3. Thermodynamic Calculation Analysis
During the reduction roasting of the samples, the possible chemical reactions included the reduction of iron oxide; generation of calcium ferrite by the combination of iron oxide and CaO; generation of tricalcium silicate (alite), C
3S (3CaO·SiO
2), and dicalcium silicate (belite), C
2S (2CaO·SiO
2), by the combination of CaO and SiO
2; and generation of C
3S by the combination of CaO and C
2S. The equations for these reactions are shown in Equations (1)–(5). The reaction module of the FactSage software package (CRCT, Montreal, Canada; GTT-Technologies, Germany) was used to calculate the standard Gibbs free energies for all the possible reactions of the main phases in the reduction roasting process between 500 and 2000 °C at 10 °C intervals.
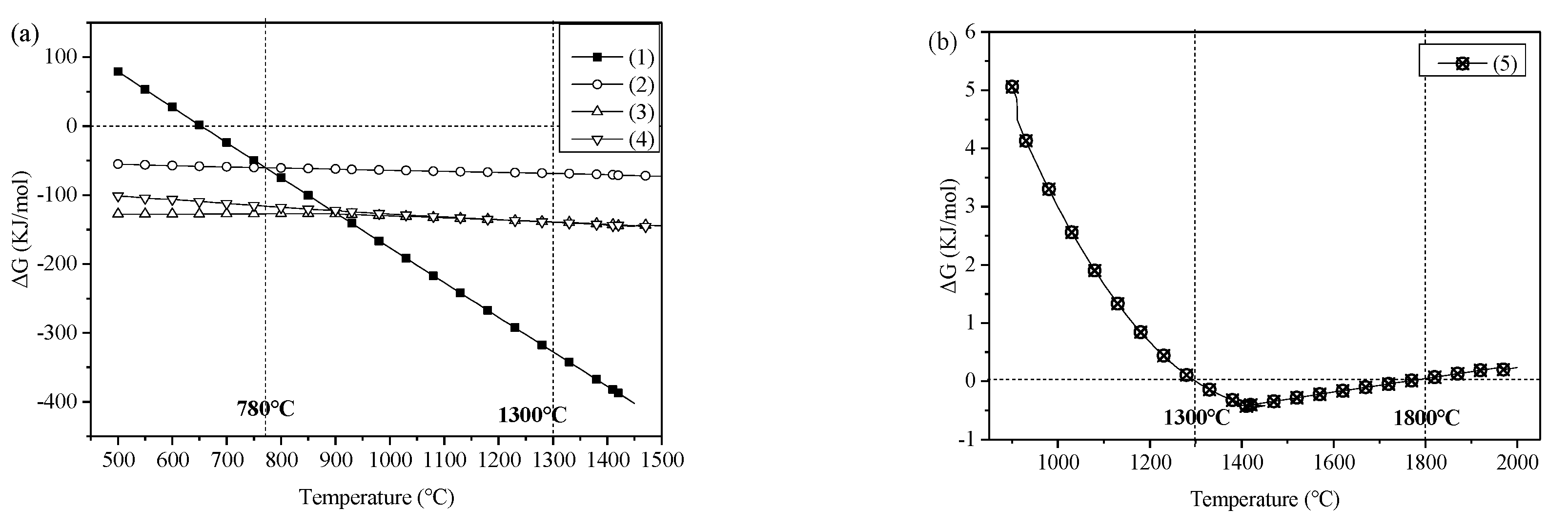
Figure 2 shows the temperature (T) and standard Gibbs free energies (ΔG) of the reactions.
Figure 2a shows that when the temperature was between 500 and 1450 °C, Equations (2)–(4) will proceed spontaneously, and tricalcium silicate, dicalcium silicate, and Ca
2Fe
2O
5 will be generated. The starting temperature for the reduction of iron in Equation (1) was 653 °C. However, when the temperature was below 780 °C, CaO and Fe
2O
3 were consumed, producing calcium ferrite through Equation (2); as this was more favorable than the reduction of iron through Equation (1), it was not conducive to the reduction of iron. When the temperature was above 780 °C, Equation (1) occurred before Equation (2), reducing Fe
2O
3 to iron. Therefore, a temperature above 780 °C is required for the reduction of Fe
2O
3.
