Effect of Trace Mg on Impact Toughness of 2.25Cr1Mo Steel Doped with 0.056% P at Medium Temperature Aging Process
Abstract
1. Introduction
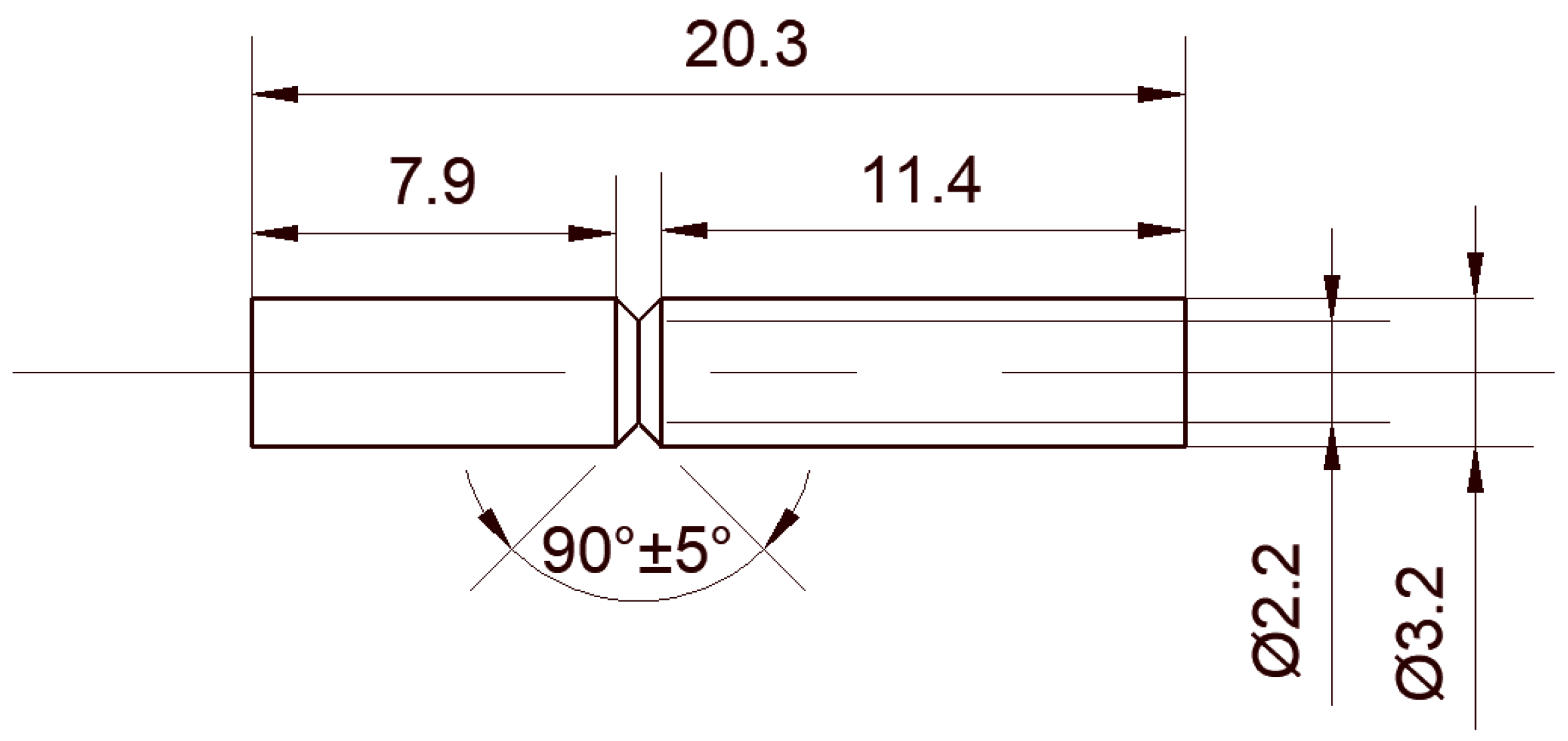
2. Materials and Methods
3. Results
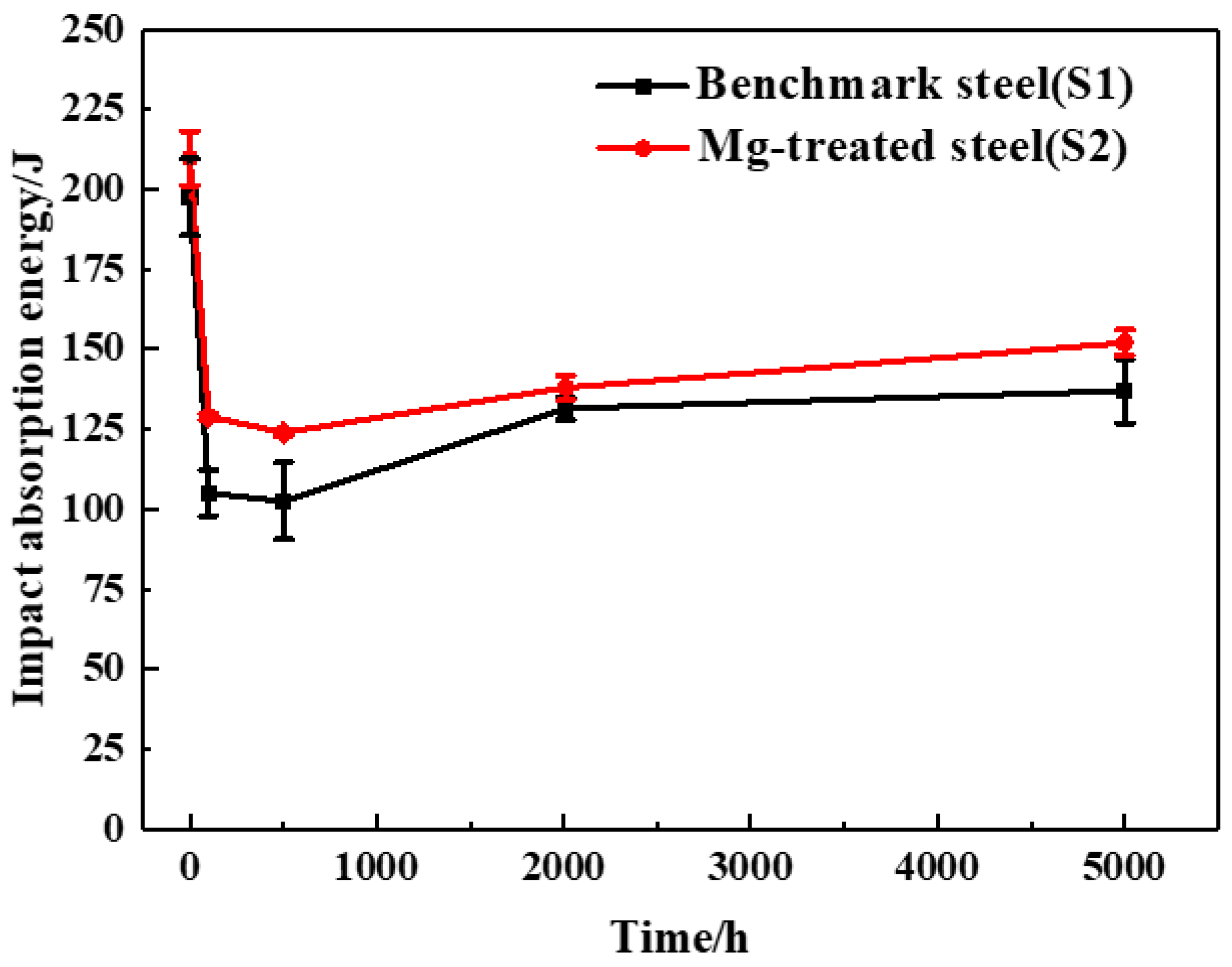
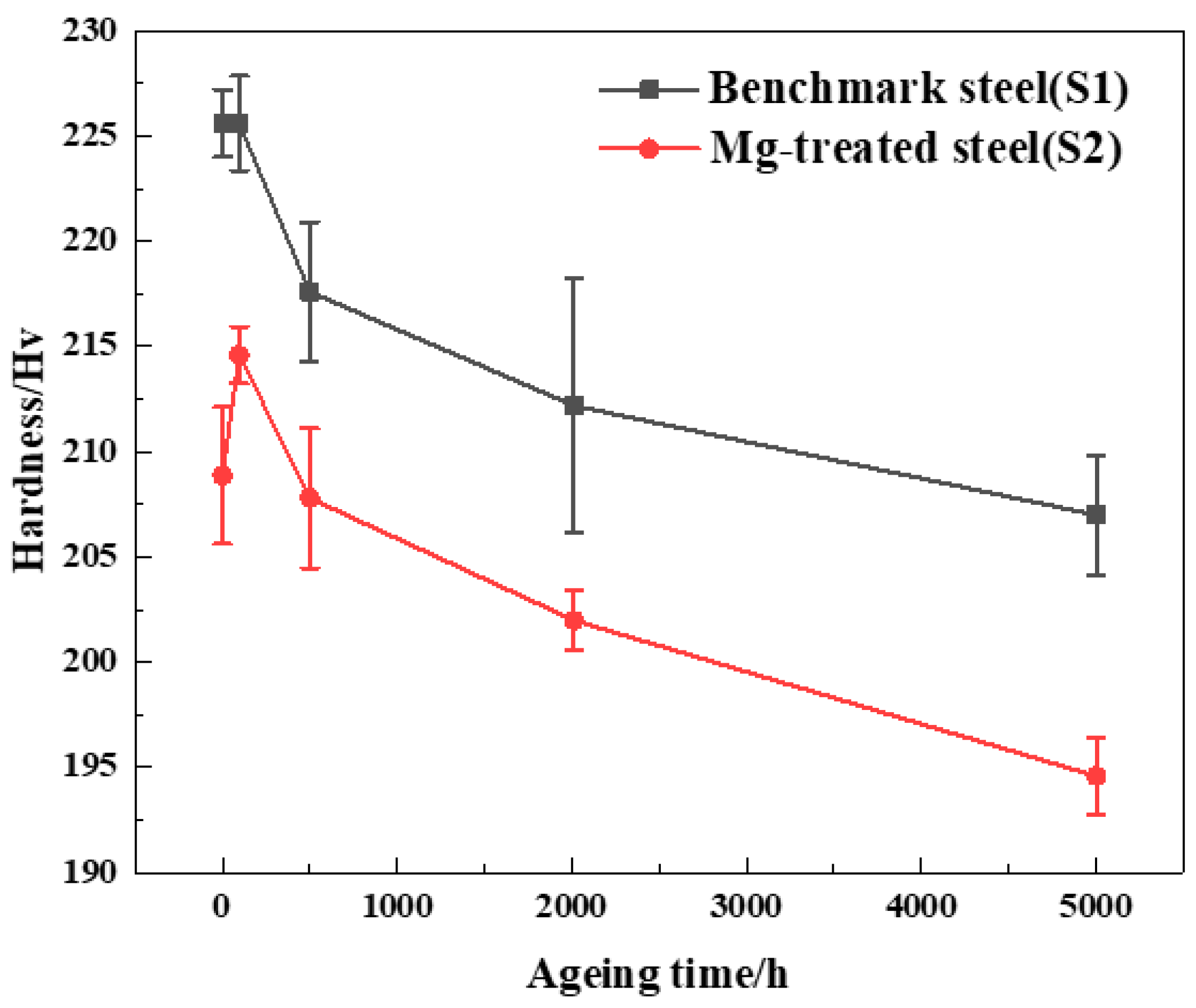
3.1. Influence of Mg on the Impact Energy Evolution after Subjected to Aging Process
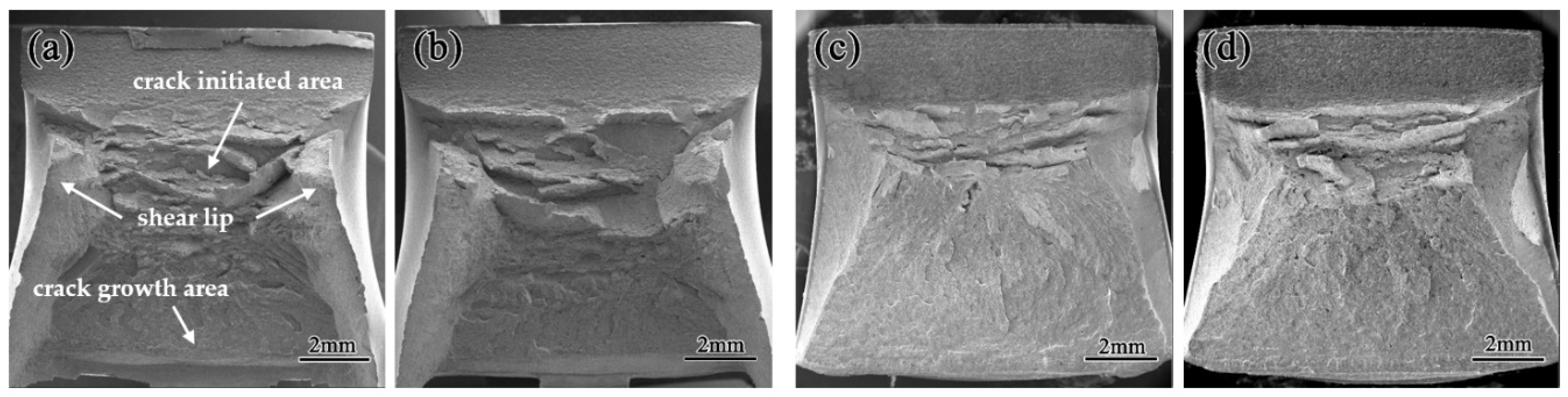
3.2. Fractographs after Charpy Impact at Room Temperature
4. Discussion
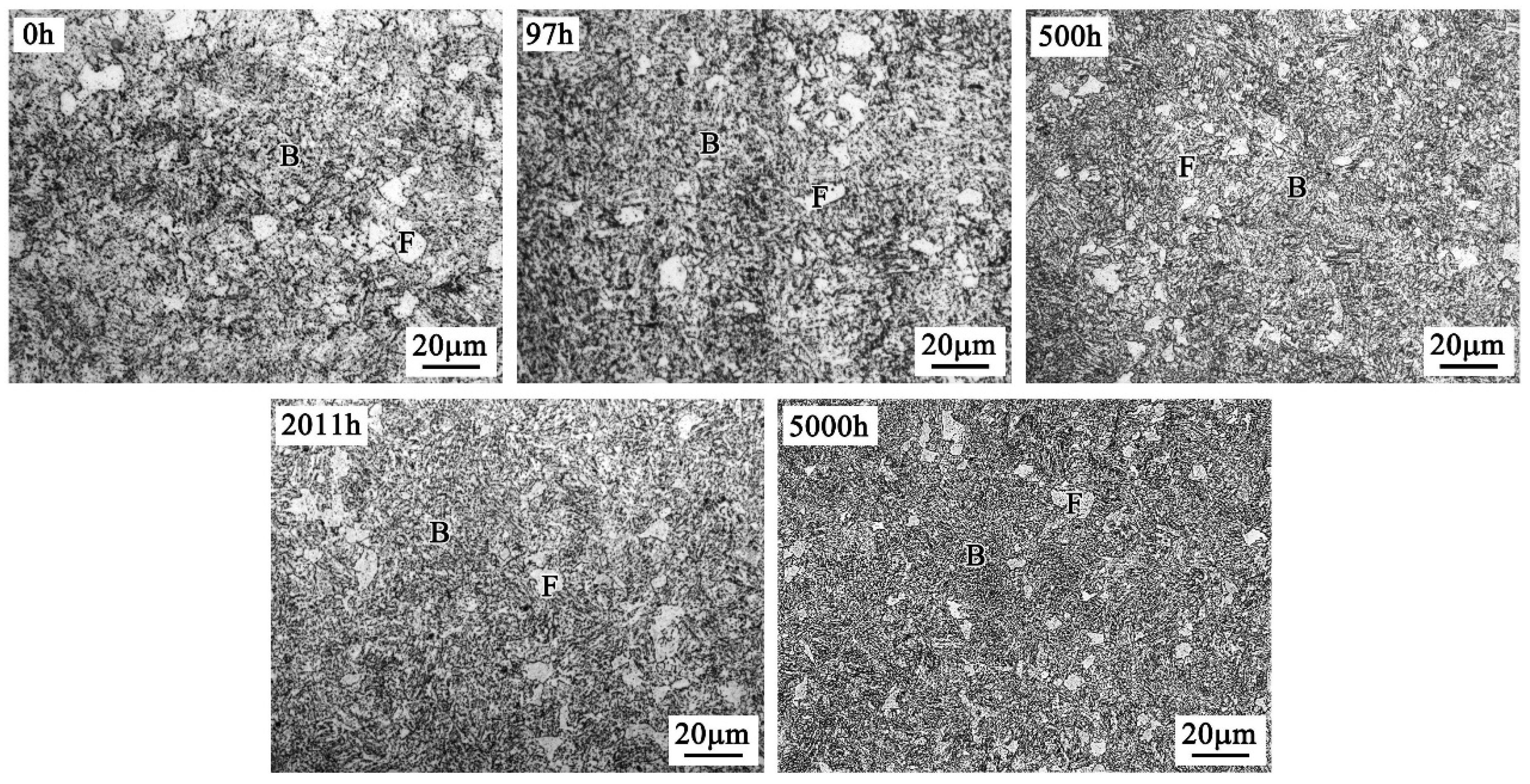
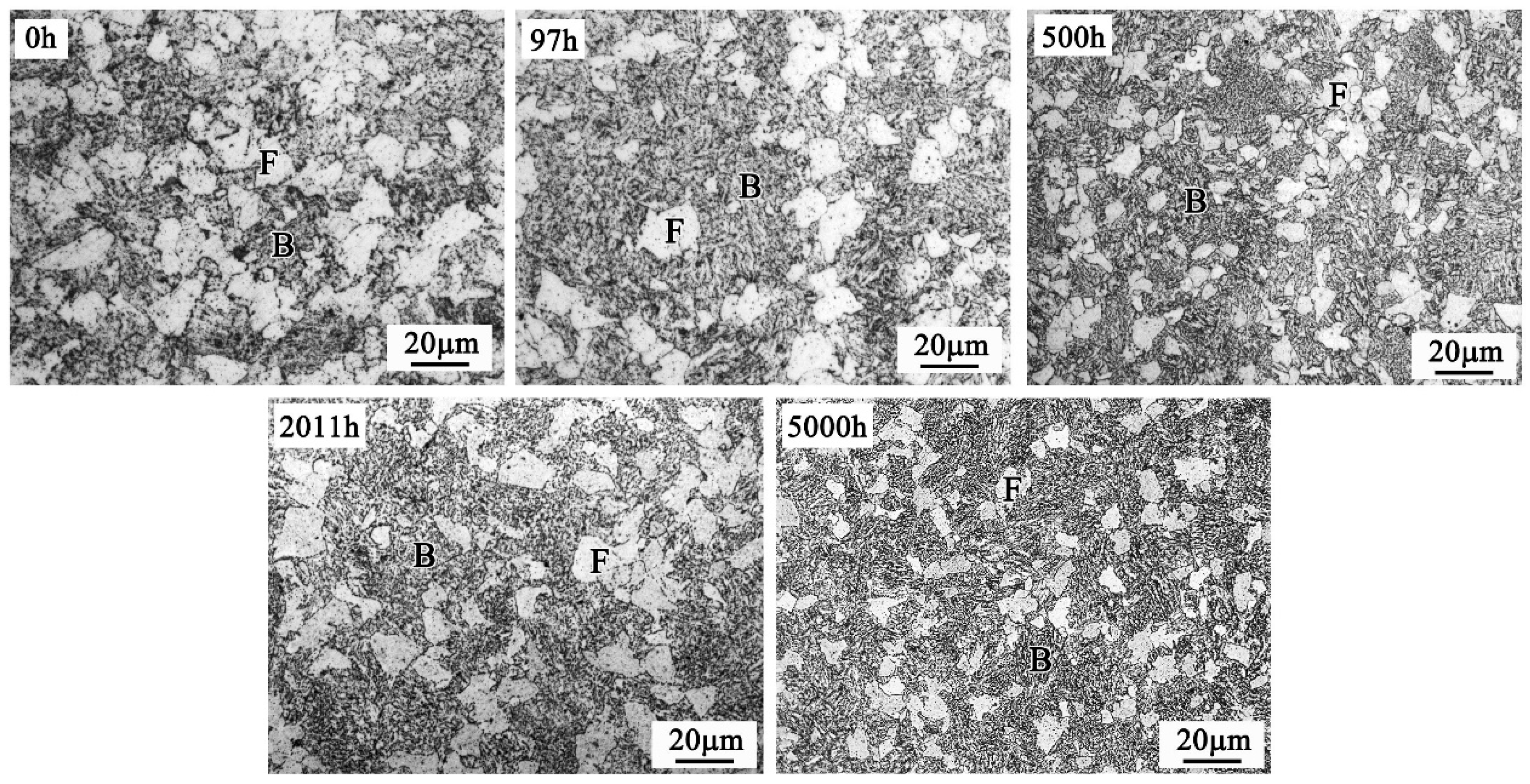
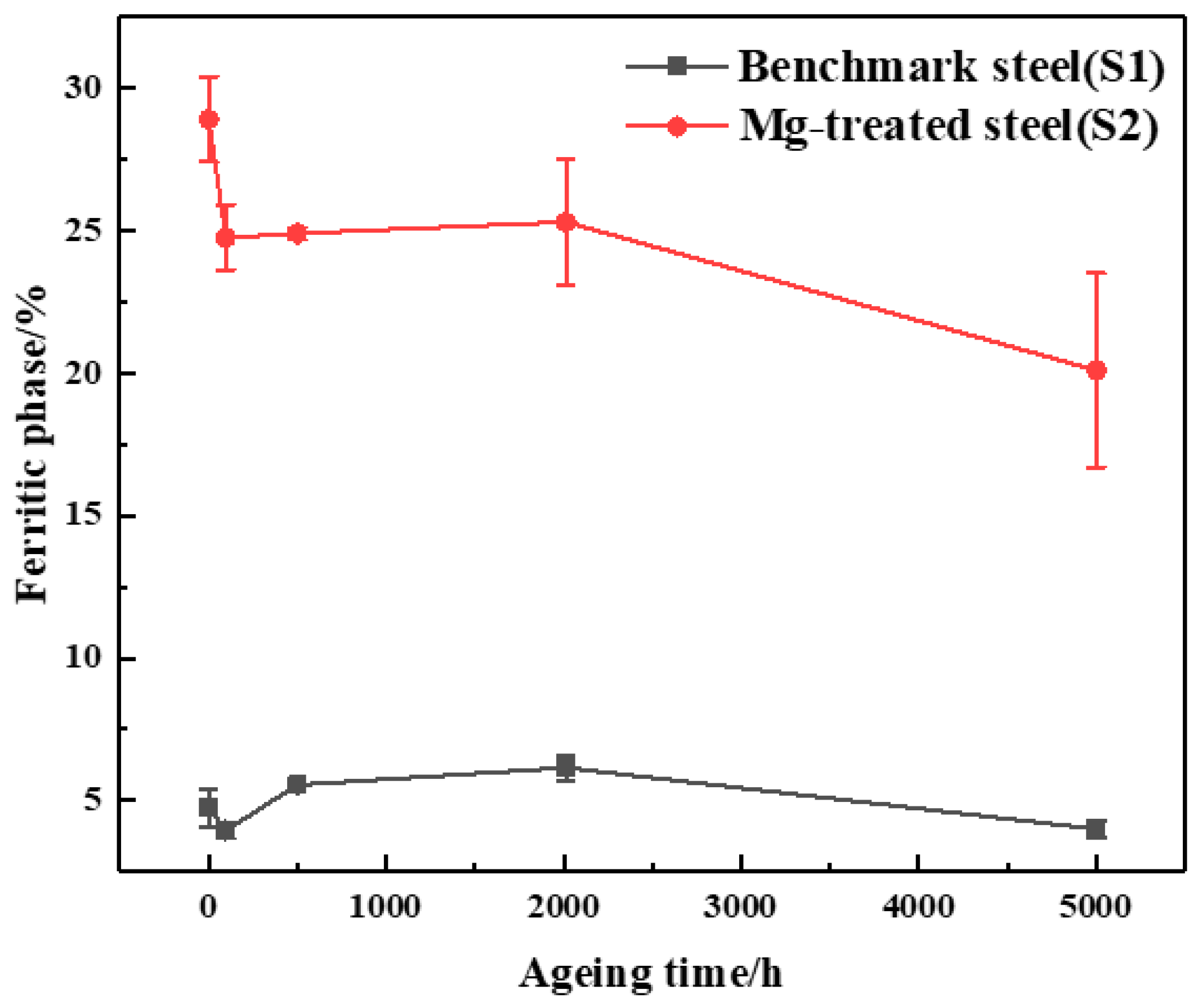
4.1. Microstructure
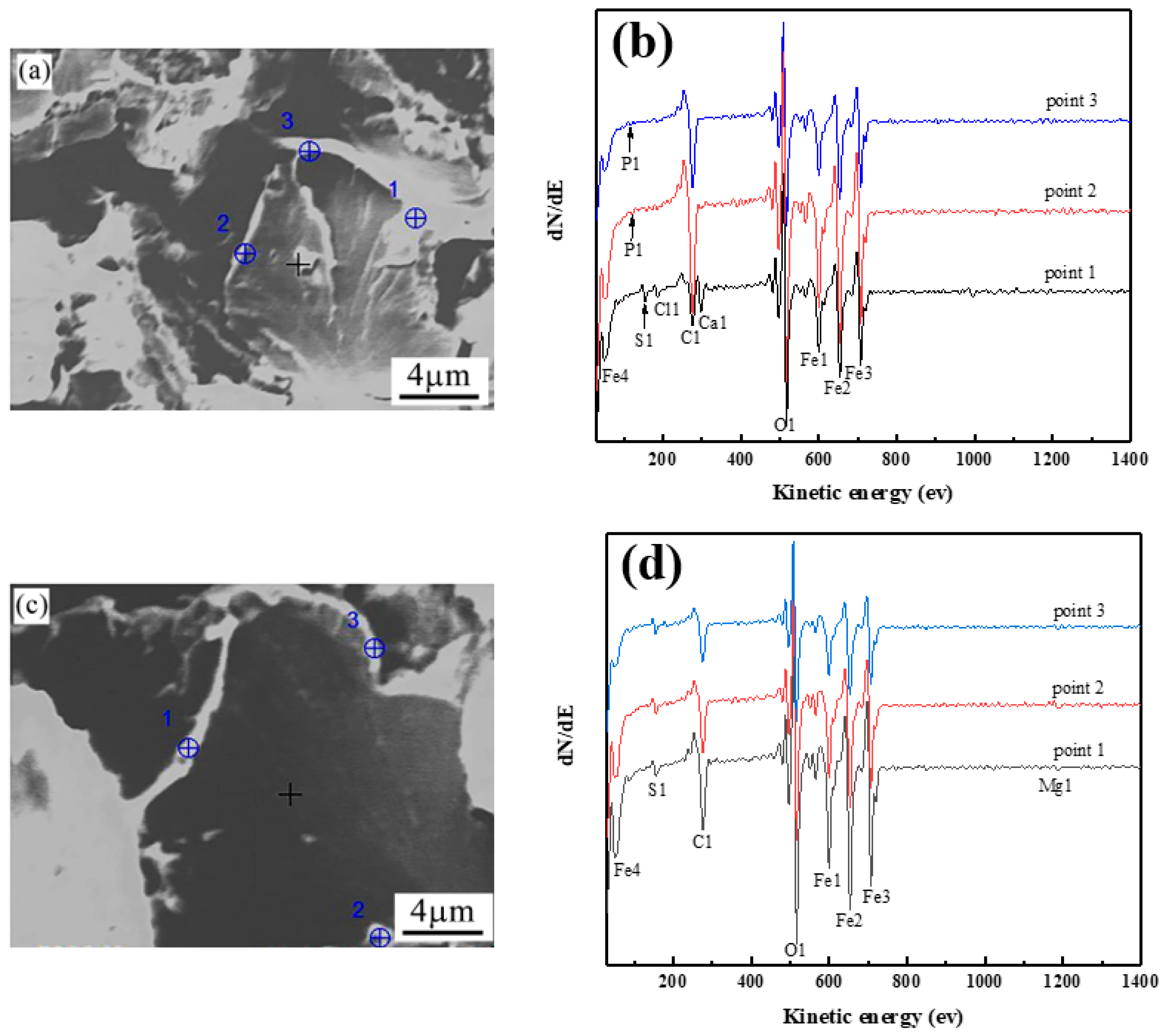
4.2. Grain Boundary Segregation of Impurity Elements
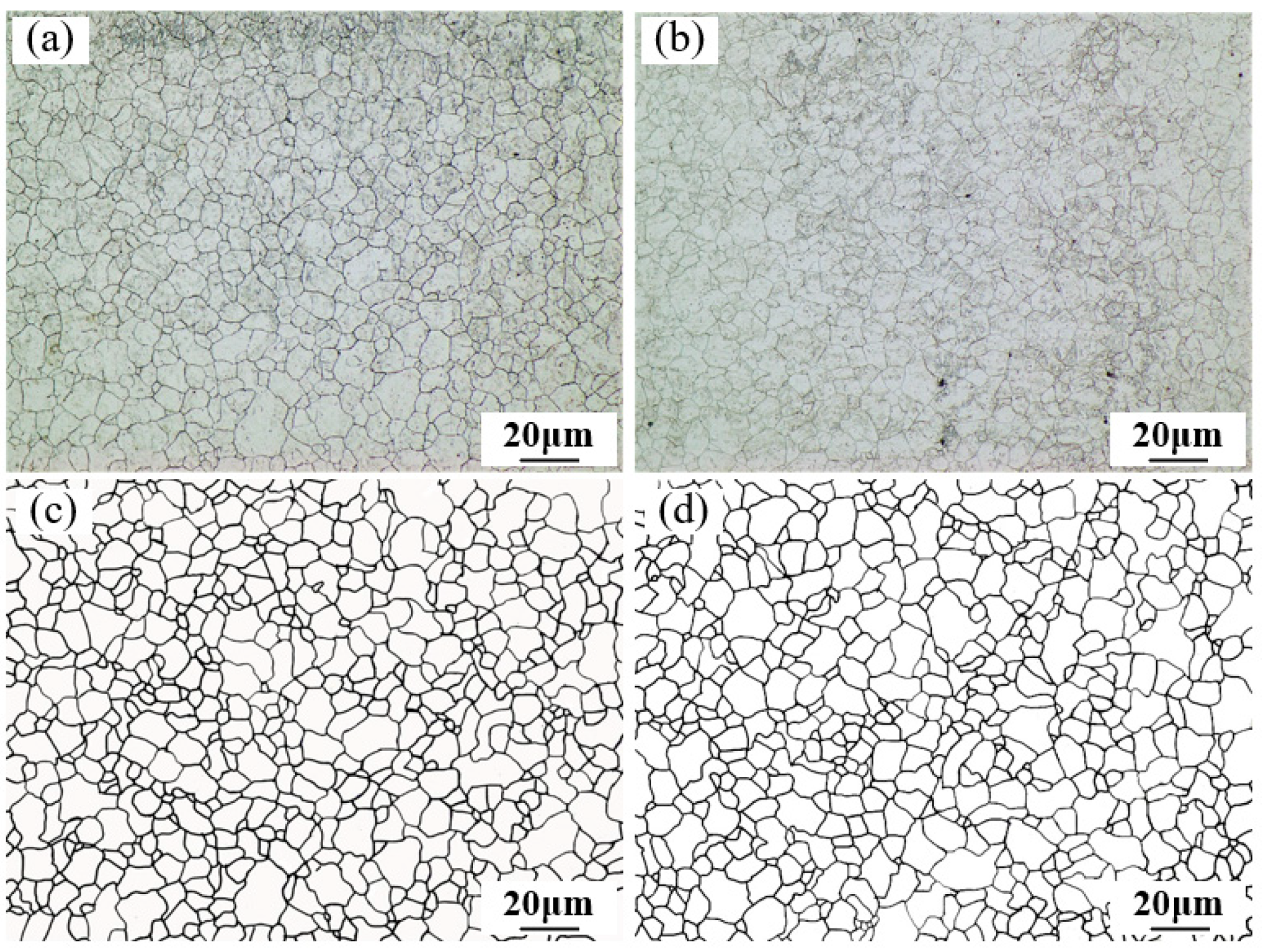
4.3. Prior Austenite Grain Size
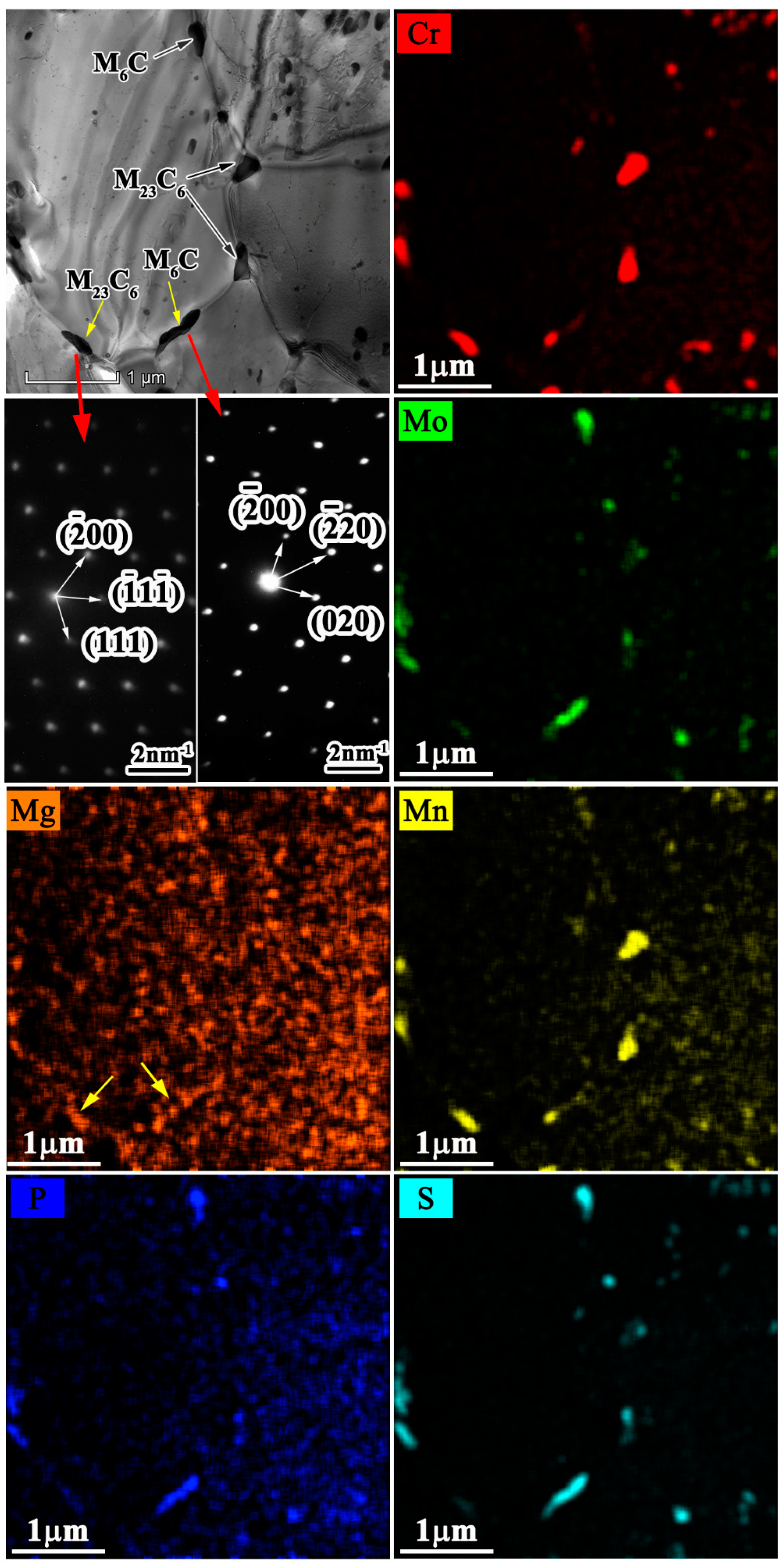
4.4. Carbide Precipitation at the Grain Boundary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B. Investigation on the pearlite spheroidizing of the 10CrMo910 steel and the rescue heat treatment technical. Hot Work. Technol. 2002, 22, 50–51. [Google Scholar]
- Pan, X.X. Development of Steam Generator Main Materials for Fast Reactor; World Nonferrous Metals: Chengdu, China, 2017; pp. 181–183. [Google Scholar]
- Xu, J.P.; Lv, B.J. Methods of preventing steel embrittlement and its application. Guide Sci. Tech. Mag. 2014, 36, 170. [Google Scholar]
