Abstract
H2SO4 was ensured to be the best candidate for Zr leaching from the eudialyte. The resulting sulfuric leach solution consisted of Zr(IV), Nb(V), Hf(IV), Al(III), and Fe(III). It was found that ordinary metal hydroxide precipitation was not feasible for obtaining a relatively pure product due to the co-precipitation of Al(III) and Fe(III). In this reported study, a basic zirconium sulfate precipitation method was investigated to recover Zr from a sulfuric acid leach solution of a eudialyte residue after rare earth elements extraction. Nb precipitated preferentially by adjusting the pH of the solution to around 1.0. After partial removal of SO42− by adding 120 g of CaCl2 per 1L solution, a basic zirconium sulfate precipitate was obtained by adjusting the pH to ~1.6 and maintaining the solution at 75 °C for 60 min. Under the optimum conditions, the loss of Zr during the SO42− removal step was only 0.11%, and the yield in the basic zirconium sulfate precipitation step was 96.18%. The precipitate contained 33.77% Zr and 0.59% Hf with low concentrations of Fe and Al. It was found that a high-quality product of ZrO2 could be obtained from the basic sulfate precipitate.
1. Introduction
Zirconium metal is widely used in the areas of atomic energy, metallurgy, the military, petrochemicals, aerospace, new materials science as well as in medicine [1]. Zirconium is also an important alloying element in steel industry [2]. The major source for zirconium production is zircon (ZrSiO4), which is highly stable, requiring high temperature sintering for decomposition [3]. Eudialyte is a complex Na-Ca-zirconosilicate mineral that can be a potential commercial source of zirconium [4,5]. The content of Zr (5–10%) in eudialyte is much lower than in zircon, but it can be easily decomposed using acid [6,7,8].
The typical empirical chemical formula for eudialyte is Na4(Ca, Ce, Fe)2ZrSi6O17(OH, Cl)2, but it displays a wide range of chemical compositions [6]. Eudialyte is also rich in Fe, Al, Mn, Ti, K, Nb and contains significant quantities of rare earth elements (REE) [4,6,9]. As such, the comprehensive extraction of the valuable metals must be considered for practical eudialyte processing [6,10]. REE extraction from eudialyte sometimes takes priority, since these elements are more valuable [11].
To date, acid decomposition of eudialyte has been extensively studied [7,8,12,13,14]. Sulfuric acid (H2SO4) is considered to be the best candidate for Zr leaching from the eudialyte or eudialyte residue after REE extraction. However, the leaching process is not selective, so many impurities remain in the sulfuric acid leach solution; such as sodium, iron, and aluminum [6,8,15,16]. Data concerning the recovery of Zr from the sulfate media containing these impurities are scarce in the general literature. A complete treatment process to produce a Zr product can only be found in a few feasibility studies [17]. Lebedev et al. studied the ordinary precipitation by adding Na2CO3, but the resulting Zr carbonate was found to be a mixture of Zr(IV), Hf(IV), Nb(V), Fe(III), and Al(III) carbonates [6]. Further separation and purification are indispensable for the production of Nb, Hf, or Zr products. Tasman Metal Ltd. has studied the exploitation and utilization of the eudialyte from Norra Kärr, Sweden. Solvent extraction followed with precipitation was used to recover Zr from the sulfate media [18]. However, the details of the solvent and process parameters were not reported. Ion exchange can also be employed to recover Zr in eudialyte processing, but the process has a lot of operations and is very complicated [16].
On the other hand, Hf is in the same period with Zr in the periodic table, so the chemical properties of these two elements are nearly identical due to the lanthanide contraction [19]. Hf is usually present in trace quantities in minerals that contain Zr [20]. The Hf concentration in eudialyte is about 0.2%, and it follows with Zr during the treatment of eudialyte [6,11,16]. Commercial-grade Zr product containing Hf can be used in chemical process industries, but for use as a cladding material, the Zr product must be Hf-free due to their varied neutron-absorbing properties [21].
This reported study focused on the extraction process following acid leaching. Based on the solution chemistry, a basic zirconium sulfate precipitation was investigated to recover Zr from the sulfuric acid leach solution of a eudialyte residue, anticipating the production of a precipitate with a low impurity level. Furthermore, preparation of ZrO2 from the basic sulfate precipitate was also attempted.
