New Utilization of Specific Biomass: Lignin in the Iron Ore Sintering Process
Abstract
:1. Introduction
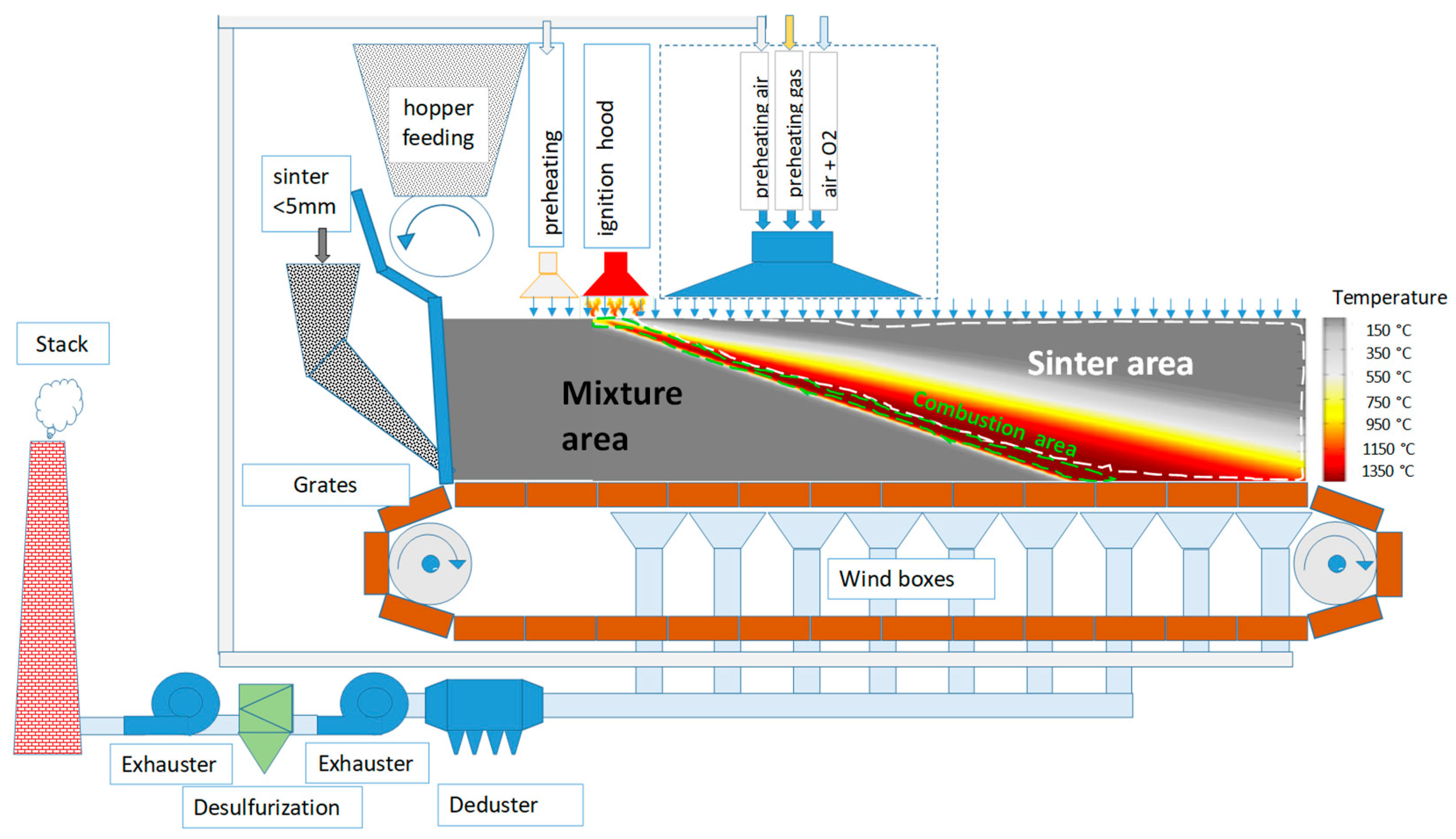
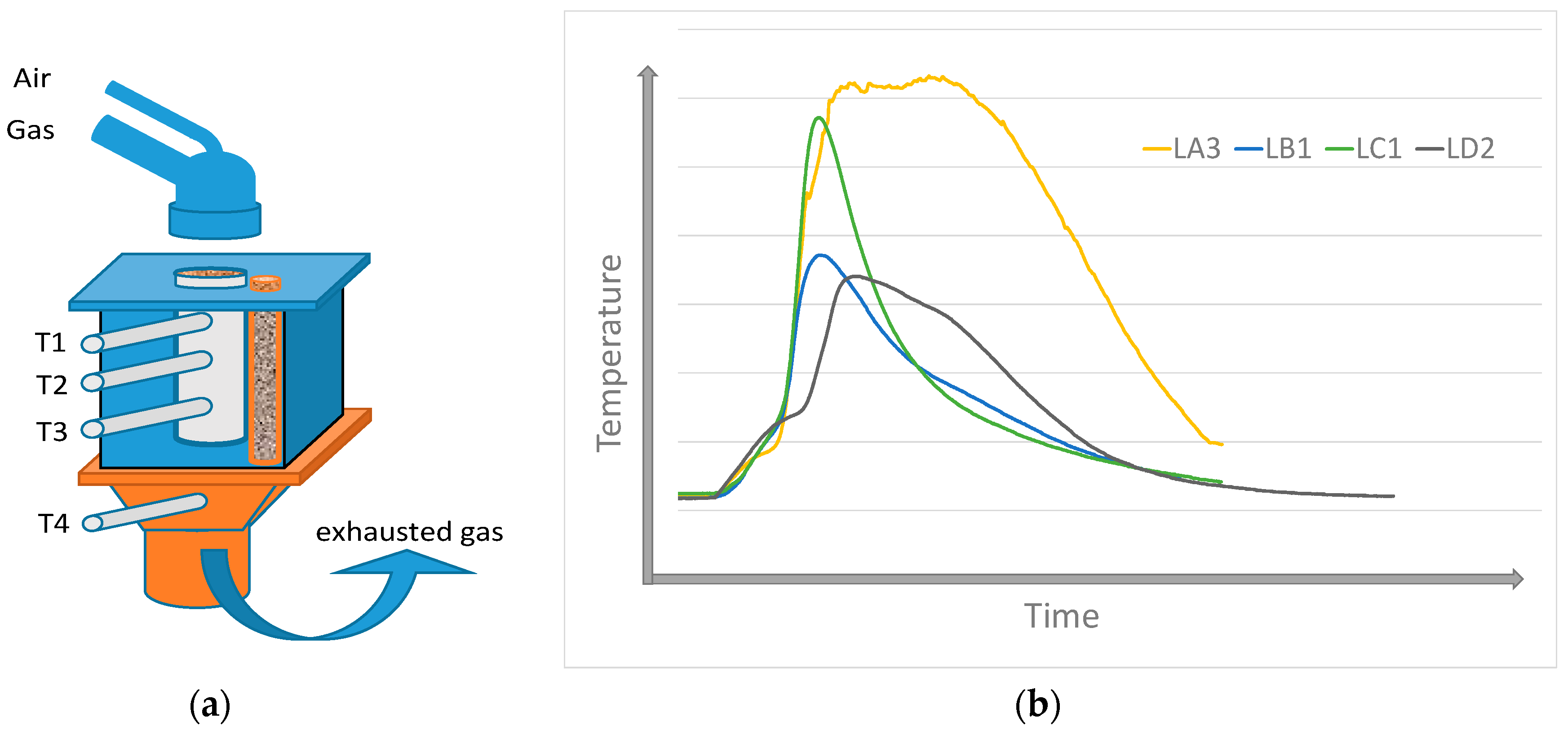
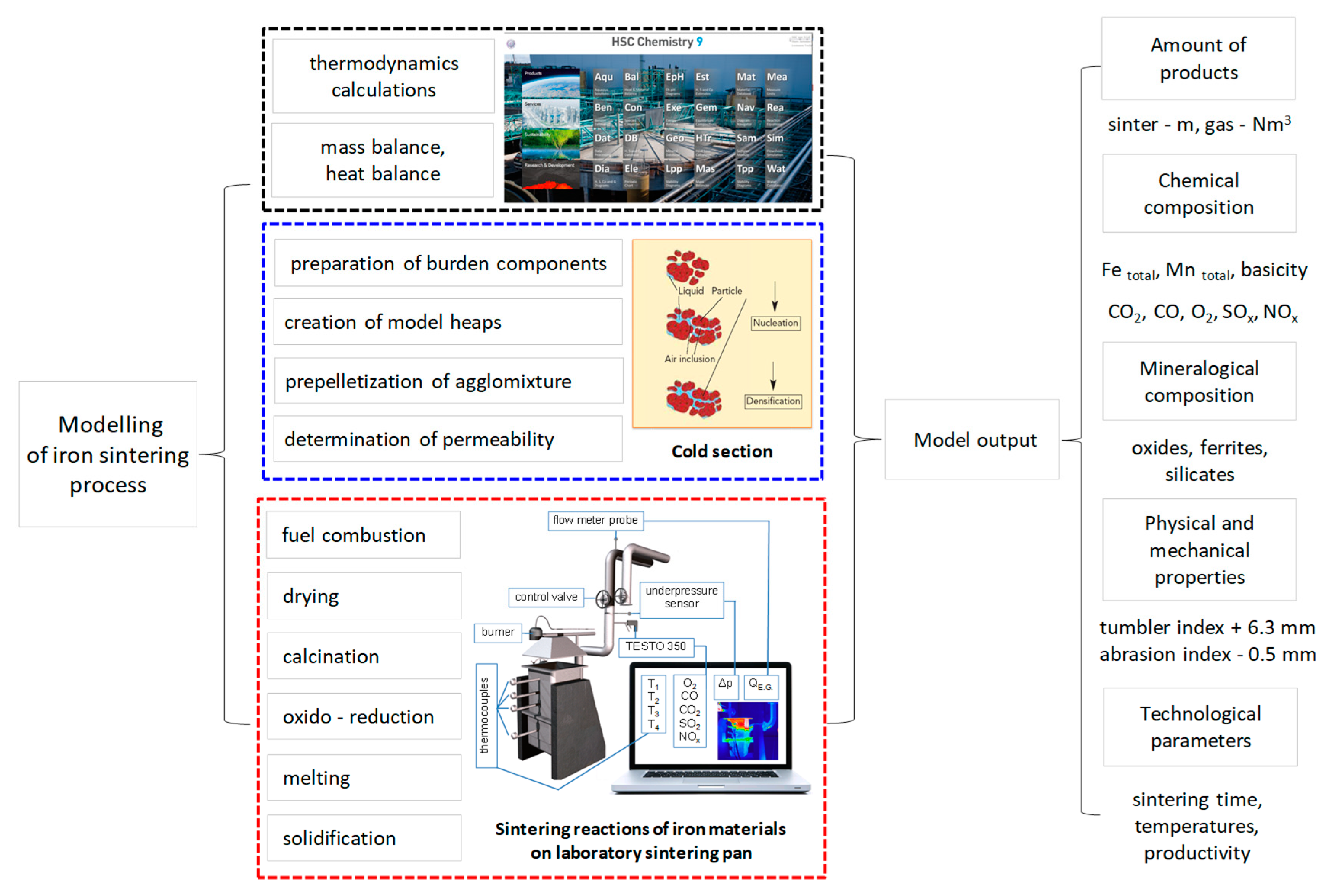
2. Materials and Methods
3. Results and Discussion
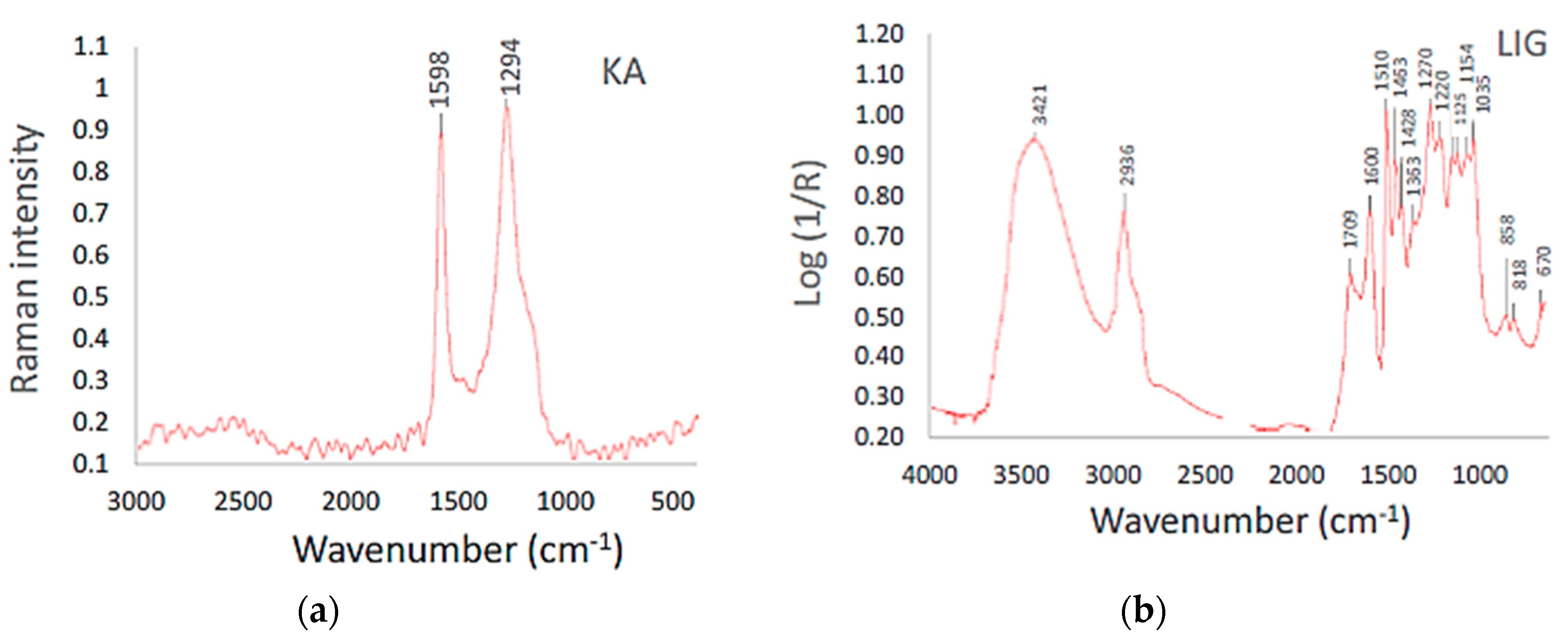
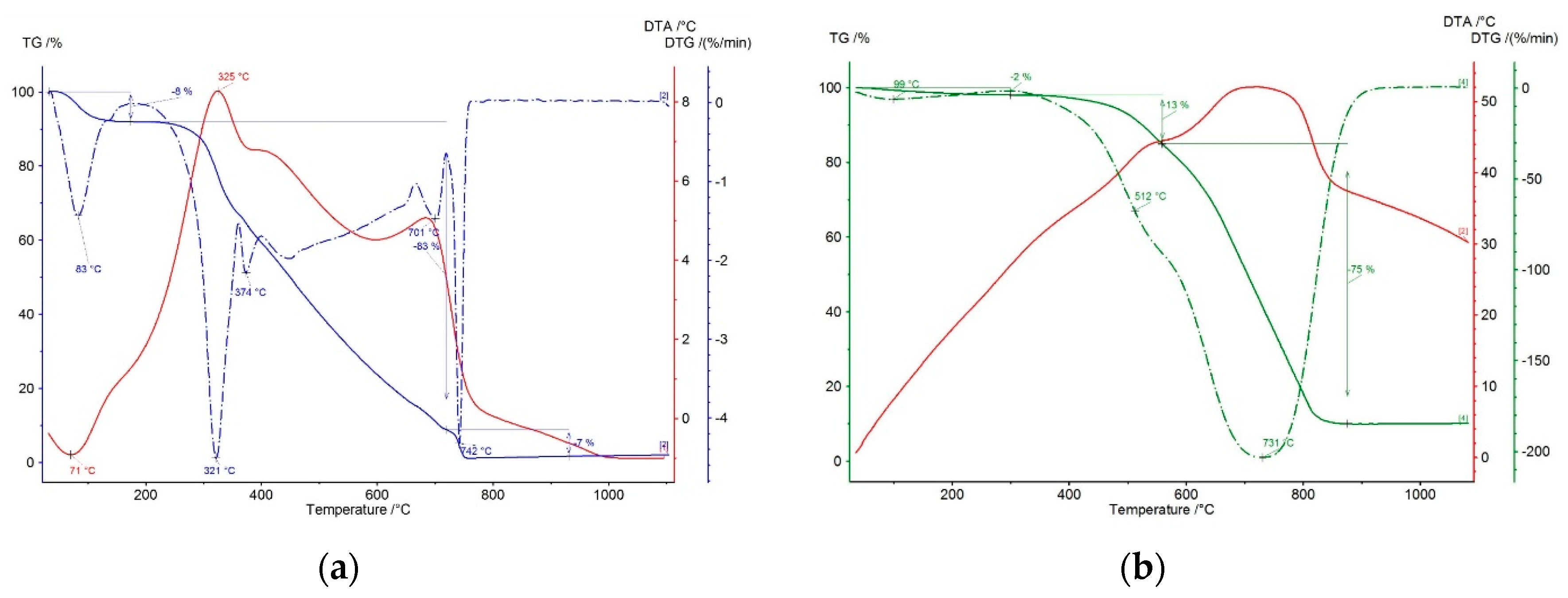
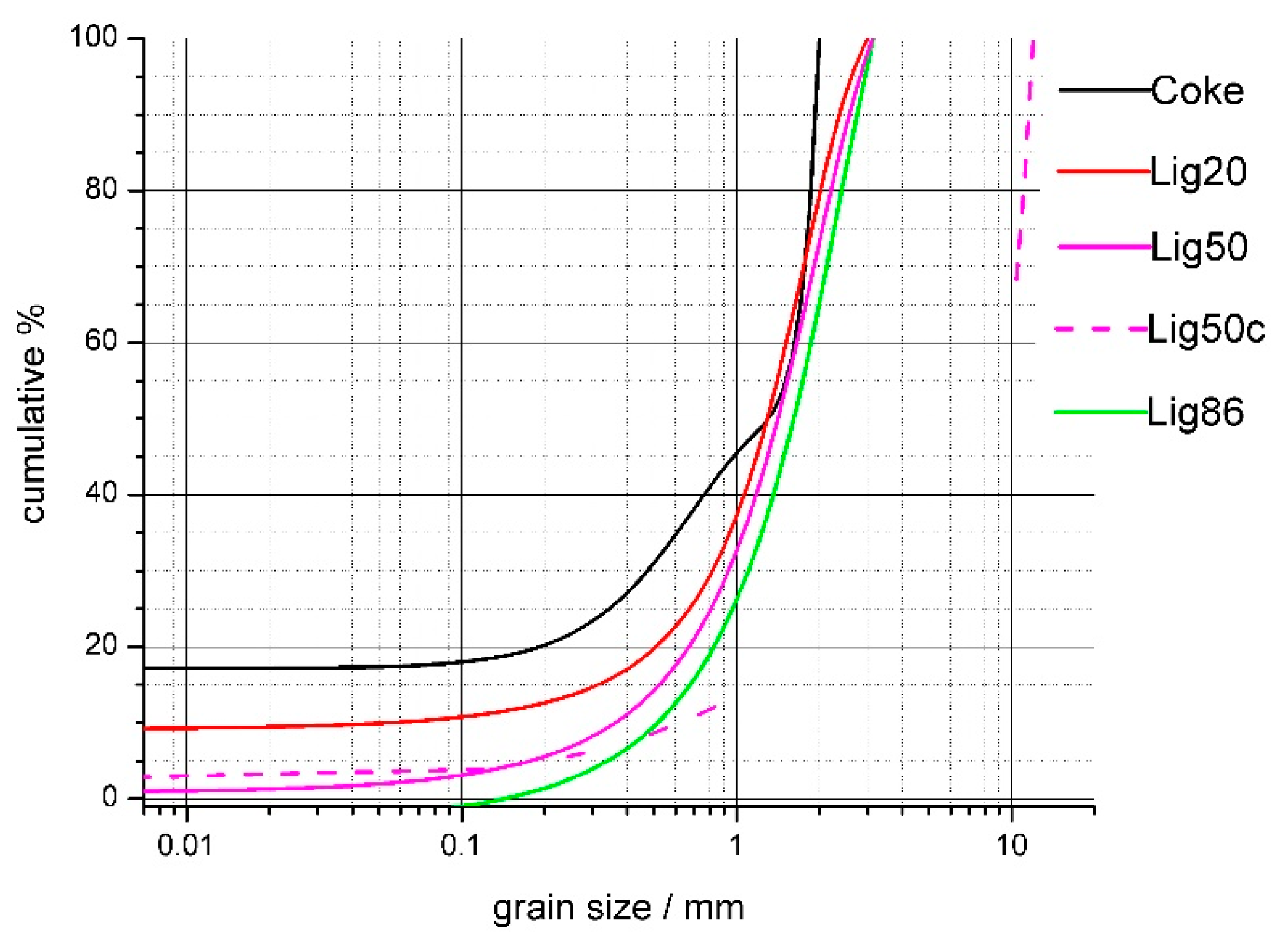
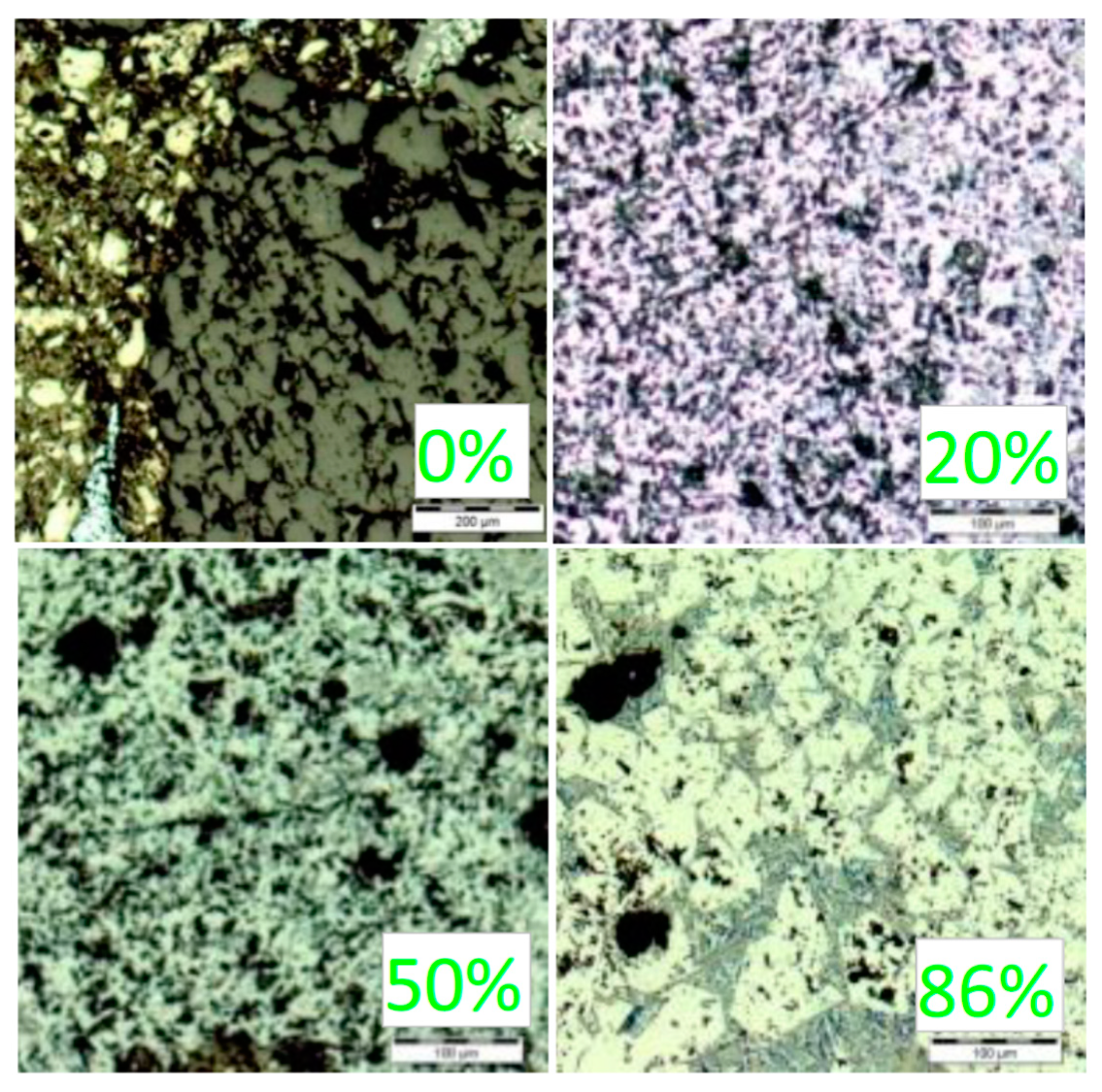
3.1. Material Research of Coke and Lignin
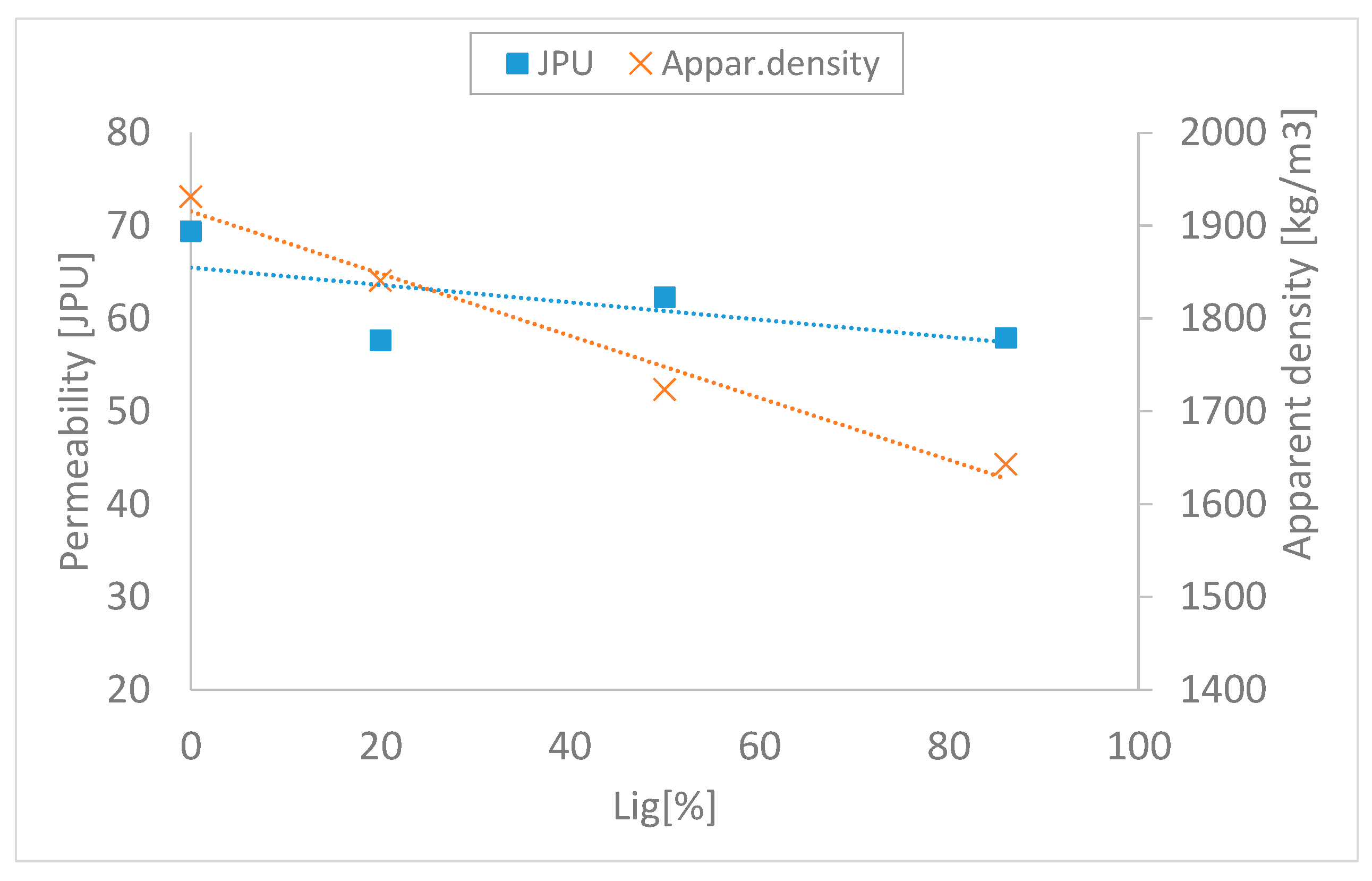
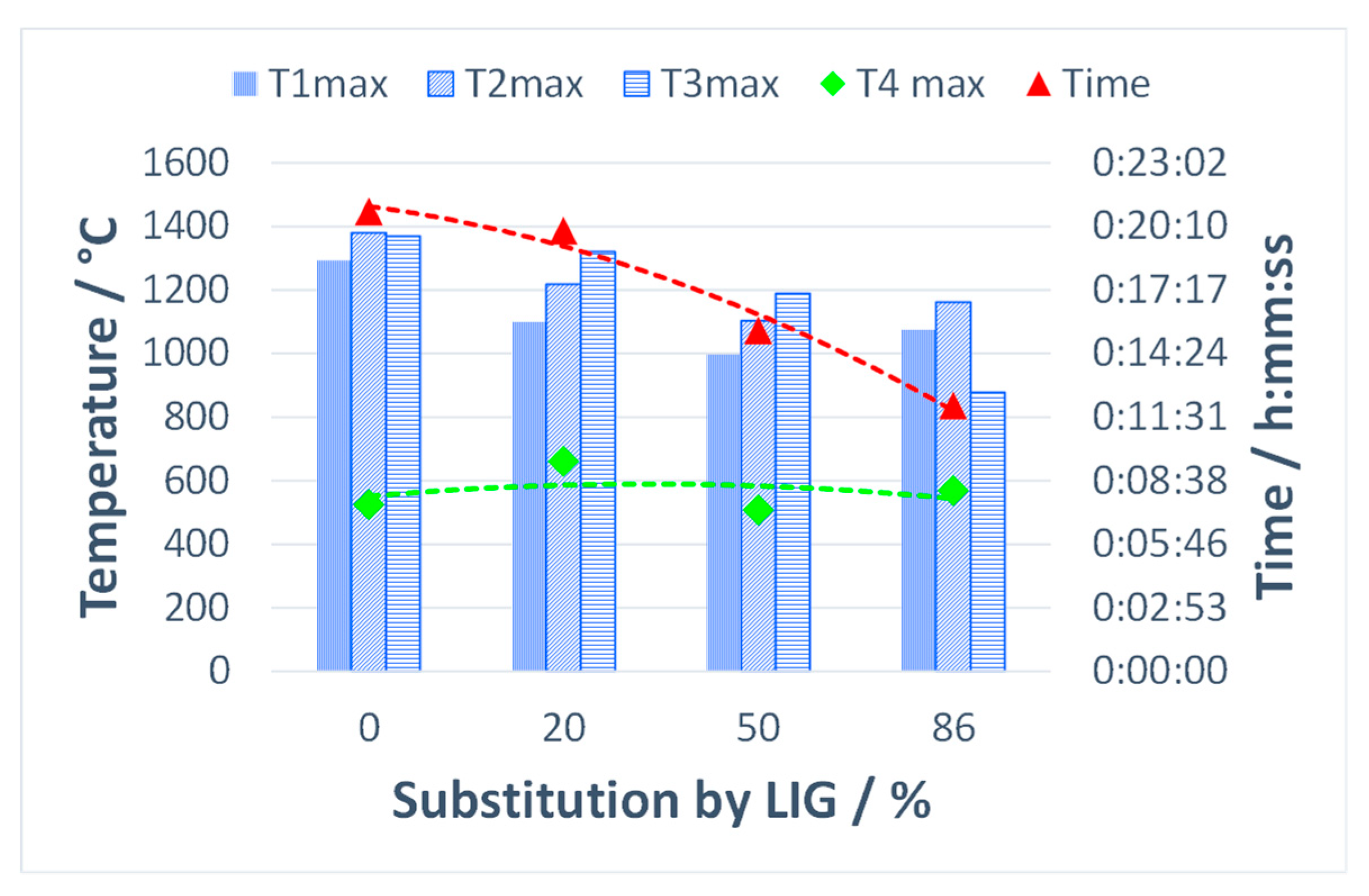
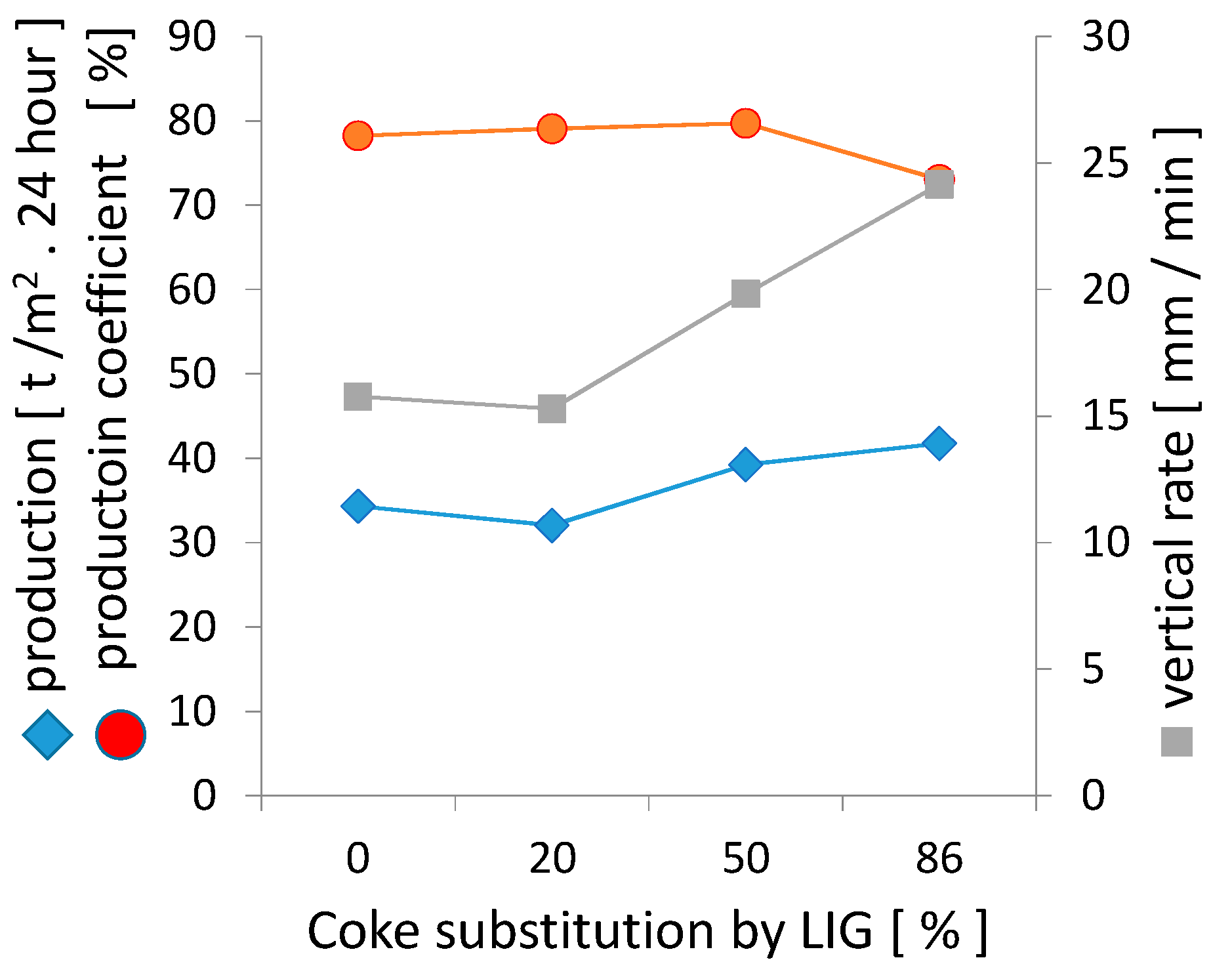
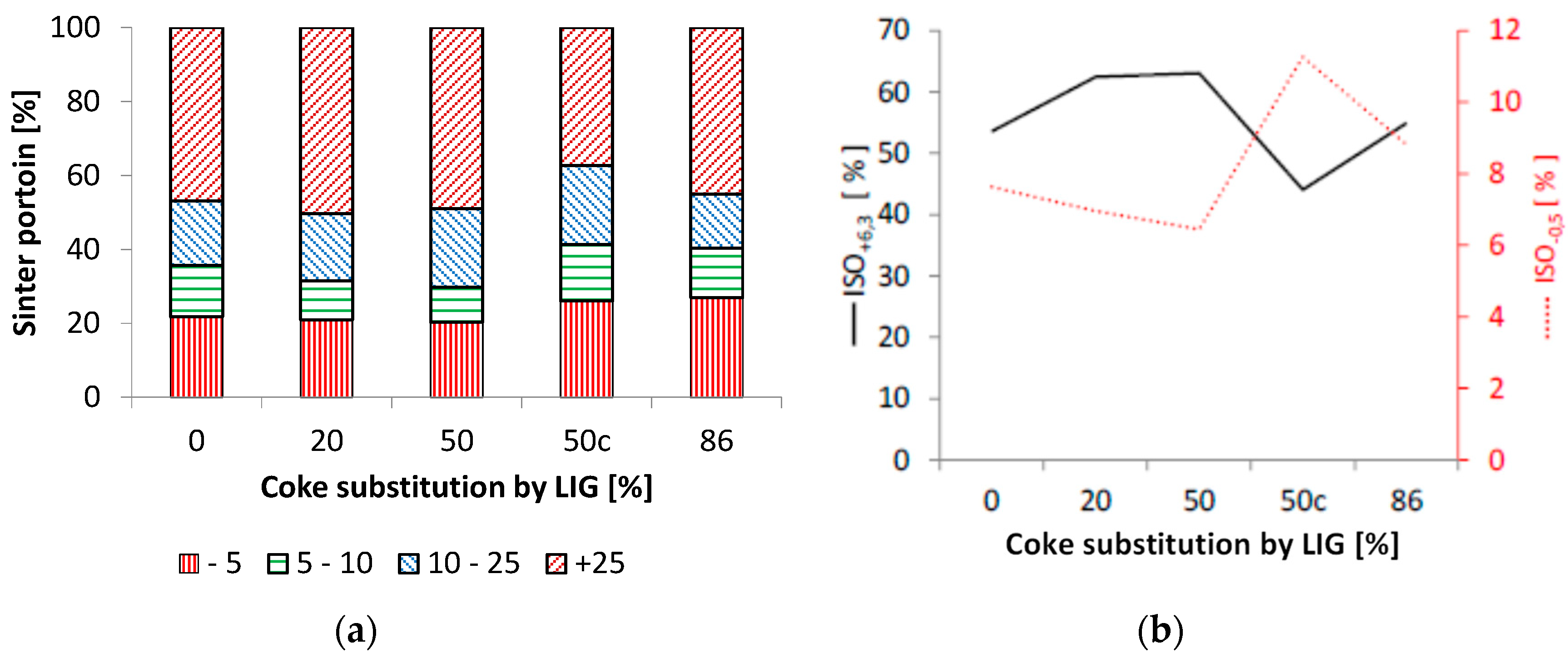
3.2. Impact of Lignin on the Agglomeration Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Findorák, R.; Frőhlichová, M.; Legemza, J. Biomasa v Aglomeračnom Procese; Technical University of Košice: Košice, Slovakia, 2020; ISBN 978-80-553-3567-4. [Google Scholar]
