Superior Hydrogen Sorption Kinetics of Ti0.20Zr0.20Hf0.20Nb0.40 High-Entropy Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Hydrogenation Performance Test
2.3. Sample Characterization
3. Results
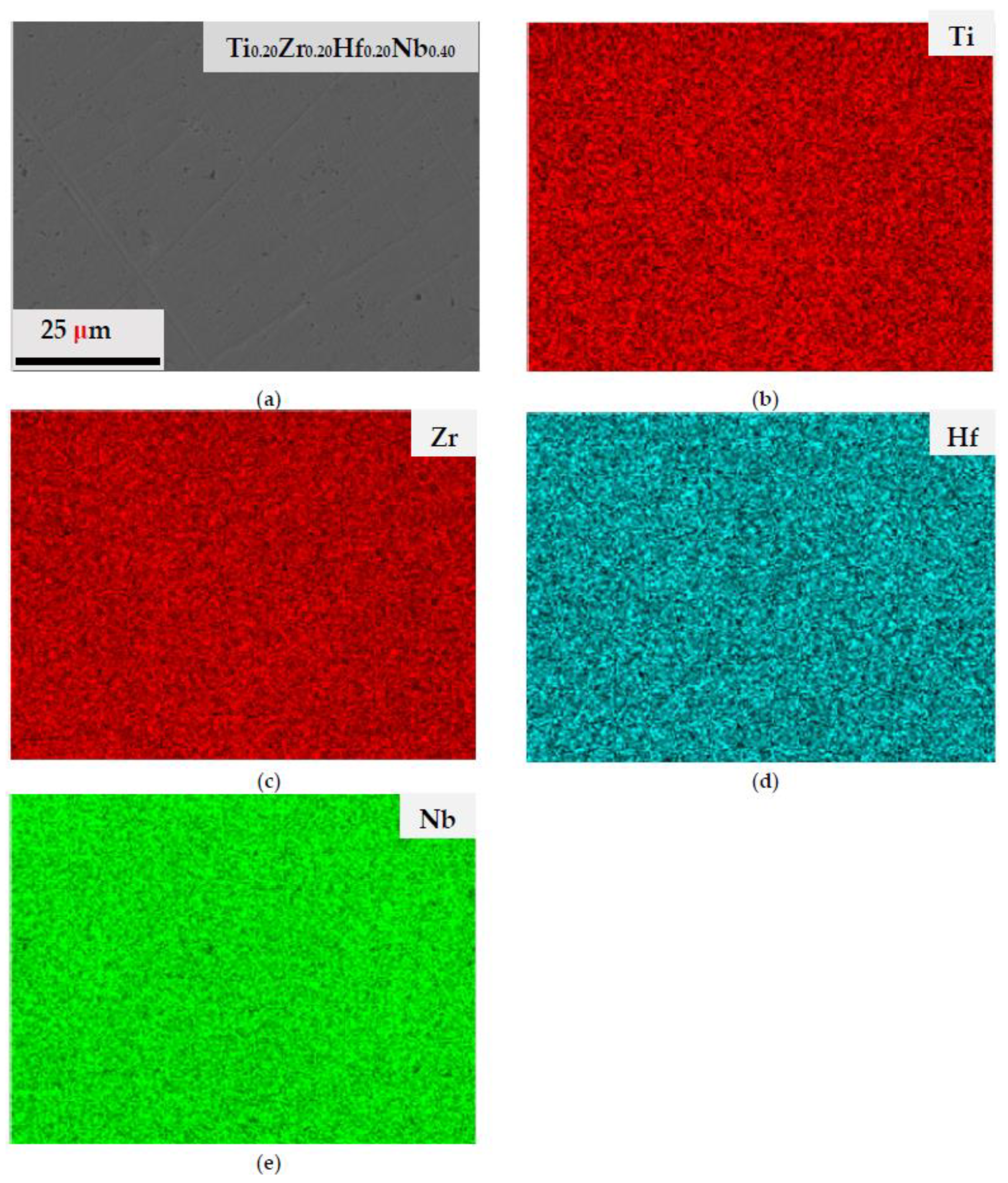
3.1. Microstructure of the As-Cast HEA Alloy
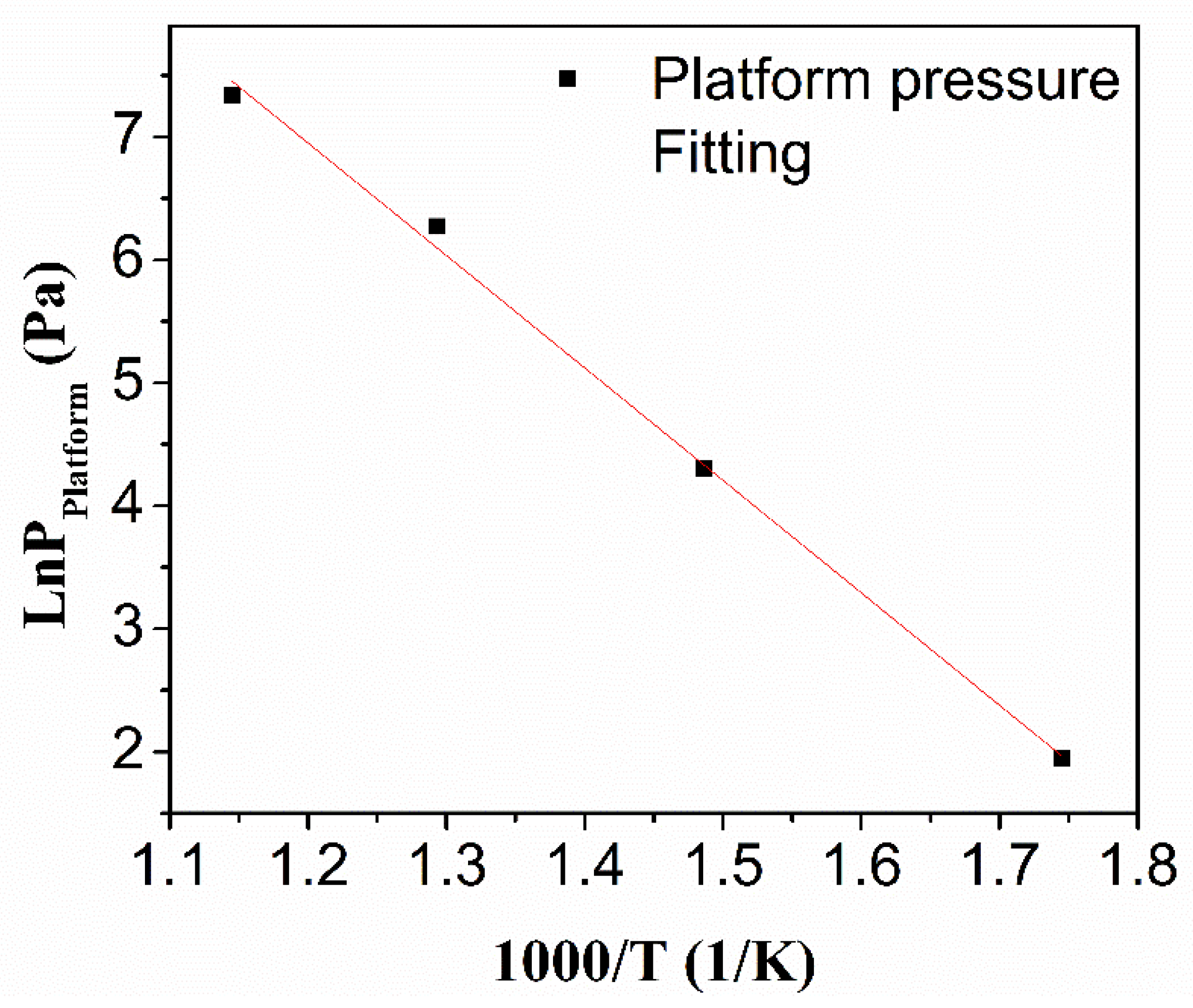
3.2. Thermodynamic Properties of the HEA Alloy
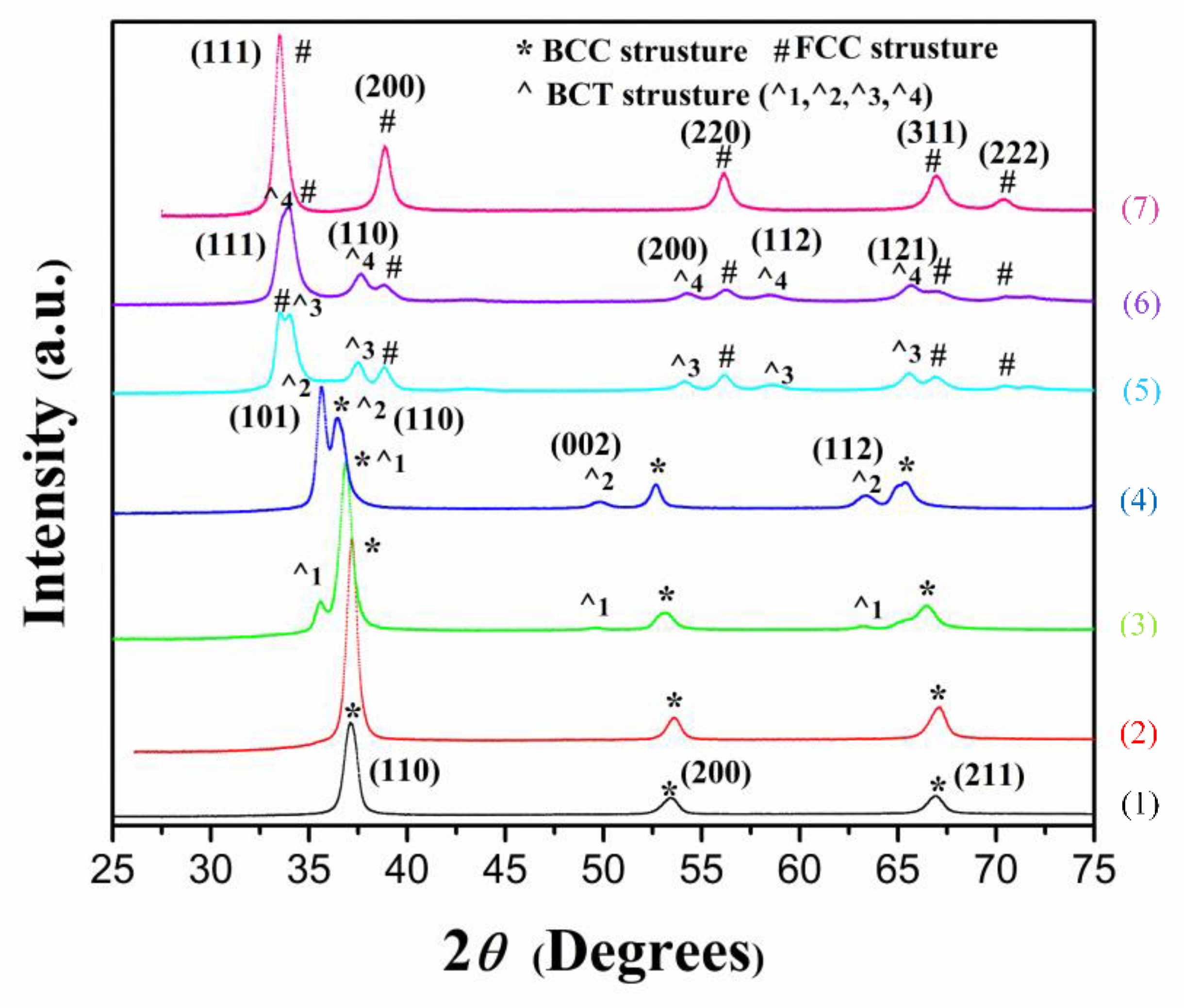
3.3. Crystal Structure Transition during the Hydrogen Absorption
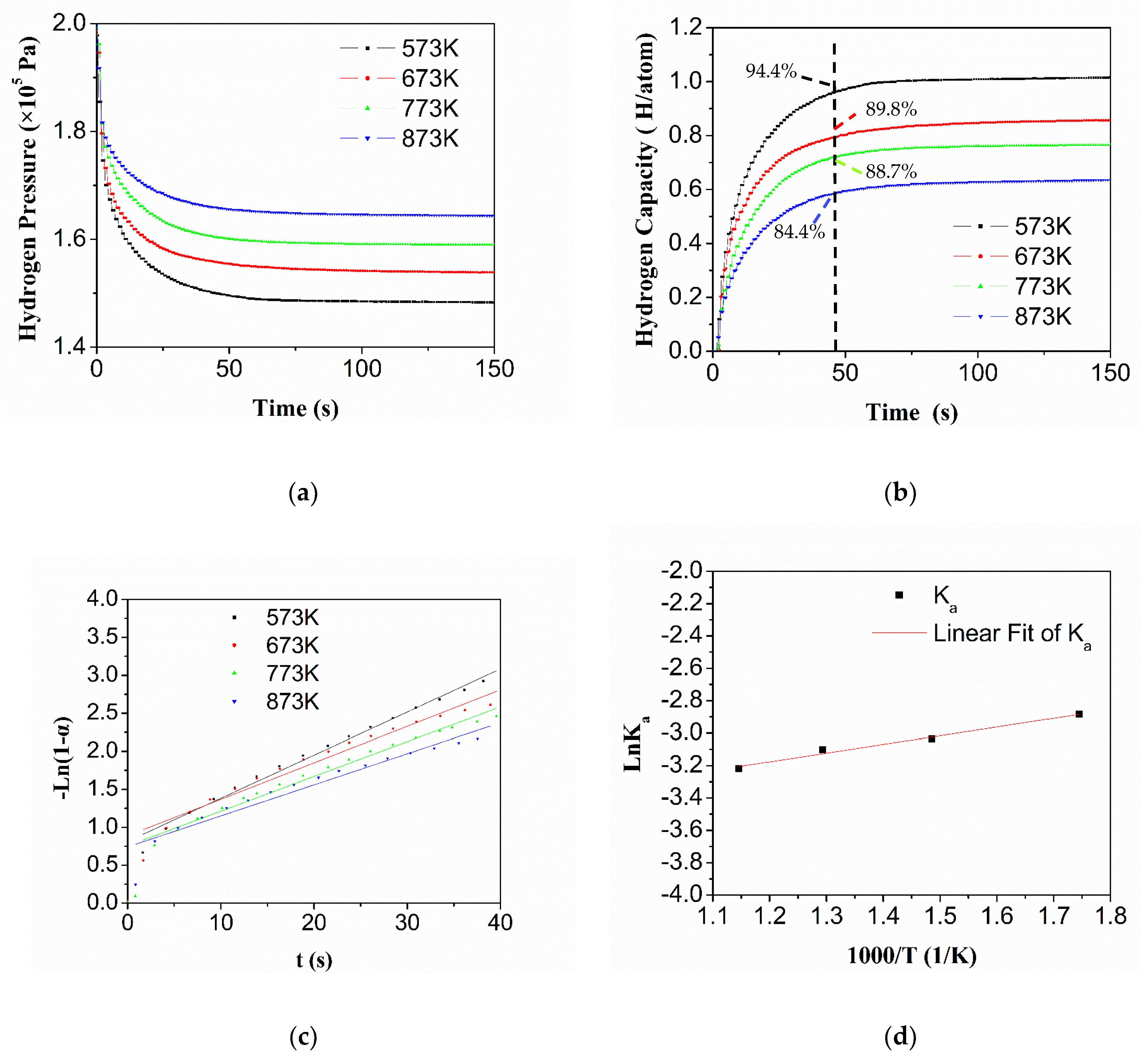
3.4. Hydrogen Sorption Kinetics of HEA Alloy
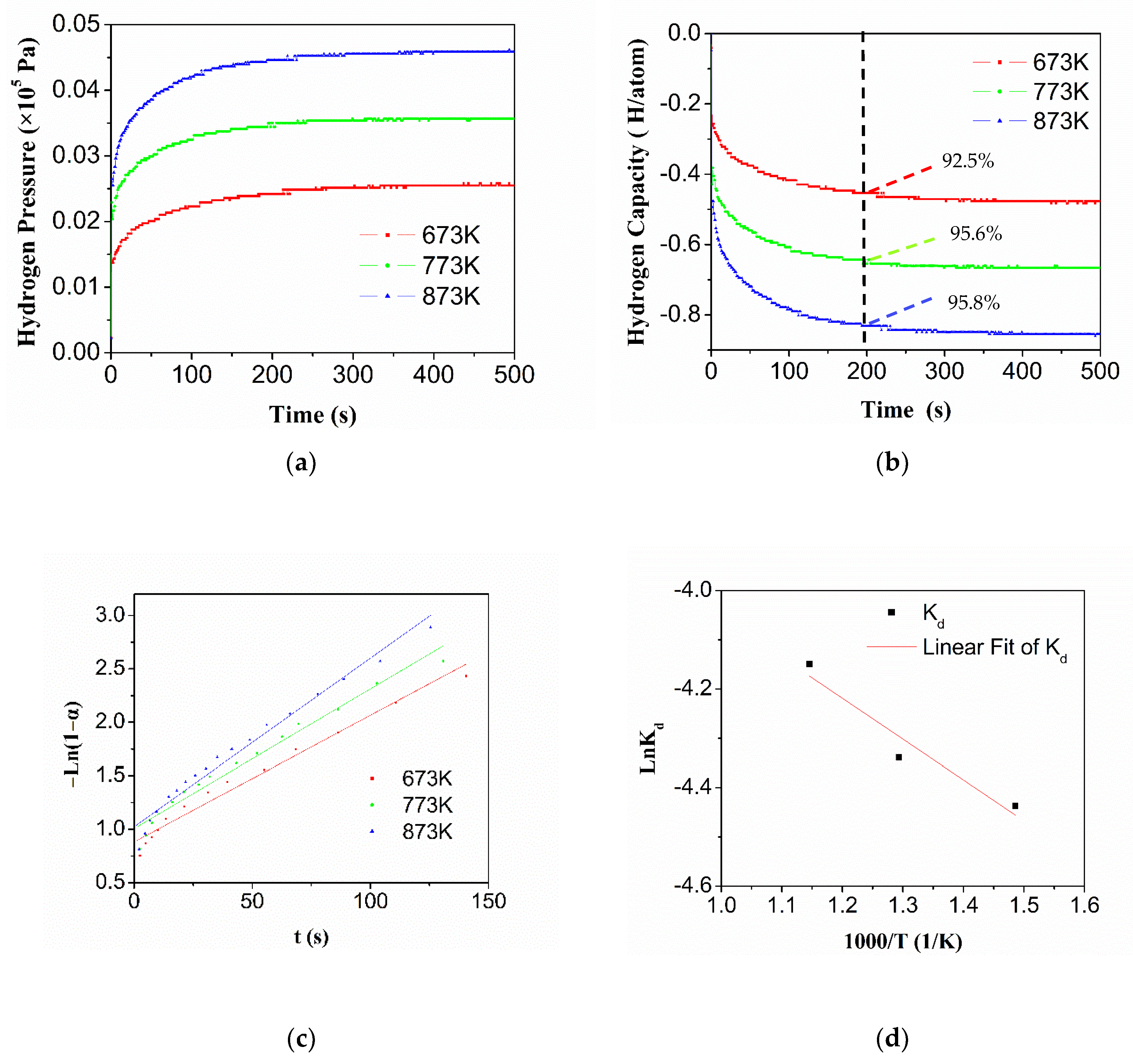