- Song, S.H.; Wu, J.; Yuan, Z.X.; Weng, L.Q.; Xu, T.H. Non-equilibrium grain boundary segregation of phosphorus under a high applied tensile stress in a 2.25CrlMo steel. Mater. Sci. Eng. Astruct. Mater. Prop. Microstruct. Process. 2007, 486, 675–679. [Google Scholar] [CrossRef]
- Wang, J.; Ni, B.H.; Cai, C.; Zhang, Z. Performance degradation of 2.25Cr1Mo steel served in hydrogenation reactor. J. Mater. Eng. 2015, 43, 82–88. [Google Scholar]
- Cao, J.C.; Liu, C.L.; Gao, P.; Zhou, X.L.; Zhou, H. Research status and development trend of elemental segregation in steel. Iron Steel 2019, 54, 11–19. [Google Scholar]
- Wang, Q.J. Investigation on Impact Toughness and Step Cooling Embrittlement Law for 2.25Cr1Mo Steel Electrode Deposited Metal; China Academy of Machinery Science and Technology: Beijing, China, 2015. [Google Scholar]
- Shen, D.D.; Yuan, Z.X. Analysis of factors affecting the ductile-brittle transition temperature of 2.25Cr1Mo steel. J. Wuhan Univ. Sci. Technol. 2011, 34, 12–15. [Google Scholar]
- Liu, R.L. Phosphorus Equilibrium Grain Boundary Segregation and Its Effect on the Ductile-To-Brittle Transition Temperature for a 2.25Cr1Mo Steel; Harbin Institute of Technology: Harbin, China, 2006. [Google Scholar]
- Zhao, Y.; Song, S. Combined Effect of Phosphorus Grain Boundary Segregation, Yield Strength, and Grain Size on Embrittlement of a Cr-Mo Low-Alloy Steel. Steel Res. Int. 2018, 89, 6–12. [Google Scholar] [CrossRef]
