A First-Principles Study of Hydrogen Desorption from High Entropy Alloy TiZrVMoNb Hydride Surface
Abstract
1. Introduction
2. Computational Details
3. Results and Discussions
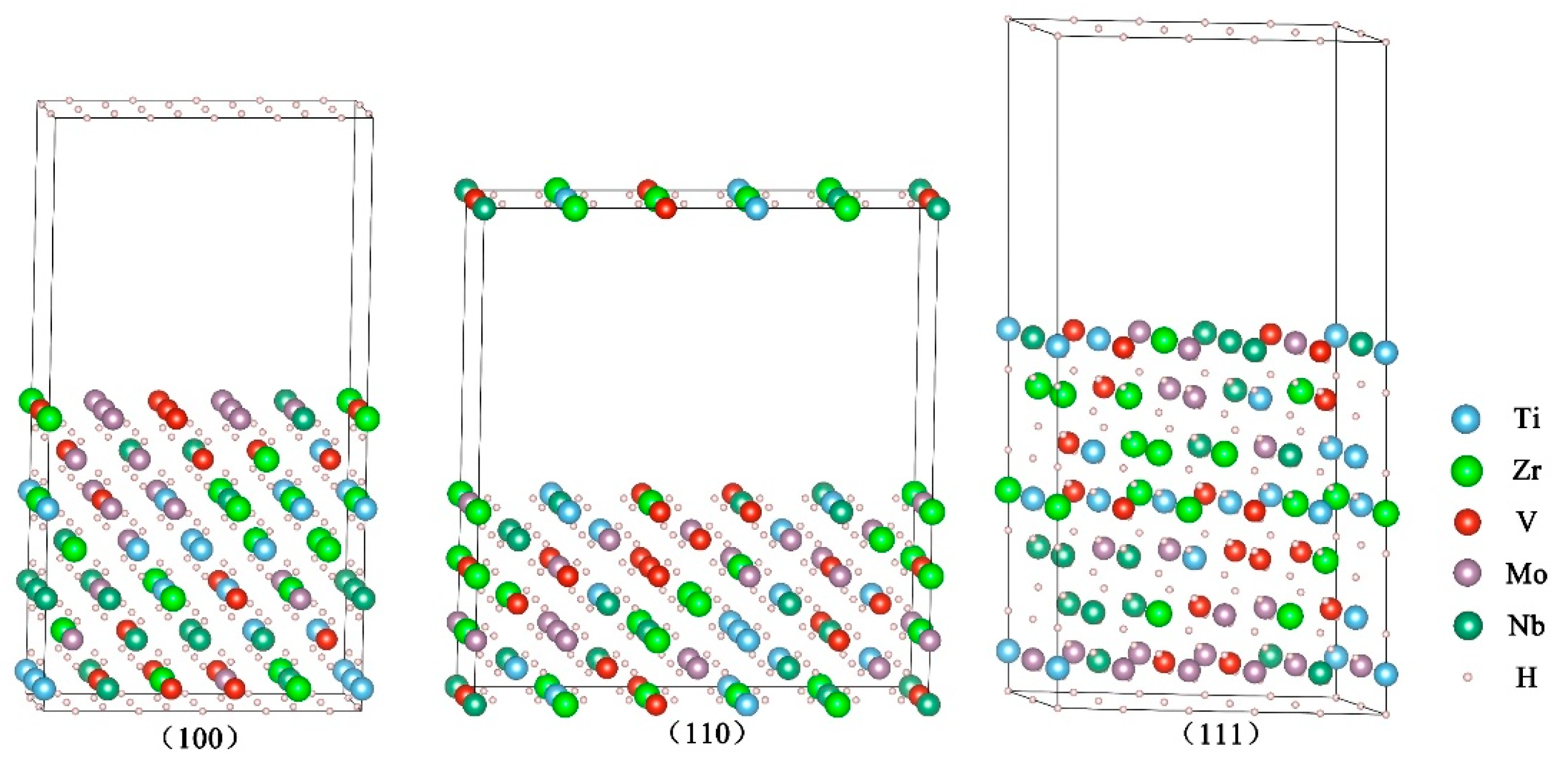
3.1. Surface Energies of the Clean HEA TiZrVMoNb Hydrides
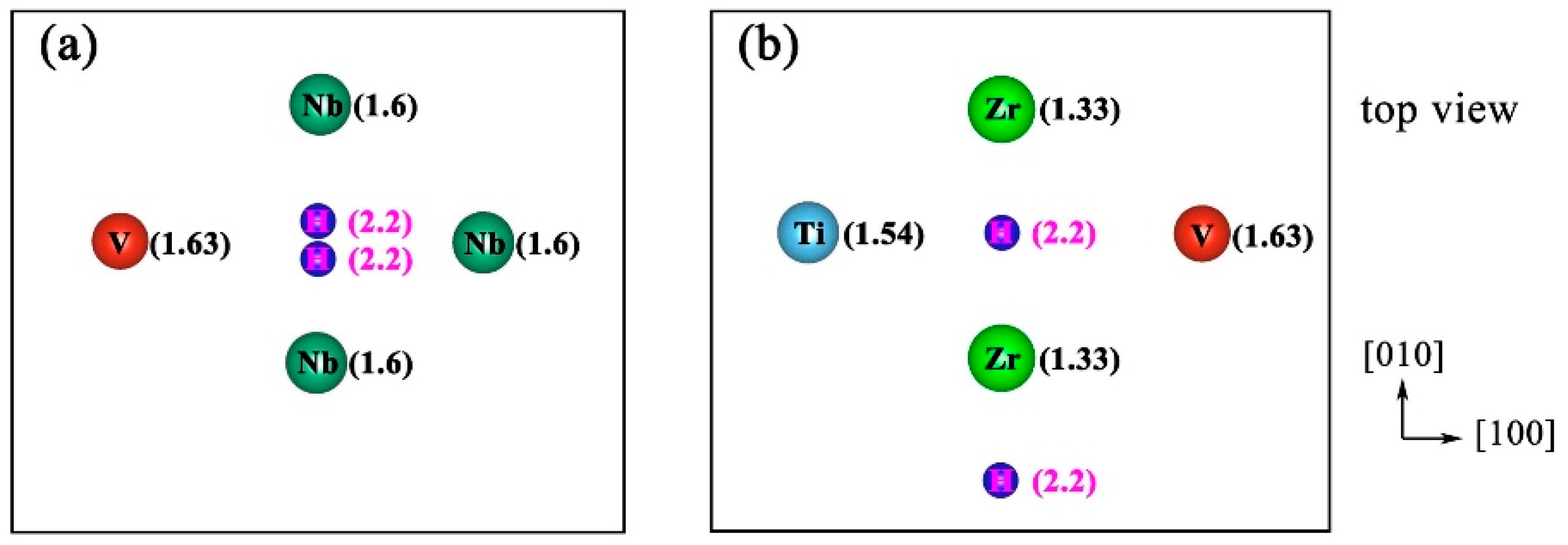
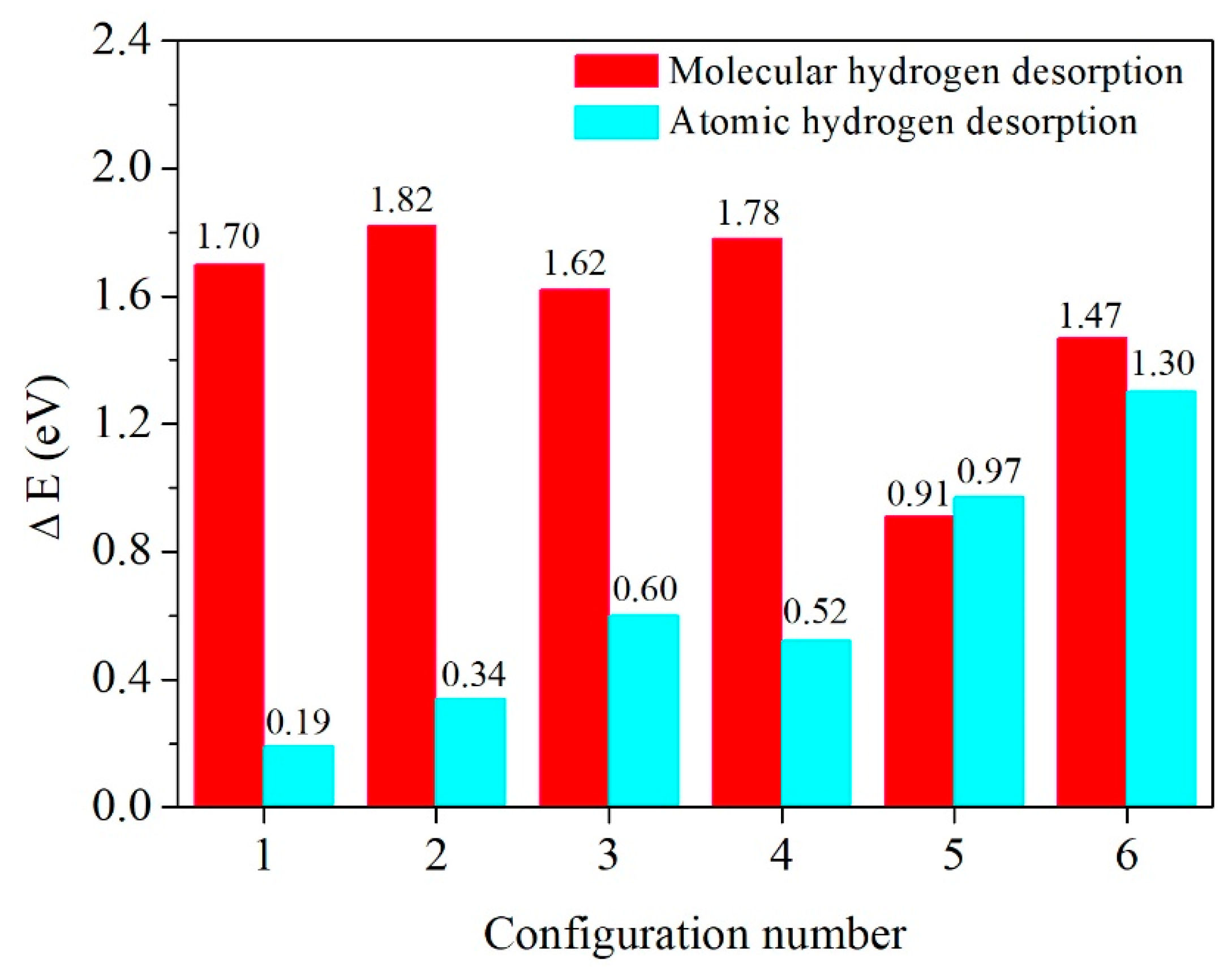
3.2. Molecular Hydrogen Desorption
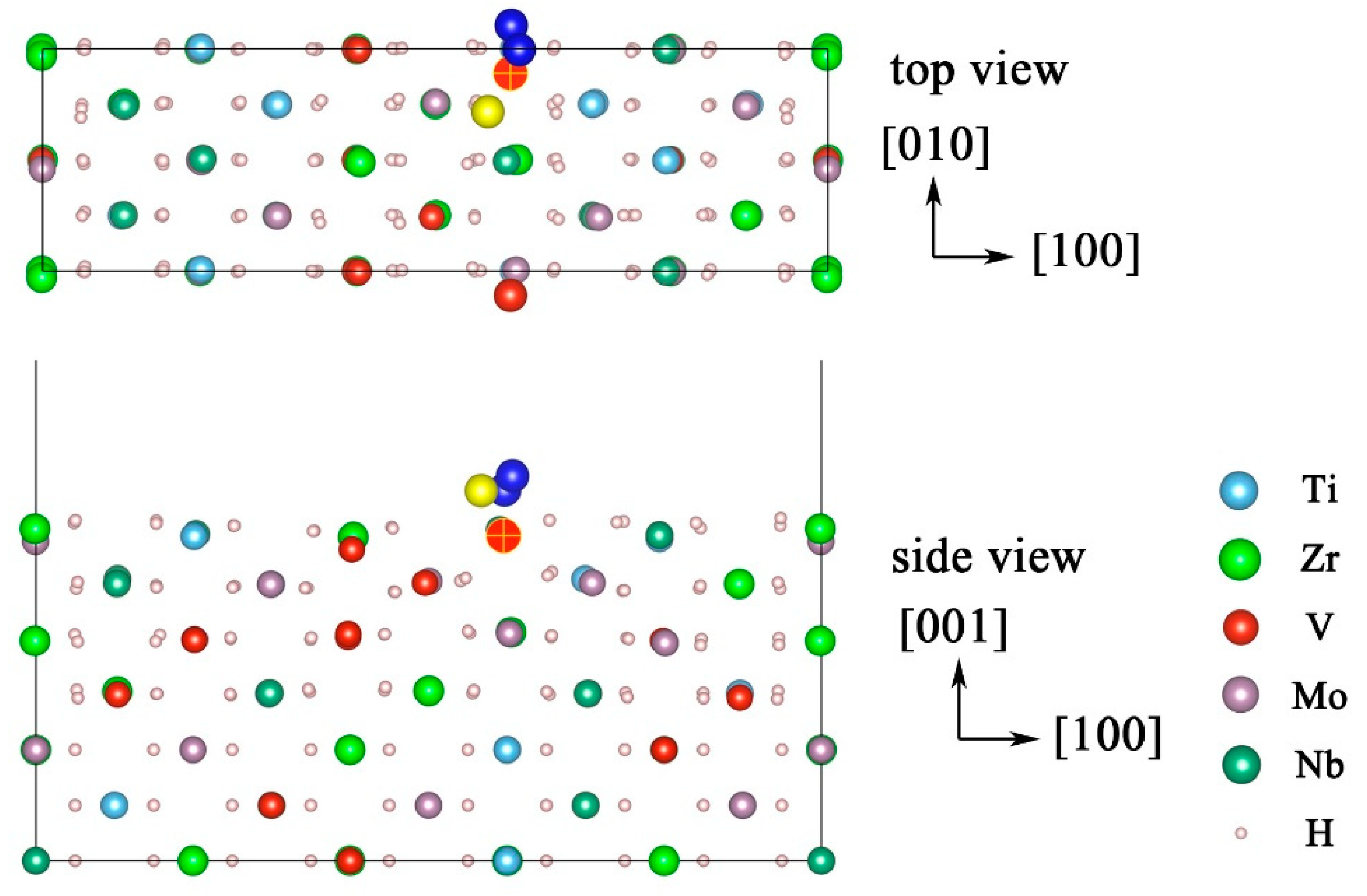
3.3. Atomic Hydrogen Desorption
3.4. A Comparison of Molecular and Atomic Hydrogen Desorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, I.P. Hydrogen the fuel for 21st century. Int. J. Hydrog. Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrog. Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Lai, Q.W.; Sun, Y.H.; Wang, T.; Modi, P.; Cazorla, C.; Demirci, U.B.; Ares Fernandez, J.R.; Leardini, F.; Aguey-Zinsou, K.F. How to Design Hydrogen Storage Materials? Fundamentals, Synthesis, and Storage Tanks. Adv. Sustain. Syst. 2019, 3, 64. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Conv. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Li, X.Q.; Li, W.; Irving, D.L.; Varga, L.K.; Vitos, L.; Schonecker, S. Ductile and brittle crack-tip response in equimolar refractory high-entropy alloys. Acta Mater. 2020, 189, 174–187. [Google Scholar] [CrossRef]