3.5. Hydrogen Desorption Kinetics of HEA Alloy
4. Discussion
4.1. Controllability of Thermodynamic Stability
4.2. Unique Feature of PCI Curve of HEA Alloy
4.3. Fast Hydrogen Sorption Kinetic Performance
5. Conclusions
- The hydrogenation performance of Ti0.20Zr0.20Hf0.20Nb0.40 HEA was systematically evaluated. The maximum equilibrium hydrogen storage capacity of the Ti0.20Zr0.20Hf0.20Nb0.40 HEA is 1.5 H/atom at 573 K. The thermal stability of HEA hydrides can be changed by the adjustment of their elements, and some HEA hydrides may exhibit a phase separation phenomenon of hydrogen elements, and this phenomenon may be beneficial in reducing its hydrides’ thermal stability.
- Ti0.20Zr0.20Hf0.20Nb0.40 HEA has a unique feature in its PCI curve. Before the full hydrogenation of the HEA, the increase in hydrogen content leads to a gradual increase in its plateau pressure. Furthermore, with the increase in temperature, the plateau width of the HEA becomes shorter. During the hydrogen absorption process, the structure of Ti0.20Zr0.20Hf0.20Nb0.40 HEA hydride undergoes a transition from BCC to FCC with a BCT intermediate state with the increasing hydrogen content. A series of BCT hydrides with different cell volumes precipitate consecutively with the increasing hydrogen content, which causes the increased plateau pressure during hydrogen absorption.
- Ti0.20Zr0.20Hf0.20Nb0.40 HEA exhibits outstanding hydrogenation kinetics properties. Almost full hydrogen absorption (94.4%, 573 K) is completed in 50 s, which is less than that of other synthesized hydrogen storage materials in the literature. Moreover, in contrast with the hydrogenation reaction, the decomposition process is more significant when the temperature is higher than 573 K. The hydrogen absorption reaction rate constant of the HEA gradually decreases with increasing temperature.
