Dissolution of Palladium Metal in Solvent Leaching System with the Presence of Oxidizing Agent
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Chemicals
2.2. Experimental Procedure and Analytical Methods
3. Results and Discussion
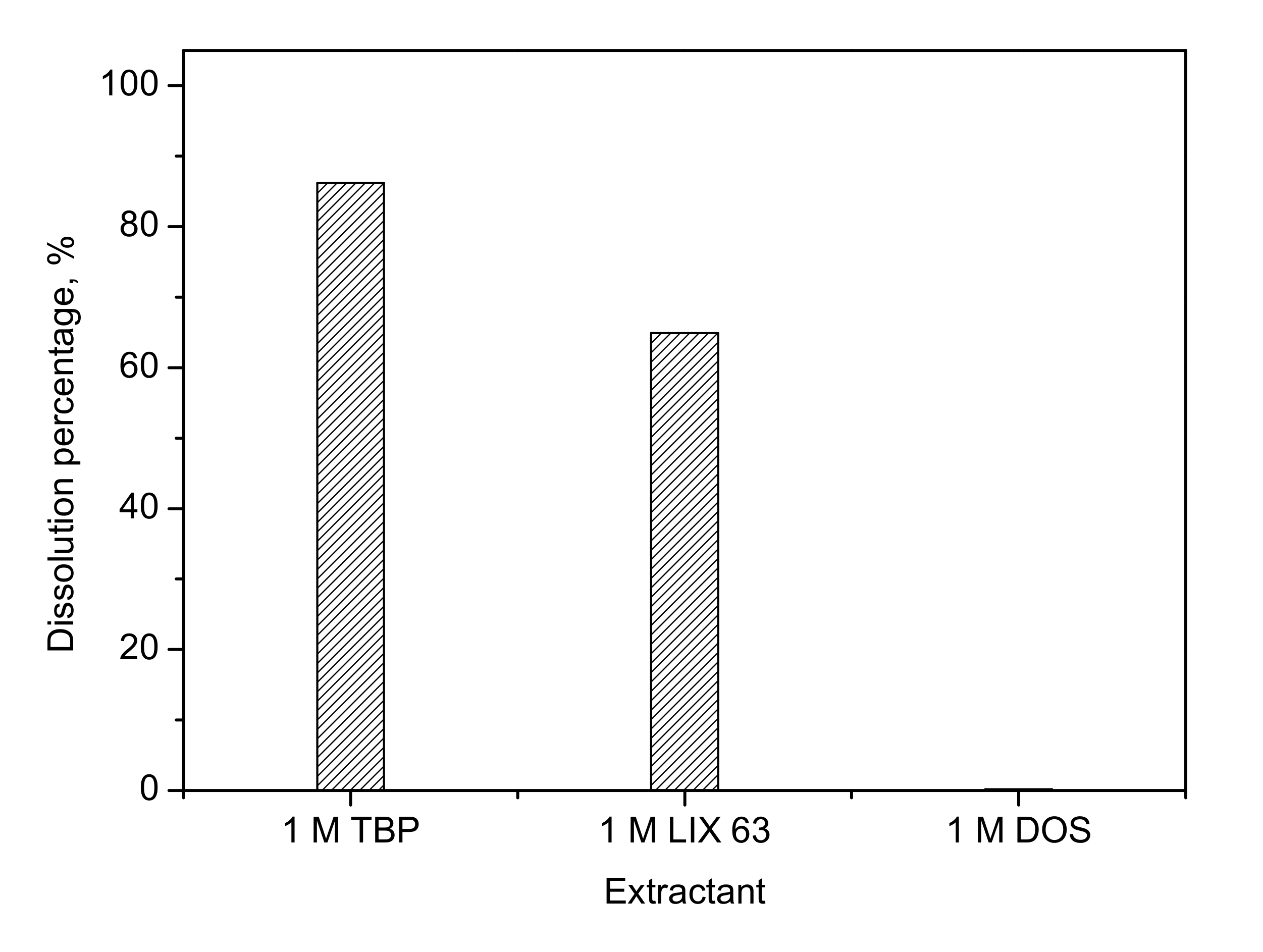
3.1. Effect of Extractant on Dissolution of Palladium
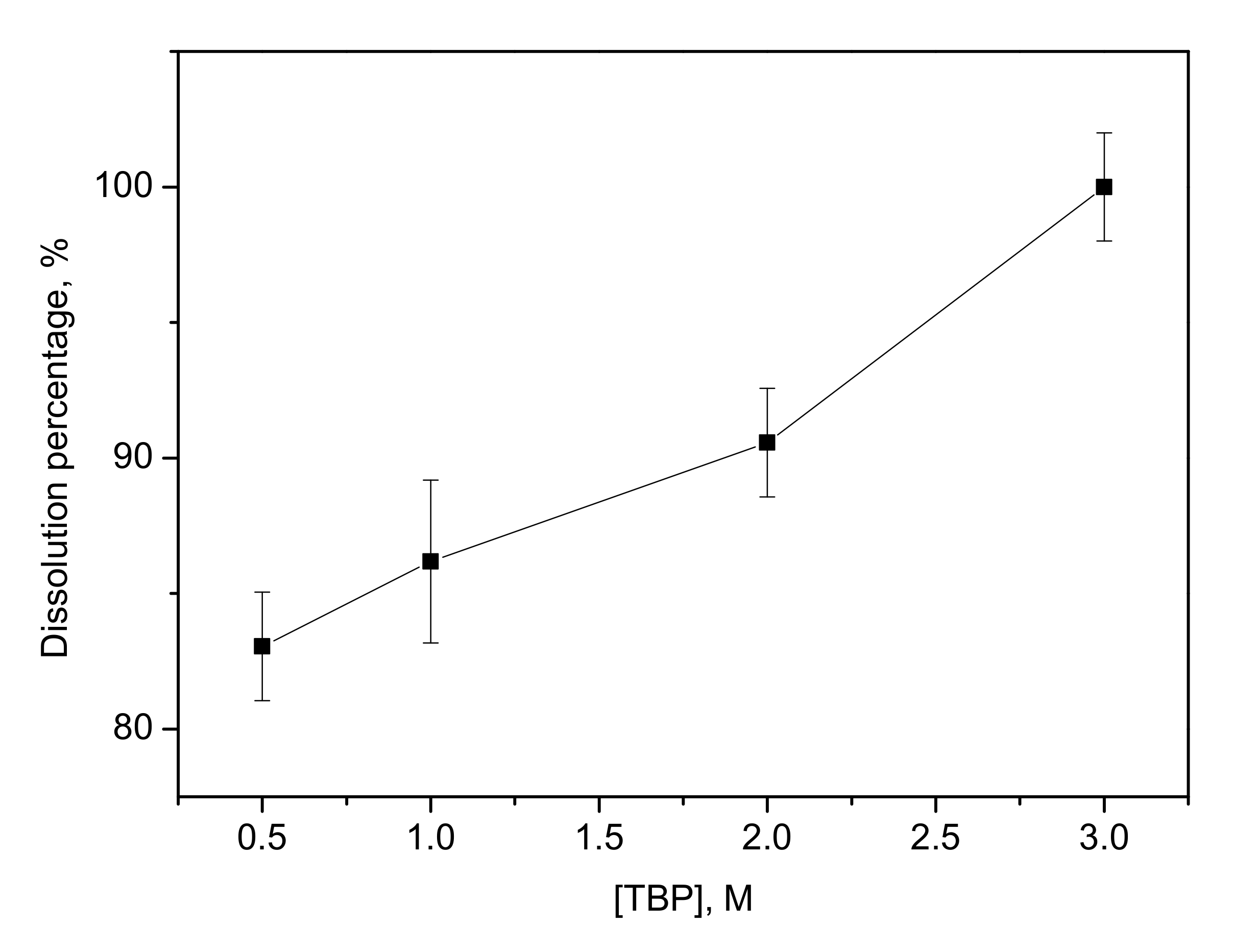
3.2. Effect of TBP Concentration
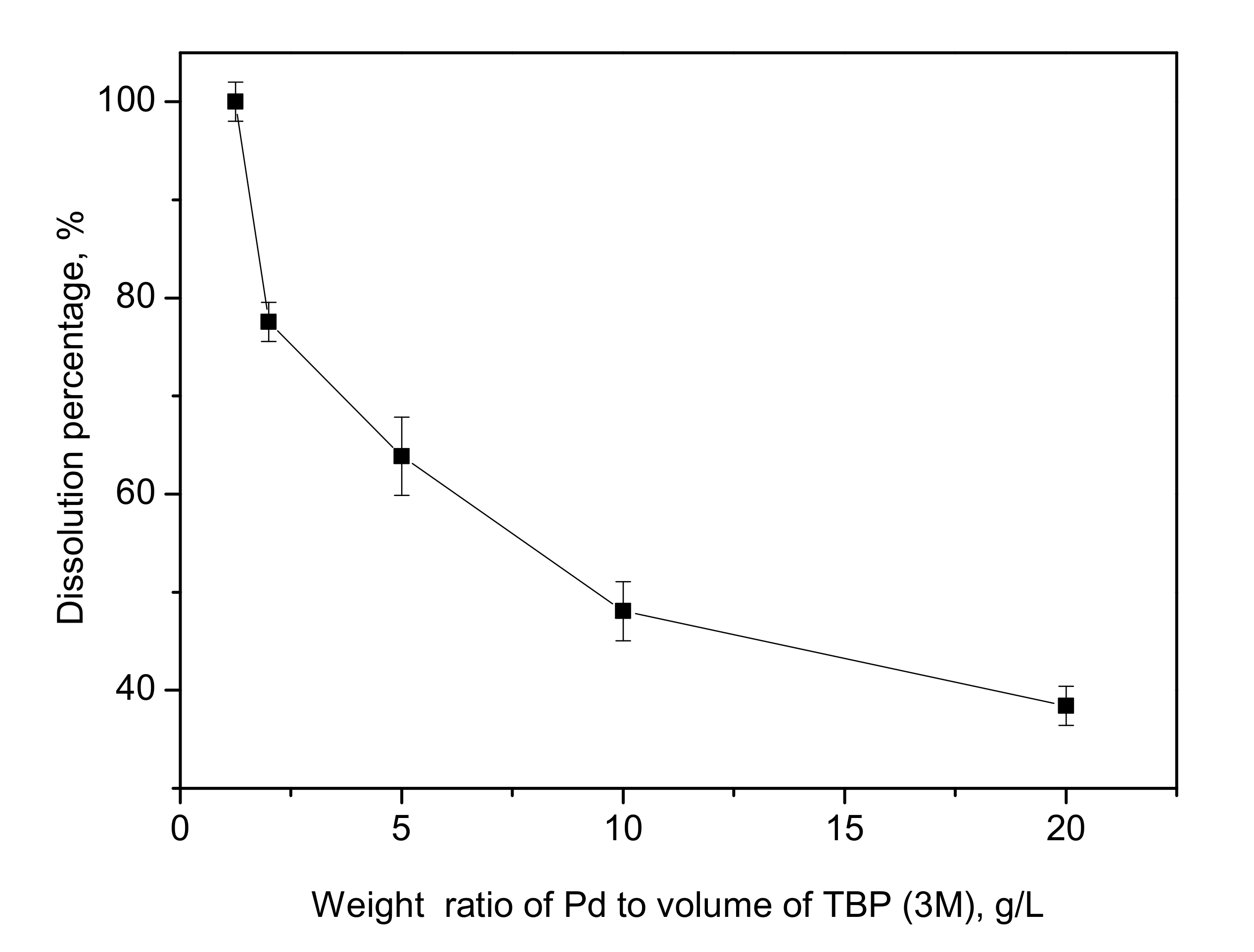
3.3. Effect of the Weight Ratio of Palladium to TBP
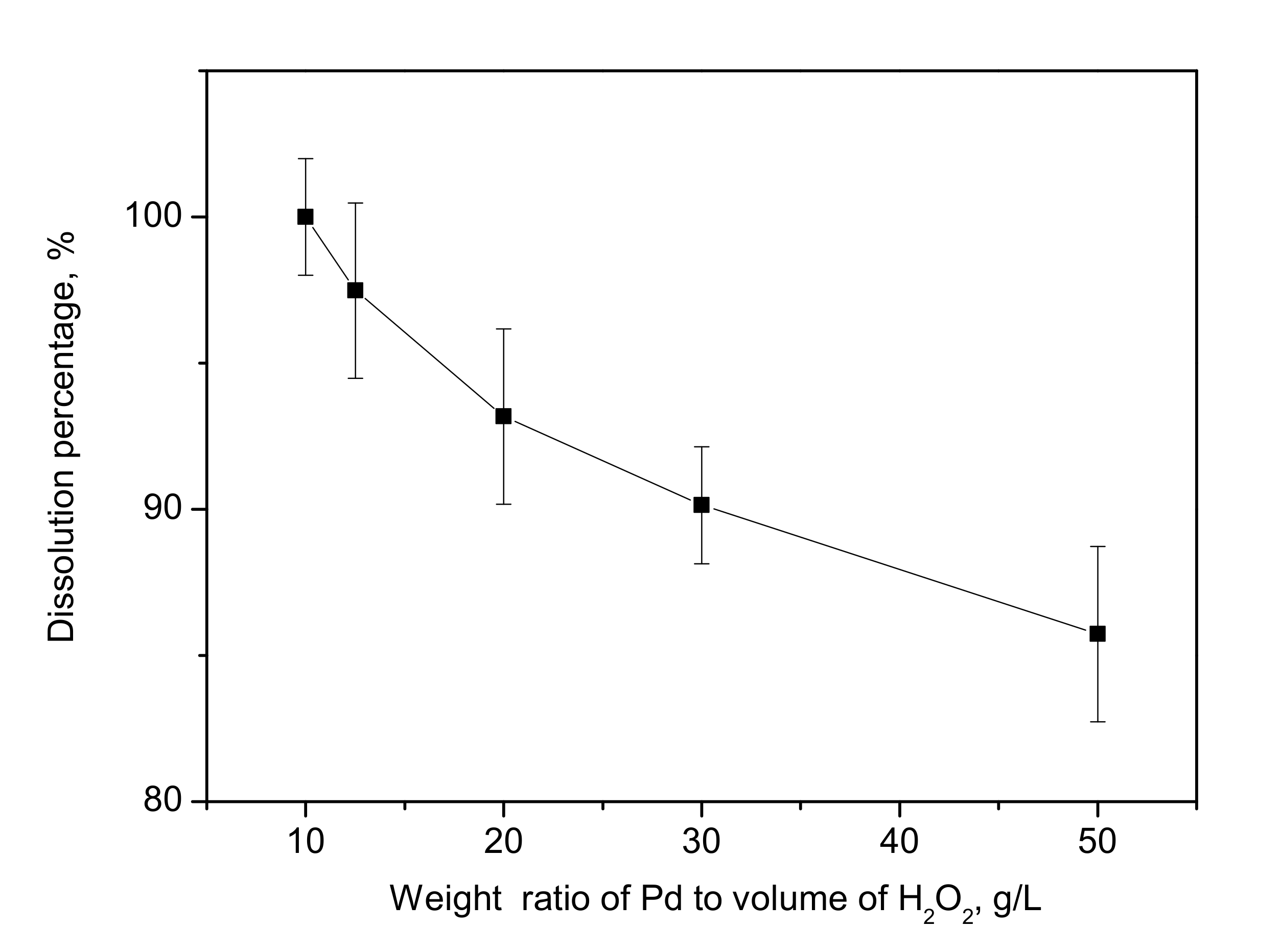
3.4. Effect of Weight Ratio of Palladium to H2O2
3.5. Effect of Weight Ratio of Palladium to Concentrated HCl
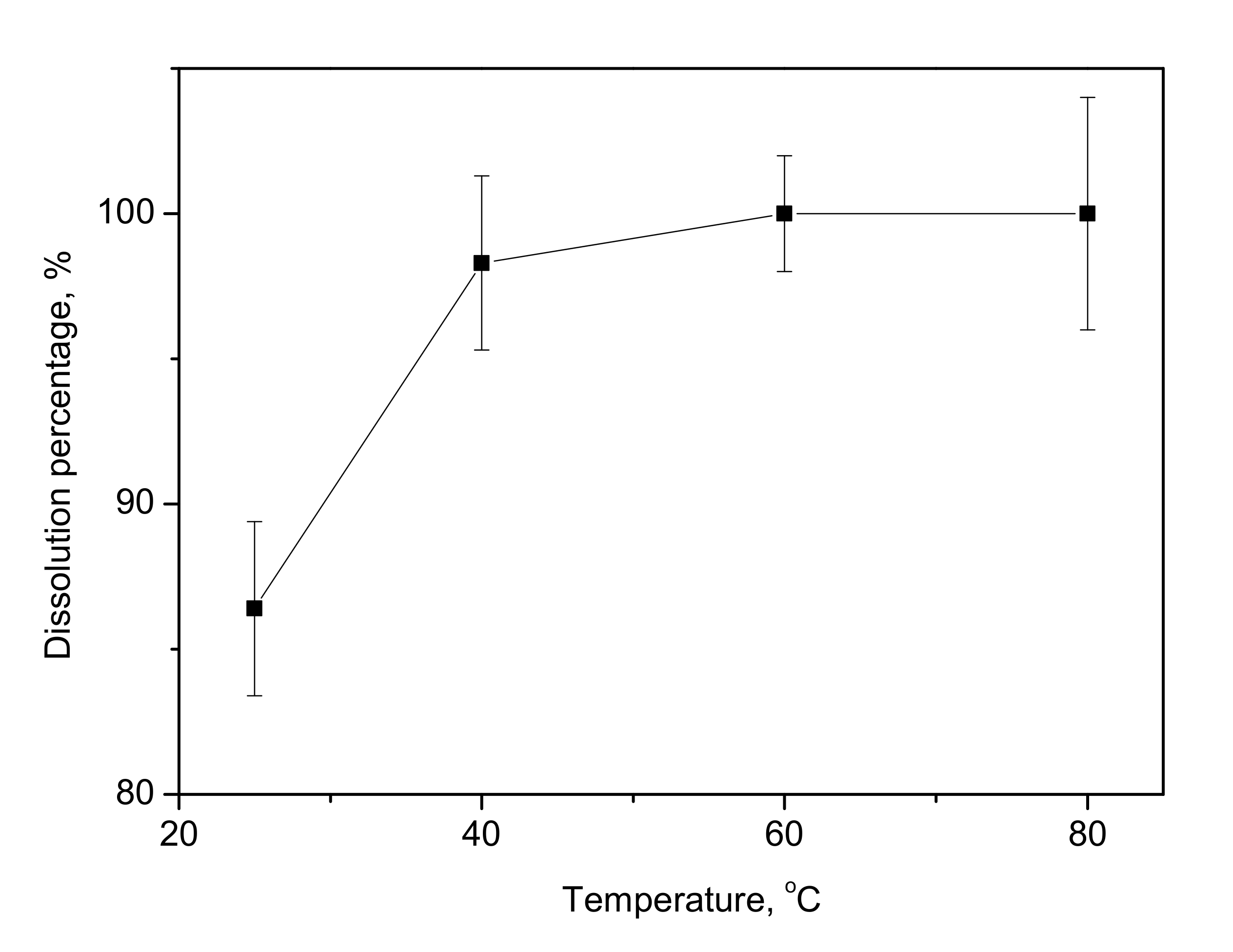
3.6. Effect of Reaction Temperature
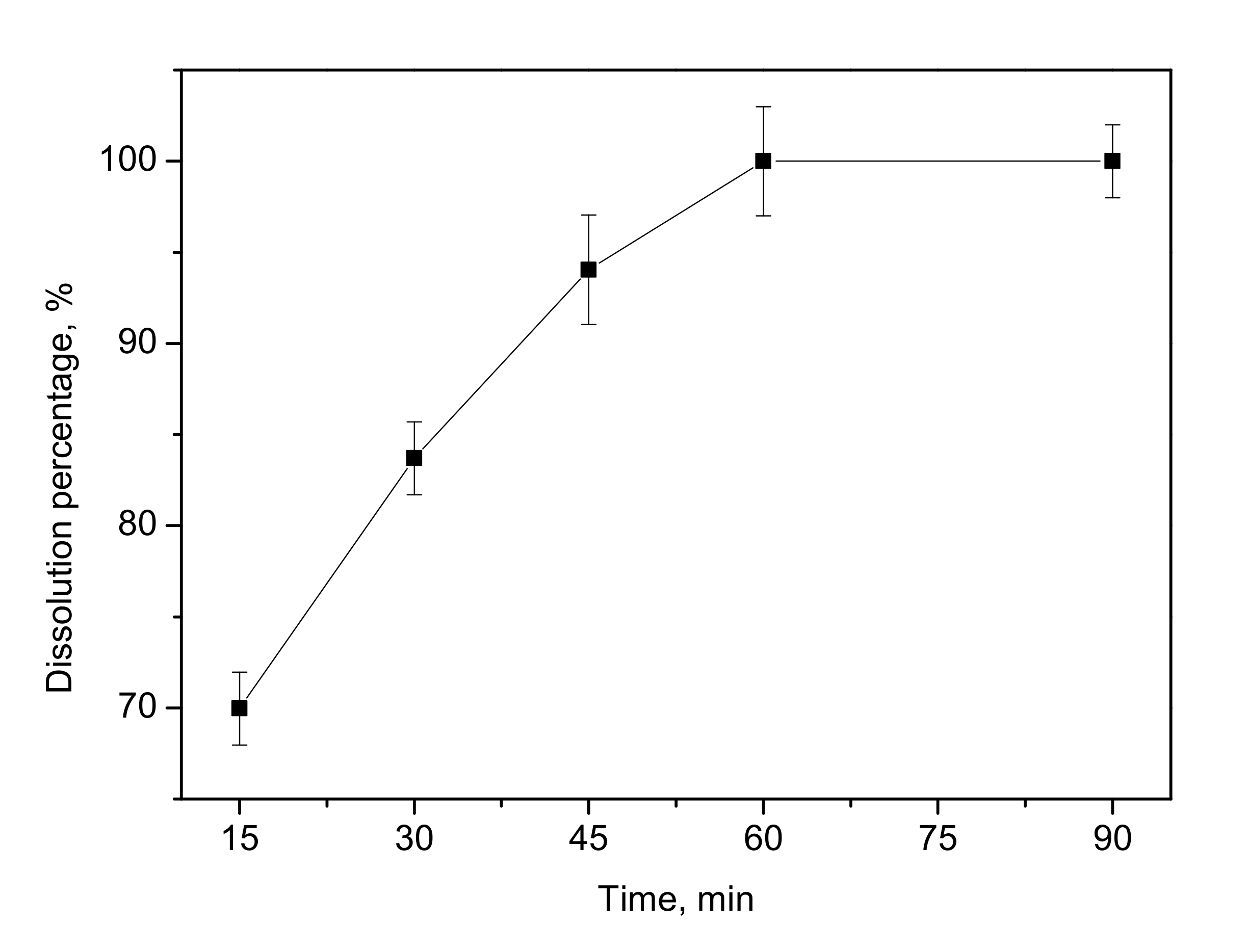
3.7. Effect of Reaction Time and Stirring Speed
3.8. Comparison between the Solvent Leaching and HCl–H2O2–H2O Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Afolabi, A.S.; Nkobane, M.P.; Abdulkareem, A.S. Development of PGMs and chrome extraction circuit from UG-2 ore. In Proceedings of the World Congress on Engineering, London, UK, 4–6 July 2012; Volume 3. [Google Scholar]