By comparing the temperature versus standard Gibbs free energy curves for Equations (3) and (4), shown in
Figure 2a, it can be seen that Equation (3) was more favorable than Equation (4) at temperatures below 1300 °C, and dicalcium silicate is easily generated. The formation of tricalcium silicate is more favorable when the temperature is above 1300 °C. It can also be seen from
Figure 2b that Equation (5) only proceeds spontaneously when the temperature is between 1300 and 1800 °C, and tricalcium silicate can be generated through the combination of the dicalcium silicate and calcium oxide formed in the previous reactions.
Figure 2a,b show that to generate cementing materials comprising predominantly tricalcium silicate, the temperature should be between 1300 and 1800 °C.
In order to investigate the phase composition of the reactants at high temperatures, when the iron was insufficiently reduced, the phase module of the FactSage software package was used to calculate and analyze the equilibrium phase diagram of the Fe
2O
3-CaO-SiO
2 ternary system at 1350 °C. This is shown in
Figure 3.
Figure 3 shows that at 1350 °C, Fe
2O
3 reacts with CaO, generating Ca
2Fe
2O
5, and coexists with C
2S and C
3S in the high-CaO content regions (regions 2 and 3 in
Figure 3); here, Ca
2Fe
2O
5 becomes an impurity in the cementing materials. When Fe
2O
3 has low concentrations or is absent (region 1 in
Figure 3), the main phases present in the system are C
3S and excess free CaO. Therefore, Fe
2O
3 should be sufficiently reduced to iron, by the roasting process, to prevent the formation of Ca
2Fe
2O
5. In the regions of high Fe
2O
3 and SiO
2 content (regions 4, 5, and 6 in
Figure 3), there are low melting point liquid phases containing three components—namely, Fe
2O
3, CaO, and SiO
2—and C
3S is not generated.
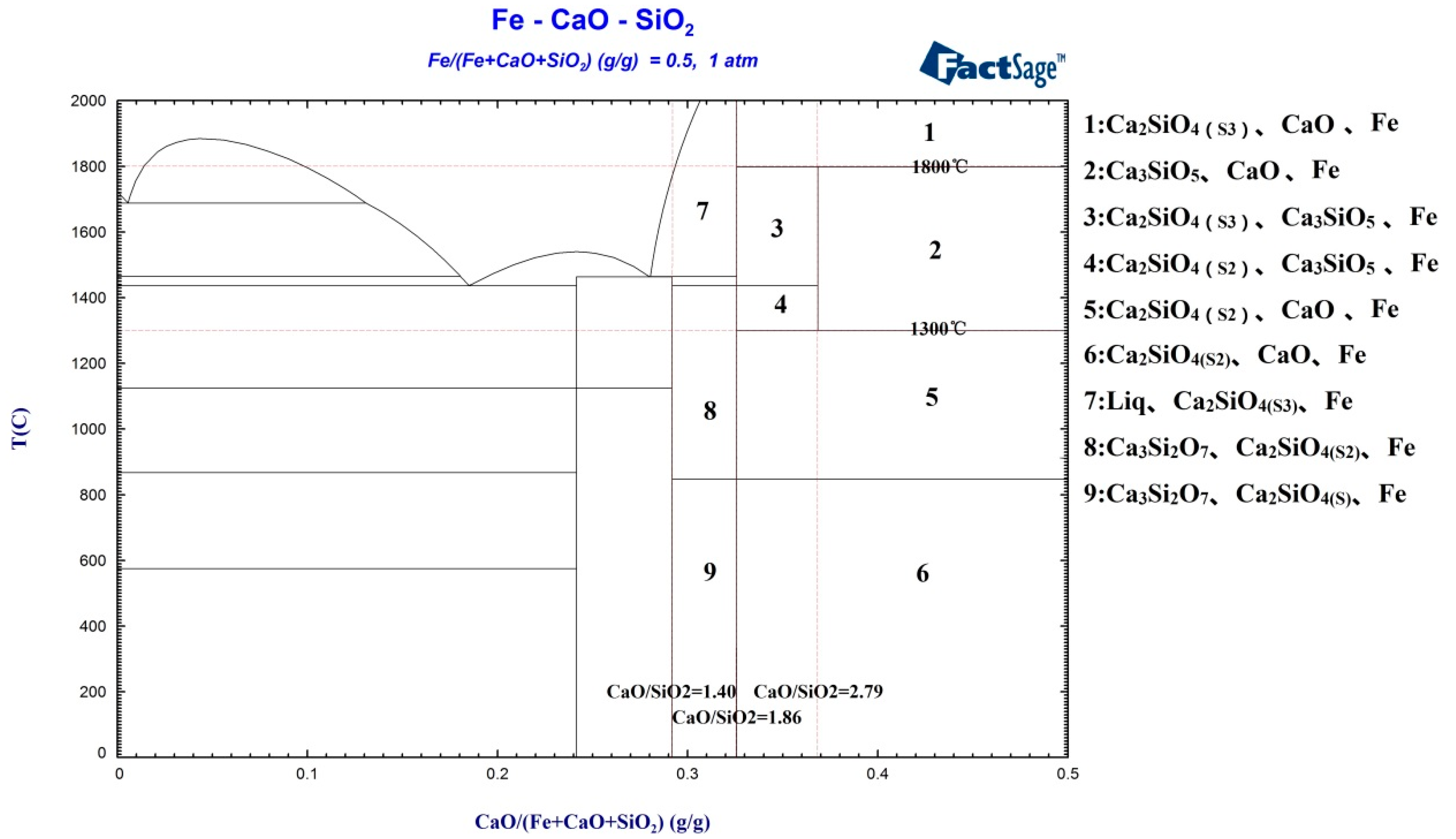
