Investigation of Thermophysical Properties of Three Barrel Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results
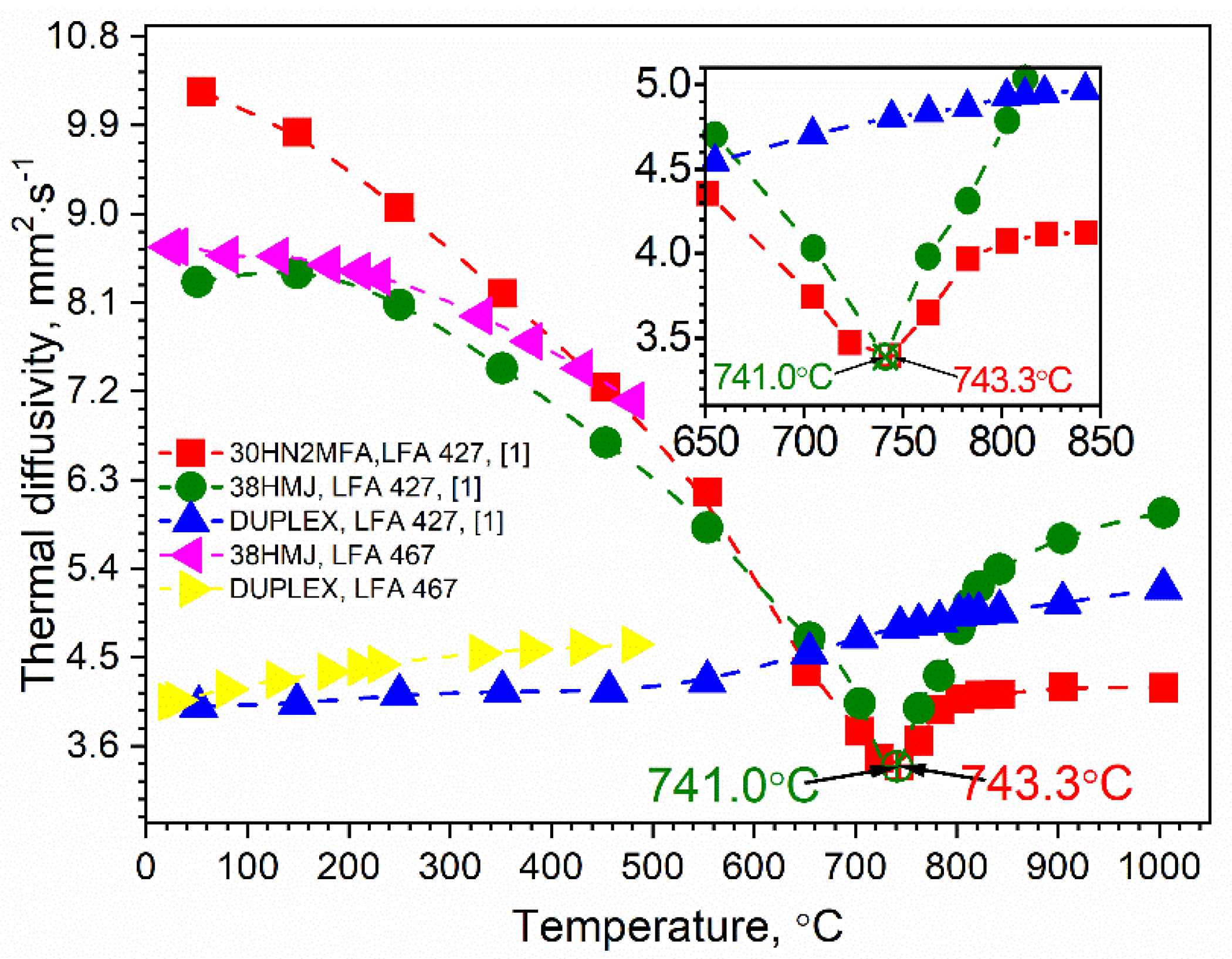
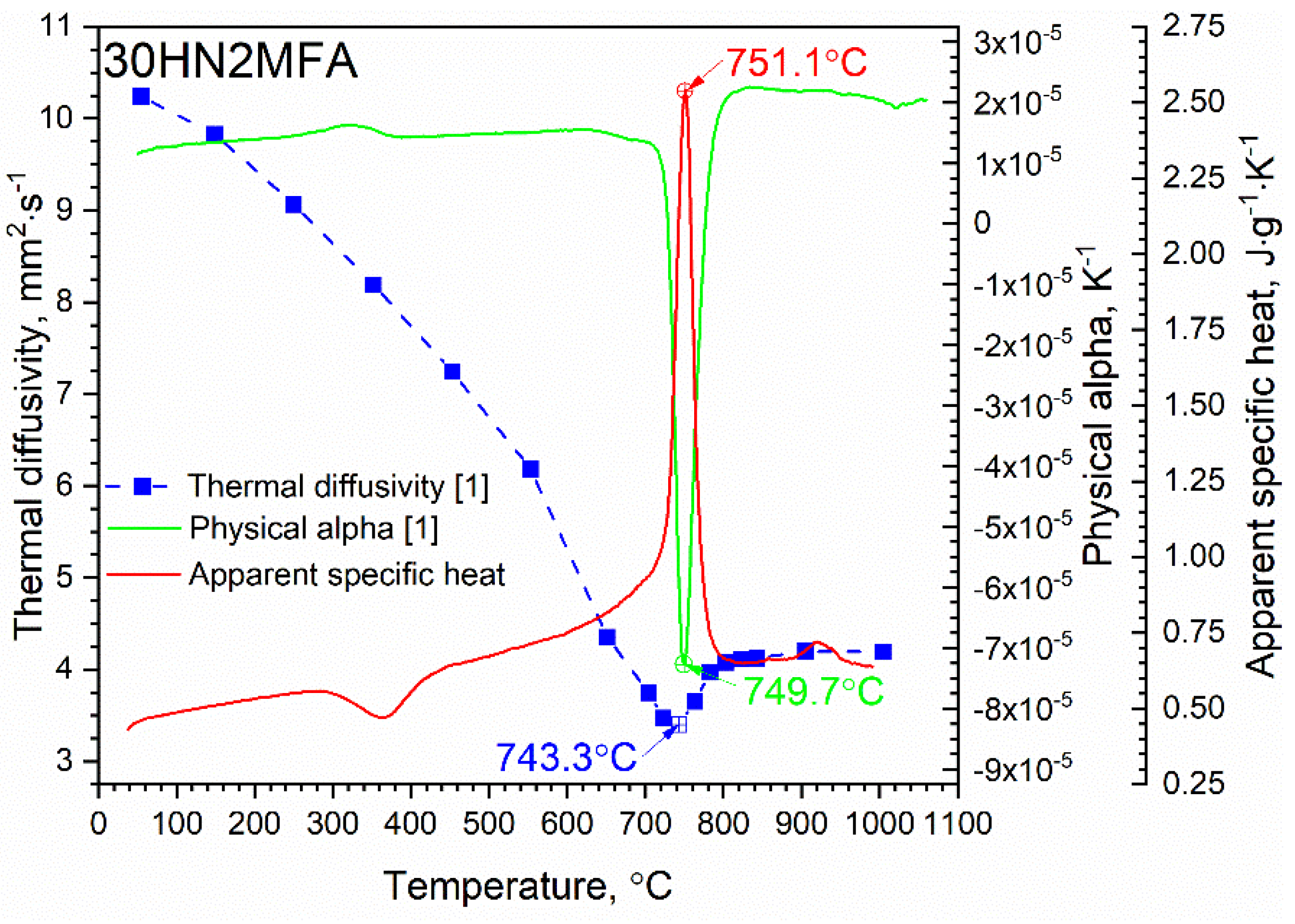
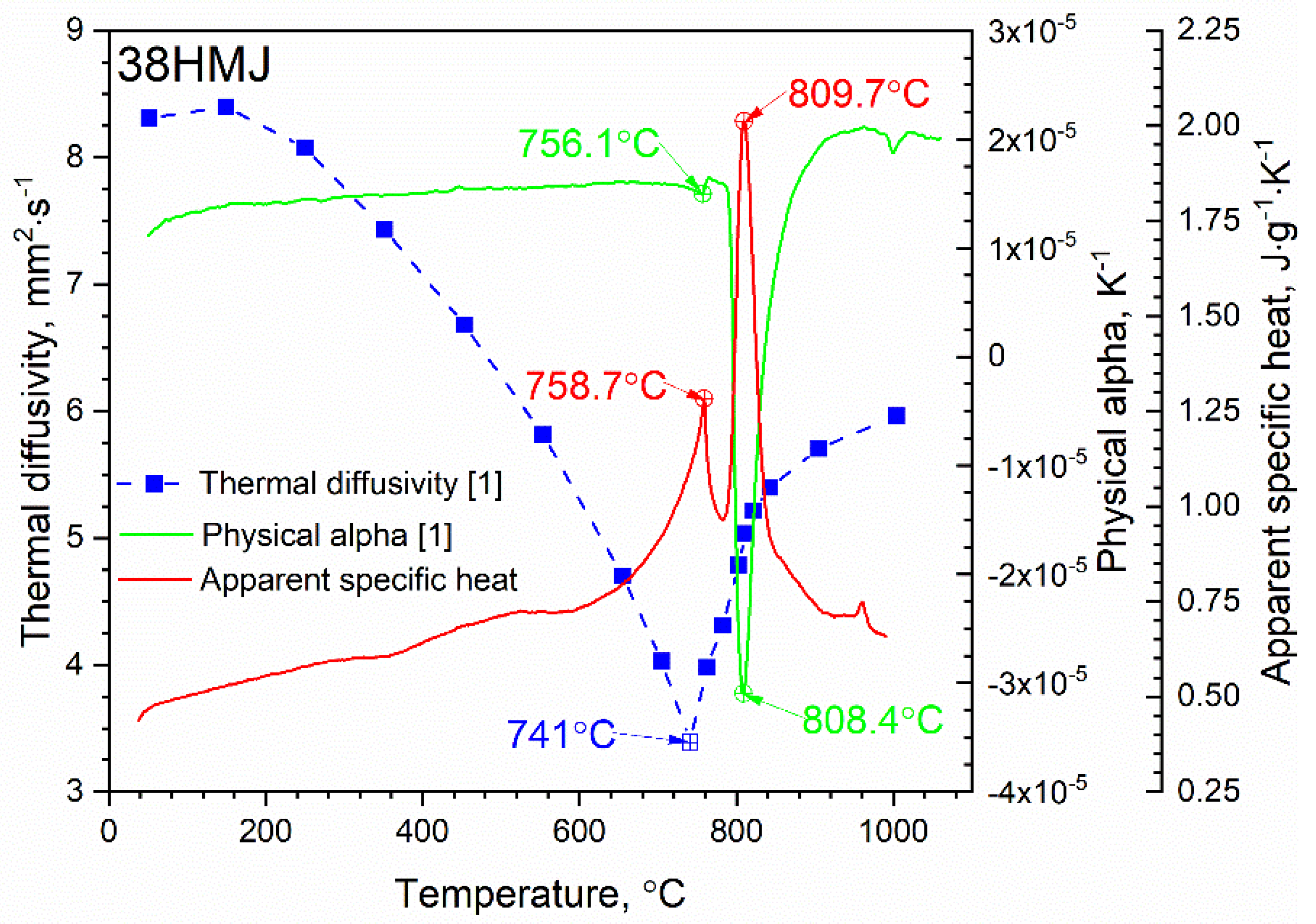
3.1. Thermal Diffusivity Results
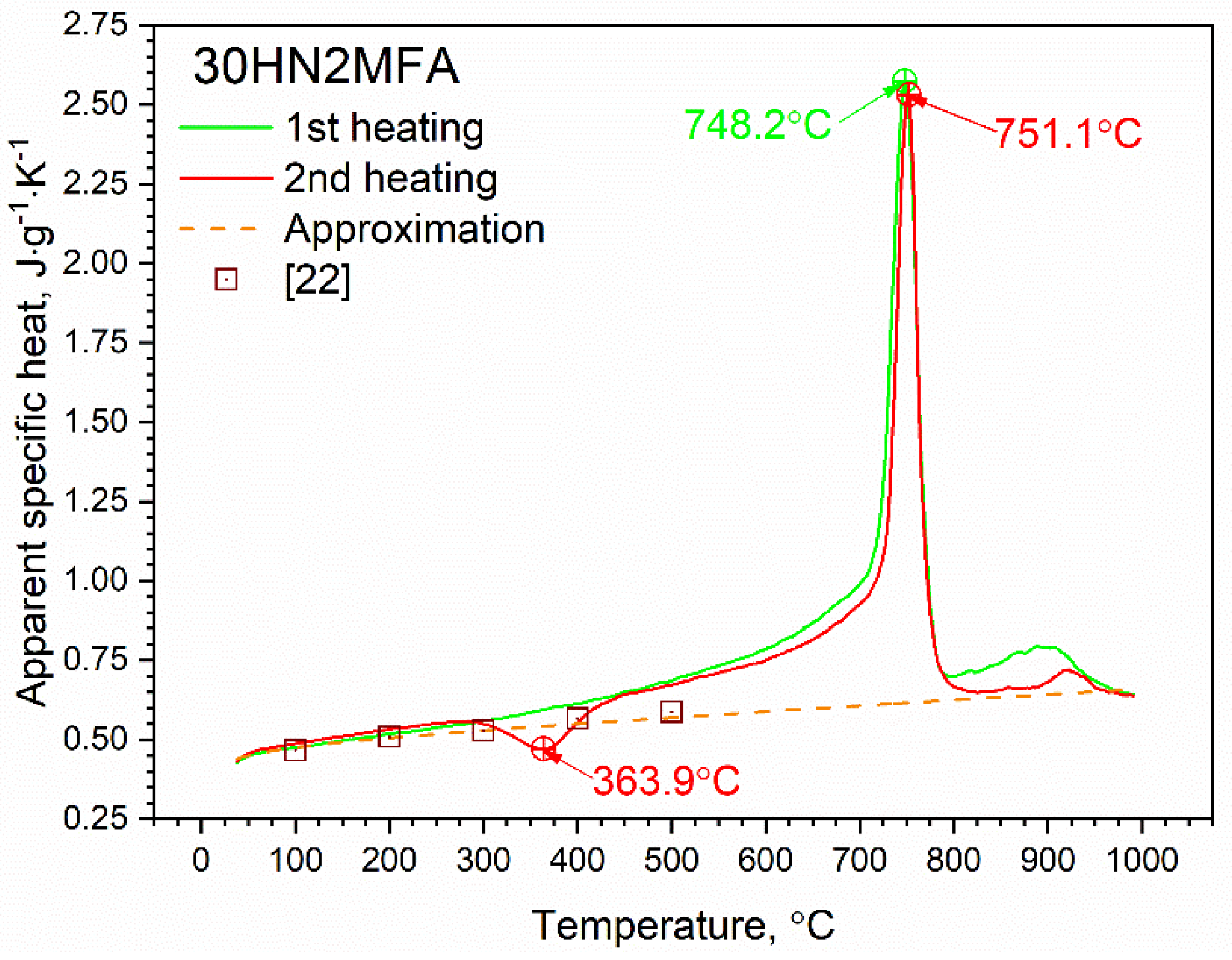
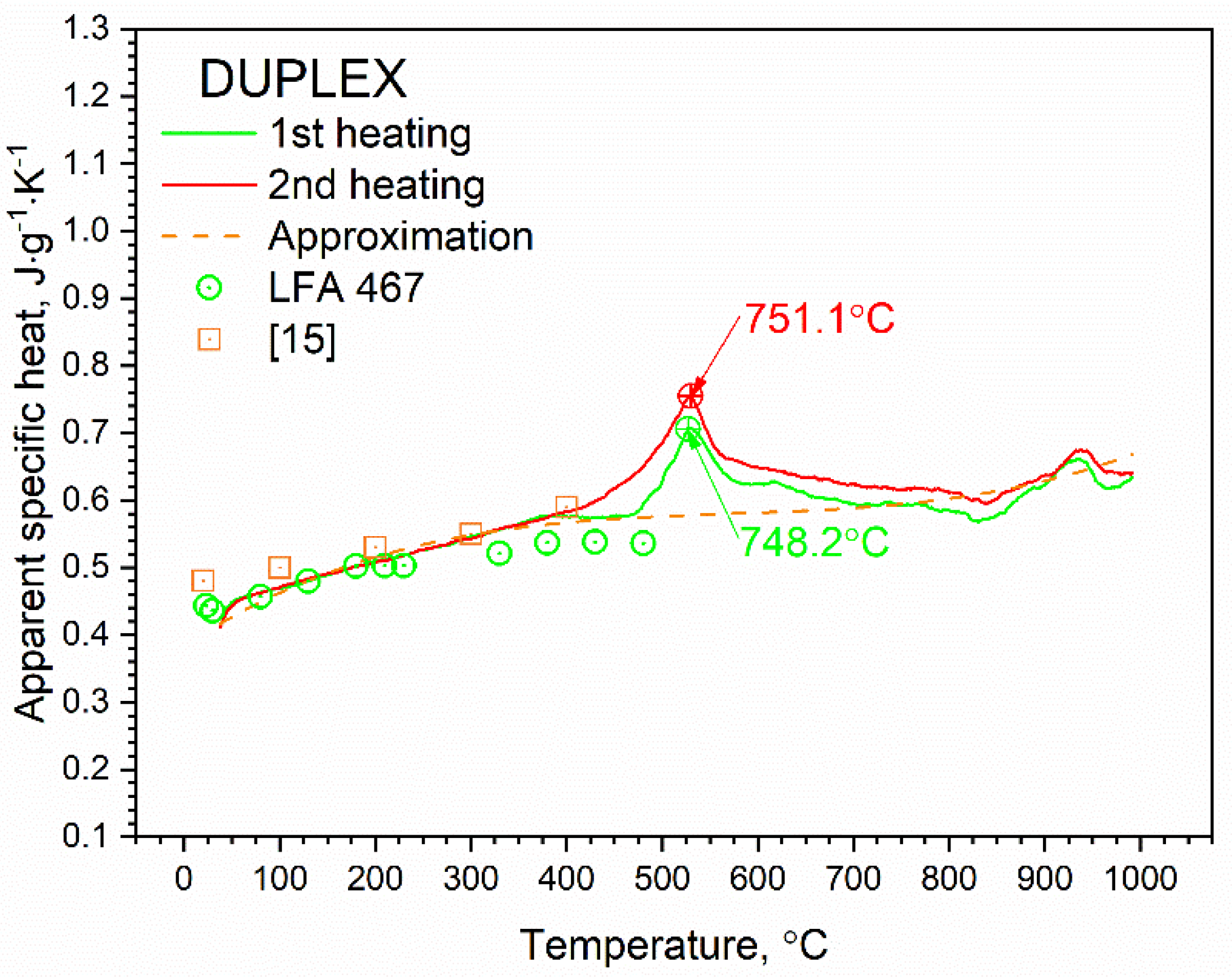
3.2. Specific Heat Results
3.3. Thermal Conductivity Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koniorczyk, P.; Zmywaczyk, J.; Dębski, A.; Zieliński, M.; Cegła, M. Investigations of thermal diffusivity for three types of the barrel steel. AIP Conf. Proc. 2019, 2170, 020006. [Google Scholar] [CrossRef]