2. Materials and Methods
2.1. Materials and Analysis
As a resource for REE in the EURARE project, which was funded by the European Commission for the development of a sustainable exploitation scheme for Europe’s rare earth ore deposits, eudialyte ore was mined in South Greenland, and after beneficiation, the eudialyte concentrate was the initial material for REE extraction. After REE extraction, the eudialyte residue was known to contain attractive quantities of Zr. H2SO4 was used to leach Zr from the eudialyte residue, and a detailed description of leaching process has been reported [10] by our group. The composition of the resulting sulfuric acid leach solution is listed in Table 1. Among the metal ions, iron ions existed only in the form of Fe(III), because H2O2 was added in the leaching process. This study focused on the subsequent step of Zr recovery from the sulfate leachate. As can be seen from the data in Table 1, the concentration of SO42− in the leach solution was high and the main impurities in the leach solution were found to be Al and Fe. The CaCl2, Na2CO3, and HCl used in this work were of analytical grade, and all aqueous solutions were prepared using distilled water. The elemental content of the solution was determined by inductively coupled plasma emission spectroscopy (ICP) using a PS-6 PLASMA SPECTROVAC (Baird, Waltham, MA, USA). The composition of the precipitate was determined by X-ray fluorescence (AXIOS, PANalytical, Eindhoven, The Netherlands), and Zr in the basic sulfate precipitate was analyzed from solution after it was solubilized. The content of the basic sulfate precipitate was also characterized using X-ray diffraction (XRD, PANalytical X’PERT-PRO diffractometer, Malvern PANalytical, Eindhoven, The Netherlands) and Fourier-transform infrared spectroscopy (FT-IR, Spectrum 100, Perkin Elmer, Inc., Waltham, MA, USA). The prepared ZrO2 was characterized by XRD and the composition of ZrO2 was evaluated using ICP analysis. The data of chemical compositions and concentrations in this paper are the averages of 3 analysis results.

Table 1.
Chemical composition of experimental solutions, g/L.
2.2. Thermodynamic Analysis and Methods
Metal precipitation can be defined as a process where metal ions are reacted with other compounds to form a low solubility product. Metal hydroxide precipitation is the most common example of this [22]. The log [Men+]-pH diagram based on the solubility product constants of metals hydroxide at 298.15 K is shown in Figure 1, where Nb5+ in the solution is readily hydrolyzed at low pH, and the pH ranges for the hydrolysis of Zr4+ and Hf4+ are between the pH ranges for hydrolysis of Fe3+ and Al3+. However, some anions can act as ligands in the solution, which was ignored in this representation. In the absence of SO42−, the hydrolysis of Zr4+ can be initiated even in high acidity. If complexation in the aqueous solution is considered, the pH ranges for precipitation will increase.
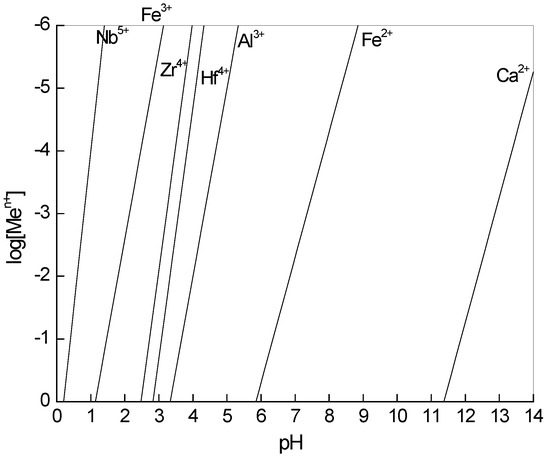
Figure 1.
log[Men+]-pH diagram based on the solubility product constants (Ksp) of metals hydroxide at 298.15 K (Ksp data obtained from the literature [22]).
For the test solution in this study, a complex reaction with SO42− inhibits the hydrolysis of Zr4+ and Hf4+ when the pH is increased, because the use of SO42− produces competition between SO42− and OH− ions to react with Zr4+ and Hf4+. The complex reaction of Zr4+ and Hf4+ in the acidic sulfate solution can be represented by Equation (1), and the corresponding stability constants (βi) are listed in Table 2 [23]. In other words, the formation of complexes with SO42− can expand the stable region of the dissolved Zr and Hf. In addition, NbO(SO4)2− is also exist in the acidic sulfate solution, and the relevant equation is represented by Equation (3) [24]. While sulfate ions have little effect on other metal ions in the sulfuric acid leach solution.
Me4+(aq) + i HSO4− = Me(SO4)i4−2i (aq) + i H+
Nb(OH)5 + 3H+ + 2SO42− = NbO(SO4)2− + 4H2O (logK = 7.24)

Table 2.
