Abstract
The formation of Zn and Mg segregations at a tilt Σ5{013} <100> grain boundary (GB) in Al and the effects of these solutes on deformation behavior of polycrystalline Al were investigated using ab initio total energy calculations. Using a step-by-step modeling of the segregation process, we found that the formation of a thick segregation layer of Zn at the GB is energetically preferable, while the formation of an atomically thin segregation layer is expected in the case of Mg. To reveal the effect of segregation on the cohesive properties of Al GBs, we calculated the energy of cleavage decohesion and the shear resistance for GB sliding. We show that the segregation of Zn results in a substantial decrease in barriers for GB sliding, while the segregation of Mg increases the barriers. The results obtained allow us to explain experimental findings and demonstrate a strong relationship between chemical bonding of solute atoms, their segregation ability, and GB strength.
1. Introduction
Grain boundary (GB) segregation frequently occurs in polycrystalline materials, affecting their properties, such as strength and plasticity, and influencing the kinetics of important processes such as recrystallization, solid solution decomposition, new phase nucleation, etc. [1]. Scientific interest in the segregation of solute elements to GBs was recently revived in connection with the development of new ultra-fine-grained (UFG) Al-based alloys [2,3,4,5]. Commercial aluminum alloys containing Cu, Zn, Mg, and other alloying additions were therefore the focus of numerous research studies [5,6,7,8,9]. In particular, using scanning transmission electron microscopy and atom probe tomography, it was observed that Mg atoms tend to form heterogeneous agglomerations at GBs [10,11], and this results in extra strengthening. In contrast, Al-Zn alloys exhibit different behaviors. Layers of Zn atoms are distributed homogeneously along GBs in an alloy [5,12]. These specific segregations are thought to be one reason for the increased strain rate sensitivity and GB sliding enhancement at relatively low temperatures in UFG Al-Zn alloys, which result in super-ductility [6,13,14].
These observations clearly suggest that the formation of segregations and their effect on mechanical properties could change drastically depending on the chemistry of alloying elements in a particular alloy. In some alloys, a realistic picture of solute–GB interactions may be rather complex, since both deformation (strain-induced) and electronic (chemical) contributions can play important roles [15,16]. Therefore, the implementation of a modeling approach based on ab initio calculations using density functional theory (DFT) methods is necessary for a consistent description of these phenomena [6,15,16,17,18,19,20,21,22,23,24,25,26,27]. As was shown in Refs. [16,17], the deformation contribution is dominant in the interaction between Mg and GBs in Al, wherein the solute atoms have the strongest energy preference when they substitute Al in the center of the GB. The electronic contribution is dominant in the solute–GB interaction in the case of Zn, and it can lead to the formation of a segregation layer of Zn atoms near the GB. However, for a correct description of segregation in this case, it is necessary to consider not only the solute–GB but also the solute–solute interactions and their possible changes near GBs.
In this paper, we present the results of ab initio modeling of the segregation of one, two, or multiple numbers of Mg or Zn atoms to a tilt Σ5{013} <100> GB, considering it as a representative GB in Al alloys (see [28,29], for example). By using a consistent computational approach for simulating the processes of segregation, we find that the formation of a thick layer of Zn at the GB is energetically preferable, while the appearance of a monoatomic segregation layer of solutes should be expected in the case of Mg. The energy barrier for shearing in the GB plane is determined using ab initio calculations, which show that the segregation of Zn promotes GB sliding, in contrast to Mg segregations, which make such sliding more difficult.