- Sopok, S.; Rickard, C.; Dunn, S. Thermal–chemical–mechanical gun bore erosion of an advanced artillery system part one: Theories and mechanisms. WEAR 2005, 258, 659–670. [Google Scholar] [CrossRef]
- Sopok, S.; Rickard, C.; Dunn, S. Thermal–chemical–mechanical gun bore erosion of an advanced artillery system part one: Modeling and predictions. WEAR 2005, 258, 671–683. [Google Scholar] [CrossRef]
- Dennis, J.K.; Such, T.E. Nickel and Chromium Plating, 3th ed.; Woodhead Publishing: Cambrige, UK, 1993; pp. 238–240. [Google Scholar]
- Jones, A.R. Corrosion of Electroplated Hard Chromium, ASM Handbook Corrosion: Materials; Cramer, S.D., Covino, B.S., Jr., Eds.; ASM International: Materials Park, OH, USA, 2006; Volume 13B, pp. 434–441. [Google Scholar]
- Li, H.; Chen, G.; Zhang, K.; Luo, G.; Ye, Z. Degradation failure features of chromium-plated gun barrels with a laser-discrete-quenched substrate. Surf. Coat. Technol. 2007, 201, 9558–9564. [Google Scholar] [CrossRef]
- Gervaise, C.; Gagliano, O.; Serra, J.-J.; Claudet, B.; Commandré, M.; Serror, S. Local thermal characterization of inner gun barrel refractory metallic coatings. Microscale Thermophys. Eng. 2001, 5, 209–223. [Google Scholar] [CrossRef]
