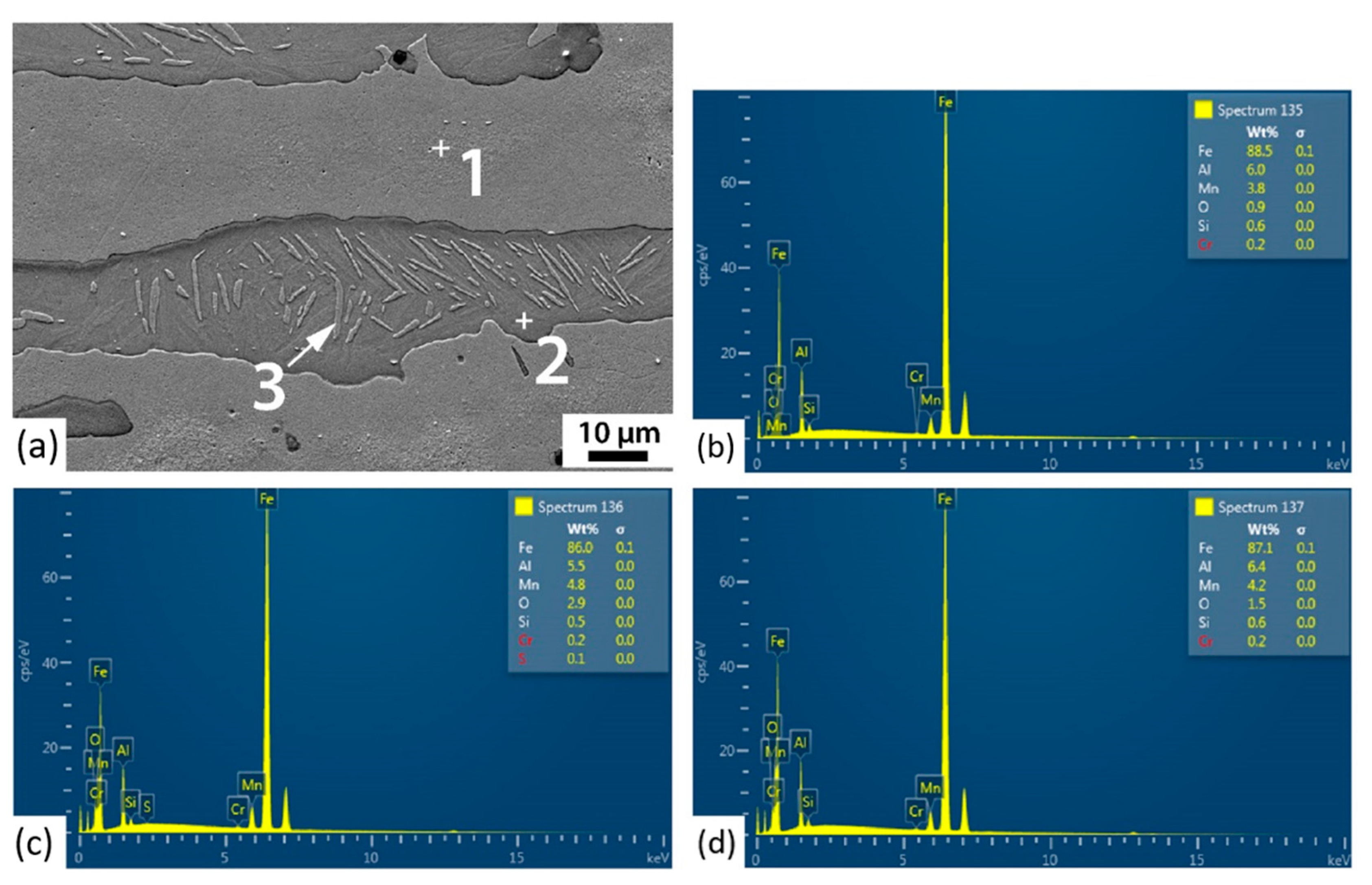
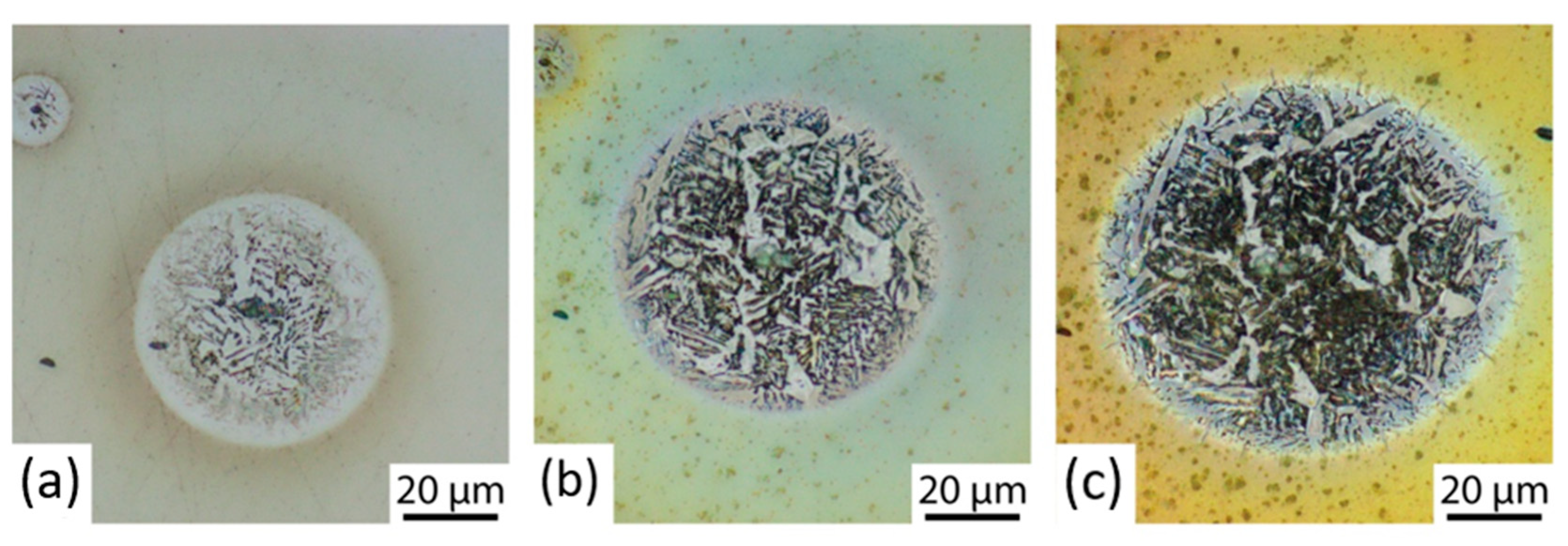
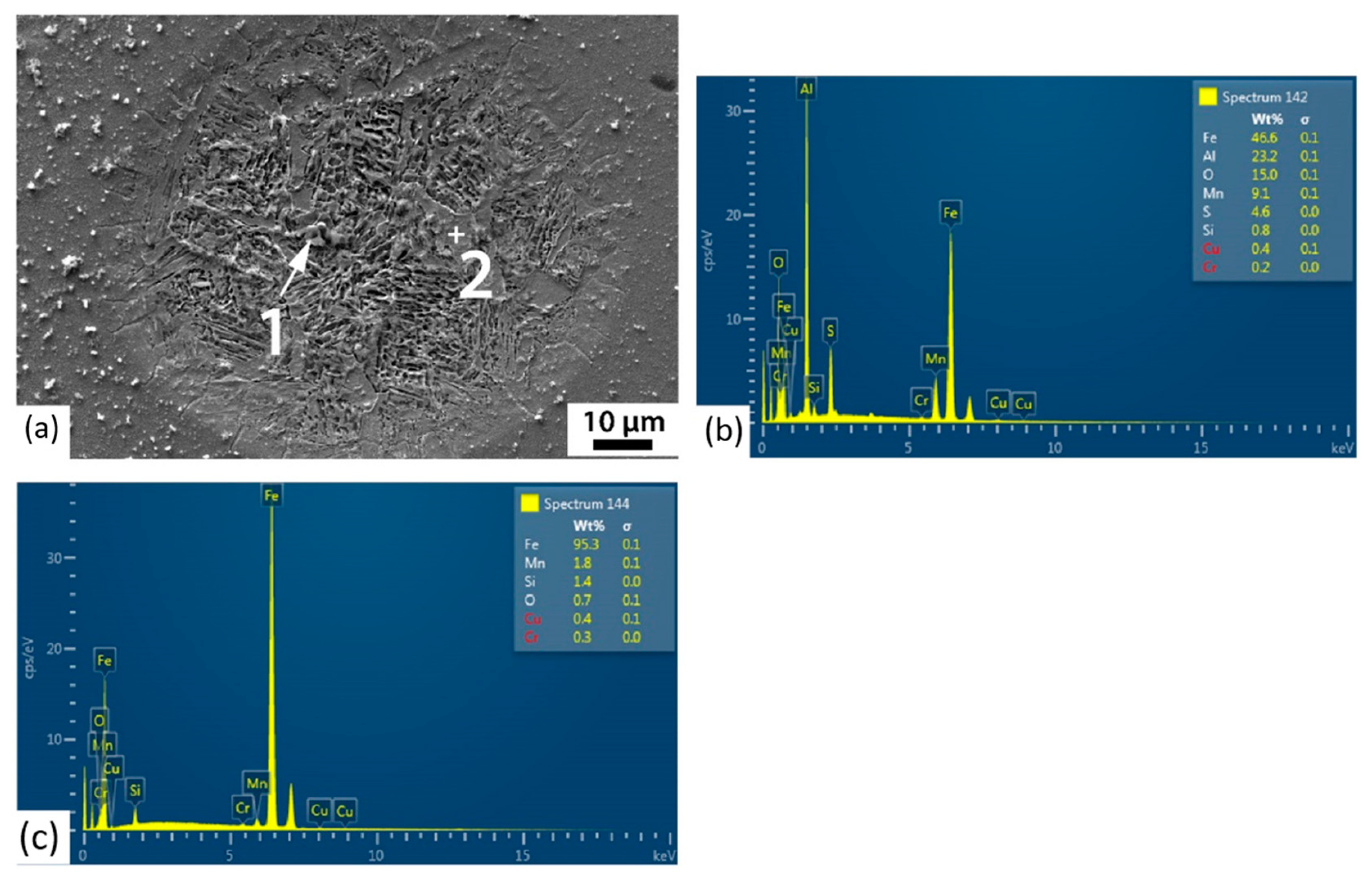
3.3. Corrosion Initiation Analysis
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keeler, S.; Kimchi, M.; Mooney, P.J. Advanced High-Strength Steels Application Guidelines. World Auto Steel. 2017, 6. Available online: http://www.worldautosteel.org/download_files/AHSS%20Guidelines%20V6/00_AHSSGuidelines_V6_20170430.pdf (accessed on 23 November 2020).