- Zhang, W.R.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China-Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Li, X.Q.; Wei, D.X.; Vitos, L.; Lizarraga, R. Micro-mechanical properties of new alternative binders for cemented carbides: CoCrFeNiWx high-entropy alloys. J. Alloy. Compd. 2020, 820, 8. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Li, X.Q. First-principles study of the third-order elastic constants and related anharmonic properties in refractory high-entropy alloys. Acta Mater. 2018, 142, 29–36. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage-past, recent progress and future outlook. J. Alloy. Compd. 2020, 827, 39. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, S.K.; Sheu, J.H.; Lin, J.T.; Lin, W.E.; Yeh, J.W.; Lin, S.J.; Liou, T.H.; Wang, C.W. Hydrogen storage properties of multi-principal-component CoFeMnTixVyZrz alloys. Int. J. Hydrog. Energy 2010, 35, 9046–9059. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Structure and hydrogen storage properties of a high entropy ZrTiVCrFeNi alloy synthesized using Laser Engineered Net Shaping (LENS). Int. J. Hydrog. Energy 2013, 38, 12180–12189. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef]
- Hu, J.T.; Shen, H.H.; Jiang, M.; Gong, H.F.; Xiao, H.Y.; Liu, Z.J.; Sun, G.A.; Zu, X.T. A DFT Study of Hydrogen Storage in High-Entropy Alloy TiZrHfScMo. Nanomaterials 2019, 9, 461. [Google Scholar] [CrossRef]