- McMahon, C.J.; Cianelli, A.K.; Feng, H.C. The influence of Mo on P-induced temper embrittlement in Ni-Cr steel. Metall. Trans. A 1977, 8, 1055–1057. [Google Scholar] [CrossRef]
- Qu, Z.; McMahon, C.J. The effects of tempering reactions on temper embrittlement of alloy steels. Metall. Trans. A 1983, 14, 1101–1108. [Google Scholar] [CrossRef]
- Janovec, J.; Výrostková, A.; Perhácová, J.V.; Homolová, V.; Grabke, H.J.; Ševc, P.; Lucas, M. Effect of vanadium on grain boundary segregation of phosphorus in low alloy steels. Steel Res. 1999, 70, 269–273. [Google Scholar] [CrossRef]
- Song, S.-H.; Guo, A.-M.; Shen, D.-D.; Yuan, Z.-X.; Liu, J.; Xu, T.-D. Effect of boron on the hot ductility of 2.25Cr1Mo steel. Mater. Sci. Eng. A 2003, 360, 96–100. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Park, K.S.; Lee, S. Effects of boron addition on tensile and Charpy impact properties in high-phosphorous steels. Mater. Sci. Eng. A 2014, 589, 165–173. [Google Scholar] [CrossRef]
- Wu, C.J.; Tang, X.L. Grain boundary segregation of Ce, Mo and P and temper embrittlement of steel. Iron Steel 1991, 26, 31–35. [Google Scholar]
- Jiang, X.; Song, S. Enhanced hot ductility of a Cr–Mo low alloy steel by rare earth cerium. Mater. Sci. Eng. A 2014, 613, 171–177. [Google Scholar] [CrossRef]