- Billur, E.; Taylan, A. Three generations of advanceed high strength steel for automotive applications, Part 1. Stamp. J. 2013, 16–17. Available online: https://ercnsm.osu.edu/sites/ercnsm.osu.edu/files/uploads/S_FormingAHSS/664-1.pdf (accessed on 23 November 2020).
- Kučerová, L.; Jirková, H.; Volkmannová, J.; Vrtáček, J. Effect of Aluminum nad Manganese Contents on the Microstructure Development of Forged and Annealed TRIP Steel. Manuf. Technol. 2018, 12, 605–610. [Google Scholar]
- Schmitt, J.-H.; Iung, T. New developments of advanced high-strength steels for automotive applications. Comptes Rendus Phys. 2018, 19, 641–656. [Google Scholar] [CrossRef]
- Billur, E.; Taylan, A. Three generations of advanced high strength steels, part 3. Stamp. J. 2014, 12–13. Available online: https://ercnsm.osu.edu/sites/ercnsm.osu.edu/files/uploads/S_FormingAHSS/664-3.pdf (accessed on 24 November 2020).
- Zhao, P. The potential significance of microalloying with niobium in governing very high cycle fatigue behavior of bainite/martensite multiphase steels. Mater. Sci. Eng. A 2016, 650, 438–444. [Google Scholar] [CrossRef]
- Yan, H.; Bi, H.; Li, X.; Xu, Z. Precipitation and mechanical properties of Nb-modified ferritic stainless steel during isothermal aging. Mater. Charact. 2009, 60, 204–209. [Google Scholar] [CrossRef]
- Kucerova, L.; Jirkova, H.; Mašek, B. Continuous Cooling of CMnSi TRIP Steel. Mater. Today Proc. 2015, 2, S677–S680. [Google Scholar] [CrossRef]
- Zambrano, O.A. A general perspective of Fe–Mn–Al–C steels. J. Mater. Sci. 2018, 53, 14003–14062. [Google Scholar] [CrossRef]
- Zhu, X.M.; Zhang, Y.S. Investigation of the Electrochemical Corrosion Behavior and Passive Film for Fe-Mn, Fe-Mn-Al, and Fe-Mn-Al-Cr Alloys in Aqueous Solutions. Corrosion 1998, 54, 3–12. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S.; Khodam, S. Comparative studies on the corrosion properties of a Fe–Mn–Al–Si steel and an interstitial-free steel. Corros. Sci. 2008, 50, 2879–2884. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X. Electrochemical polarization and passive film analysis of austenitic Fe–Mn–Al steels in aqueous solutions. Corros. Sci. 1999, 41, 1817–1833. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Li, Y. Observation of the pitting corrosion and uniform corrosion for X80 steel in 3.5 wt.% NaCl solutions using in-situ and 3-D measuring microscope. Corros. Sci. 2016, 111, 508–517. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.H. Influence of Inclusions on Early Corrosion Development of Ultra-Low Carbon Bainitic Steel in NaCl Solution. Corrosion 2015, 71, 1467–1480. [Google Scholar] [CrossRef]
- Nam, N.D.; Kim, J.G. Effect of niobium on the corrosion behaviour of low alloy steel in sulfuric acid solution. Corros. Sci. 2010, 52, 3377–3384. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Shetata, O.S.; Tantawy, N.S. Effects of NB and Cr on the Corrosion Characterization of Al-containing Trasformation-Induced Plasticity Steels in Neutral Cloride Solutions. Corrosion 2011, 9, 67. [Google Scholar] [CrossRef]
- Qiao, Q.; Lu, L.; Fan, E.; Zhao, J.; Liu, Y.; Peng, G.; Huang, Y.; Li, X. Effects of Nb on stress corrosion cracking of high-strength low-alloy steel in simulated seawater. Int. J. Hydrog. Energy 2019, 44, 27962–27973. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, W.; Cheng, L.; Liu, J.; Wu, K.; Liu, M. Effects of niobium and rare earth elements on microstructure and initial marine corrosion behavior of low-alloy steels. Appl. Surf. Sci. 2019, 475, 83–93. [Google Scholar] [CrossRef]
- Kadowaki, M.; Muto, I.; Katayama, H.; Masuda, H.; Sugawara, Y.; Hara, N. Effectiveness of an intercritical heat-treatment on localized corrosion resistance at the microstructural boundaries of medium-carbon steels. Corros. Sci. 2019, 154, 159–177. [Google Scholar] [CrossRef]
- Kučerová, L.; Bystrianský, M.; Jeníček, Š. The Effect of Annealing Temperature on Microstructure and Mechanical Properties of Lightweight Steel with Increased Aluminium Content. Manuf. Technol. 2017, 17, 881–887. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Z. Thermomechanical processing of advanced high strength steels. Prog. Mater. Sci. 2018, 94, 174–242. [Google Scholar] [CrossRef]
- Fu, B.; Yang, W.; Li, L.; Sun, Z. Effect of bainitic transformation temperature on the mechanical behavior of cold-rolled TRIP steels studied with in-situ high-energy X-ray diffraction. Mater. Sci. Eng. A 2014, 603, 134–140. [Google Scholar] [CrossRef]