- Shen, H.H.; Zhang, J.W.; Hu, J.T.; Zhang, J.C.; Mao, Y.W.; Xiao, H.Y.; Zhou, X.S.; Zu, X.T. A Novel TiZrHfMoNb High-Entropy Alloy for Solar Thermal Energy Storage. Nanomaterials 2019, 9, 248. [Google Scholar] [CrossRef]
- Zhang, C.; Song, A.N.; Yuan, Y.; Wu, Y.; Zhang, P.L.; Lu, Z.P.; Song, X.P. Study on the hydrogen storage properties of a TiZrNbTa high entropy alloy. Int. J. Hydrog. Energy 2020, 45, 5367–5374. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sorby, M.H.; Sahlberg, M.; Hauback, B.C. Counting electrons—A new approach to tailor the hydrogen sorption properties of high-entropy alloys. Acta Mater. 2019, 175, 121–129. [Google Scholar] [CrossRef]
- Zepon, G.; Leiva, D.R.; Strozi, R.B.; Bedoch, A.; Figueroa, S.J.A.; Ishikawa, T.T.; Botta, W.J. Hydrogen-induced phase transition of MgZrTiFe0.5Co0.5Ni0.5 high entropy alloy. Int. J. Hydrog. Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinie, J.P.; Perriere, L.; Guillot, I.; Bourgon, J.; Moller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloy. Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sahlberg, M.; Sorby, M.H.; Hauback, B.C. Hydrogen storage in high-entropy alloys with varying degree of local lattice strain. Int. J. Hydrog. Energy 2019, 44, 29140–29149. [Google Scholar] [CrossRef]