- Shengwei, T.; Xilou, D.; Kai, Z. Study on the craft technology scheme of the sinter flue gas desulphurization of Ma’anshan. In Metal World; Iron & Steel Co. Ltd.: Xi’an, China, 2008; Volume 6, pp. 20–23. [Google Scholar]
- Legemza, J.; Frohlichova, M.; Findorak, R. The process of simulating the agglomerate laboratory production under laboratory conditions. Acta Metall. Slov. 2010, 1, 70–75. [Google Scholar]
- Murakami, K.; Sugawara, K.; Kawaguchi, T. Analysis of combustion rate of various carbon materials for iron ore sintering process. ISIJ Int. 2013, 53, 1580–1587. [Google Scholar] [CrossRef] [Green Version]
- Lovel, R.; Vining, K.; Dell’Amico, M. Iron ore sintering with charcoal. Min. Process. Extractr. Metall. 2007, 116, 85–92. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Hara, M. Utilization of biomass for iron ore sintering. ISIJ Int. 2013, 53, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Ji, Z.; Gan, M.; Chen, X.; Li, W.; Yu, Z. Strengthening refractory iron ore sintering with biomass fuel. In Proceedings of the 3rd International Symposium on High-Temperature Metallurgical Processing–TSM 2012, Orlando, FL, USA, 3 April 2012; pp. 357–364. [Google Scholar]
- Gan, M.; Fan, X.; Chen, X.; Ji, Z.; Lv, W.; Wang, Y.; Yu, Z.; Jiang, T. Reduction of pollutant emission in iron ore sintering process by applying biomass fuels. ISIJ Int. 2012, 52, 1574–1578. [Google Scholar] [CrossRef] [Green Version]
- Findorák, R.; Fröhlichová, M.; Legemza, J. The effect of charcoal addition on iron-ore sintering performance. In Proceedings of the 13th International Multidisciplinary Scientific Geoconference SGEM, Albena, Bulgaria, 16–22 June 2013; Volume 2, pp. 637–642. [Google Scholar]