- According to the fitting calculation of the average plateau pressure at different temperatures, the platform pressure of Ti0.20Zr0.20Hf0.20Nb0.40 HEA at room temperature is extrapolated to be 1.6 × 10−8 Pa. The activation energy of the reaction between the alloy and hydride is negative (−4.37 kJ/mol), indicating that the hydrogenation of Ti0.20Zr0.20Hf0.20Nb0.40 HEA is spontaneous after primary activation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.C.; Niu, M.; Maddy, J. The Survey of Key Technologies in Hydrogen Energy Storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Colbe, J.B.V.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of Hydrides in Hydrogen Storage and Compression: Achievements, Outlook and Perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, M.; Ding, X.; Liu, Z.R.; Tian, H.; Shen, H.H.; Zu, X.T.; Li, S.; Qiao, L. One-Step Colloid Fabrication of Nickel Phosphides Nanoplate/Nickel Foam Hybrid Electrode for High-Performance Asymmetric Supercapacitors. Chem. Eng. J. 2019, 373, 1132–1143. [Google Scholar]
- Principi, G.; Agresti, F.; Maddalena, A.; Russo, S.L. The Problem of Solid State Hydrogen Storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- Jena, P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A Review on the Current Progress of Metal Hydrides Material for Solid-State Hydrogen Storage Applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Yu, X.B.; Tang, Z.W.; Sun, D.L.; Ouyang, L.Z.; Zhu, M. Recent Advances and Remaining Challenges of Nanostructured Materials for Hydrogen Storage Applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Billur, S.; Farida, L.D.; Michael, H. Metal Hydride Materials for Solid Hydrogen Storage:A Review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen Storage: Materials, Methods and Perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Nassar, A.E.; Nassar, E.E.; Younis, M.A. Hydrogen Storage Alloys: Types and Characteristics. In Handbook of Ecomaterials; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1–19. [Google Scholar]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements_ Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent Progress in High-Entropy Alloys. Eur. J. Control 2016, 31, 633–648. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Miracle, D.B. Critical Assessment 14: High Entropy Alloys and Their Development as Structural Materials. Mater. Sci. Technol. 2015, 31, 1142–1147. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-Entropy Alloy Challenges and Prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Zhang, W.R.; Liaw, P.K.; Zhang, Y. Science and Technology in High-Entropy Alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Diao, H.Y.; Feng, R.; Dahmen, K.A.; Liaw, P.K. Fundamental Deformation Behavior in High-Entropy Alloys: An Overview. Curr. Opin. Solid State Mater. Sci. 2017, 21, 252–266. [Google Scholar] [CrossRef]
- Harrington, T.J.; Gild, J.; Sarker, P.; Toher, C.; Rost, C.M.; Dippo, O.F.; McElfresh, C.; Kaufmann, K.; Marin, E.; Borowski, L.; et al. Phase Stability and Mechanical Properties of Novel High Entropy Transition Metal Carbides. Acta Mater. 2019, 166, 271–280. [Google Scholar] [CrossRef]