- Marques, F.; Pinto, H.C.; Figueroa, S.J.A.; Winkelmann, F.; Felderhoff, M.; Botta, W.J.; Zepon, G. Mg-containing multi-principal element alloys for hydrogen storage: A study of the MgTiNbCr0.5Mn0.5Ni0.5 and Mg0.68TiNbNi0.55 compositions. Int. J. Hydrog. Energy 2020, 45, 19539–19552. [Google Scholar] [CrossRef]
- Strozi, R.B.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Synthesis and hydrogen storage behavior of Mg-V-Al-Cr-Ni high entropy alloys. Int. J. Hydrog. Energy 2021, 46, 2351–2361. [Google Scholar] [CrossRef]
- De Marco, M.O.; Li, Y.T.; Li, H.W.; Edalati, K.; Floriano, R. Mechanical Synthesis and Hydrogen Storage Characterization of MgVCr and MgVTiCrFe High-Entropy Alloy. Adv. Eng. Mater. 2020, 22, 9. [Google Scholar] [CrossRef]
- Shen, H.; Hu, J.; Li, P.; Huang, G.; Zhang, J.; Zhang, J.; Mao, Y.; Xiao, H.; Zhou, X.; Zu, X.; et al. Compositional dependence of hydrogenation performance of Ti-Zr-Hf-Mo-Nb high-entropy alloys for hydrogen/tritium storage. J. Mater. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Mohammadi, A.; Li, Y.T.; Zepon, G.; Li, H.W.; Edalati, K. Reversible room temperature hydrogen storage in high-entropy alloy TiZrCrMnFeNi. Scr. Mater. 2020, 178, 387–390. [Google Scholar] [CrossRef]
- Montero, J.; Ek, G.; Laversenne, L.; Nassif, V.; Zepon, G.; Sahlberg, M.; Zlotea, C. Hydrogen storage properties of the refractory Ti-V-Zr-Nb-Ta multi-principal element alloy. J. Alloy. Compd. 2020, 835, 7. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Xiao, H.; Xie, L.; Shen, H.; Li, P.; Zhang, J.; Gong, H.; Zu, X. A Density Functional Theory Study of the Hydrogen Absorption in High Entropy Alloy TiZrHfMoNb. Inorg. Chem. 2020, 59, 9774–9782. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Gommel, C.; Borkhart, C.; Fromm, E. Absorption and desorption kinetics of hydrogen storage alloys. J. Alloy. Compd. 1996, 238, 193–201. [Google Scholar] [CrossRef]
- Chou, K.C.; Li, Q.; Lin, Q.; Jiang, L.J.; Xu, K.D. Kinetics of absorption and desorption of hydrogen in alloy powder. Int. J. Hydrog. Energy 2005, 30, 301–309. [Google Scholar] [CrossRef]
- German, E.; Verdinelli, V.; Luna, C.R.; Juan, A.; Sholl, D. A Theoretical Study of the Effect of Zr-, Nb-Doped and Vacancy-like Defects on H Desorption on MgH2 (110) Surface. J. Phys. Chem. C 2014, 118, 4231–4237. [Google Scholar] [CrossRef]
- Ri, S.I.; Um, K.J.; Wi, J.H.; Kim, J.C.; Kim, N.H. Effects of single- and co-substitution of Ti and Fe on vacancy formation and dehydrogenation from MgH2 (110) surface: Ab initio study. Int. J. Hydrog. Energy 2019, 44, 22761–22769. [Google Scholar] [CrossRef]
- Dai, J.H.; Song, Y.; Yang, R. First Principles Study on Hydrogen Desorption from a Metal (=Al, Ti, Mn, Ni) Doped MgH2 (110) Surface. J. Phys. Chem. C 2010, 114, 11328–11334. [Google Scholar] [CrossRef]