- Bor, H.; Chao, C.; Ma, C. The influence of magnesium on carbide characteristics and creep behavior of the Mar-M247 superalloy. Scr. Mater. 1997, 38, 329–335. [Google Scholar] [CrossRef]
- Feng, H.; Jia, H.Y.; Song, Z.G.; Guo, H.S. Effect of mid-temperature aging on precipitation and impact toughness of 022Cr21Ni2Mn5N duplex stainless steel. J. Iron Steel Res. 2017, 29, 137–143. [Google Scholar]
- Li, Z.J.; Xiao, N.M.; Li, D.Z.; Zhang, J.Y.; Luo, Y.J.; Zhang, R.X. Influence of microstructure on impact toughness of G18CrMo2-6 steel during tempering. Acta Metall. Sin. 2014, 50, 777–786. [Google Scholar]
- Zheng, Z.J. The analysis of considerably reduction to HR3C’s aging impact ductility. Boil. Technol. 2011, 42, 46–48. [Google Scholar]
- Ma, D.Q.; Song, Y.H.; Zhao, X.Q.; Lang, Q.B.; Zhang, G.W. Research on impact toughness of 2.25Cr-1Mo-0.25V steel for hydrogenation forgings. J. Mater. Sci. Eng. 2020, 38, 625–628. [Google Scholar]
- Viswanathan, R.; Joshi, A. Effect of microstructure on the temper embrittlement of Cr-Mo-V steels. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 1975, 6, 2289–2297. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; He, X.-K.; Qiao, S.; Xie, C. Effect of microstructure on the impact toughness and temper embrittlement of SA508Gr.4N steel for advanced pressure vessel materials. Sci. Rep. 2018, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Q.; Zhao, J.Q.; Wan, R.D.; Yang, G. Effect of microstructure on long-term aging stability of 2.25Cr-1Mo steel. Heat Treat. Met. 2020, 45, 11–18. [Google Scholar]