2. Methods of Calculations
The total energy of a bicrystal containing a symmetric Σ5{013} <100> tilt GB and solute atoms was calculated using projector-augmented plane wave (PAW) method pseudopotentials as implemented in the Vienna ab initio simulation package (VASP) [30,31,32]. The exchange-correlation energy was calculated in the generalized gradient approximation (GGA) by using the formalism proposed in Ref. [33]. The kinetic-energy cutoff of plane waves was 520 eV. The reciprocal space was sampled with a Γ-centered 2 × 4 × 8 k-point mesh [34]. The convergence criterion for total energy was 1 × 10−5 eV. After atomic position optimization, the forces on each atom were less than 1 × 10−2 eV/Å and the stress tensor components were less than 20 MPa. The calculated lattice parameter of Al agreed with the experimental value nm to within 0.2%. As an additional test, we calculated the excess volume of considered GB and found the value to be 0.32 Å, which agrees with results from previous calculations [35].
An 80-atom supercell with dimensions of was used in the calculations (Figure 1a); it contained two crystallites misoriented (tilted) with respect to each other by 36.87° on the [100] axis, as the coincident site lattice (CSL) model [19] dictates. As in Ref. [28], the GB with and without solute atoms was simulated by a slab model, which can minimize the effect of interactions between GB images. Periodic boundary conditions were used along the Y and Z directions in the GB plane, while free boundary conditions were imposed along the X direction, perpendicular to the GB; a vacuum region of 10 Å thickness was added on both sides.
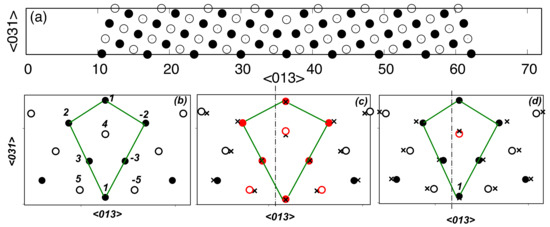
Figure 1.
The (100) plane projection of a bicrystal containing a symmetric Σ5{013} <100> tilt grain boundary (GB) (a). Both the atomic cell and the vacuum layer are shown; numbers indicate distances in Angstroms. Open and closed circles correspond to atoms in the two nearest (100) planes. Structural units characterizing the local atomic configuration [36] are marked with solid lines for Al (b), Al-Zn (c), and Al-Mg (d) alloys. Dash-dotted lines show the position of the planes, parallel to the GB plane, along which the shear or cleavage of the bicrystal was performed. Red circles correspond to impurity atoms; crosses mark the positions of atoms before relaxation.
When simulating segregation, the crystallite was subjected to complete relaxation (atomic positions, cell shape, and volume optimization) both before and after the addition of a substitutional atom. In the simulation of sliding, after the shift, the calculations were carried out without any optimization; in this case, full relaxation (with the optimization of the atomic positions perpendicular to the shear plane) occurred only at the shifts corresponding to the maximum energy. When simulating cleavage, a rigid shift of one of the grains was carried out perpendicular to the grain boundary and then full relaxation was carried out.
As in previous papers (see [16]), the segregation energy of a single solute atom was determined as the energy difference
where is the total energy of the crystallite containing an impurity X at a distance of R from the GB plane and is the energy of the crystallite with the same impurity situated on a bulk-like site , far from the GB. Equation (1) determines the energy gain for a single solute atom to move from the bulk to the GB. However, this single-site energy is insufficient for describing the formation of concentrated segregations, where the value of depends on the presence of solutes previously segregated to the GB. The segregation energy in this case was determined as follows,
where is the energy of the supercell containing solute atoms on sites near the GB; and is the energy of the supercell with solutes on sites near the GB and one solute atom on the bulk-like site . When using Equation (2) as a definition of the segregation energy, we assumed that each next solute reaching the GB occupies the site most energetically preferred among the sites available. Note that this definition of segregation energy differs from the one used in Refs. [29,37], where the formation of segregation from isolated solute atoms was considered.