- Peng, Z.; Li, Z.; Lin, X.; Tang, H.; Ye, L.; Ma, Y.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical Recovery of Platinum Group Metals from Spent Catalysts. JOM 2017, 69, 1553–1562. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Cox, M. Solvent extraction in hydrometallurgy. In Solvent Extraction Principles and Practice, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 455–505. [Google Scholar]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An Emerging Branch of Extractive Metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, R.W.; Jang, S.S.; Wong, C.P.; Hong, J.I. “Organic Aqua Regia”-Powerful Liquids for Dissolving Noble Metals. Angew. Chem. Int. 2010, 49, 7929–7932. [Google Scholar] [CrossRef] [PubMed]
- Forte, F.; Riaño, S.; Binnemans, K. Dissolution of noble metals in highly concentrated acidic salt solutions. Chem. Commun. 2020, 56, 8230–8232. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y. Dissolution of Noble Metals in Halogen-Halide-Polar Organic Solvent Systems. J. Chem. Soc. Chem. Commun. 1992, 5, 426–427. [Google Scholar] [CrossRef]
- Van den Bossche, A.; De Witte, E.; Dehaen, W.; Binnemans, K. Trihalide ionic liquids as non-volatile oxidizing solvents for metals. Green Chem. 2018, 20, 3327–3338. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sonu, C.H.; Lee, M.S. Separation of platinum(IV) and palladium(II) from concentrated hydrochloric acid solutions by mixtures of amines with neutral extractants. J. Ind. Eng. Chem. 2015, 32, 238–245. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Lee, M.S. Solvent extraction of Hydrochloric Acid Using Commercial Extractants and Synthesized Ionic Liquids. J. Korean Inst. Resour. Recycl. 2020, 9, 79–87. [Google Scholar]
- Sarangi, K.; Padhan, E.; Sarma, P.V.R.B.; Park, K.H.; Das, R.P. Removal/recovery of hydrochloric acid using Alamine 336, Aliquat 336, TBP and Cyanex 923. Hydrometallurgy 2006, 84, 125–129. [Google Scholar] [CrossRef]
- Truong, H.T.; Lee, M.S. Separation of Pd(II) and Pt(IV) from hydrochloric acid solutions by solvent extraction with Cyanex 301 and LIX 63. Miner. Eng. 2018, 115, 13–20. [Google Scholar] [CrossRef]
- Rajiv Gandhi, M.; Yamada, M.; Haga, K.; Shibayama, A. Synthesis of pincer-type extractants for selective extraction of palladium from PGMs: An improved liquid-liquid extraction approach to current refining processes. Sci. Rep. 2017, 7, 8709. [Google Scholar] [CrossRef]