- Almotairi, A.; Warkentin, A.; Farhat, Z. Mechanical damage of hard chromium coatings on 416 stainless steel. Eng. Fail. Anal. 2016, 66, 130–140. [Google Scholar] [CrossRef]
- Almotairi, A.; Farhat, Z.; Warkentin, A. Thermal damage of conventional hard chromium coatings on 416 stainless steel. Eng. Fail. Anal. 2019, 105, 1118–1130. [Google Scholar] [CrossRef]
- Hirvonen, J.K.; Derek Demaree, J.; Marble, D.K.; Conroy, P.; Leveritt, C.; Montgomery, J.; Bujanda, A. Gun barrel erosion studies utilizing ion beams. Surf. Coat. Technol. 2005, 196, 167–171. [Google Scholar] [CrossRef]
- Okamato, H.; Schlesinger, M.E.; Mueller, E.M. ASM Handbook Alloy. Phase Diagram; ASM International: Materials Park, OH, USA, 2016; Volume 3, pp. 625–643. [Google Scholar]
- Żółciak, T.; Łataś, Z.; Dębski, A. New Materials and Thermo-Chemical Treatment Technologies of Small Arms Barrels. In Problems of Mechatronics, Armament, Aviation, Safety Engineering; Military University of Technology: Warsaw, Poland, 2010; Volume 1, pp. 43–53. [Google Scholar]
- Dębski, A.; Surma, Z.; Koperski, W. Material and Technological Optimization Research in Terms of Increasing the Durability of Small Arms; Research Report; Military University of Technology: Warsaw, Poland, 2009. (In Polish) [Google Scholar]
- Dębski, A.; Żółciak, T.; Łataś, Z.; Ciski, A.; Kowalski, S. Application of Unconventional Steel Grades and Surface Treatments for Increase in the Lifetime of 5.56-Caliber Small Arms Barrels; Bulletin of the Military University of Technology: Warsaw, Poland, 2009; Volume LVIII, pp. 345–354. [Google Scholar]
- Sandvik. Available online: www.materials.sandvik/en/materials-center/material-datasheets (accessed on 2 March 2020).