In order to study the interaction between the reduced iron and the CaO and SiO
2 in the system, the FactSage software package was used to calculate and analyze the phase diagram of the Fe-CaO-SiO
2 ternary system, in which the iron accounts for 50% of the total mass. These results are shown in
Figure 4.
Figure 4 shows that tricalcium silicate is an unstable compound. Dicalcium silicate can form stable tricalcium silicate through combination with calcium oxide (regions 2, 3, and 4 in
Figure 4) only within a temperature range of 1300–1800 °C; when the temperature is below 1300 °C or above 1800 °C (regions 1 and 5 in
Figure 4), C
3S decomposes into C
2S and free CaO—this is in line with the thermodynamic calculation results given in
Figure 2b. At the same time, it can also be seen that the reduced iron had no obvious effect on the CaO-SiO
2 system, meaning that iron can coexist with the CaO-SiO
2 system. When the concentration of CaO was such that CaO/SiO
2 < 1.40 and the temperature was between 1300 and 1800 °C, there were no C
2S or C
3S phases present in the system, and CaO mainly formed CaSiO
3 and Ca
3Si
2O
7 by combining with SiO
2. When CaO/SiO
2 was between 1.40 and 1.86, C
2S was present in the system (regions 7 and 8 in
Figure 4). When CaO/SiO
2 was between 1.86 and 2.79, C
2S coexisted with C
3S (regions 3 and 4 in
Figure 4). When CaO/SiO
2 exceeded 2.79, the main phases in the system were C
3S, free CaO, and coexistent iron (region 2 in
Figure 4). Thus, CaO/SiO
2 = 1.40 is the composition point for Ca
3Si
2O
7, CaO/SiO
2 = 1.86 is the composition point for C
2S, and CaO/SiO
2 = 2.79 is the composition point for C
3S. Therefore, when cementing materials with a tricalcium silicate main phase are being prepared, the masses of CaO and SiO
2 used in the blending process should have a ratio of CaO/SiO
2 = 2.79, and the roasting temperature should be between 1300 and 1800 °C.