To determine the effect of segregation on GB strength, we calculated (i) the grain boundary generalized staking fault (GB-SF) energy to characterize the shear resistance inherent in this type of GB [38] and (ii) the GB decohesion energy spent during the separation of the two crystallites in the direction perpendicular to the GB plane. The GB-SF energy was calculated as the energy variation when one crystallite is shifted relative to another in the GB plane by a given vector f; the resulting set of values forms a GB-SF surface. Each saddle point on the GB-SF surface at a certain vector f corresponds to so an unstable stacking fault, and its height characterizes the GB shear resistance [39]. To evaluate the effect of segregation on GB cleavage, the corresponding energy of decohesion was calculated by cleaving the bicrystal along the GB plane and separating the two obtained grains far enough from each other (up to 7 Å).
3. Results
The results of calculations of the segregation energy, Equation (1), for individual Zn and Mg solutes at sites one to five (see Figure 1) near the Σ5(013) <100> GB are given in Table 1. The calculations showed that Zn segregation is energetically favorable for all considered positions except site four. In contrast to Zn, site four is the only position where the segregation of Mg is energetically favorable. Similar observations were previously reported in Reference [16]; some numerical differences with the results presented here may be ascribed to a different GB being considered in Reference [16]. As discussed in Refs. [16,17], the electronic contribution dominates in solute–GB interaction in the case of Zn, whereas the atomic size mismatch plays the decisive role in the case of Mg. Local distortions at site i near a GB can be characterized using the following parameter,
where is the number of the nearest neighbors of ith atom; and are the distances between the nearest neighbors i and j with and without GB, respectively. For relaxed structure of the Σ5(013) <100> GB in Al, the value of reaches the absolute maximum (of about 0.1) for site i = 4, where it is an order of magnitude larger than at any other site. This means that the excess volume is concentrated mostly in the center of the GB. As Table 1 shows, the biggest gain in energy is achieved exactly when an Mg atom segregates to site four (see Table 1). In contrast with Mg, segregation of Zn is energetically preferred most at sites three and one, while its segregation energy to site four is positive. Thus, calculated values of support the conclusion that the deformation mechanism of solute-GB interaction dominates in the case of Mg and is negligible in the case of Zn [16,17].

Table 1.
Segregation energy ΔEs (eV) of Zn and Mg solutes and local lattice deformation δu (%) in different positions on the Σ5(013) <100> GB in Al.
To simulate the process of GB enrichment forming concentrated solute segregations, we considered the segregation of two, three, and more solute atoms. Segregation energy of the second solute atom (Zn or Mg) to sites one to five, under the condition that the first atom has already occupied the most preferable site (one or three in the case of Zn and four in the case of Mg), was calculated using Equation (2). As seen from the results presented in Table 2, segregation of the second Zn atom leads to an additional energy gain, except for segregation to site four. In the case of Zn, the most energetically favorable configurations are (1)–(3/−3) and (3)–(−3). In contrast to Zn, the segregation of the second Mg atom has an energy cost, and the formation of a thin layer of Mg should be expected at the special type Σ5(013) <100> GB. This feature of the segregation behavior is due to the deformation contribution being dominant in the Mg–GB interaction [16], as site four provides sufficient extra volume for Mg solutes substituting Al atoms at the central plane of the considered GB.

Table 2.
Segregation energy (eV) of the second atom (Zn or Mg) in different positions near the Σ5{013}<100> GB, which contains a Zn atom (at site 1 or 3) or a Mg atom (at site 4).
In Figure 2, the variation in energy is shown as a function of the number n of segregated Zn atoms for various possible fillings of atomic positions. Curve 3 corresponds to the case when the atoms are located at the most energetically preferred sites, according to the sequence (−3)–(2)–(1)–(3)–(−2)–(4)–(5)–(−5). Other possible methods of filling positions (1)–(5)–(2), curve 1, and 3–(−3)–(1)–(2), curve 2, have higher energies. As Figure 2 shows, the value changes non-monotonically, and the domain of GB segregation (n ≤ 5) is clearly separated from a bulk-like behavior (n > 5). When filling the sites with the solutes at n ≤ 5, Zn atoms substitute Al atoms mainly at the sites closest to the boundary plane; while for n > 5, some GB reconstruction occurs, which is determined by the interactions between the segregated Zn atoms. In particular, we found that the initially energetically costly site four becomes favorable when six neighboring sites become occupied by Zn. The monotonic decrease in segregation energy for n > 5 corresponds to the growth of a bulky Zn precipitate after its nucleation at the GB; the slope of the curve in this case is about , where is the interaction energy between two Zn atoms in the Al bulk (−0.015eV [40]) and is the number of nearest Zn–Zn neighbors for the Zn atom attaching to the precipitate.