- Kuroda, T.; Ikeuchi, K.; Kitagawa, Y. Microstructure Control for Joining Advanced Stainless Steel. In Proceedings of the International Symposium on Novel Materials Processing by Advanced Electromagnetic Energy Sources, Osaka, Japan, 19–22 March 2004; pp. 419–422. [Google Scholar]
- Nippon Yakin Kogyo Co., LTD. High-Performance Alloys. Available online: www.nyk.co.jp/en/products/alloys (accessed on 2 March 2020).
- Żółciak, T.; Ciski, A.; Płociński, T. Influence of carbonitriding and nitriding atmosphere compositions on the corrosion resistance of 30HN2MFA (-DIN 30CrNiMo8) and 38HMJ (DIN 41 CrALMo7-10) constructional steels. Surf. Eng 2009, 14, 17–24. [Google Scholar]
- Database of Steel and Alloy—Marochnik. Available online: www.splav-kharkov.com/en/index.php (accessed on 2 March 2020).
- Parker, W.J.; Jenkins, R.J.; Butler, C.P.; Abbott, G.L. Flash Method of Determining Thermal Diffusivity, Heat Capacity, and Thermal Conductivity. J. Appl. Phys. 1961, 32, 1679. [Google Scholar] [CrossRef]
- Netzsch Proteus ver. 7.1 Software Manual. Available online: https://www.netzsch-thermal-analysis.com/en/products-solutions/software/proteus/ (accessed on 2 March 2020).
- Central Metal’s Portal of the Russian Federation. Available online: Metallicheckiy-portal.ru (accessed on 2 March 2020).

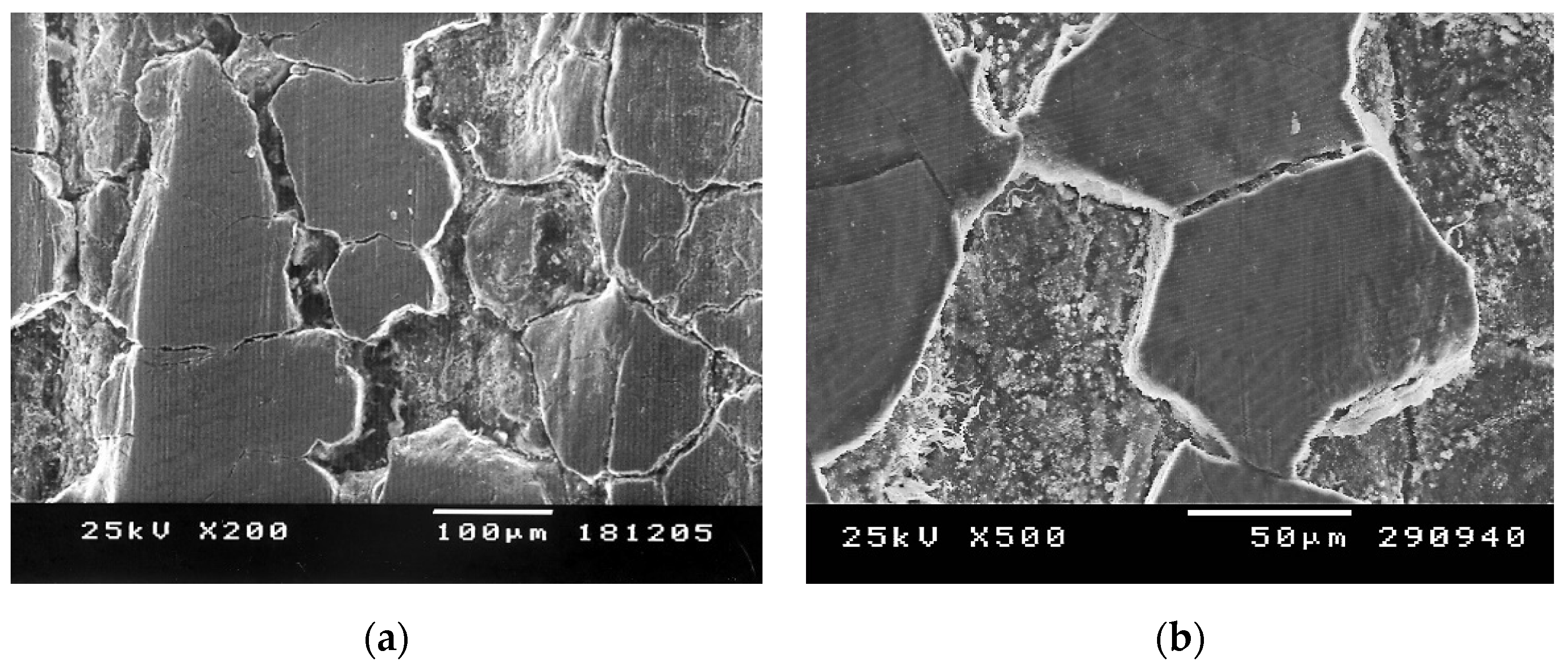


| Steel Grade | Chemical Composition, (wt. %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | Cr | Mo | Ni | Al | V | |
| Duplex | 67.99 | 0.04 | 0.33 | 1.80 | 21.83 | 3.14 | 4.45 | - | 0.11 |