- Tomijima, H.; Tsukui, K.; Okoshi, Y. Direct Dissolution of Water-Insoluble Uranium Compounds by Contact with Neutral Organic Solvents Pretreated with Nitric Acid. U.S. Patent 3,288,568, 29 November 1966. [Google Scholar]
- Duan, W.H.; Zhu, L.Y.; Jing, S.; Zhu, Y.J.; Chen, J. Study on properties of TBP-HNO3 complex used for direct dissolution of lanthanide and actinide oxides in supercritical fluid CO2. Chin. J. Chem. 2007, 25, 319–322. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, S.; Sampath, M.; Sivakumar, D.; Mudali, U.K.; Natarajan, R. Direct dissolution of UO2 and in situ extraction by TBP-HNO3 and TiAP-HNO3 organic solutions at atmospheric pressure. J. Radioanal. Nucl. Chem. 2011, 288, 443–445. [Google Scholar] [CrossRef]
- Tong, Q.L.; Fan, Z.F.; Yang, J.W.; Li, Q.; Chen, Y.X.; Cheng, M.S.; Liu, Y. The Selective Oxidation of Sulfides to Sulfoxides or Sulfones with Hydrogen Peroxide Catalyzed by a Dendritic Phosphomolybdate Hybrid. Catalysts 2019, 9, 791. [Google Scholar] [CrossRef]