Stability constants of sulphate complexes of zirconium and hafnium.
Figure 2 shows the fraction of ions of Nb and Zr in the sulfate aqueous solution at different pH values. As can be seen, the pH for Nb(V) precipitation via hydrolysis was as low as the pH in the absence of SO42−. However the predominant forms of Zr and Hf in the acidic sulfate solution with pH > 0 are Zr(SO4)32− and Hf(SO4)32− and the pH range for hydrolysis increased. These conditions are consistent with those reported in the literature. The selective recovery of Zr(IV) from sulfuric acid leach solution via ordinary neutralization is not feasible due to the co-precipitation of Fe(III) and Al(III) [6].
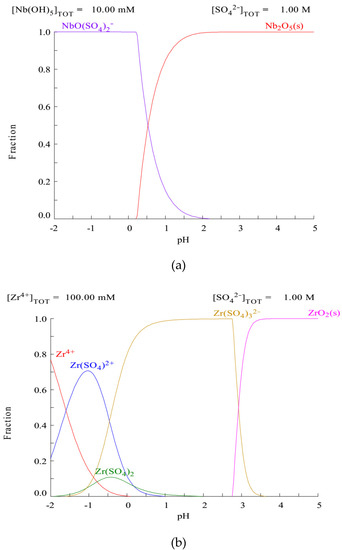
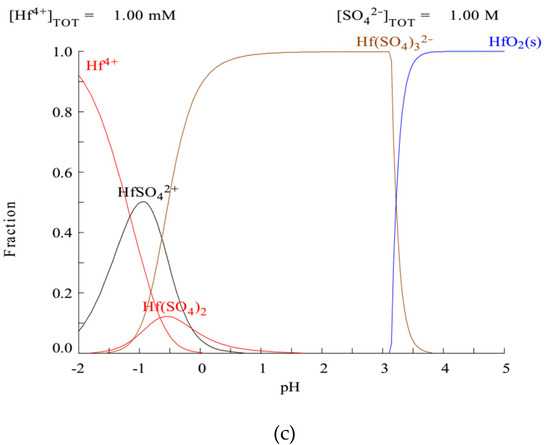
Figure 2.
Predicted fraction diagrams of (a) Nb, (b) Zr, and (c) Hf in aqueous solutions using MEDUSA software (Make Equilibrium Diagrams Using Sophisticated Algorithms, 32 bit version 2010, Royal Institute of Technology, Stockholm, Sweden).
Precipitation using basic zirconium sulfate (Zr5O8(SO4)2·xH2O) in place of Zr(OH)4 is another alternative for producing zirconate. According to the literature [25,26], basic zirconium sulfate can be obtained when zirconium chloride solution is mixed with sulfuric acid solution at a set temperature (60–90 °C) and pH (1.2–2) with a zirconium to sulfate ratio of 5:2. This reaction can be expressed by Equation (4).
Since this reaction occurs at a pH < 2.0, co-precipitation of Fe(III) and Al(III) will be low.
5Zr4+ + 2HSO4− + (8 + x)H2O → Zr5O8(SO4)2·xH2O (s) + 18H+
A flow chart for the recovery of Zr using the basic sulfate precipitation method is shown in Figure 3. As shown, first, Nb is preferentially precipitated by neutralization. After separating the Nb from the solution, CaCl2 is added to remove some SO42−, and then zirconium is selectively precipitated using basic sulfate zirconium at low pH without the co-precipitation of Fe(III) and Al(III). A small amount of Hf in the solution would follow Zr in the precipitation process, since it has chemical properties that are very similar to Zr. In the process, Na2CO3 and CaCl2 was added in solid form little by little.
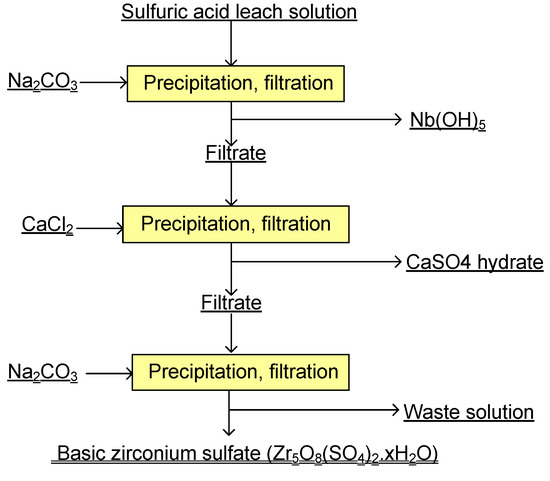
Figure 3.