- Zou, Y.; Wheeler, J.M.; Ma, H.; Okle, P.; Spolenak, R. Nanocrystalline High-Entropy Alloys: A New Paradigm in High-Temperature Strength and Stability. Nano Lett. 2017, 17, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Z.; Yang, B.; Liaw, P. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, S.K.; Sheu, J.H.; Lin, J.T.; Lin, W.E.; Yeh, J.W.; Lin, S.J.; Liou, Z.H.T.; Wang, C.W. Hydrogen Storage Properties of Multi-Principal-Component Cofemntixvyzrz Alloys. Int. J. Hydrogen Energy 2010, 35, 9046–9059. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, K.S.; Hwang, K.T.; Kim, B.G.; Kang, Y.M. Effect of Heat Treatment on Microstructure and Hydrogen Storage Properties of Mass-Produced Ti0.85zr0.13(Fex–V)0.56mn1.47ni0.05. Alloy. Int. J. Hydrogen Energy 2013, 38, 6215–6220. [Google Scholar] [CrossRef][Green Version]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and Hydrogen Storage Properties of a Tizrnbmov High Entropy Alloy Synthesized Using Laser Engineered Net Shaping (Lens). Int. J. Hydrogen Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior Hydrogen Storage in High Entropy Alloys. Scientificreports 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.J.; Liu, Y. Microstructure of Nb-Ta-V Based Hydrogen Permeation High Entropy Alloys. Mater. Sci. Forum 2016, 849, 3–7. [Google Scholar] [CrossRef]
- Karlsson, D.; Ek, G.; Cedervall, J.; Zlotea, C.; Møller, K.T.; Hansen, T.C.; Bednarčík, J.; Paskevicius, M.; Sørby, M.H.; Jensen, T.R.; et al. Structure and Hydrogenation Properties of a Hfnbtivzr High-Entropy Alloy. Inorg. Chem. 2017, 57, 2103–2110. [Google Scholar] [CrossRef]
- Kunce, I.; Polański, M.; Czujko, T. Microstructures and Hydrogen Storage Properties Of la Ni Fe V Mn Alloys. Int. J. Hydrogen Energy 2017, 42, 27154–27164. [Google Scholar] [CrossRef]
- Zepon, G.; Leiva, D.R.; Strozi, R.B.; Bedoch, A.; Figueroa, S.J.A.; Ishikawa, T.T.; Botta, W.J. Hydrogen-Induced Phase Transition of Mgzrtife0.5co0.5ni0.5 High Entropy Alloy. Int. J. Hydrogen Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen Sorption in Tizrnbhfta High Entropy Alloy. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Hu, J.T.; Shen, H.H.; Jiang, M.; Gong, H.F.; Xiao, H.Y.; Liu, Z.J.; Sun, G.A.; Zu, X.T. A Dft Study of Hydrogen Storage in High-Entropy Alloy Tizrhfscmo. Nanomaterials 2019, 9, 461. [Google Scholar] [CrossRef]
- Shen, H.H.; Zhang, J.W.; Hu, J.T.; Zhang, J.C.; Mao, Y.W.; Xiao, H.Y.; Zhou, X.S.; Zu, X.T. A Novel Tizrhfmonb High-Entropy Alloy for Solar Thermal Energy Storage. Nanomaterials 2019, 9, 248. [Google Scholar] [CrossRef]
- Shen, H.H.; Hu, J.T.; Li, P.C.; Huang, G.; Zhang, J.W.; Zhang, J.C.; Mao, Y.W.; Xiao, H.Y.; Zhou, X.S.; Zu, X.T.; et al. Compositional Dependence of Hydrogenation Performance of Ti-Zr-Hf-Mo-Nb High-Entropy Alloys for Hydrogen/Tritium Storage. J. Mater. Sci. Technol. 2020, 55, 116–125. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Sharma, V.K.; Sahu, P.; Kumar, V. Synthesis and Characterization of Hydrogenated Novel Alcrfemnniw High Entropy Alloy. Int. J. Hydrogen Energy 2020, 45, 16984–16991. [Google Scholar] [CrossRef]
- Ek, G.; Nygard, M.M.; Pavan, A.F.; Montero, J.; Henry, P.F.; Sorby, M.H.; Witman, M.; Stavila, V.; Zlotea, C.; Hauback, B.C.; et al. Elucidating the Effects of the Composition on Hydrogen Sorption in Tivzrnbhf-Based High-Entropy Alloys. Inorg. Chem. 2021, 60, 1124–1132. [Google Scholar] [CrossRef]
- Strozi, R.B.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Synthesis and Hydrogen Storage Behavior of Mg–V–Al–Cr–Ni High Entropy Alloys. Int. J. Hydrogen Energy 2021, 46, 2351–2361. [Google Scholar] [CrossRef]
- Wang, C.Z.; Wang, H.X.; Shankar, K.; Morozov, E.V.; Hazell, P.J. On the Mechanical Behaviour of Steel Wire Mesh Subjected to Low-Velocity Impact. Thin-Walled Struct. 2021, 159, 107281. [Google Scholar] [CrossRef]