- Mašlejová, A. Evaluation of iron ore sinter structure using a various types of biomass. In Proceedings of the 13th SGEM GeoConference on Science and Technologies, Albena, Bulgaria, 16–22 June 2013; Volume 2, pp. 581–588. [Google Scholar]
- Frőhlichová, M.; Findorák, R.; Legemza, J.; Džupková, M. The fusion characteristics of ashes from lignin and the coke breeze. Arch. Metall. Mater. 2018, 3, 1523–1530. [Google Scholar]
- Legemza, J.; Frőhlichová, M.; Findorák, R.; Džupková, M. Modelling of mass and thermal balance and simulation of iron sintering process with biomass. Metals 2019, 9, 1010. [Google Scholar] [CrossRef] [Green Version]
- Findorak, R.; Frohlichova, M.; Legemza, J.; Dzupkova, M. Utilization of Infrared Thermometry for Analyse of Biomass Burning Process. In Proceedings of the International Multidisciplinary Scientific GeoConference: SGEM: Surveying Geology & Mining Ecology Management, Albena, Bulgaria, 30 June–9 July 2018; Volume 18, pp. 1017–1022. [Google Scholar]
- Majerčák, Š.; Majerčáková, A. Vysokopecná Vsádzka; ALFA Bratislava: Bratislava, Slovakia, 1986. [Google Scholar]
- Kruk, M.; Kohlhaas, K.M.; Dufour, B.; Celer, E.B.; Jaroniec, M.; Matyjaszewski, K.; Ruoff, R.S.; Kowalewski, T. Partially graphitic, high-surface-area mesoporous carbons from polyacrylonitrile templated by ordered and disordered mesoporous silicas. Microporous Mesoporous Mater. 2007, 102, 178–187. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Koizumi, N.; Urabe, Y.; Inamura, K.; Itoh, T.; Yamada, M. Investigation of carbonaceous compounds deposited on NiMo catalyst used for ultra-deep hydrodesulfurization of gas oil by means of temperature-programmed oxidation and Raman spectroscopy. Catal. Today 2005, 106, 211–218. [Google Scholar] [CrossRef]