- Fu, G.Q.; Zhu, M.Y. Phosphorus segregation on steel grain boundary. Angang Technol. 2006, 339, 5–9. [Google Scholar]
- Guttmann, M.; McLean, D. Interfacial Segregation; Johnsonand, W.C., Blakely, J.M., Eds.; American Society for Metals: Karlsruhe, Germany; Metal Park, OH, USA, 1979; pp. 261–347. [Google Scholar]
- Kenji, A.; Shigeru, J.; Hiroshi, K. Chemical state of phosphorus segregated at grain boundaries of iron. Iron Steel Nippon Steel Corp. 1983, 69, 625–630. [Google Scholar]
- Yin, L.; Sridhar, S. Effects of Residual Elements Arsenic, Antimony, and Tin on Surface Hot Shortness. Met. Mater. Trans. A 2011, 42, 1031–1043. [Google Scholar] [CrossRef]
- Jones, R.H.; Baer, D.R.; Charlot, L.A.; Thomas, M.T. Grain boundary segregation of sulfur and antimony in iron alloys. Met. Mater. Trans. A 1988, 19, 2005–2011. [Google Scholar] [CrossRef]
- Seah, M. Adsorption-induced interface decohesion. Acta Met. 1980, 28, 955–962. [Google Scholar] [CrossRef]
- Lejček, P.; Šob, M.; Paidar, V. Interfacial segregation and grain boundary embrittlement: An overview and critical assessment of experimental data and calculated results. Prog. Mater. Sci. 2017, 87, 83–139. [Google Scholar] [CrossRef]