- MacRuary, K.J.; Gordon, R.J.; Grant, R.A.; Woollam, S.; Ellis, R.J.; Tasker, P.A.; Love, J.B.; Morrison, C.A. On the Extraction of HCl and H2PtCl6 by Tributyl Phosphate: A Mode of Action Study. Solvent Extr. Ion Exch. 2017, 35, 531–548. [Google Scholar] [CrossRef]
- Harjanto, S.; Cao, Y.; Shibayama, A.; Naitoh, I.; Nanami, T.; Kasahara, K.; Okumura, Y.; Liu, K.; Fujita, T. Leaching of Pt, Pd and Rh from Automotive Catalyst Residue in Various Chloride Based Solutions. Mater. Trans. 2006, 47, 129–135. [Google Scholar] [CrossRef]
- Sadeghi, N.; Keshavarz Alamdari, E.; Saeedi, M. Extraction mechanism of palladium from chloride solution by tbp-kerosene. Metall. Eng. 2014, 17, 3–8. [Google Scholar]
- Debethune, A.J.; Loud, N.A.S. Standard Aqueous Electrode Potentials and Temperature Coefficients at 25 °C; Hampel, C.A., Ed.; The Electrochemical Society: Philadelphia, PA, USA, 1965. [Google Scholar]
- Silberberg, M.S. Principles of General Chemistry, 1st ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Wei, W.; Cho, C.W.; Kim, S.; Song, M.H.; Bediako, J.K.; Yun, Y.S. Selective recovery of Au(III), Pt(IV), and Pd(II) from aqueous solutions by liquid–liquid extraction using ionic liquid Aliquat-336. J. Mol. Liq. 2016, 216, 18–24. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kumar, B.N.; Lee, M.S. Selective recovery of Fe(III), Pd(II), Pt(IV), Rh(III) and Ce(III) from simulated leach liquors of spent automobile catalyst by solvent extraction and cementation. Korean J. Chem. Eng. 2016, 33, 2684–2690. [Google Scholar] [CrossRef]
- Barakat, M.A.; Mahmoud, M.H.H.; Mahrous, Y.S. Recovery and separation of palladium from spent catalyst. Appl. Catal. A Gen. 2006, 301, 182–186. [Google Scholar] [CrossRef]
- Prasetyo, E.; Anderson, C. Platinum Group Elements Recovery from Used Catalytic Converters by Acidic Fusion and Leaching. Metals 2020, 10, 485. [Google Scholar] [CrossRef]
- Kim, Y.E.; Byun, M.Y.; Baek, J.H.; Lee, K.Y.; Lee, M.S. Recovery of Metallic Pd with High Purity from Pd/Al2O3 Catalyst by Hydrometallurgy in HCl. Clean Technol. 2020, 26, 270–278. [Google Scholar]
- Ding, Y.; Zheng, H.; Li, J.; Zhang, S.; Liu, B.; Ekberg, C. An Efficient Leaching of Palladium from Spent Catalysts through Oxidation with Fe(III). Materials 2019, 12, 1205. [Google