Proposed flowchart for recovery of Zr using precipitation methods.
In order to prepare a Zr product, the next stage of the process can be connected to the conventional technology in the Zr production from zircon [27]. The prepared basic zirconium sulfate was dissolved with 4 mol/L HCl. Evaporative crystallization was then used to prepare ZrOCl2·8H2O. After obtaining the ZrOCl2·8H2O, the ZrO2 can be obtained by calcination at 600 °C in a muffle furnace (Thermo Scientific™ M110) for 2 h.
3. Results and Discussion
3.1. Effect of pH on the Precipitation of Metal Ions by Neutralization
Figure 4 shows the effect of pH on the precipitation of the main elements in the leach solution. Based on the aqueous solution chemistry analysis and precipitation behavior, Nb, Zr, Hf, Al, and Fe were precipitated from the solution by hydrolysis at a suitable pH. The quantity of dissolved Ca2+ in the sulfate solution was very low, and it also partially precipitated in the form of CaSO4·2H2O when the pH was increased. Zr and Hf precipitated until the pH was increased to 3.5, but Fe and Al precipitated simultaneously, so that it was not feasible to selectively recover Zr and Hf via the ordinary precipitation method from the sulfuric acid leach solution. Figure 5 shows a precipitate in a difficult state of filtration at pH 4.0.
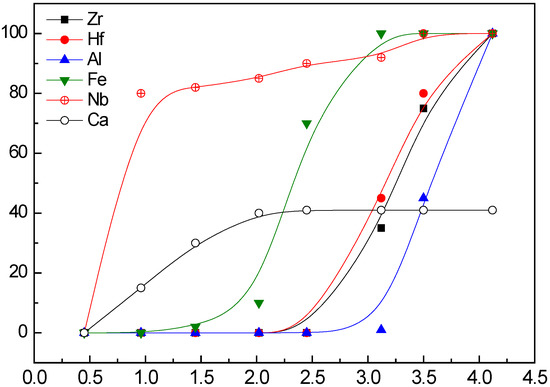
Figure 4.
Effect of pH on the precipitation of elements from the leach solution.
Figure 5.
The precipitate in a difficult state of filtration at pH 4.0 after adding Na2CO3.
The precipitation method for the selective recovery of Zr, Hf, and Nb included precipitation of Nb by hydrolysis and precipitation of Zr and Hf using basic zirconium sulfate. The optimum pH for the precipitation of Nb was 1.0 based on the results shown in Figure 4. Table 3 shows the composition of the resulting precipitate. As can be seen, the content of Nb was 40.1%, and the concentration of other impurities was low, but a further purification was necessary to obtain the Nb product. After Nb precipitation, Zr and Hf were recovered using basic zirconium sulfate precipitation or ion exchange.

Table 3.
Composition of Nb precipitate.
3.2. Effect of the Quantity of CaCl2 on the Basic Sulfate Precipitation
In order to control a suitable zirconium to sulfate ratio for the Zr precipitation process, an amount of CaCl2 was added to remove a portion of the sulfate ions from the Zr-bearing solution after the Nb recovery. As can be seen in Figure 6, the Zr precipitate yield increased with the addition of CaCl2 from 100 to 120 g per 1 L solution, and further increases in the amount of CaCl2 resulted in a decrease in the yield of Zr precipitation. The Hf precipitation yield exhibited the same trend, but the precipitate yields of Fe(III) and Al(III) were always very low. Hence, 120 g/L of CaCl2 was chosen as the optimum quantity for removing the sulfate ions. After calculation, the concentration of sulfate in the solution was decreased from 112.0 g/L to 12.4 g/L, and the suitable molar ratio of zirconium to sulfate was about 2.5:1.
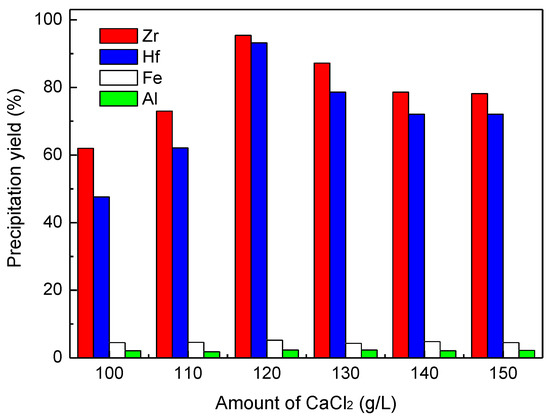
Figure 6.