- Jia, S.J.; Qu, P.; Wen, Y.; Zhang, J.B.; Chen, H.J.; Liu, Q.Y. Research on influence of phosphorus and grain size on mechanical properties of low carbon steels. Iron Steel 2005, 40, 59–63. [Google Scholar]
- Zhao, Y.; Song, S.; Si, H.; Wang, K. Effect of Grain Size on Grain Boundary Segregation Thermodynamics of Phosphorus in Interstitial-Free and 2.25Cr-1Mo Steels. Metals 2017, 7, 470. [Google Scholar] [CrossRef]
- Bu, Y.; Yin, F.Z.; Hu, B.F.; Chen, X. Effect of rare-earth metal, Ca and Mg on microstructure and toughness of heat affected zone of high heat-input welding. Iron Steel 2006, 41, 71–76. [Google Scholar]
- Cui, T.; Lv, J.Y.; Wang, L.; Yang, H.C.; Zhao, G.P. Effect of grain boundary carbide on impact ductility of superalloy GH586. J. Iron Steel Res. 2003, 15, 24–28. [Google Scholar]
- Zhang, X.F.; Komizo, Y. Direct observation of thermal stability of M23C6 carbides during reheating using in situsynchrotron X-ray diffraction. Philos. Mag. Lett. 2013, 93, 9–17. [Google Scholar] [CrossRef]
- Tariq, F.; Naz, N.; Baloch, R.A.; Ali, A. Evolution of microstructure and mechanical properties during quenching and tempering of ultrahigh strength 0.3C Si–Mn–Cr–Mo low alloy steel. J. Mater. Sci. 2010, 45, 1695–1708. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Sridharan, K.; Allen, T.R. Evolution of Carbide Precipitates in 2.25Cr-1Mo Steel during Long-Term Service in a Power Plant. Met. Mater. Trans. A 2010, 41, 1441–1447. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, K.H.; Chang, L.Z.; Chen, J.S.; Zheng, F.Z. Effect of Mg on carbides in M2 high speed tool steel. J. Iron Steel Res. 2019, 31, 1046–1052. [Google Scholar]
- Lu, Q.L.; Zheng, S.B.; Qiu, X.D.; Li, Z. Effect of trace Mg in steel on carbide of bearing steel. Shanghai Met. 2008, 30, 28–32. [Google Scholar]



| No. | C | Cr | Mo | Mn | Ti | Ni | Ca | P | S | O | Mg | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 (wt.%) | 0.12 | 2.22 | 1.01 | 0.51 | 0.06 | 0.88 | <0.001 | 0.050 | 0.0015 | 0.0005 | - | Bal. |
| S1 (at.%) | 0.559 | 2.386 | 0.588 | 0.517 | 0.067 | 0.834 | <0.001 | 0.090 | 0.00349 | 0.00105 | - | Bal. |
| S2 (wt.%) | 0.11 | 2.12 | 0.97 | 0.49 | 0.05 | 0.84 | <0.001 | 0.056 | 0.002 | 0.0003 | 0.006 | Bal. |
| S2 (at.%) | 0.512 | 2.279 | 0.565 | 0.498 | 0.056 | 0.796 | <0.001 | 0.101 | 0.0034 | 0.00105 | 0.0139 | Bal. |
| - | S1 | S2 |
|---|---|---|
| No-aged | 25.3 ± 2.1% | 30.9 ± 1.8% |
| Aged 97 h | 12.1 ± 2.4% | 16.2 ± 1.5% |
| Aging Time/h | Segregated Number of Elements for S1 | Segregated Number of Elements for S2 | |||||
|---|---|---|---|---|---|---|---|
| P | S | Ca | P | S | Ca | Mg | |
| 0 (at.%) | ND 1 | ND | ND | ND | ND | ND | 3.68 ± 0.47 |
| 0 (wt.%) | ND 1 | ND | ND | ND | ND | ND | 1.64 ± 0.21 |