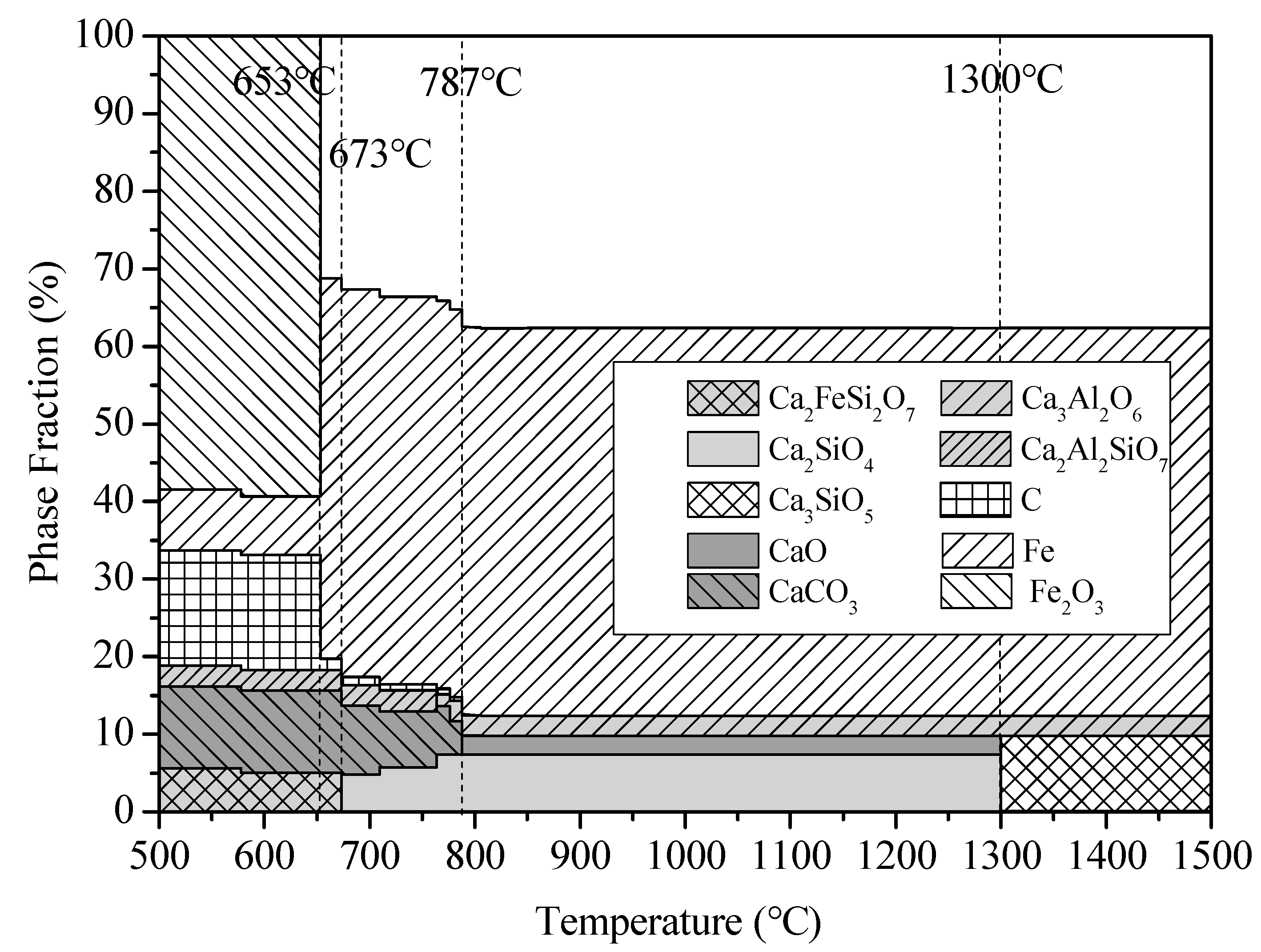
The equipment module of the FactSage software package was used to perform a theoretical analysis of changes in the reactant phase composition during the temperature ramping, with hematite as the raw material, powdered carbon as the reducing agent, and CaO as the additive, under a protective CO atmosphere. The ratio of the reactants used was as shown in
Table 2. The phase change of the system between 500 and 1500 °C was analyzed, and the temperature difference was 50 °C. The Origin software package was used to prepare the plot. The theoretical reaction products, as determined by the calculations, are shown in
Figure 5.
From the calculation and analysis results shown in
Figure 5, it can be seen that the reactions occur in the low-temperature region (<653 °C). The generated phases included Ca
2Al
2SiO
7, CaCO
3, Ca
2FeSi
2O
7, and a small amount of reduced iron, and there are large amounts of the reactants C and Fe
2O
3. When the temperature exceeds 653 °C, Fe
2O
3 is largely reduced by C to iron, and most of the reactants C and Fe
2O
3 are consumed. When the temperature is 673 °C, C
2S is produced and the intermediate product, Ca
2FeSi
2O
7, is consumed. When the temperature is 787 °C, CaCO
3 completely decomposes into CaO and CO
2. When the temperature is between 787 and 1300 °C, the main phases are Fe, CaO, C
2S, and Ca
3Al
2O
6. When the temperature exceeds 1300 °C, CaO combines with C
2S to form C
3S; the other phases do not change, and the final products, under high-temperature reaction conditions, are iron, C
3S, and Ca
3Al
2O
6.