- De Cooman, B. Structure–properties relationship in TRIP steels containing carbide-free bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 285–303. [Google Scholar] [CrossRef]
- Jacques, P. Transformation-induced plasticity for high strength formable steels. Curr. Opin. Solid State Mater. Sci. 2004, 8, 259–265. [Google Scholar] [CrossRef]
- Muránsky, O.; Šittner, P.; Zrník, J.; Oliver, E. In situ neutron diffraction investigation of the collaborative deformation–transformation mechanism in TRIP-assisted steels at room and elevated temperatures. Acta Mater. 2008, 56, 3367–3379. [Google Scholar] [CrossRef]
- Grajcar, A.; Skrzypczyk, P.; Wozniak, D. Thermomechanically rolled medium-Mn steel containing retained austenite. Arch. Metall. Mater. 2014, 59, 1691–1697. [Google Scholar] [CrossRef]
- Gau, Y.J.; Wu, J.K. Galvanic corrosion behaviour of Fe-Mn-Al alloys in sea water. J. Mater. Sci. Lett. 1992, 11, 119–121. [Google Scholar] [CrossRef]
- Shih, S.T.; Peng, T.P. Corrosion Behaviour of Two-Phase Fe-Mn-Al alloys in 3,5% NaCl Solution. Corrosion 1993, 49, 130–134. [Google Scholar] [CrossRef]
- Lee, J.W.; Tsai, S.Y. Corrosion Resistance and Microstructural Evaluation of the Chromized Coating Process in a Dual Phase Fe-Mn-Al-Cr Alloy. Surf. Coat. Technol. 2002, 153, 59–66. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, C.-L.; Chao, C.-G.; Liu, T.-F. Excellent enhancement of corrosion properties of Fe–9Al–30Mn–1.8C alloy in 3.5% NaCl and 10% HCl aqueous solutions using gas nitriding treatment. J. Alloys Compd. 2015, 633, 137–144. [Google Scholar] [CrossRef]
- Yeganeh, M.; Alavi-Zaree, S.R. A Comparison Between Corrosion Behaviors of Fine-Grained and Coarse-Grained Structures of High-Mn Steel in NaCl Solution. J. Mater. Eng. Perform. 2017, 26, 2484–2490. [Google Scholar] [CrossRef]
- Jablonska, M.; Michalik, R. Studies on the Corrosion Porperties of High-Mn austenitic steels. Solid State Phenom. 2014, 227, 75–78. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S.; Khoddam, S.; Liyanaarachchi, S. Corossion Behaviour of Twinning-Induced Plasticity (TWIP) steel. Mater. Corro. 2013, 63, 231–235. [Google Scholar] [CrossRef]