Effect of CaCl2 quantity on the Zr precipitation yield, Zr precipitation conditions: 75 °C, final pH ~1.6, 60 min.
3.3. Effect of pH on the Basic Sulfate Precipiation
During the basic zirconium sulfate precipitation process, Na2CO3 was used to adjust the solution pH, and the effect of varying the final pH was also examined. As shown in Figure 7, the Zr precipitation yield increased from 62.1% to 96.1% when the final pH was increased from 1.2 to 1.6, and above 1.6, the precipitation yield of Fe(III) and Al(III) noticeably increased. Thus, the optimum final pH for the basic zirconium precipitation process was 1.6.
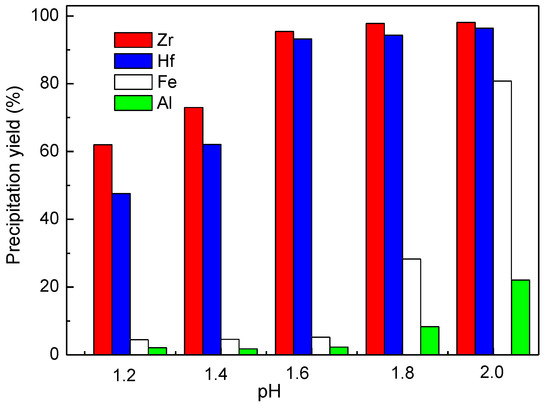
Figure 7.
Effect of pH on the Zr precipitation yield, Zr precipitation conditions: 75 °C, C(SO42−) = 12.4 g/L, 60 min.
3.4. Effect of Temperature on the Basic Sulfate Precipiation
The effect of temperature on the precipitation yields of metals was also determined experimentally. It can be seen from the results in Figure 8 that the precipitation yields of Zr and Hf both increased with the increase in temperature, and 75 °C was the best for the recovery of Zr and Hf. Most of Fe and Al could be kept in the solution by controlling the pH at ~1.6.
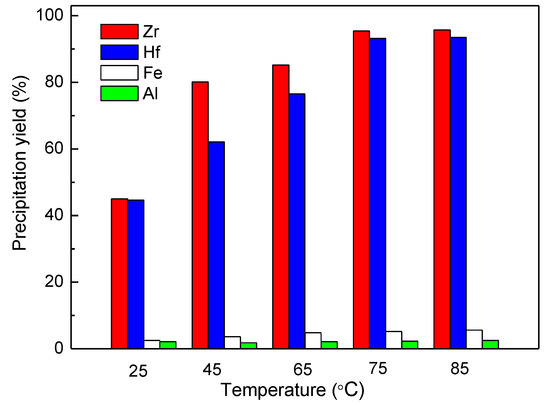
Figure 8.
Effect of temperature on the Zr precipitation yield, Zr precipitation conditions: final pH ~1.6, C(SO42−) = 12.4 g/L, 60 min.
3.5. Effect of Time on the Basic Sulfate Precipiation
The experimental results shown in Figure 9 indicate that the precipitation process was very fast. The precipitation yields of Zr and Hf reached 96.1% and 91.5% when the precipitation time was 60 min. No further increase in yield was noted with longer reaction time. Thus, 60 min was considered to be the optimum reaction time for the basic zirconium precipitation here. It may be considered to shorten the residence time when this method is applied in industry, because the solution after precipitation with a small amount of Zr could be returned to the previous process.
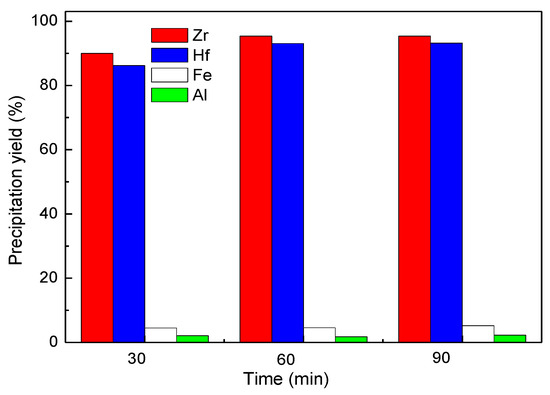
Figure 9.
Effect of time on Zr precipitation yield, Zr precipitation conditions: final pH ~1.6, 75 °C, C(SO42−) = 12.4 g/L.