Based on the above analysis, the preparation of elemental iron, C3S, C2S, and other cementing materials by reduction roasting of hematite, after apportioning the reactants according to the thermomechanical analysis, is feasible. At the same time, in order to ensure sufficient reduction of the iron and effective formation of the cementing materials (such as C3S and C2S), the reaction temperature needs to be controlled in stages.
In order to prevent Fe2O3 and CaO combining to form Ca2Fe2O5, which affects the reduction of iron, the reaction temperature, for Fe2O3, should be between 780 and 1220 °C. This temperature should be maintained for a sufficient time to fully reduce the iron and prevent formation of the low melting point liquid phase (the melting point of fayalite is 1220 °C) from Fe2O3 and SiO2. By this stage, C2S has been generated by the combination of CaO and SiO2. To achieve sufficient reduction of the iron, the temperature should be raised for a second time, and C3S generated through the reaction of C2S with CaO (1300–1800 °C). Finally, the samples should be cooled by fast cooling. During fast cooling, the decomposition rate of tricalcium silicate is slow enough that it can exist as a metastable state at ambient temperature.
4. Analysis of Experimental Results
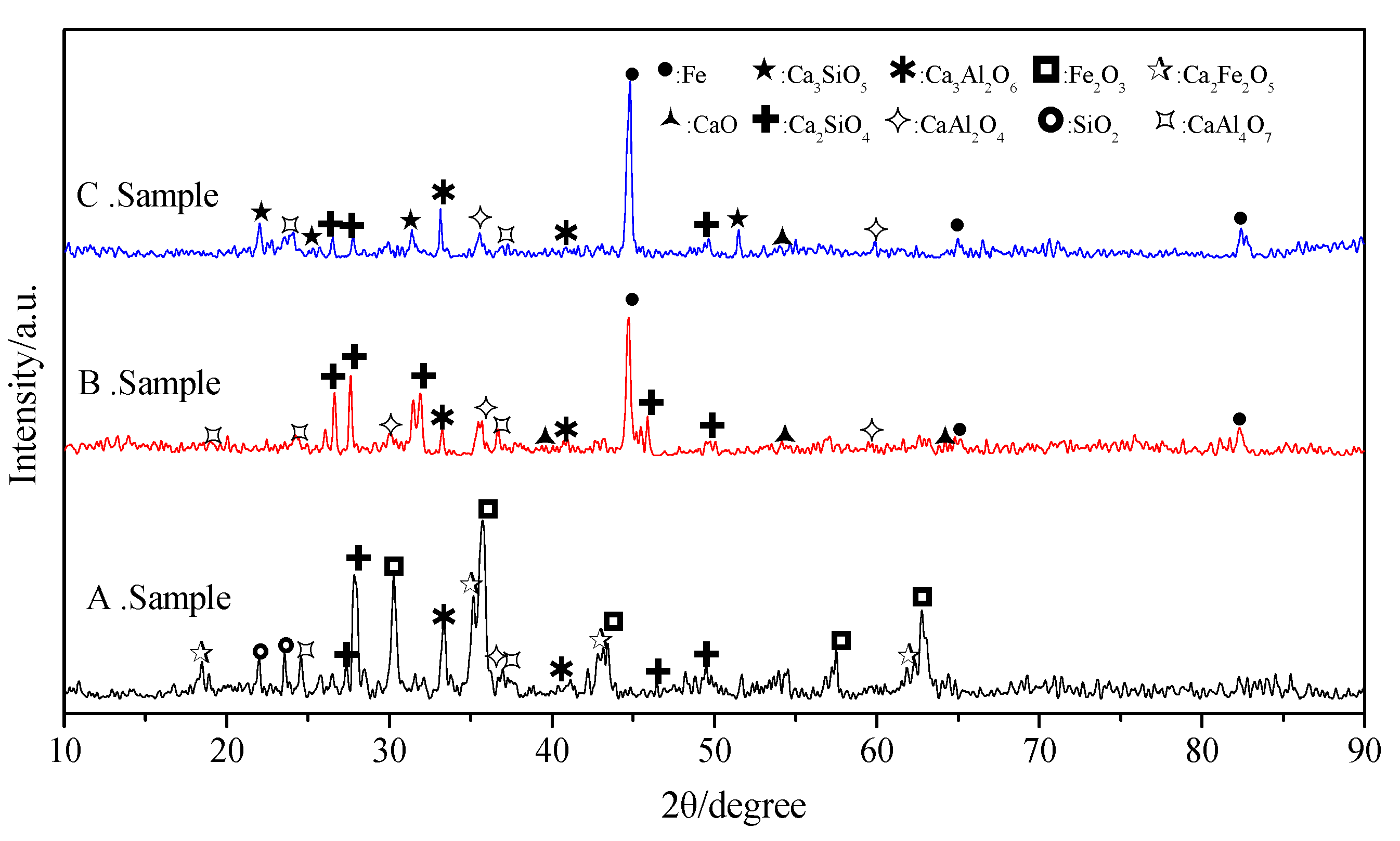
In this experiment, to verify the effects of the different reaction conditions on the results of the thermodynamic calculations, three different control processes were designed, as follows: (1) 8 g of sample was placed in a corundum crucible in an airtight, box-type resistance furnace. Without a protective atmosphere, the temperature was continuously raised from room temperature to 