| 500 (at.%) | 2.35 ± 0.76 | 1.27 ± 0.63 | ND | 0.55 ± 0.42 | 1.88 ± 0.54 | ND | 6.70 ± 1.02 |
| 500 (wt.%) | 1.32 ± 0.43 | 0.74 ± 0.37 | ND | 0.32 ± 0.24 | 1.13 ± 0.32 | ND | 3.06 ± 0.47 |
| 5000 (at.%) | 1.12 ± 0.21 | 2.07 ± 0.80 | 12.48 ± 8.6 | ND | 2.60 ± 0.58 | ND | 5.12 ± 1.26 |
| 5000 (wt.%) | 0.56 ± 0.11 | 1.25 ± 0.48 | 9.42 ± 6.49 | ND | 1.56 ± 0.35 | ND | 2.32 ± 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Li, X.; Xing, W.; Ding, L.; Ma, Y.; Liu, K.; Zhang, N. Effect of Trace Mg on Impact Toughness of 2.25Cr1Mo Steel Doped with 0.056% P at Medium Temperature Aging Process. Metals 2021, 11, 10. https://doi.org/10.3390/met11010010
Dong X, Li X, Xing W, Ding L, Ma Y, Liu K, Zhang N. Effect of Trace Mg on Impact Toughness of 2.25Cr1Mo Steel Doped with 0.056% P at Medium Temperature Aging Process. Metals. 2021; 11(1):10. https://doi.org/10.3390/met11010010
Chicago/Turabian StyleDong, Xin, Xiaobing Li, Weiwei Xing, Leilei Ding, Yingche Ma, Kui Liu, and Nannan Zhang. 2021. "Effect of Trace Mg on Impact Toughness of 2.25Cr1Mo Steel Doped with 0.056% P at Medium Temperature Aging Process" Metals 11, no. 1: 10. https://doi.org/10.3390/met11010010
APA StyleDong, X., Li, X., Xing, W., Ding, L., Ma, Y., Liu, K., & Zhang, N. (2021). Effect of Trace Mg on Impact Toughness of 2.25Cr1Mo Steel Doped with 0.056% P at Medium Temperature Aging Process. Metals, 11(1), 10. https://doi.org/10.3390/met11010010