3.6. Characterization of Precipitates
Table 4 shows the chemical composition of calcium sulfate precipitate as a result of the addition of 120 g/L CaCl2. As can be seen, the loss of Zr in the SO42− removal step was low. After washing, the calcium sulfate was a byproduct.

Table 4.
Chemical composition of calcium sulfate precipitate.
Table 5 shows the chemical composition of the basic zirconium sulfate precipitate prepared using the optimized conditions. As can be seen, the contents of Fe and Al in the precipitate were very low. The Zr basic sulfate prepared by this method was easily dissolved in acids, thus it could be used in conventional techniques to produce a final product, such as ZrOCl2 and ZrO2. Figure 10 shows the XRD pattern of the basic zirconium sulfate precipitate. Qual-X software was used to analyze the data. It was observed that some of the peaks matched those of the calcium sulfate anhydride (bassanite, CaSO4·0.5H2O) phase, and the basic zirconium sulfate was amorphous. The presence of bassanite can be explained by the fact that a few Ca2+ in the sulfate media were not stable and precipitated when the temperature or time was increased.

Table 5.
Chemical composition of the basic zirconium sulfate precipitate.
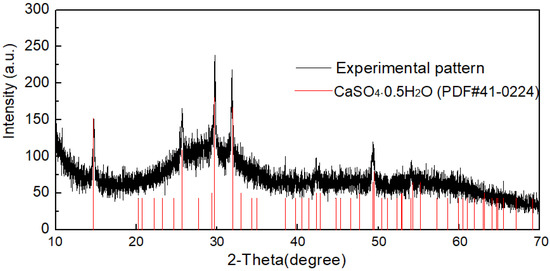
Figure 10.
XRD pattern of the basic zirconium sulfate precipitate.
Fourier-transform infrared spectroscopy analysis (FT-IR) was used to study the structure of basic zirconium sulfate precipitate further, and the FT-IR spectra of the resulting precipitate, Zr(SO4)2·4H2O, and ZrOSO4·4H2O are shown in Figure 11. The FT-IR spectra of the precipitate agreed well with those of ZrOSO4·4H2O, which confirmed that they had a same structure. It was speculated that the adsorption bands at 1000 cm−1 and 1220 cm−1 corresponded to Zr-O-S stretching vibrations [26].
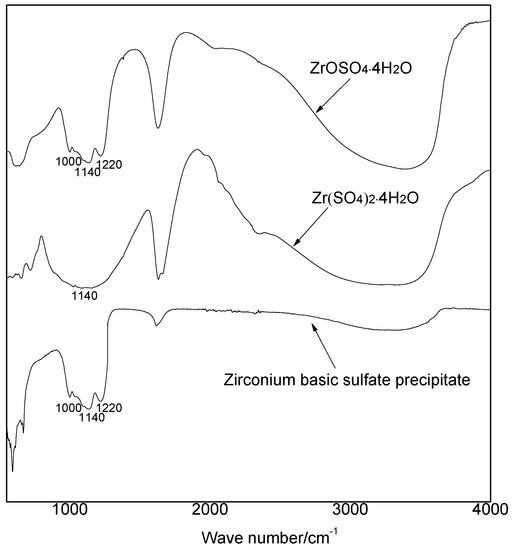
Figure 11.
IR spectra of the basic zirconium sulfate precipitate and pure zirconium sulfate salts.
3.7. Flowchart with Metal Balance
Figure 12 shows the proposed flowchart for the recovery of Zr, Hf, and Nb from the sulfuric acid leach solution using selective precipitation, and it also reveals the directions of the different metals. As shown, although the basic zirconium sulfate precipitation could achieve selective recovery of Zr and Hf, the concentration of sulfate in the solution needed to be controlled, and the resulting precipitate still contained some impurities. Further purification of the product was needed for Zr production.
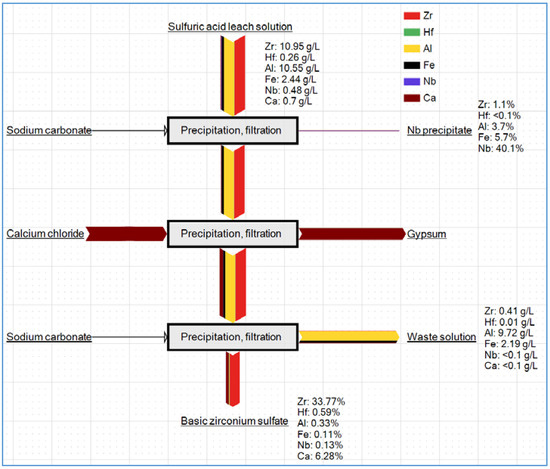
Figure 12.