Scholar] [CrossRef]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Tran, T.T.; Liu, Y.; Lee, M.S. Recovery of pure molybdenum and vanadium compounds from spent petroleum catalysts by treatment with ionic liquid solution in the presence of oxidizing agent. Sep. Purif. Technol. 2021, 255, 117734. [Google Scholar] [CrossRef]



| Leachate | Metal | Dissolution | Ref. |
|---|---|---|---|
| Organic Aqua Regia—SOCl2 and some organic solvents/reagents (pyridine, N,N-dimethylformamide, and imidazole) (3:1 v/v) | Au, Pd, Ag | Selective leaching Pd over Pt. Dissolution speed was the following: Au: 2.5 mg/180 s Ag: 1.5 mg/80 s Pd: 1.5 mg/120 s Pt: 0 mg at room temperature or even at 70 °C (reflux) for 1 week. | [6] |
| Highly concentrated solutions of AlCl3·6H2O and Al(NO3)3·9H2O (T = 80 °C, S/W = 35 g/g, 22 wt. % water added, stirring speed = 350 rpm) | Au and PGMs | 95% Pd was leached in 15 min at 80 °C. Au dissolution from wires in 24 h. 64% Pt was dissolved after a couple of days. | [7] |
Halogen–Halide–Polar Organic Solvent Systems
| Pd, Ag and Au | Chlorine–acetonitrile systems, quaternary ammonium chlorides and amine hydrogen chlorides are effective systems. | [8] |