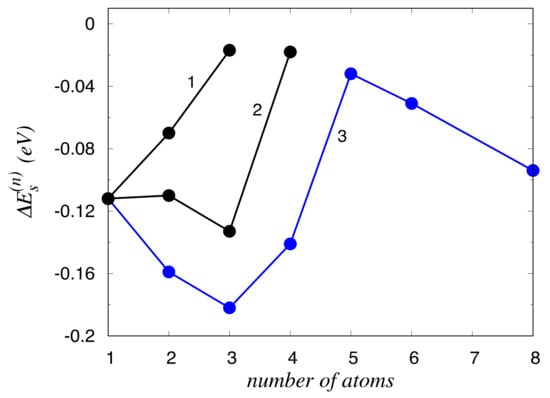
Figure 2.
Segregation energy as a function of the number of atoms in Zn segregation for various possible fillings of atomic positions. The curves 1,2 and 3 correspond to the sequence of filling nodes (1)–(5)–(2), 3–(−3)–(1)–(2), and (−3)–(2)–(1)–(3)–(−2)–(4)–(5)–(−5), respectively.
As a result of successive steps of Zn atoms segregating at the GB, a rather wide enrichment region can be formed. Thus, the segregation behaviors of Zn and Mg were found to be completely opposite to each other; they originate from the features of both solute–GB and solute–solute interactions. Although this result was obtained for a particular special type of GB, one may expect that this conclusion is quite general, since the electronic mechanism of solute–GB interaction is usually more long–ranged than the deformation mechanism (see the discussion in Reference [16]).
To reveal the effect of segregation on the cohesive properties of GB in Al, we calculated the barriers for GB sliding and the energy of cleavage decohesion for GBs enriched with Zn or Mg solutes as well as for the GB in pure Al. As in Reference [38], here we determined the barrier for GB sliding as the change in energy when one part of the crystallite is shifted relative to the other along a given direction f in the GB plane (the plane is indicated by a dashed line in Figure 1c,d). The GB-SF energy, which characterizes the shear resistance of the considered GB in Al, was obtained in Reference [38] using molecular dynamics (MD) simulations. Although the use of interatomic potentials in MD simulations does not provide a completely reliable description, we think that the results in Reference [38] correctly reproduce the main topological features of the GB-SF surface (Figure 3). In particular, there are two easy GB sliding directions: O–O1 (along the tilt axis <100>) and O–A (at an angle to the tilt axis, Figure 3), for which the energy barrier is relatively small.
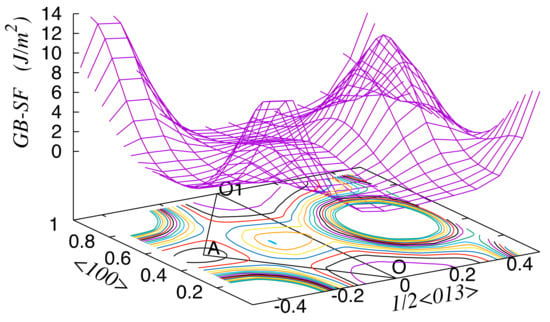
Figure 3.
Unrelaxed GB–staking fault (SF) energy for the Σ5{013} <100> GB in Al calculated by the molecular dynamics (MD) method [38].