Proposed flowchart for the recovery of Zr, Hf, and Nb from the sulfuric acid leach solution using selective precipitation.
3.8. ZrO2 Preprared from Basic Zirconium Sulfate
XRD results in Figure 13 confirmed that the powder prepared by the basic zirconium sulfate precipitate was ZrO2.
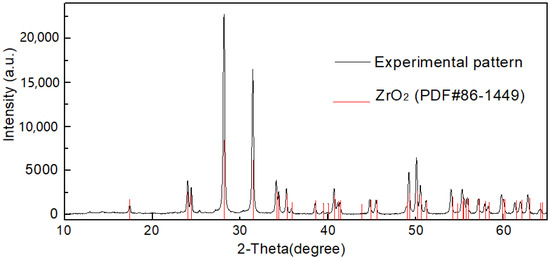
Figure 13.
XRD pattern of the ZrO2 prepared from the basic zirconium sulfate precipitate.
Table 6 shows the chemical composition of ZrO2 prepared after selective precipitation. As can be seen, the contents of ZrO2 + HfO2 in the product were higher than 99%, which met the requirements of industrial ZrO2.

Table 6.
Chemical composition of ZrO2 prepared from the basic zirconium sulfate precipitate.
4. Conclusions
In this study, the treatment of Zr-bearing sulfuric acid leach solution to yield ZrO2 was successfully carried out. After Nb was preferentially precipitated by adjusting pH to around 1.0, selective precipitation via basic zirconium sulfate (Zr5O8(SO4)2·xH2O) was the novel method used to selectively recover Zr. This method achieved removal of the main impurities, such as Fe and Al, and enrichment of Zr from the sulfuric acid leach solution. After partial removal of SO42− by adding 120g CaCl2 per 1L solution, 96.18% Zr precipitation yield was obtained by adjusting the pH to ~1.6 and keeping the temperature at 75 °C for 60 min. The resulting precipitate contained 33.77% Zr and 0.59% Hf with low Fe and Al. A high-quality product of ZrO2 can be obtained from the basic sulfate precipitate. The present study showed a promising process to recover Zr from sulfuric acid leach solution.
Author Contributions
Y.M. performed the experiments and wrote the paper; X.W. and S.S. contributed the reagents/materials/analysis tools; Y.M., K.F., X.W., and B.F. analyzed the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
One of the authors (Yiqian Ma) is grateful to the Chinese Government for providing a scholarship. Authors are thankful to the EURARE (www.eurare.eu) project for providing the initial material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nielsen, R.H.; Schlewitz, J.H.; Nielsen, H. Zirconium and Zirconium Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley online Library, John Wiley & Sons, Inc.: New York, NY, USA, 2000; Volume 1, pp. 1–46. [Google Scholar]
- Zirconium in Steels. Available online: http://ispatguru.com/zirconium-in-steels/ (accessed on 5 March 2018).