- Guo, P.; La Plante, E.C.; Wang, B.; Chen, X.; Balonis, M.; Bauchy, M.; Sant, G. Direct observation of pitting corrosion evolutions on carbon steel surfaces at the nano-to-micro- scales. Sci. Rep. 2018, 8, 7990. [Google Scholar] [CrossRef] [PubMed]
- Grajcar, A.; Grzegorczyk, B.; Kozłowska, A. Corrosion Resistance and Pitting Behaviour of Low-Carbon High-Mn Steels in Chloride Solution. Arch. Met. Mater. 2016, 61, 825–832. [Google Scholar] [CrossRef][Green Version]
- Park, I.-J.; Lee, S.-M.; Kang, M.; Lee, S.; Lee, Y.-K. Pitting corrosion behavior in advanced high strength steels. J. Alloys Compd. 2015, 619, 205–210. [Google Scholar] [CrossRef]
- BBaker, M.; Castle, J. The initiation of pitting corrosion at MnS inclusions. Corros. Sci. 1993, 34, 667–682. [Google Scholar] [CrossRef]
- Shi, W.; Yang, S.; Dong, A.; Li, J. Understanding the Corrosion Mechanism of Spring Steel Induced by MnS Inclusions with Different Sizes. JOM J. Miner. Met. Mater. Soc. 2018, 70, 2513–2522. [Google Scholar] [CrossRef]
- Yanjun, G.; Jinchneg, H.; Liazhu, J.; Tianwei, L.; Yanping, W. Effect of annealing temperature on the mechanical and corrosion behaviour of newly developed novel lean duplex steel. Materials 2014, 7, 6604–6619. [Google Scholar]
- Kim, B.; Kim, S.; Kim, H. Effects of Alloying Elements (Cr, Mn) on Corrosion Properties of the High-Strength Steel in 3.5% NaCl Solution. Adv. Mater. Sci. Eng. 2018, 2018, 7638274. [Google Scholar] [CrossRef]
- Jirková, H.; Vrtáček, J.; Peković, M.; Janda, T.; Kučerová, L. Influence of Chromium and Niobium on the Press-Hardening Process of Multiphase Low-Alloy TRIP Steels. Mater. Sci. Forum 2021, 1016, 636–641. [Google Scholar] [CrossRef]
- Jirková, H.; Opatová, K.; Jeníček, Š.; Kučerová, L.; Meza-García, E. The use of advanced high-strength steels in the press-hardening technology. In Proceedings of the 7th International Conference on Hot Sheet Metal Forming of High-Performance Steel CHS2-2019, Lulea, Sweden, 2–5 June 2019; Verlag Wissenschaftliche Scripten: Auerbach, Germany, 2019; pp. 465–473. ISBN 978-3-95735-104-3. [Google Scholar]