- AlMatrouk, H.S.; Chihaia, V. Theoretical study on the effects of the magnesium hydride doping with cobalt and nickel on the hydrogen release. Int. J. Hydrog. Energy 2015, 40, 5319–5325. [Google Scholar] [CrossRef]
- Kumar, E.M.; Rajkamal, A.; Thapa, R. Screening based approach and dehydrogenation kinetics for MgH2: Guide to find suitable dopant using first-principles approach. Sci. Rep. 2017, 7, 11. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Project augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- White, J.A.; Bird, D.M. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys. Rev. B 1994, 50, 4954–4957. [Google Scholar] [CrossRef]
- Wu, Z.G.; Cohen, R.E. More accurate generalized gradient approximation for solids. Phys. Rev. B 2006, 73, 6. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, J.; Zhang, J.; Xiao, H.; Xie, L.; Shen, H.; Li, P.; Sun, G.; Zu, X. Theoretical Combined Experimental Study of Unique He Behaviors in High-Entropy Alloys. Inorg. Chem. 2021, 60, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kivy, M.B.; Zaeem, M.A. Competition between formation of Al2O3 and Cr2O3 in oxidation of Al0.3CoCrCuFeNi high entropy alloy: A first-principles study. Scr. Mater. 2019, 168, 139–143. [Google Scholar] [CrossRef]
- Schlapbach, L. Surface properties and activation. In Hydrogen in Intermetallic Compounds II; Schlapbach, L., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 72. [Google Scholar] [CrossRef]