1450 °C at a ramp rate of 10 °C/min; the product, A, was obtained after heating for 1 h and cooling to room temperature. (2) 8 g of sample was placed in a corundum crucible and then, placed in a tube-type resistance furnace. Under a protective CO atmosphere, with a flow rate of 60 mL/h, the temperature was continuously raised from room temperature to 1000 °C and, after heating for 2 h, continuously raised to 1450 °C at a ramp rate of 10 °C/min; the product, B, was obtained after heating for 1 h at 1450 °C and cooling to room temperature. (3) 8 g of sample was reduced and roasted, and the same atmosphere and temperature ramp to 1450 °C were used as for sample B; the difference was that, after heating for 1 h at 1450 °C, the product, C, was obtained using the fast cooling method. Finally, XRD and scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM–EDS) analyses were conducted on the samples obtained. The same diffractometer was used as for the raw material analysis; the scanning electron microscope used was a VEGA II XMU from TESCAN, Czech Republic, and the EDS was type 7718 from Oxford Instruments, UK. The analysis results are shown in
Figure 6 and
Figure 7.
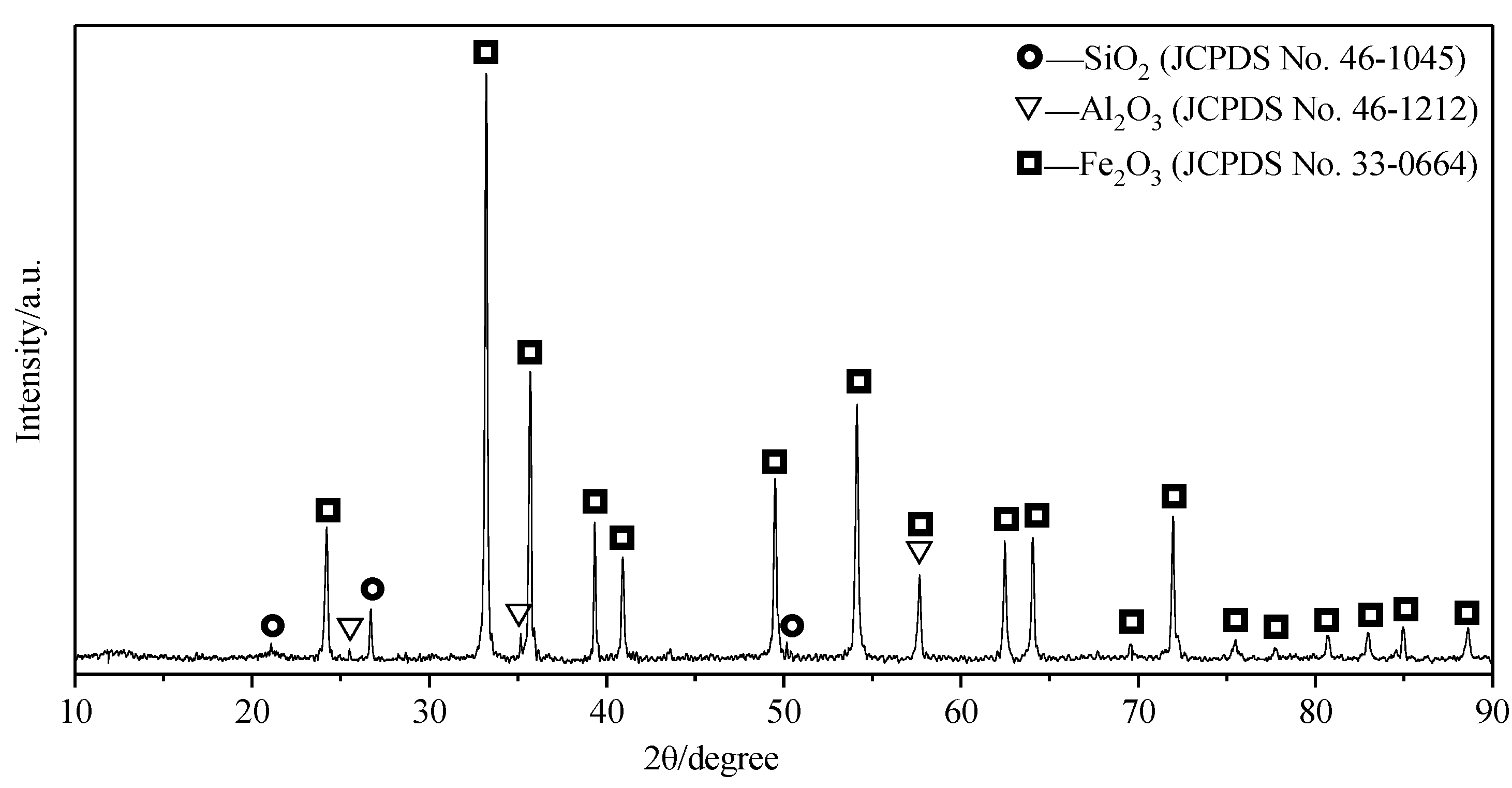
The iron oxide accounted for 70.58% of the mass of the sample, and the elemental iron in the theoretical reaction product accounted for 80.27% of the total mass. Therefore, strong peaks from elemental iron and iron oxide appear in the XRD patterns of the samples after the reaction, and the X-ray diffraction peaks of the other phases are generally low.
From the results of the XRD analysis, it can be seen that, for sample A, the reduction of iron could not be ensured, due to the lack of a protective atmosphere; the iron was present in the form of Fe