- Cai, C.; Wang, M.Y.; Han, S.B.; Wang, Q.; Zhang, Q.; Zhu, Y.M.; Yang, X.M.; Wu, D.J.; Zu, X.T.; Sterbinsky, G.E.; et al. Ultrahigh Oxygen Evolution Reaction Activity Achieved Using Ir Single Atoms on Amorphous Coox Nanosheets. ACS Catal. 2020, 11, 123–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Lin, J.; Chen, G.; Liaw, P. Solid-Solution Phase Formation Rules for Multi-Component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Bing, W.Z.; Long, X.G.; Zhu, Z.L.; Hao, W.L.; Luo, S.Z.; Peng, S.M. Structure and Thermodynamic Aspect of Ti-Hf Alloys and Their Deuterides. Chin. J. Inorganal Chem. 2010, 26, 1008–1012. [Google Scholar]
- Zhang, C.; Song, A.; Yuan, Y.; Wu, Y.; Zhang, P.L.; Lu, Z.P.; Song, X.P. Study on the Hydrogen Storage Properties of a TiZrNbTa High Entropy Alloy. Int. J. Hydrogen Energy 2020, 45, 5367–5374. [Google Scholar] [CrossRef]
- Xiong, W.; Li, B.Q.; Wang, L.; Li, J.; Kong, F.Q.; Yan, H.Z. Investigation of the Thermodynamic and Kinetic Properties of La–Fe–B System Hydrogen-Storage Alloys. Int. J. Hydrogen Energy 2014, 39, 3805–3809. [Google Scholar] [CrossRef]
- Ruz, P.; Banerjee, S.; Halder, R.; Kumar, A.; Sudarsan, V. Thermodynamics, Kinetics and Microstructural Evolution of Ti0.43zr0.07cr0.25v0.25 Alloy Upon Hydrogenation. Int. J. Hydrogen Energy 2017, 42, 11482–11492. [Google Scholar] [CrossRef]
- Martin, M.; Gommel, C.; Borkhart, C.; Fromm, E. Absorption and Desorption Kinetics of Hydrogen Storage Alloys. J. Alloys Compd. 1996, 238, 193–201. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Ichikawa, T. Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism. Catalysts 2018, 8, 651. [Google Scholar] [CrossRef]
- Pang, Y.P.; Li, Q. A Review on Kinetic Models and Corresponding Analysis Methods for Hydrogen Storage Materials. Int. J. Hydrogen Energy 2016, 41, 18072–18087. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-State Kinetic Models: Basics and Mathematical Fundamentals. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Gao, M.C.; Gao, P.; Hawk, J.A.; Ouyang, L.Z.; Alman, D.E.; Widom, M. Computational Modeling of High-Entropy Alloys: Structures, Thermodynamics and Elasticity. J. Mater. Res. 2017, 32, 3627–3641. [Google Scholar] [CrossRef]
- Zhao, J.W.; Ding, H.; Tiana, X.F.; Zhao, W.J.; Hou, H.L. Thermodynamic Calculation on the Formation of Titanium Hydride. Chin. J. Chem. Phys. 2008, 21, 569. [Google Scholar] [CrossRef]
- Huang, G.; Cao, X.H.; Long, X.G.; Yang, B.F.; Liu, W.K. Research on the Kinetics Isotope Effects of Tritium Absorbing Hydrogen, Deuterium and Titanium. J. Mater. Sci. Eng. 2005, 23, 850–853. [Google Scholar]
- Park, C.N.; Lee, J.Y. Kinetic Properties of the Hydrogenation of the Feti Intermetallic Compound. J. Less-Common Met. 1983, 91, 189–201. [Google Scholar]
- Israel, A.; Jacob, I. Kinetics and Thermodynamics of Hydrogen Absorption in the Zr(Al0.1fe0.9)2 Intermetallic Compound. J. Alloys Compd. 1997, 260, 131–134. [Google Scholar] [CrossRef]
- Kitada, M. Absorption-Desorption Characteristics of Hydrogen for Lani5, Ceco5, and Smco5. J. Jpn. Inst. Met. 1977, 41, 412–420. [Google Scholar] [CrossRef][Green Version]
- Simičić, M.V.; Zdujić, M.; Dimitrijević, R.; Nikolić-Bujanović, L.; Popović, N.H. Hydrogen Absorption and Electrochemical Properties of Mg2ni-Type Alloys Synthesized by Mechanical Alloying. J. Power Sources 2006, 158, 730–734. [Google Scholar]
- He, J.X.; Shen, Y.; Li, B.; Xiang, X.; Li, S.; Fang, X.; Xiao, H.Y.; Zu, X.T.; Qiao, L. A Comparative Study of the Structural and Optical Properties of Si-Doped Gaas under Different Ion Irradiation. Opt. Mater. 2021, 111, 110611. [Google Scholar] [CrossRef]
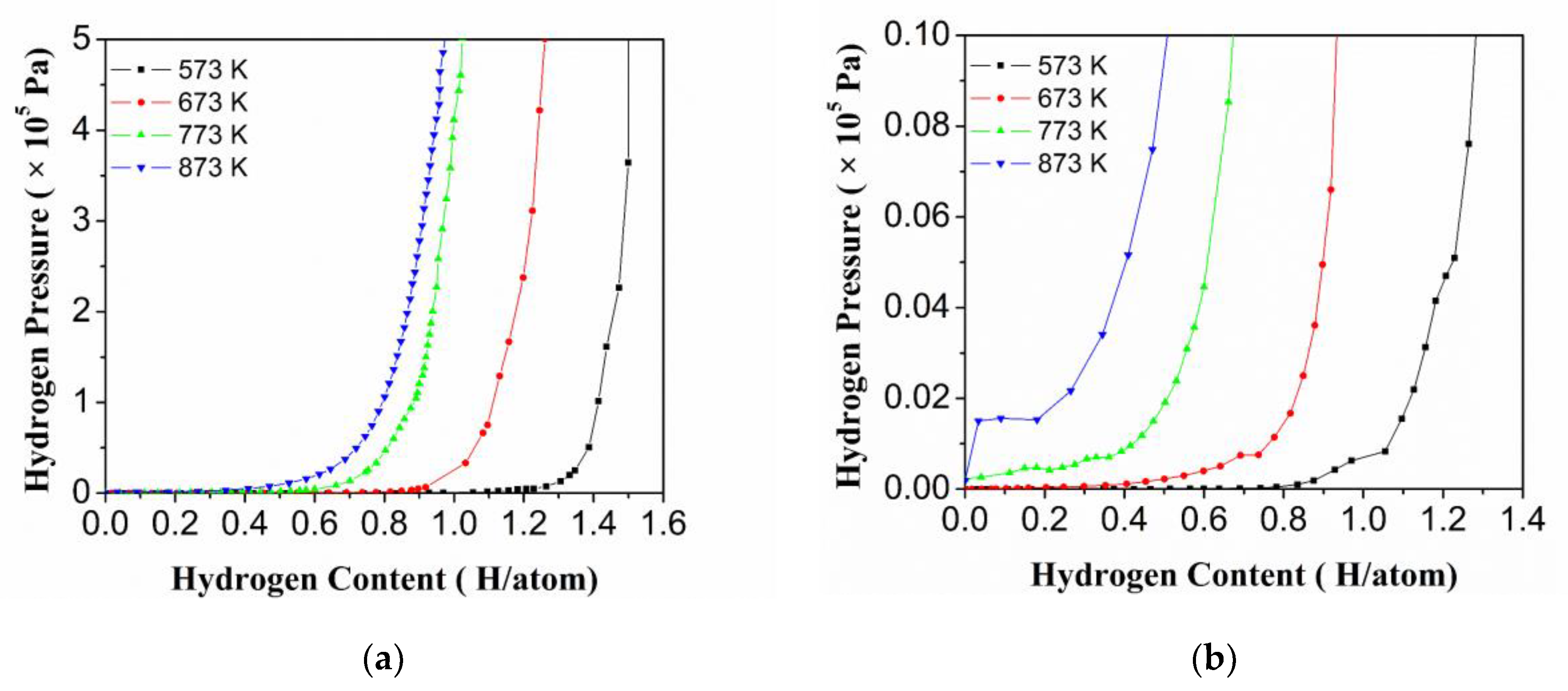
| Temperature (K) | Plateau Pressure (Pa) | Hydrogen Storage Capacity (<5 × 105 Pa, H/atom) |
|---|---|---|
| 573 | 7 | 1.50 |
| 673 | 74 | 1.27 |