The results of the ab initio calculation of the energy variation (without structural relaxation) upon sliding of one part of the bicrystal relative to the other along the considered easy directions are shown in Figure 4. The structural relaxation in the direction perpendicular to the GB plane results in a drastic decrease in GB–SF energy. In particular, the relaxed value of GB–SF at point A is 0.22 J/m2 in Al GB and 0.29 J/m2 in the case of Al GB with segregation of Mg. The smallest value of γ(A) = 0.11 J/m2 was obtained for the seven-layer segregation of Zn, whose formation process is illustrated in Figure 2. Note, such magnitude of γ is close to the intrinsic stacking fault energy typical of many face-centered cubic metals.
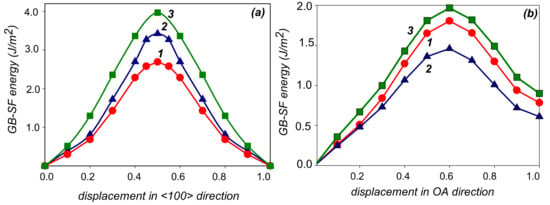
Figure 4.
Ab initio GB–SF energy without relaxation for shear along O–O1 <100> (a) and O–A (b) directions in the plane of the Σ5{013} <100> GB. Curves 1, 2, and 3 correspond to pure Al, Al–Zn alloy, and Al–Mg alloy, respectively.
The energies of unstable stacking faults γus (corresponding to saddle points on the GB–SF surface and characterizing the GB resistance against sliding) are presented in Table 3 for the two easy shear directions. As one can see, the segregation of Mg increases the resistance to GB sliding compared to Al in both directions. The effect of Zn is somewhat ambiguous: the formation of Zn segregation decreases the value of γus in the O–A direction and increases it in the O–O1 <100> direction. However, it should be expected that sliding in the <100> direction can be carried out more easily, following the scheme <100> = O–A + A–O1 (Figure 3), which is energetically more favorable. The results of the calculation for partial shear O–A (Table 3) lead to the conclusion that segregations of Zn will effectively decrease γus and thus facilitate GB sliding.

Table 3.
The unstable stacking fault energies γus (J/m2) for GB sliding in two easy directions, with and without structural relaxation.
To reveal the effect of alloying elements on a GB fracture, we simulated the cleavage decohesion process by cleaving the two grains along the GB and separating them away from each other in the supercell; the cleavage plane is shown in Figure 1 by a dash-dotted line. A similar position of the cleavage plane with respect to the GB was considered previously in Refs. [28,29,41], where calculations were carried out for ∑5(012)[100] GB in Al. The results of our calculation for a ∑5{013}[100] GB are shown in Table 4, together with previously published results [28,29,41]. We found that the separation energy upon this process resulting in the formation of two free surfaces is close to the values obtained by a similar method in Refs. [28,29,41]. Here, the Mg segregation is found to increase the cleavage energy in contrast to Zn, whose segregation decreases the cleavage energy.

Table 4.
Cleavage energy for the ∑5{013}[100] GB in Al and Al alloys. The results of previous calculations are shown in brackets.
4. Discussion
The segregation of Zn and Mg to the Σ5{013} <100> tilt GB in Al was studied using ab initio total energy calculations. We showed that interactions between Mg solutes and the GB in Al lead to the formation of a thin monatomic layer (Figure 1d) at the GB plane. In contrast with Mg, the Zn–GB and Zn–Zn interactions result in a rather wide Zn segregation region at the GB (Figure 1c and Figure 2). Different segregation behaviors are due to (also discussed in Refs. [16,17]) the deformation contribution to solute–GB interactions dominating in the case of Mg, whereas the electronic contribution dominates in the case of Zn. As a result, it should be expected that the morphological features of Mg and Zn segregations will differ. However, the information about energy gain due to moving a single solute from the bulk to GB is insufficient for judging the formation of concentrated segregations, since the energy is dependent on the presence of solute atoms that previously segregated to the GB.