- Opatová, K.; Jirková, H.; Meza-García, E.; Jeníček, Š.; Vrtáček, J. Mechanical behavior of new press hardening steels at elevated temperatures and technological modeling of their processing. In Proceedings of the 7th International Conference on Hot Sheet Metal Forming of High-Performance Steel CHS2-2019, Lulea, Sweden, 2–5 June 2019; Verlag Wissenschaftliche Scripten: Auerbach, Germany, 2019; pp. 719–726. ISBN 978-3-95735-104-3. [Google Scholar]
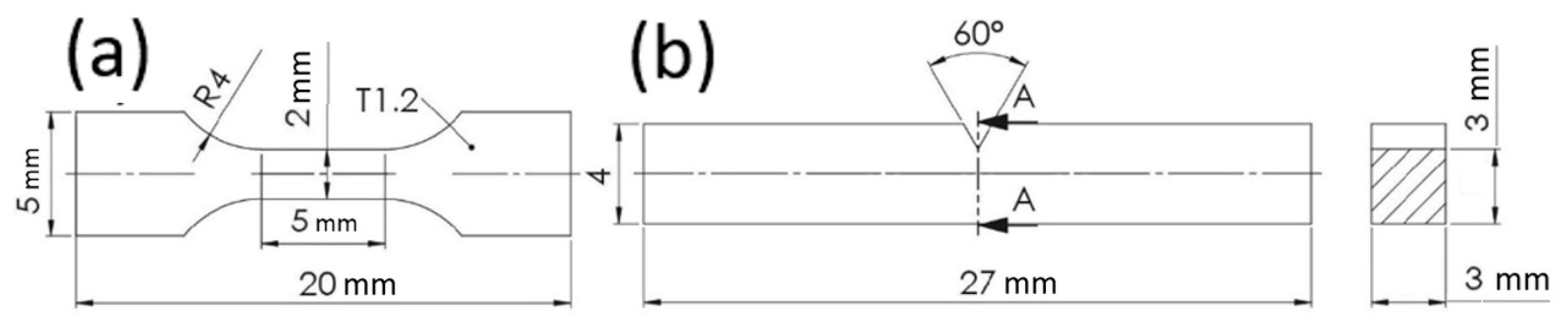
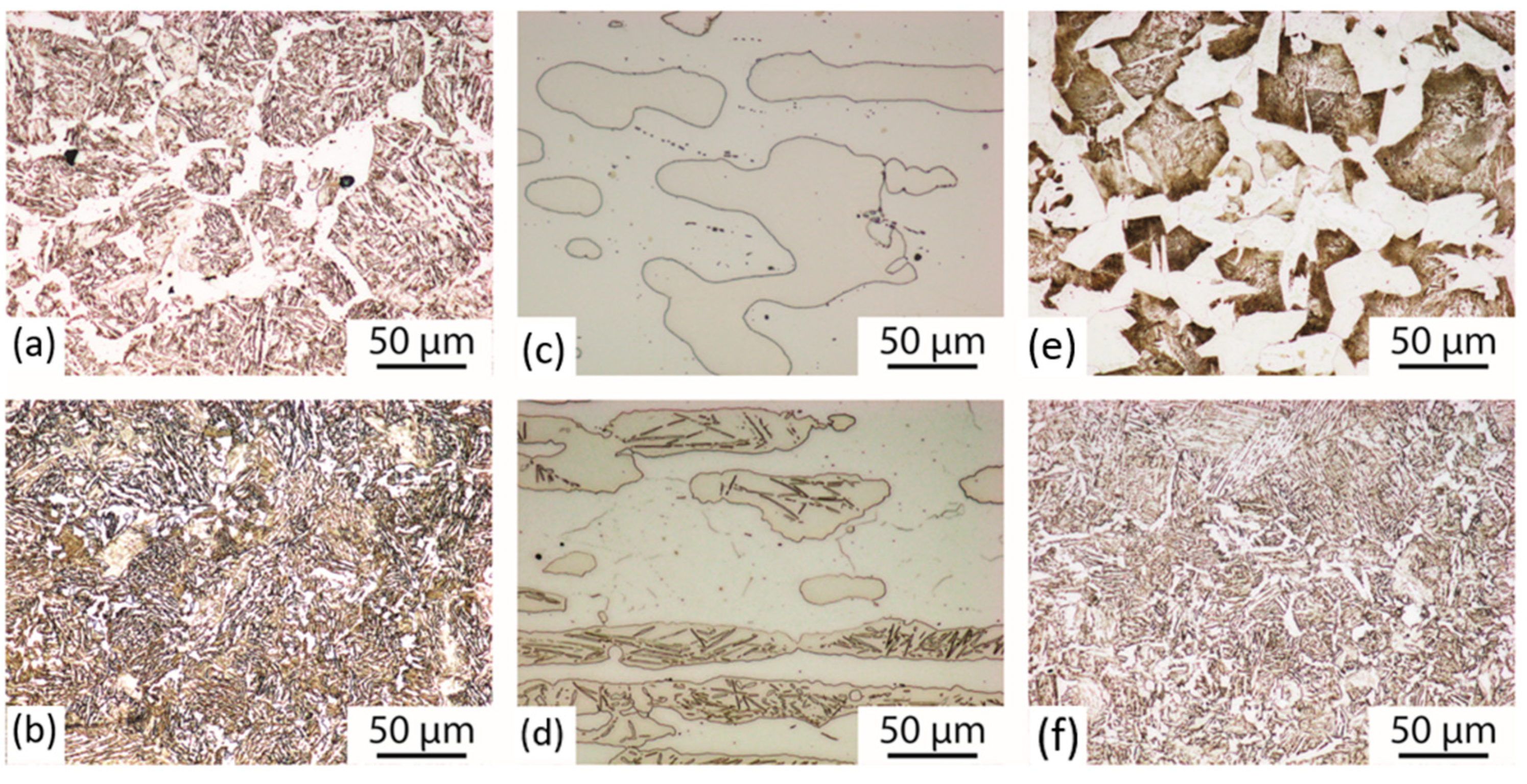
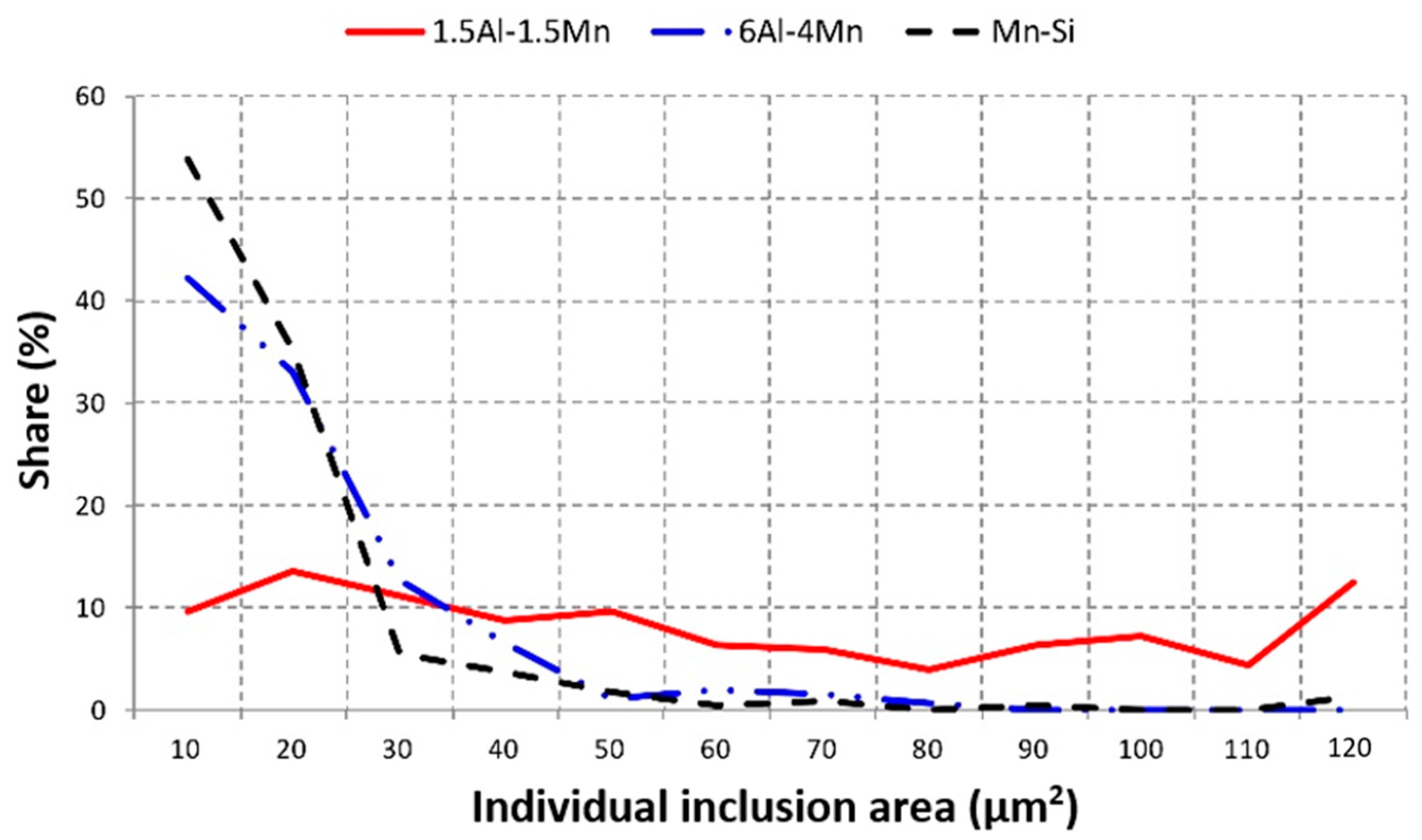
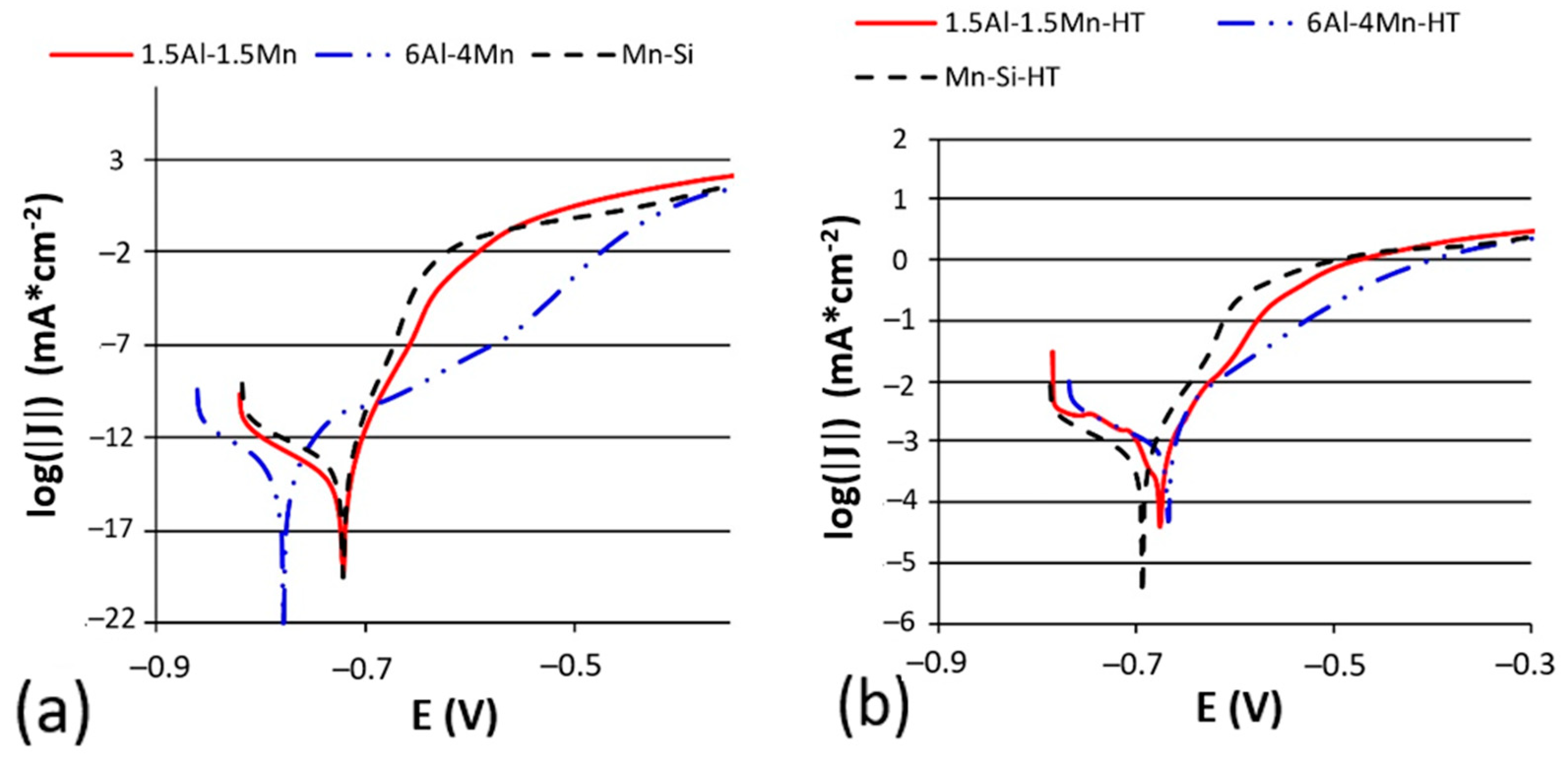



| Group | Material | C | Si | Mn | P | Cr | Al | Nb | Ti | N | Mn/Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Mn | 1.5Al-1.5Mn | 0.2 | 0.6 | 1.5 | 0.008 | 0.19 | 1.5 | 0.06 | 0.0009 | 0.02 | 1 |
| 1.5Al-3Mn | 0.2 | 0.6 | 3.0 | 0.008 | 0.19 | 1.5 | 0.06 | 0.004 | 0.02 | 2 | |
| 2Al-3Mn | 0.2 | 0.6 | 3.0 | 0.008 | 0.17 | 2.0 | 0.06 | 0.0002 | 0.01 | 1.5 | |
| 6Al-4Mn | 6Al-4Mn | 0.2 | 0.6 | 4.0 | 0.008 | 0.14 | 6.5 | 0.06 | 0.002 | 0.02 | 0.6 |
| Mn-Si | Mn-Si | 0.2 | 1.8 | 1.5 | 0.008 | 0.008 | 0.008 | 0.03 | 0.0002 | 0.004 | - |
| Mn-Si-Nb | 0.2 | 1.8 | 1.5 | 0.008 | 0.008 | 0.008 | 0.06 | 0.0002 | 0.007 | - |
| Group | Material | Forging Temperature | Annealing Conditions |
|---|---|---|---|
| Al-Mn | 1.5Al-1.5Mn | 1050 °C/60 min dwell, air cooled to RT | Furnace-cooled only |
| 1.5Al-3Mn | - | 950 °C/2 h (argon) | |
| 2Al-3Mn | - | 1000 °C/2 h (argon) | |
| 6Al-4Mn | 6Al-4Mn | 1050 °C/60 min dwell, air cooled to RT | Furnace-cooled only |
| Mn-Si | Mn-Si | 1150 °C/60 min dwell | 950 °C/2 h (argon) |
| Mn-Si-Nb | air cooled to RT | - |
| Group | Material | Condition | Ecorr (mV) | Jcorr (mA/cm2) | Corrosion Rate (µm·year−1) |
|---|---|---|---|---|---|
| Al-Mn | 1.5Al-1.5Mn | F&A | −680 ± 22 | 5 ± 2 | 6.8 ± 0.7 |
| HT | −694 ± 14 | 4.4 ± 0.6 | 4 ± 2 | ||
| 1.5Al-3Mn | F&A | −690 ± 15 | 7 ± 2 | 10 ± 7 | |
| HT | −673 ± 8 | 4 ± 2 | 8 ± 3 | ||
| 2Al-3Mn | F&A | −701 ± 16 | 6 ± 2 | 6 ± 2 | |
| HT | −718 ± 9 | 5 ± 1 | 5.0 ± 0.9 | ||
| 6Al-4Mn | 6Al-4Mn | F&A | −733 ± 17 | 5 ± 1 | 6 ± 2 |
| HT | −715 ± 37 | 8 ± 5 | 7 ± 4 | ||
| Mn-Si | Mn-Si | F&A | −712 ± 29 | 3.2 ± 0.1 | 4 ± 2 |
| HT | −702 ± 9.5 | 6 ± 2 | 6 ± 2 | ||
| Mn-Si-Nb | F&A | −685 ± 22 | 6 ± 2 | 12 ± 7 | |
| HT | −712 ± 25 | 5 ± 2 | 5 ± 1 |