| 38HMJ | 95.21 | 0.44 | 0.24 | 0.54 | 1.61 | 0.26 | 0.19 | 1.20 | - |
| 30HN2MFA | 96.42 | 0.29 | 0.26 | 0.36 | 0.65 | 0.24 | 2.21 | - | 0.23 |
| Coefficient | Value | Coefficient | Value |
|---|---|---|---|
| 5.39 × 10−1 | −1.74 × 10−7 | ||
| 3.25 × 10−4 | −0.31 × 10−9 |
| Coefficient | Value | Coefficient | Value |
|---|---|---|---|
| 4.94 × 10−1 | −1.46 × 10−8 | ||
| 1.92 × 10−4 | −0.38 × 10−9 |
| Coefficient | Value | Coefficient | Value |
|---|---|---|---|
| 3.84 × 10−1 | −1.54 × 10−6 | ||
| 9.31 × 10−4 | 9.01 × 10−10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koniorczyk, P.; Zmywaczyk, J.; Dębski, A.; Zieliński, M.; Preiskorn, M.; Sienkiewicz, J. Investigation of Thermophysical Properties of Three Barrel Steels. Metals 2020, 10, 573. https://doi.org/10.3390/met10050573
Koniorczyk P, Zmywaczyk J, Dębski A, Zieliński M, Preiskorn M, Sienkiewicz J. Investigation of Thermophysical Properties of Three Barrel Steels. Metals. 2020; 10(5):573. https://doi.org/10.3390/met10050573
Chicago/Turabian StyleKoniorczyk, Piotr, Janusz Zmywaczyk, Andrzej Dębski, Mateusz Zieliński, Marek Preiskorn, and Judyta Sienkiewicz. 2020. "Investigation of Thermophysical Properties of Three Barrel Steels" Metals 10, no. 5: 573. https://doi.org/10.3390/met10050573
APA StyleKoniorczyk, P., Zmywaczyk, J., Dębski, A., Zieliński, M., Preiskorn, M., & Sienkiewicz, J. (2020). Investigation of Thermophysical Properties of Three Barrel Steels. Metals, 10(5), 573. https://doi.org/10.3390/met10050573