We found that the formation of Zn segregation at the considered GB includes two stages. At the first stage, during the nucleation of a small Zn cluster (in our case, with n ≤ 5), the segregation energy changes non-monotonically (Figure 2), whereas its specific structure depends on the order in which the sites are occupied by each next solute atom. For n > 5, the nucleation stage is finished and the cluster begins to grow by adjoining of more Zn atoms. This process is controlled by bulk-like interactions of Zn atoms and is accompanied by a monotonic decrease in . Note that the initially energetically unfavorable segregation site four becomes energetically preferable only at the second stage of cluster growth, so that this position can remain unoccupied by a Zn atom. In such a case, a mixed Zn–Al or Zn–Mg precipitate can form at the GB.
As a result of the formation of Zn or Mg segregations, the cohesive properties of GBs change since bonds between Al atoms are replaced by Zn–Zn or Mg–Al bonds. We simulated the cleavage decohesion process by separating the two grains away from the GB plane and found that Mg segregation slightly increases the GB cleavage energy, in contrast to Zn whose segregation decreases the GB cohesion. To elucidate the mechanisms controlling the mobility of GBs in a Zn–or Mg-alloyed Al polycrystal, the energy barriers for the sliding process along the special-type, tilt Σ5{013} <100>, GB were calculated by the ab initio method. We found that for easy sliding directions, the segregation of Zn results in a substantial decrease in the GB sliding barrier, whereas the segregation of Mg increases it (Table 3). This conclusion is in agreement with experimental observations of enhanced GB sliding in Al–Zn alloys [6] and the GB strengthening phenomenon realized in Al–Mg alloys [11].
In real fine-grained polycrystals, the deformation behavior is much more complicated. A more realistic modeling must consider the presence of different types of grain boundaries, as well as the occurrence of concurrent accommodation processes. However, we think that the results obtained in this work clearly demonstrate a strong relationship between chemical bonding of solute atoms, their segregation behavior, and GB strength.
Author Contributions
Conceptualization, Y.G. and P.K.; Methodology, L.K., and A.K.; Software, A.K.; Validation, L.K., and Y.G.; Formal Analysis, A.K.; Investigation, L.K., and A.K.; Data Curation, Y.G., and P.K.; Writing—Original Draft Preparation, L.K.; Writing—Review & Editing, Y.G. and P.K., visualization, L.K. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out within the state assignment of the Ministry of Science and Higher Education of the Russian Federation (topic Structure, No. AAA-A18-118020190116-6, and Pressure, No. AAA-A18-118020190104-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was carried out using computing resources of the federal collective usage center Complex for Simulation and Data Processing for Mega-science Facilities at NRC Kurchatov Institute, http://ckp.nrcki.ru/ (accessed on 7 April 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lejček, P. Grain Boundary Segregation in Metals; Springer Series in Materials Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Gayle, F.W.; Goodway, M. Precipitation hardening in the first aerospace aluminum alloy: The wright flyer crankcase. Science 1994, 266, 1015–1017. [Google Scholar] [CrossRef]