- Biswas, R.K.; Habib, M.A.; Karmakar, A.K.; Islam, M.R. A novel method for processing of Bangladeshi zircon: Part I: Baking, and fusion with NaOH. Hydrometallurgy 2010, 10, 124–129. [Google Scholar] [CrossRef]
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Pekov, I.V. The nomenclature of eudialyte-group minerals. Can. Mineral. 2003, 4, 785–794. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K. Structural Mineralogy of the Eudialyte Group: A Review. Crystallogr. Rep. 2007, 52, 47–64. [Google Scholar] [CrossRef]
- Lebedev, V.N. Sulfuric acid technology for processing of eudialyte concentrate. Russ. J. Appl. Chem. 2003, 76, 1559–1563. [Google Scholar] [CrossRef]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of rare earth elements from Eudialyte concentrate by suppressing silicon dissolution. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Voßenkaul, D.; Birich, A.; Müller, N.; Stoltz, N.; Friedrich, B. Hydrometallurgical processing of eudialyte bearing concentrates to recover rare earth elements via low-temperature dry digestion to prevent the silica gel formation. J. Sustain. Met. 2017, 3, 79–89. [Google Scholar]
- Balomenos, E.; Davris, P.; Deady, E.; Yang, J.; Panias, D.; Friedrich, B.; Binnemans, K.; Seisenbaeva, G.A.; Dittrich, C.; Kalvig, P.; et al. The EURARE Project: Development of a Sustainable Exploitation Scheme for Europe’s Rare Earth Ore Deposits. Johns. Matthey Technol. Rev. 2017, 61, 142–153. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Gronen, L.; Friedrich, B. Recovery of Zr, Hf, Nb from eudialyte residue by sulfuric acid dry digestion and water leaching with H2O2 as a promoter. Hydrometallurgy 2018, 181, 206–214. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Friedrich, B. Hydrometallurgical Treatment of a Eudialyte Concentrate for Preparation of Rare Earth Carbonate. Johns. Matthey Technol. Rev. 2019, 63, 2–13. [Google Scholar] [CrossRef]
- Zakharov, V.I.; Maiorov, D.V.; Alishkin, A.R.; Matveev, V.A. Causes of insufficient recovery of zirconium during acidic processing of lovozero eudialyte concentrate. Russ. J. Non Ferr. Met. 2011, 52, 423–428. [Google Scholar] [CrossRef]
- Dibrov, I.A.; Chirkst, D.E.; Litvinova, T.E. Experimental study of zirconium(IV) extraction from fluoride-containing acid solutions. Russ. J. Appl. Chem. 2002, 75, 195–199. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Minenko, V.G.; Samusev, A.L.; Chanturia, E.L.; Koporulina, E.V.; Bunin, I.Z.; Ryazantseva, M.V. The Effect of Energy Impacts on the Acid Leaching of Eudialyte Concentrate. Min. Proc. Ext. Met. Rev. 2020. [Google Scholar] [CrossRef]
- Lebedev, V.N.; Schur, T.E.; Maiorov, D.V.; Popova, L.A.; Serkova, R.P. Specific Features of Acid Decomposition of Eudialyte and Certain Rare-Metal Concentrates from Kola Peninsula. Russ. J. Appl. Chem. 2003, 76, 1191–1196. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Huang, Z.; Friedrich, B. Selective recovery and separation of Zr and Hf from sulfuric acid leach solution using anion exchange resin. Hydrometallurgy 2019, 189, 105143. [Google Scholar] [CrossRef]
- Gates, P.A.; Horlacher, C.F.; Reed, G. Preliminary Economic Assessment NI 43-101 Technical Report for the Norra Kärr (REE-Y-Zr) Deposit Gränna; Tasman Metals Limited: Norra Kärr, Sweden, 2012. [Google Scholar]
- Davidson, T.; Thompson, J.; Short, M.; Moseley, G.; Mounde, M.; La Digges Touche, G. Norra Kärr Project PFS Prefeasibility Study-NI 43-101-Technical Report for the Norra Kärr Rare Earth Element Deposite; GBM Minerals Engineering Consultants Limited: London, UK, 2015. [Google Scholar]
- Kozak, C.M.; Mountford, P. Encyclopedia of Inorganic Chemistry, Zirconium & Hafnium: Inorganic—Coordination Chemistry; John Wiley & Sons: Oxford, UK, 2006. [Google Scholar]
- Wang, L.Y.; Lee, M.S. A review on the aqueous chemistry of Zr(IV) and Hf(IV) and their separation by solvent extraction. J. Ind. Eng. Chem. 2016, 39, 1–9. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Dean, J. Langes’s Handbook of Chemistry, 13th ed.; McGraw-Hill, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Nielsen, R.H.; Govro, R.V. Zirconium Purification: Using a Basic Sulfate Precipitation; U.S. Dept. of the Interior, Bureau of Mines: Washington, DC, USA, 1956. [Google Scholar]
- Ryabchikov, D.I.; Marov, I.N.; Ermakov, A.N.; Belyaeva, V.K. Stability of some inorganic and organic complex compound of zirconium and hafnium. J. Inorg. Nucl. Chem. 1964, 26, 965–980. [Google Scholar] [CrossRef]
- Berg, R.W. Progress in niobium and tantalum coordination chemistry. Coord. Chem. Rev. 1992, 113, 1–130. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ray, J.; Chatterjee, A.; Ganguli, D. Characterization of basic zirconium sulphate, a precursor for zirconia. J. Mater. Sci. Lett. 1989, 8, 548–553. [Google Scholar] [CrossRef]
- Xiong, B.; Wen, W.; Yang, X.; Li, H.; Luo, F.; Zhang, W. Zirconium and Hafnium Metallurgy; Metallurgical Industry Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).