| Various ionic liquids containing a trihalide anion [X3]− (X = Cl, Br or I), e.g., tribromide IL ([P44410][Br3]) | Au, Pd, Pt, Rh | Selective leaching of Au and Pd (47 h of contact with the IL at room temperature). The dissolution of the metals was checked visually. | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.N.H.; Song, S.J.; Lee, M.S. Dissolution of Palladium Metal in Solvent Leaching System with the Presence of Oxidizing Agent. Metals 2021, 11, 575. https://doi.org/10.3390/met11040575
Nguyen VNH, Song SJ, Lee MS. Dissolution of Palladium Metal in Solvent Leaching System with the Presence of Oxidizing Agent. Metals. 2021; 11(4):575. https://doi.org/10.3390/met11040575
Chicago/Turabian StyleNguyen, Viet Nhan Hoa, Si Jeong Song, and Man Seung Lee. 2021. "Dissolution of Palladium Metal in Solvent Leaching System with the Presence of Oxidizing Agent" Metals 11, no. 4: 575. https://doi.org/10.3390/met11040575
APA StyleNguyen, V. N. H., Song, S. J., & Lee, M. S. (2021). Dissolution of Palladium Metal in Solvent Leaching System with the Presence of Oxidizing Agent. Metals, 11(4), 575. https://doi.org/10.3390/met11040575