- Andersen, S.J.; Marioara, C.D.; Friis, J.; Wenner, S.; Holmestad, R. Precipitates in aluminium alloys. Adv. Phys. X 2018, 3, 1479984. [Google Scholar] [CrossRef]
- Valiev, R.Z. Nanostructuring of metals by severe plastic deformation for advanced properties. Nat. Mater. 2004, 3, 511. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, X.; Wilde, G.; Divinski, S.V.; Horita, Z.; Valiev, R.Z. Grain boundaries in ultrafine grained materials processed by severe plastic deformation and related phenomena. Mater. Sci. Eng. A 2012, 540, 1–12. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z.; Valiev, R.Z. Transition from poor ductility to room-temperature superplasticity in a nanostructured aluminum alloy. Sci. Rep. 2018, 8, 6740. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Starink, M. Precipitates and intermetallic phases in precipitation hardening Al-Cu-Mg-(Li) based alloys. Int. Mater. Rev. 2005, 50, 193–215. [Google Scholar] [CrossRef]
- Nicolas, M.; Deschamps, A. Characterisation and modelling of precipitate evolution in an Al-Zn-Mg alloy during non-isothermal heat treatments. Acta Mater. 2003, 51, 6077–6094. [Google Scholar] [CrossRef]
- Yi, G.; Cullen, D.A.; Littrell, K.C.; Golumbfskie, W.; Sundberg, E.; Free, M.L. Characterization of Al-Mg alloy aged at low temperatures. Metall. Mater. Trans. A 2017, 48, 2040–2050. [Google Scholar] [CrossRef]
- Valiev, R.Z. Nanomaterial Advantage. Nature 2002, 419, 887. [Google Scholar] [CrossRef]
- Sauvage, X.; Enikeev, N.; Valiev, R.; Nasedkina, Y.; Murashkin, M. Atomic-scale analysis of the segregation and precipitation mechanisms in a severely deformed Al–Mg alloy. Acta Mater. 2014, 72, 125–136. [Google Scholar] [CrossRef]
- Li, H.Q.; Ebrahimi, F. An investigation of thermal stability and microhardness of electrodeposited nanocrystalline nickel-21% iron alloys. Acta Mater. 2003, 51, 3905–3913. [Google Scholar] [CrossRef]
- Chinh, N.Q.; Valiev, R.Z.; Sauvage, X.; Varga, G.; Havancsak, K.; Kawasaki, M.; Straumal, B.B.; Langdon, T.G. Grain Boundary Phenomena in an Ultrafine-Grained Al–Zn Alloy with Improved Mechanical Behavior for Micro-Devices. Adv. Eng. Mater. 2014, 16, 1000–1009. [Google Scholar] [CrossRef]
- Bobruk, E.V.; Sauvage, X.; Enikeev, N.A.; Straumal, B.B.; Valiev, R.Z. Mechanical behavior of ultrafine-grained Al-5Zn, Al-10Zn, Al-30Zn alloys. Rev. Adv. Mater. Sci. 2015, 43, 45–51. [Google Scholar]
- Lozovoi, A.Y.; Paxton, A.T.; Finnis, M.W. Structural and chemical embrittlement of grain boundaries by impurities: A general theory and first-principles calculations for copper. Phys. Rev. B 2006, 74, 155416. [Google Scholar] [CrossRef]
- Karkina, L.E.; Karkin, I.N.; Kuznetsov, A.R.; Razumov, I.K.; Korzhavyi, P.A.; Gornostyrev, Y.N. Solute–grain boundary interaction and segregation formation in Al: First principles calculations and molecular dynamics modeling. Comput. Mater. Sci. 2016, 112, 18–26. [Google Scholar] [CrossRef]
- Petrik, M.V.; Kuznetsov, A.R.; Enikeev, N.; Gornostyrev, Y.N.; Valiev, R.Z. Peculiarities of Interactions of Alloying Elements with Grain Boundaries and the Formation of Segregations in Al–Mg and Al–Zn Alloys. Phys. Met. Metallogr. 2018, 119, 607–612. [Google Scholar] [CrossRef]
- Wu, R.; Freeman, A.J.; Olson, G.B. First principles determination of the effects of phosphorus and boron on iron grain boundary cohesion. Science 1994, 265, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.T.; Freeman, A.J.; Wu, R.; Olson, G.B. Effect of Mo and Pd on the grain-boundary cohesion of Fe. Phys. Rev. B 2000, 62, 6208–6215. [Google Scholar] [CrossRef]