- WebElements. Available online: https://www.webelements.com (accessed on 19 March 2021).
- Ren, X.L.; Shi, P.H.; Zhang, W.W.; Wu, X.Y.; Xu, Q.; Wang, Y.X. Swamps of hydrogen in equiatomic FeCuCrMnMo alloys: First-principles calculations. Acta Mater. 2019, 180, 189–198. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]

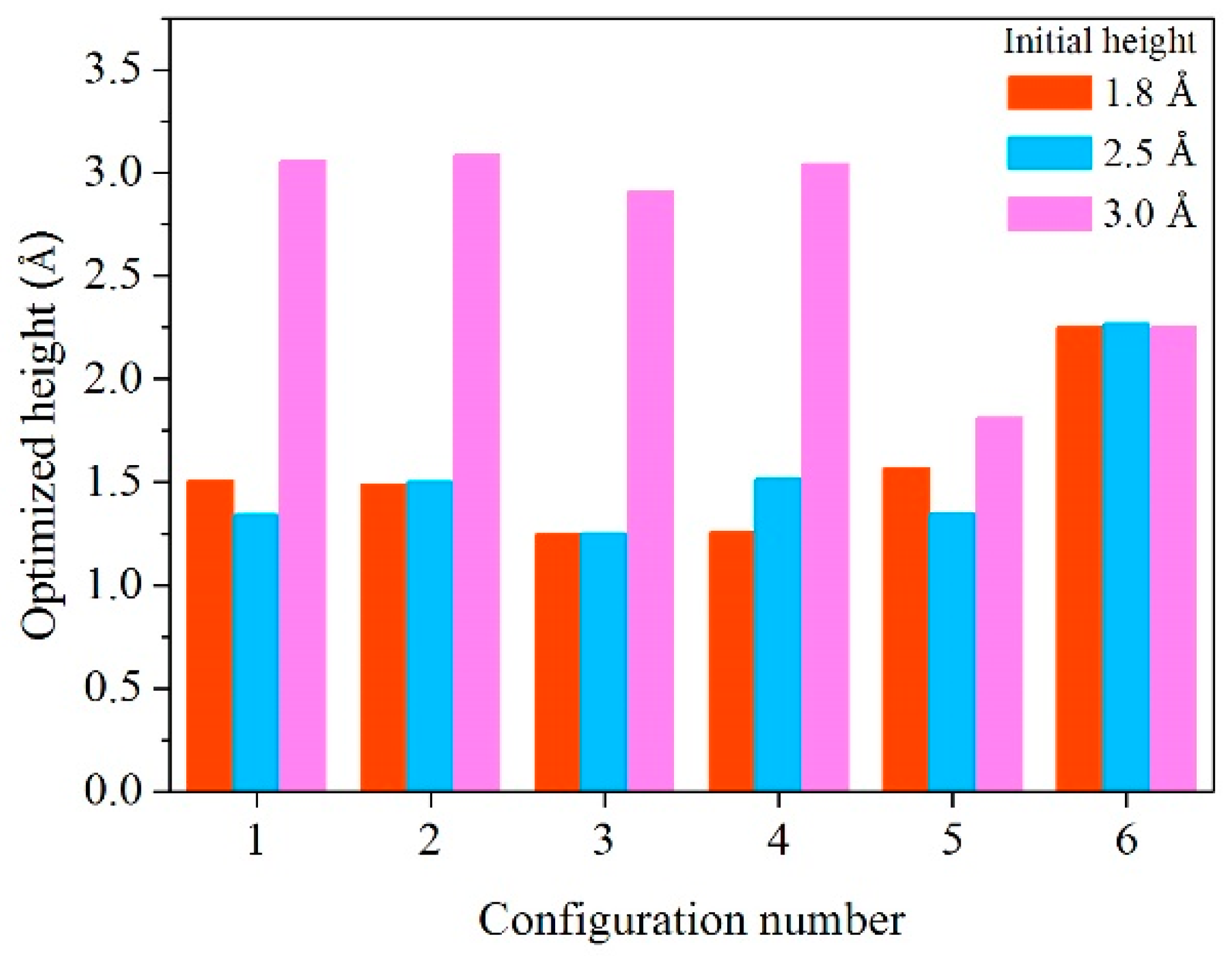
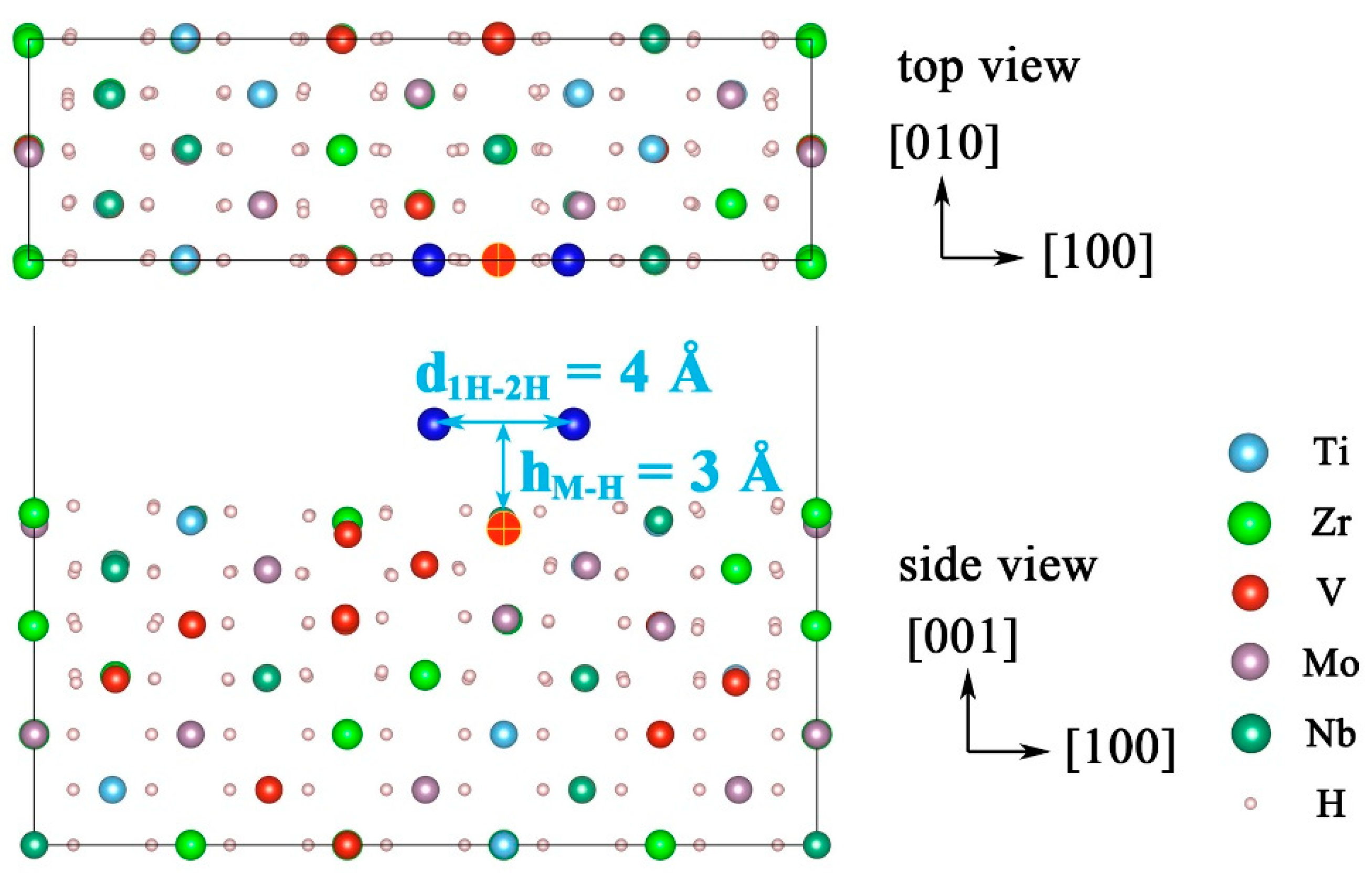

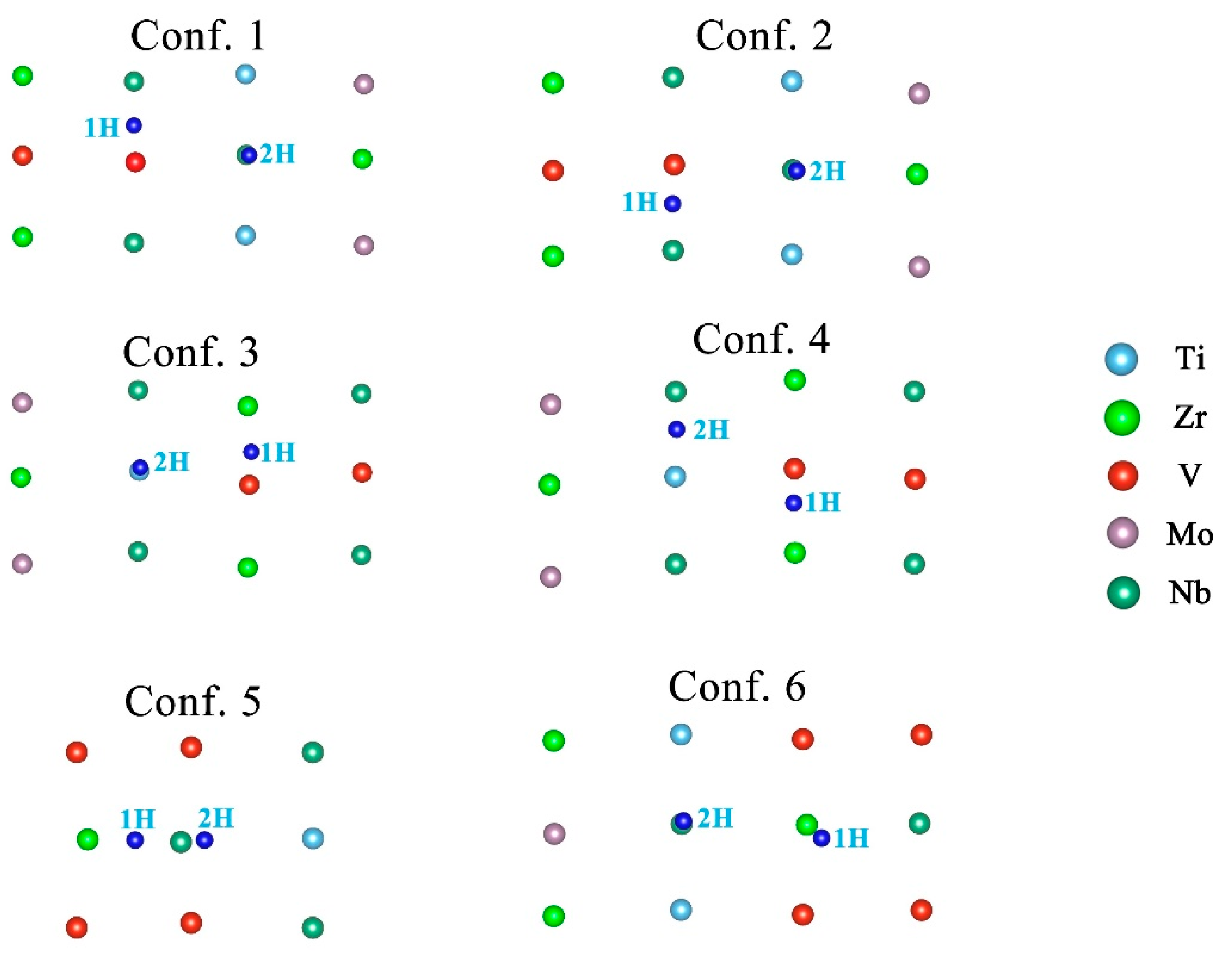
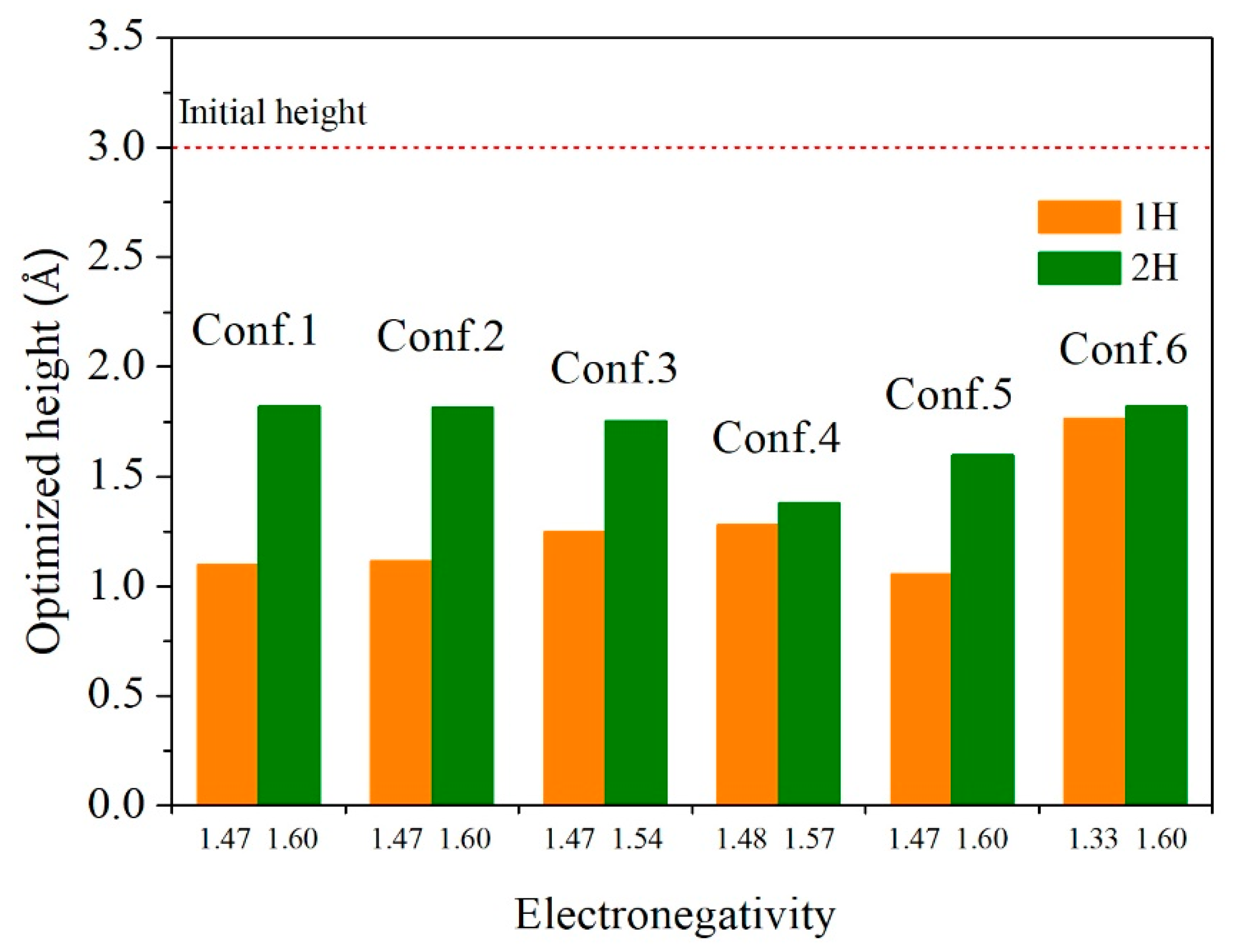
| Configuration | hM-H (Å) | h’M-1H (Å) | h’M-2H (Å) | d’1H-2H (Å) | d’3H-1H (Å) | d’3H-2H (Å) |
|---|---|---|---|---|---|---|
| 1 | 1.8 | 1.727 | 1.282 | 0.816 | 1.992 | 2.617 |
| 2.5 | 1.546 | 1.141 | 0.835 | 2.553 | 2.030 | |
| 3.0 | 3.039 | 3.077 | 0.754 | - | - | |
| 2 | 1.8 | 1.704 | 1.276 | 0.856 | 1.937 | 2.590 |
| 2.5 | 1.705 | 1.299 | 0.855 | 1.970 | 2.592 | |
| 3.0 | 3.102 | 3.069 | 0.755 | - | - | |
| 3 | 1.8 | 1.399 | 1.095 | 2.727 | - | - |
| 2.5 | 1.397 | 1.105 | 2.717 | - | - | |
| 3.0 | 2.939 | 2.879 | 0.753 | - | - | |
| 4 | 1.8 | 1.364 | 1.149 | 2.614 | - | - |
| 2.5 | 1.674 | 1.351 | 0.976 | - | - | |
| 3.0 | 3.006 | 3.083 | 0.757 | - | - | |
| 5 | 1.8 | 1.656 | 1.472 | 0.826 | 2.763 | 2.153 |
| 2.5 | 1.643 | 1.048 | 0.843 | 2.731 | 2.230 | |
| 3.0 | 1.981 | 1.641 | 0.805 | 2.819 | 2.230 | |
| 6 | 1.8 | 2.238 | 2.243 | 0.780 | - | - |
| 2.5 | 2.267 | 2.267 | 0.777 | - | - | |