- Fröhlichová, M.; Findorák, R.; Legemza, J.; Džupková, M. Impact of substituting coke with biomass on the mineralogical composition of the iron ore agglomerate. Metals 2020, 10, 909. [Google Scholar] [CrossRef]
- Yang, W.; Ryu, C.; Choi, E.S. Modelling of combustion and heat transfer in an iron ore sintering bed with considerations of multiple solid phases. ISIJ Int. 2004, 44, 492–499. [Google Scholar] [CrossRef]
- Blaskett, D.R. The sintering symposium, Port Pirie. Aust. Inst. Min. Metall. 1960, 66, 61–70. [Google Scholar]
- Jursova, S.; Pustejovska, P.; Brozova, S. Study on reducibility and porosity of metallurgical sinter. Alex. Eng. J. 2018, 57, 1657–1664. [Google Scholar] [CrossRef]





| Fuel | Proximate Analysis (wt%) | Ultimate Analysis (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2O (W) | Ash (A) | Volatile (CV) | Fixed Carbon (C FIX) | C | H | O | N | S | Caloric Value (MJ/kg) | |
| Coke dust | 5.5 | 12.10 | 1.50 | 80.9 | 85.4 | 0.30 | 0.60 | 1.30 | 0.30 | 28.16 |
| Lignin | 8.6 | 3.4 | 67.90 | 20.1 | 62.9 | 5.75 | 27.60 | 0.2 | 0.15 | 23.14 |
| Iron material | Humidity | Fe | FeO | Fe2O3 | SiO2 | Al2O3 | CaO | MgO | Mn | P |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (wt%) | |||||||||
| Ore | 4.20 | 60.39 | 0.52 | 85.38 | 11.07 | 0.90 | 0.07 | 0.21 | 0.027 | 0.028 |
| Concentrate | 9.79 | 67.95 | 27.80 | 66.16 | 4.89 | 0.16 | 0.12 | 0.24 | 0.030 | 0.011 |
| Iron material | Basicity | S | Na2O | K2O | TiO2 | Pb | Zn | As | Cl | C |
| (-) | (wt%) | |||||||||
| Ore | 0.024 | 0.015 | 0.154 | 0.053 | 0.038 | 0.001 | 0.001 | 0.001 | 0.210 | 0.070 |
| Concentrate | 0.070 | 0.123 | 0.029 | 0.060 | 0.015 | 0.001 | 0.001 | 0.002 | 0.050 | 0.152 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Findorák, R.; Legemza, J.; Fröhlichová, M.; Fabriciová, G.; Džupková, M. New Utilization of Specific Biomass: Lignin in the Iron Ore Sintering Process. Metals 2020, 10, 1170. https://doi.org/10.3390/met10091170
Findorák R, Legemza J, Fröhlichová M, Fabriciová G, Džupková M. New Utilization of Specific Biomass: Lignin in the Iron Ore Sintering Process. Metals. 2020; 10(9):1170. https://doi.org/10.3390/met10091170
Chicago/Turabian StyleFindorák, Róbert, Jaroslav Legemza, Mária Fröhlichová, Gabriela Fabriciová, and Martina Džupková. 2020. "New Utilization of Specific Biomass: Lignin in the Iron Ore Sintering Process" Metals 10, no. 9: 1170. https://doi.org/10.3390/met10091170
APA StyleFindorák, R., Legemza, J., Fröhlichová, M., Fabriciová, G., & Džupková, M. (2020). New Utilization of Specific Biomass: Lignin in the Iron Ore Sintering Process. Metals, 10(9), 1170. https://doi.org/10.3390/met10091170