| Point | O | Fe | Al | Mn | Cr | S |
|---|---|---|---|---|---|---|
| 1 | 1.4 | 92.8 | - | 4.7 | 1.0 | - |
| 2 | 3.1 | 31.8 | - | 41.4 | 0.4 | 23.2 |
| 3 | 2.0 | 91.8 | - | 4.6 | 1.1 | - |
| 4 | 8.3 | 80.8 | 5.9 | 2.9 | 0.6 | 0.2 |
| Point | O | Fe | Al | Mn | Si |
|---|---|---|---|---|---|
| 1 | 0.9 | 88.5 | 6.5 | 3.8 | 0.6 |
| 2 | 2.9 | 86.0 | 5.5 | 4.8 | 0.5 |
| 3 | 1.5 | 87.1 | 6.4 | 4.2 | 0.6 |
| Point | O | Fe | Al | Mn | S | Si |
|---|---|---|---|---|---|---|
| 1 | 15.0 | 46.6 | 23.2 | 9.1 | 4.6 | 0.8 |
| 2 | 0.7 | 95.3 | - | 1.8 | - | 1.4 |
| Group | Material | Condition | Rm (MPa) | A (%) | KCV (J·cm−2) |
|---|---|---|---|---|---|
| Al-Mn | 1.5Al-1.5Mn | F&A | 819 ± 3 | 30 | 37.4 ± 0.5 |
| HT | 802 ± 3 | 36 ± 1 | 71 ± 3 | ||
| 1.5Al-3Mn | F&A | 1086 ± 2 | 25 ± 2 | 23 ± 2 | |
| HT | 1354 ± 27 | 14 ± 1 | 6.3 ± 0.5 | ||
| 2Al-3Mn | F&A | 1088 ± 27 | 23 ± 1 | 13 ± 1 | |
| HT | 1300 ± 4 | 15 ± 1 | 8 ± 1 | ||
| 6Al-4Mn | 6Al-4Mn | F&A | 608 ± 15 | 2 | 1.1 |
| HT | 693 ± 51 | 2 ± 1 | 1.1 | ||
| Mn-Si | Mn-Si | F&A | 669 ± 1 | 34 ± 1 | 16 ± 5 |
| HT | 852 ± 2 | 33 ± 1 | 50 ± 2 | ||
| Mn-Si-Nb | F&A | 765 ± 3 | 24 ± 3 | 21 ± 5 | |
| HT | 903 ± 2 | 29 | 31 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajšman, J.; Kučerová, L.; Burdová, K. The Influence of Varying Aluminium and Manganese Content on the Corrosion Resistance and Mechanical Properties of High Strength Steels. Metals 2021, 11, 1446. https://doi.org/10.3390/met11091446
Hajšman J, Kučerová L, Burdová K. The Influence of Varying Aluminium and Manganese Content on the Corrosion Resistance and Mechanical Properties of High Strength Steels. Metals. 2021; 11(9):1446. https://doi.org/10.3390/met11091446
Chicago/Turabian StyleHajšman, Jan, Ludmila Kučerová, and Karolína Burdová. 2021. "The Influence of Varying Aluminium and Manganese Content on the Corrosion Resistance and Mechanical Properties of High Strength Steels" Metals 11, no. 9: 1446. https://doi.org/10.3390/met11091446
APA StyleHajšman, J., Kučerová, L., & Burdová, K. (2021). The Influence of Varying Aluminium and Manganese Content on the Corrosion Resistance and Mechanical Properties of High Strength Steels. Metals, 11(9), 1446. https://doi.org/10.3390/met11091446