| 3.0 | 2.251 | 2.249 | 0.784 | - | - |
| Configuration | h’M-1H (Å) | h’M-2H (Å) | d’3H-1H (Å) | d’3H-2H (Å) | ΔE (eV) |
|---|---|---|---|---|---|
| 1 | 1.100 | 1.818 | - | - | 0 |
| 2 | 1.116 | 1.815 | - | - | 0.15 |
| 3 | 1.250 | 1.755 | - | - | 0.41 |
| 4 | 1.281 | 1.379 | - | - | 0.33 |
| 5 | 1.055 | 1.600 | 2.083 | 2.519 | 0.78 |
| 6 | 1.776 | 1.818 | - | - | 1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Hu, J.; Xiao, H.; Shen, H.; Xie, L.; Sun, G.; Zu, X. A First-Principles Study of Hydrogen Desorption from High Entropy Alloy TiZrVMoNb Hydride Surface. Metals 2021, 11, 553. https://doi.org/10.3390/met11040553
Zhang J, Hu J, Xiao H, Shen H, Xie L, Sun G, Zu X. A First-Principles Study of Hydrogen Desorption from High Entropy Alloy TiZrVMoNb Hydride Surface. Metals. 2021; 11(4):553. https://doi.org/10.3390/met11040553
Chicago/Turabian StyleZhang, Jinjing, Jutao Hu, Haiyan Xiao, Huahai Shen, Lei Xie, Guangai Sun, and Xiaotao Zu. 2021. "A First-Principles Study of Hydrogen Desorption from High Entropy Alloy TiZrVMoNb Hydride Surface" Metals 11, no. 4: 553. https://doi.org/10.3390/met11040553
APA StyleZhang, J., Hu, J., Xiao, H., Shen, H., Xie, L., Sun, G., & Zu, X. (2021). A First-Principles Study of Hydrogen Desorption from High Entropy Alloy TiZrVMoNb Hydride Surface. Metals, 11(4), 553. https://doi.org/10.3390/met11040553