| 773 | 529 | 1.02 |
| 873 | 1530 | 0.98 |
| Sample Status (H/atom) | Crystal Structure | Lattice Constant (nm) | Cell Volume (A3) | Pattern No. in Figure 4 |
|---|---|---|---|---|
| Powder (P) | BCC | a = 0.3357 (1) | 37.84 | 1 |
| 0.05 (P) | BCC | a = 0.3412 (1) | 39.73 | 2 |
| Hydride 0.2 (P) | BCC | a = 0.3445 (1) | 40.9 | 3 |
| BCT1 | a = 0.3445 (1), c = 0.3675 (4) | 43.63 | ||
| Hydride 0.4 (P) | BCC | a = 0.3493 (3) | 42.37 | 4 |
| BCT2 | a = 0.3487(1), c = 0.3666 (1) | 44.44 | ||
| Hydride 0.8 (P) | FCC | a = 0.4625 (1) | 98.92 | 5 |
| BCT3 | a = 0.3378 (2), c = 0.4189 (5) | 47.81 | ||
| Hydride 1.1 (P) | FCC | a = 0.4629 (2) | 99.18 | 6 |
| BCT4 | a = 0.3379 (1), c = 0.4204 (3) | 48.01 | ||
| Fully Hydride 1.6 (P) | FCC | a = 0.4629 (1) | 99.21 | 7 |
| Temperature (K) | Hydrogen Absorption Equilibrium Pressure (Pa) | Equilibrium Hydrogen Storage Capacity (H/atom) | Hydrogen Absorption Reaction Rate Ka (1/s) | R2 (%) |
|---|---|---|---|---|
| 573 | 147838 | 1.03 | 0.056 | 99.0 |
| 673 | 152807 | 0.89 | 0.048 | 94.5 |
| 773 | 157207 | 0.82 | 0.045 | 95.8 |
| 873 | 162162 | 0.70 | 0.040 | 95.3 |
| Temperature (K) | Hydrogen Desorption Equilibrium Pressure (Pa) | Equilibrium Hydrogen Storage Capacity (H/atom) | Hydrogen Desorption Reaction Rate Kd (1/s) | R2 (%) |
|---|---|---|---|---|
| 673 | 2584 | −0.48 | 0.012 | 97.47 |
| 773 | 3655 | −0.67 | 0.013 | 97.35 |
| 873 | 4586 | −0.85 | 0.016 | 97.53 |
| Metal/Alloys | Metal Hydrides | Atomic Size Difference δ | Standard Enthalpy ΔH0 (kJ·mol−1) | Standard Entropy ΔS0 (J·mol−1·K−1) | Reference |
|---|---|---|---|---|---|
| Ti | TiH2 | / | −142.39 | −143.0 | [54] |
| Ti0.25Zr0.25Nb0.25Ta0.25 | (Nb,Ta)H | 3.75 | −6.4 | −4.3 | [46] |
| Ti0.20Zr0.20Hf0.20Nb0.40 | (HEA)H1.6 | 5.51 | −75.99 | −148.99 | This work |
| Type | Example | Pressure/ Temperature | ta (s) | Ea (kJ/mol) | Ref | |
|---|---|---|---|---|---|---|
| Metal | Ti | 4400 Pa/823 K | 400 | 55.6 | [55] | |
| Traditional Hydrogen Storage Alloys | AB | FeTi | 2.5 × 106 Pa/294 K | 270 | 7.53 | [56] |
| AB2 | Zr(Al0.1Fe0.9)2 | 5 × 106 Pa/265 K | >720 | 29 | [57] | |
| AB5 | LaNi5 | 2 × 105 Pa/293 K | 120 | 13.2 | [58] | |
| A2B | Mg2Ni | 573 K | 600 | / | [59] | |
| Solid solution alloy (BCC) | Ti0.43Zr0.07Cr0.25V0.25 | 2 × 106 Pa/293 K | 600 | 20.9 | [48] | |
| HEAs | CoFeMnTixVyZrz | 298 K | >100 s | / | [27] | |
| Ti0.25Zr0.25Nb0.25Ta0.25 | 2 × 105 Pa/290 K | 60 | 6.14 | [46] | ||
| Ti0.20Zr0.20Hf0.20Nb0.40 | 2 × 105 Pa/573 K | 50 | −4.37 | This work | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, P.; Huang, G.; Zhang, W.; Hu, J.; Xiao, H.; Zheng, J.; Zhou, X.; Xiang, X.; Yu, J.; et al. Superior Hydrogen Sorption Kinetics of Ti0.20Zr0.20Hf0.20Nb0.40 High-Entropy Alloy. Metals 2021, 11, 470. https://doi.org/10.3390/met11030470
Zhang J, Li P, Huang G, Zhang W, Hu J, Xiao H, Zheng J, Zhou X, Xiang X, Yu J, et al. Superior Hydrogen Sorption Kinetics of Ti0.20Zr0.20Hf0.20Nb0.40 High-Entropy Alloy. Metals. 2021; 11(3):470. https://doi.org/10.3390/met11030470
Chicago/Turabian StyleZhang, Jianwei, Pengcheng Li, Gang Huang, Weiguang Zhang, Jutao Hu, Haiyan Xiao, Jian Zheng, Xiaosong Zhou, Xia Xiang, Jingxia Yu, and et al. 2021. "Superior Hydrogen Sorption Kinetics of Ti0.20Zr0.20Hf0.20Nb0.40 High-Entropy Alloy" Metals 11, no. 3: 470. https://doi.org/10.3390/met11030470
APA StyleZhang, J., Li, P., Huang, G., Zhang, W., Hu, J., Xiao, H., Zheng, J., Zhou, X., Xiang, X., Yu, J., Shen, H., Li, S., & Zu, X. (2021). Superior Hydrogen Sorption Kinetics of Ti0.20Zr0.20Hf0.20Nb0.40 High-Entropy Alloy. Metals, 11(3), 470. https://doi.org/10.3390/met11030470