- Schweinfest, R.; Paxton, A.T.; Finnis, M.W. Bismuth embrittlement of copper is an atomic size effect. Nature 2004, 432, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Shiga, M.; Kaburaki, H. Grain-boundary decohesion by impurity segregation in a nickel-sulfur system. Science 2005, 307, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Wang, J.; Zhang, H.J. First-principles investigation of Mg segregation at Σ = 11(113) grain boundaries in Al. J. Phys. Condens. Matter 2005, 17, 4301–4308. [Google Scholar] [CrossRef]
- Zhang, L.; Shu, X.; Jin, S.; Zhang, Y.; Lu, G.H. First-principles study of He effects in a bcc Fe grain boundary: Site preference, segregation and theoretical tensile strength. J. Phys. Condens. Matter 2010, 22, 375401. [Google Scholar] [CrossRef]
- Christensen, M.; Angeliu, T.M.; Ballard, J.D.; Vollmer, J.; Najafabadi, R.; Wimmer, E. Effect of impurity and alloying elements on Zr grain boundary strength from first-principles computations. J. Nucl. Mater. 2010, 404, 121–127. [Google Scholar] [CrossRef]
- Yuasa, M.; Mabuchi, M.J. Effects of segregated Cu on an Fe grain boundary by first-principles tensile tests. Phys. Condens. Matter 2010, 22, 505705. [Google Scholar] [CrossRef]
- Wachowicz, E.; Kiejna, A. Effect of impurities on grain boundary cohesion in bcc iron. Comput. Mater. Sci. 2008, 43, 736–743. [Google Scholar] [CrossRef]
- Karkina, L.; Karkin, I.; Kuznetsov, A.; Gornostyrev, Y. Alloying Element Segregation and Grain Boundary Reconstruction, Atomistic Modeling. Metals 2019, 9, 1319. [Google Scholar] [CrossRef]
- Zhang, S.; Kontsevoi, O.Y.; Freeman, A.J.; Olson, G.B. First principles investigation of zinc-induced embrittlement in an aluminum grain boundary. Acta Mater. 2011, 59, 6155–6167. [Google Scholar] [CrossRef]
- Zhao, D.; Løvvik, O.M.; Marthinsen, K.; Li, Y. Segregation of Mg, Cu and their effects on the strength of Al Σ5 (210) [001] symmetrical tilt grain boundary. Acta Mater. 2018, 145, 235–246. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 1994, 6, 8245–8258. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Cao, F.; Jiang, Y.; Hu, T.; Yin, D. Correlation of grain boundary extra free volume with vacancy and solute segregation at grain boundaries: A case study for Al. Philos. Mag. 2018, 98, 464–483. [Google Scholar] [CrossRef]
- Tschopp, M.A.; Macdowell, D.L. Asymmetric tilt grain boundary structure and energy in copper and aluminium. Philos. Mag. 2007, 87, 3871. [Google Scholar] [CrossRef]
- Xiao, Z.; Hu, J.; Liu, Y.; Dong, F.; Huang, Y. Segregation of Sc and its effects on the strength of Al Σ5 (210) [100] symmetrical tilt grain boundary. Mater. Sci. Eng. A 2019, 756, 389–395. [Google Scholar] [CrossRef]
- Karkina, L.E.; Karkin, I.N.; Kuznetsov, A.R.; Gornostyrev, Y.N. Grain boundary shear-migration coupling in Al bicrystalls. Atomistic modeling. Phys. Solid State 2018, 60, 1916–1923. [Google Scholar] [CrossRef]
- Bollmann, W. Crystal Defects and Crystalline Interfaces; Springer: Berlin, Germany, 1970. [Google Scholar]
- Gorbatov, O.I.; Stroev, A.Y.; Gornostyrev, Y.N.; Korzhavyi, P.A. Effective cluster interactions and pre-precipitate morphology in binary Al-based alloys. Acta Mater. 2019, 179, 70–84. [Google Scholar] [CrossRef]
- Razumovskiy, V.I.; Ruban, A.V.; Razumovskii, I.M.; Lozovoi, A.Y.; Butrim, V.N.; Vekilov, Y.K. The effect of alloying elements on grain boundary and bulk cohesion in aluminum alloys: An ab initio study. Scr. Mater. 2011, 65, 926–929. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).