2O
3, which formed Ca
2Fe
2O
5 through combination with CaO. The consumption of CaO resulted in reductions in the CaO/SiO
2 and CaO/Al
2O
3 ratios. The X-ray diffraction peaks of phases with low calcium contents, such as CaAl
4O
7 and Ca
2SiO
4, were obvious in the reaction products, and diffraction peaks of SiO
2 and Ca
3Al
2O
6 were also present, which was consistent with the analysis results shown in
Figure 3. With the protection of a reducing atmosphere, sample B was heated at 1000 °C for 2 h, ensuring the complete reduction of iron, and then, at 1450 °C for 1 h, matching the formation conditions of C
3S; however, C
3S decomposed into C
2S and CaO phases, due to the use of furnace cooling. Thus, the final reaction products of sample B were phases such as elemental iron, Ca
2SiO
4, CaO, CaAl
4O
7, CaAl
2O
4, and Ca
3Al
2O
6; this was consistent with the analysis results shown in
Figure 4. Under a protective CO atmosphere, sample C was heated according to the same protocol as sample B, to realize the reduction of iron and formation of C
3S and C
3A phases, and fast cooling was used to avoid large-scale decomposition of C
3S. The final reaction products were Fe, C
3S, and Ca
3Al
2O
6, and the decomposition products were Ca
2SiO
4 and CaAl
2O
4, which showed substantially weaker X-ray diffraction peaks than the Ca
2SiO
4 in sample B. At the same time, from the results of the SEM–EDS analysis of sample C, it can be seen that the product morphology consisted of many iron grains, with different diameters and cementing materials among them; this was consistent with the analysis results of the theoretical products shown in
Figure 5.